Abstract
Pterygia are common ocular surface lesions thought to originate from limbal stem cells altered by chronic UV exposure. Traditionally regarded as a degenerative condition, pterygia also display tumor-like features, such as a propensity to invade normal tissue and high recurrence rates following resection, and may coexist with secondary premalignant lesions. This study was initiated to determine the rate of concurrent ocular surface diseases in patients with pterygia recruited from the practice of a single surgeon operating in a Sydney metropolitan hospital. One hundred pterygium specimens were histopathologically reviewed and selected cases were immunohistochemically assessed to confirm diagnosis. Along with previously documented typical features including epithelial proliferation, goblet cell hyperplasia, angiogenesis, inflammation, elastosis, stromal plaques, and Bowman's membrane dissolution, we identified five cases of ocular surface squamous neoplasia, six cases of primary acquired melanosis, two compound nevi (one suspect invasive melanoma), and one dermoid-like lesion. In 18 specimens, clusters of basal epithelial cells that coexpressed cytokeratin-15/-19 and p63-α were identified at the head of the pterygium, coinciding with clinical observation of Fuchs' flecks. Our data show that significant preneoplastic lesions may be associated with pterygium and that all excised pterygia should undergo histological examination. The presence of p63-α-positive epithelial cell clusters supports the hypothesis that pterygia develop from limbal epithelial progenitors.
Pterygium is a wing-shaped ocular surface lesion traditionally described as an encroachment of bulbar conjunctiva onto the cornea.1 Historically, pterygia were considered degenerative lesions, exemplified by degradation of Bowman's layer and elastosis. Currently, however, pterygia are described as a proliferative disorder resembling an aberrant wound healing response.2 Histopathologically, pterygia are characterized by a hyperplastic, centripetally directed growth of altered limbal epithelial cells accompanied by Bowman's layer dissolution, epithelial-mesenchymal transition, and an activated fibroblastic stroma with inflammation, neovascularization, and matrix remodeling, mediated through the concerted actions of cytokines, growth factors, and matrix metalloproteinases.2–9 Despite advances in understanding of its pathogenesis, pterygium remains an ophthalmic enigma. Intriguingly, pterygia have a predilection for the nasal limbus and affect only humans, possibly reflecting the unique ocular morphology of humans, compared with nonhuman primates and other animals.10 Although there is no consensus regarding the pathogenesis of pterygia, epidemiological evidence,11–14 its association with sun-related disorders such as pinguecula and cataracts,15 climatic droplet keratopathy,16 and squamous cell and basal cell carcinomas,17,18 together with our in vitro studies,5–8 support the concept that UV radiation plays a major role in development of pterygium.19 Furthermore, the limbal predilection may be explained by the phenomenon of peripheral light focusing, in which incidental light passes through the anterior chamber and is focused at the distal (nasal) limbus where limbal stem cells (LSCs) reside.20
A healthy corneal surface is maintained by self-renewing, lineage-specific stem cells (SCs) that reside in the limbus, a narrow annular transition zone that circumscribes the cornea. This regenerative capacity is regulated by exquisite programs that govern stem cell quiescence, proliferation, migration, and differentiation. Failure to maintain a normal microenvironment as a result of extrinsic (eg, UV radiation) or intrinsic (eg, cytokines) signals can result in the development of ocular disorders.2–6,19,21,22 The importance of an intact limbus and its stem cells was recognized four decades ago by Davanger and Evensen,23 who proposed that pterygia represent a specific zone of LSC deficiency. Our hypothesis for pterygium development takes into account peripheral light focusing2,9,19,20 at the nasal limbus, which activates and/or mutates LSCs, resulting in clonal expansion, local cell proliferation, and invasion into the cornea (Figure 1A). Alternatively, focal UV radiation may destroy the LSC repository, which acts as a barrier that segregates cornea from conjunctiva, thereby opening the flood gates for conjunctival ingress and pterygium formation. Furthermore, an intrinsic weakness in the LSC reserves is implied by less prominent limbal palisades in the nasal and temporal limbus,24,25 suggesting that these regions might be more susceptible to damage and less likely to undergo effective repair. An analogous mechanism may occur in patients with total LSC deficiency,26 in which the absence of LSCs allows conjunctival invasion of the cornea to occur from 360 degrees (Figure 1B). In support of this posit, consecutive rounds of limbal excision affected wound healing, encouraged neovascularization, and promoted conjunctival ingress in rabbit corneas.27
Figure 1.
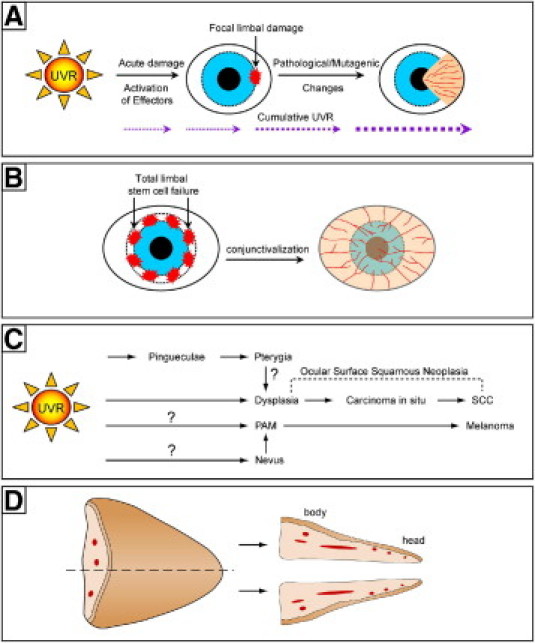
The role of cumulative UV radiation exposure in pterygium development. A: Model for the pathogenesis of pterygium: focal limbal damage from UV radiation triggers migration of altered LSCs toward the central cornea. B: In total LSC deficiency, damage to the limbal niche or depletion of stem cell reserves results in conjunctivalization of the cornea from all directions. C: Model of how ocular surface squamous neoplasia and melanoma might arise from pterygia. Question marks with pathways indicate absence of direct supporting clinical or experimental evidence. D: Bisection and orientation of pterygium specimens as assessed in the current study.
Ophthalmologists have traditionally regarded pterygia as benign lesions, because they grow slowly. Unless a pterygium is sufficiently large as to obscure the visual axis or causes astigmatism, decisions to treat are often based on a patient's cosmetic concerns. An argument against this view, however, is the local invasiveness and high rate of recurrence when pterygia are inappropriately managed.28 Current management strategies for pterygia involve surgical excision, followed by wound closure with grafts or by application of adjunctive therapy to the bare scleral bed.29,30 Once excised, pterygia are commonly discarded without histological evaluation. This practice is not recommended, in the face of reported identification of unsuspected and potentially malignant secondary disorders in association with pterygia31–33 (Table 1). These studies suggest that pterygia might have the propensity to evolve into precursors of squamous cell carcinoma and malignant melanoma of the ocular surface (Figure 1C).
Table 1.
Premalignant Ocular Disease Reported in Association with Pterygium and Pinguecula
| References | Study population | Tissues | Sample size | Associated ocular disease | % |
|---|---|---|---|---|---|
| Sevel and Sealy31 | Cape Town, South Africa | pterygia | n = 100 | squamous cell carcinoma | 12 |
| carcinoma in situ | 17 | ||||
| Clear et al17 | Malawi | pinguecula and pterygia | n = 167 | hyperplasia or mild dysplasia | 75.4 |
| moderate dysplasia | 11.4 | ||||
| carcinoma in situ | 12.6 | ||||
| Erie et al34 | Mayo Clinic, Minnesota | pterygia | n = 92 | carcinoma in situ | 9.8 |
| Perra et al33 | Ecuador | pterygia | n = 80 | PAM without atypia | 6.3 |
| PAM with atypia | 2.5 | ||||
| nevi | 2.5 | ||||
| Hirst et al32 | Queensland, Australia | pterygia | n = 533 | OSSN | 9.8 |
PAM, primary acquired melanosis; OSSN, ocular surface squamous neoplasia.
In this study, we examined the histopathology of pterygia from patients treated by a single surgeon operating in a metropolitan hospital in Sydney, Australia. Histological features of pterygia and concurrent ocular diseases were recorded. All unusual cases were reviewed by an experienced anatomical pathologist and were further investigated by immunohistochemical methods. Additionally, we describe novel cell clusters in some pterygia that expressed putative LSC markers. These cell clusters may provide the first histological evidence supporting the view that pterygium is a disease of stem cell origin.
Materials and Methods
Patients
Patients undergoing routine pterygium excision surgery by a single surgeon (M.T.C.) were recruited from Prince of Wales Hospital, Randwick, Sydney, Australia, and from the surgeon's private practice. Clinicodemographic features recorded included patient age and sex and location (nasal or temporal) and type of lesion (primary or recurrent) (Table 2). The study population was of mixed ethnic background, but the majority of patients were of European continental origin. Patients underwent routine ophthalmic examination and documentation of the pterygium, including anterior segment photography (iPIX camera; Designs For Vision, Ronkonkoma, NY) and in vivo confocal microscopy (HRT 3 Rostock cornea module; Heidelberg Engineering, Heidelberg, Germany). There were no clinical signs of dysplasia in any of the patients, although one patient demonstrated obvious pigmentation in the head of his pterygium. All patients underwent pterygium excision with reconstruction of the resulting wound using an autologous free limbal-conjunctival graft.35 Informed consent was obtained from each patient before tissue and data collection. This study was approved by the institutional Human Research Ethics Committee and adheres to the tenets of the Declaration of Helsinki.
Table 2.
Clinicodemographic Data for 100 Patients with Pterygia
| Variable | Value |
|---|---|
| Age (years) | |
| Mean ± standard deviation | 50 ± 15 |
| Range | 21–83 |
| Sex (no. of cases) | |
| Male | 62⁎ |
| Female | 35⁎ |
| Eye (no. of cases) | |
| Right | 45 |
| Left | 55 |
| Location of lesion (no. of cases) | |
| Nasal | 96 |
| Temporal | 4† |
| Type of lesion (no. of cases) | |
| Primary | 59 |
| Recurrent | 41 |
Two females and one male had bilateral disease and pterygium surgery on separate occasions.
All temporal pterygia were recurrent.
Histopathological Evaluation
Pterygia (n = 100) were FFPE and oriented such that sections were cut longitudinally through the head and the body of the pterygium (Figure 1D). Sections (4 μm) were stained with H&E, then evaluated by two experienced senior ocular scientists (J.C. and N.D.). Unusual, suspect, and atypical cases were reviewed by a pathologist (R.C.) to provide a histopathological diagnosis.
Immunohistochemistry
Pterygia with atypical features were investigated further by immunohistochemistry. Tissues were stained with putative markers for melanocytes, LSCs, or cytokeratins (Table 3). Briefly, 4-μm paraffin sections were dewaxed in xylene and rehydrated through a graded series of ethanol baths. Sections were subjected to antigen retrieval by heating in a microwave oven in 0.1 mol/L sodium citrate buffer (pH 6.0), followed by incubation in 3% H2O2 in methanol to block endogenous peroxidase activity. After blocking in 20% normal goat serum in Tris-buffered saline (pH 7.6) for 30 minutes, sections were incubated overnight in primary antibody (Table 3) at 4°C. Tissues were next incubated in biotinylated goat anti-rabbit or goat anti-mouse IgG (1:200 dilution; Dako, Glostrup, Denmark) for 30 minutes, followed by streptavidin-conjugated horseradish peroxidase (1:100 dilution; Dako) for 1 hour at room temperature. Sections were thoroughly washed with Tris-buffered saline between each step. Immunoreactivity was visualized using 3-amino-9-ethylcarbazole (AEC; Sigma-Aldrich, St. Louis, MO), and nuclei were counterstained with Mayer's hematoxylin (Dako). Sections were mounted in aqueous mounting medium (Crystal Mount; Biomeda Corporation, Foster City, CA), then coverslipped in DPX mounting medium (VWR International, Poole, UK). For double-labeling, tissues were incubated in a mixture of primary antibodies (Table 3), followed by incubations in goat anti-mouseAlexa488 and goat anti-rabbitAlexa594 (Invitrogen, Carlsbad, CA) and counterstained with DAPI (0.3 μmol/L final). Sections were coverslipped in Vectashield anti-fade mounting medium (Vector Laboratories, Burlingame, CA), then imaged. Negative control reactions included tissues that were incubated with an isotype antibody instead of an epitope-specific primary antibody. Photomicrographs were taken with a DP70 digital camera system mounted on an Olympus BX51 microscope (Olympus; Sydney, Australia) and processed with Photoshop version 9 (Adobe Systems, San Jose, CA).
Table 3.
Primary Antibodies Used for Immunohistochemistry
| Targeted epitope | Antibody type | Host⁎ | Clone | Manufacturer† | Catalog no. | Dilution |
|---|---|---|---|---|---|---|
| Human melanosome | IgG1, κ | M | HMB-45 | DAKO | M0634 | 1:50 |
| Melan A | IgG1, κ | M | A103 | DAKO | M7196 | 1:100 |
| S100B | IgG | R | — | DAKO | Z0311 | 1:900 |
| P63 (pan) | IgG2a | M | 4A4 | DAKO | M7247 | 1:50 |
| P63-alpha | IgG | R | — | CST | 4892 | 1:20 |
| Keratin-15 | IgG2a | M | MS-1068 | TFS | LHK15 | 1:150 |
| Keratin-19 | IgG1 | M | 4A36 | USB | C9097-24B | 1:150 |
| Ki-67 | IgG | R | — | TFS | RB-1510R7 | 1:200 |
| Connexin 43 | IgG1, κ | M | CX-1B1 | Zymed | 13-8300 | 1:100 |
| Isotype | IgG1 | M | — | DAKO | X0931 | 1:100 |
| Isotype | IgG | R | — | DAKO | X0903 | 1:900 |
M, mouse; R, rabbit.
DAKO, DakoCytomation; CST, Cell Signaling Technology; TFS, Thermo Fisher Scientific; USB, United States Biological; Zymed, Zymed Laboratories.
Results
Typical and Common Histopathological Findings in Pterygia
Common histological features observed included a proliferative and locally invasive front of pterygium epithelium that abruptly transitioned into corneal epithelium at the advancing edge (Figure 2A). At the junction between the pterygium epithelium and normal cornea, the stroma was often characterized by feeder blood vessels (Figure 2A, asterisk) that preceded the fibroblastic stroma. The advancing pterygium edge was demarcated by a fragmented Bowman's layer (Figure 2A, arrows). Goblet cell hyperplasia was prominent in pterygium epithelium (Figure 2B), compared with autologous normal conjunctiva (Figure 2C). Feeder vessels extending the length of the lesion were regularly noted (Figure 2D), as well as subepithelial neovascularization (Figure 2D, inset). Stromal elastosis (Figure 2E, double asterisk) and both intra- and subepithelial (Figure 2F) and intravascular inflammation were present in 60% of cases.
Figure 2.
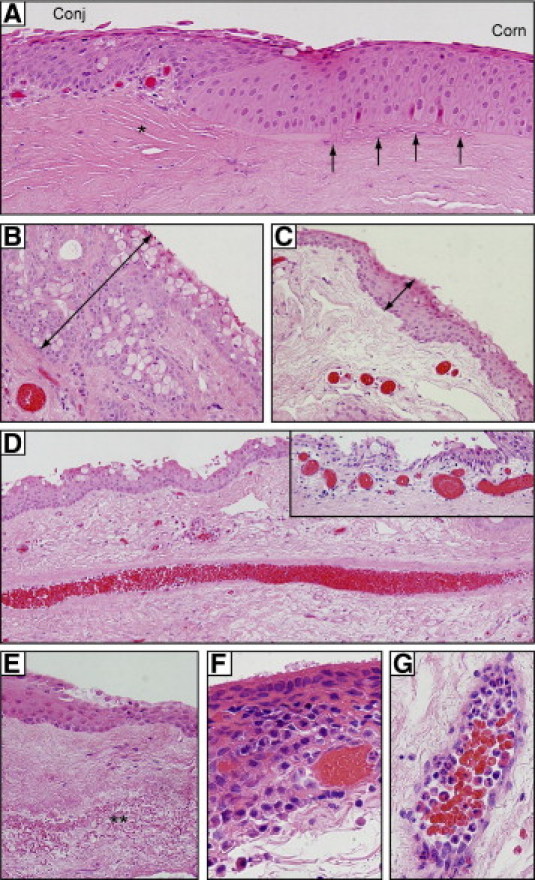
Typical histological features of pterygia. A: In the advancing head of a pterygium, conjunctival-like epithelium (Conj) merges abruptly into corneal epithelium (Corn). The underlying Bowman's layer (arrows) is fragmented and precedes a fibrovascular stroma (asterisk). B, C: Goblet cell hyperplasia is apparent in pterygium (B), compared with donor-matched conjunctiva (C). Note the thickness of the epithelial layer (double-headed arrows in B and C). D: Prominent central feeder vessel; inset shows dilated subepithelial vessels. E: Elastotic changes (double asterisk) in pterygium stroma. F: Inflammatory infiltrates in the epithelium. G: Stromal vessels loaded with polymorphonuclear leukocytes. All sections were stained with H&E. Original magnification: ×200 (A and D); ×400 (B, C, D inset, and E); ×1000 oil emersion (F and G).
Uncommon and Novel Histopathological Findings in Pterygia
Uncommon histological features included basophilic stromal plaques within the pterygium body in 6% of cases (Table 4) that localized to elastotic zones (Figure 3, arrows). These plaques varied in size and shape, and were generally lilac in color after H&E staining (Figure 3, A and B, arrows) or dark blue when stained with phosphotungstic acid (Figure 3C, asterisk). However, we could not identify their composition further with other histological stains, including tetrachrome, Safranin O, von Kossa stain, or alizarin crimson (data not shown).
Table 4.
Histological Findings in 100 Cases of Pterygia
| Pterygia (no.) |
|||
|---|---|---|---|
| Histological findings | Primary (n = 59) | Recurrent (n = 41) | Total |
| PAM without atypia | 4 | 1 | 5 |
| PAM with atypia with a subconjunctival nevus | 1 | 0 | 1 |
| Epidermolysis bullosa nevus | 0 | 1 | 1 |
| OSSN | 3 | 2 | 5 |
| Dermoid–like lesion | 0 | 1 | 1 |
| Plaques | 4 | 2 | 6 |
| Basal stem cell–like clusters | 8 | 10 | 18 |
PAM, primary acquired melanosis; OSSN, ocular surface squamous neoplasia.
Figure 3.
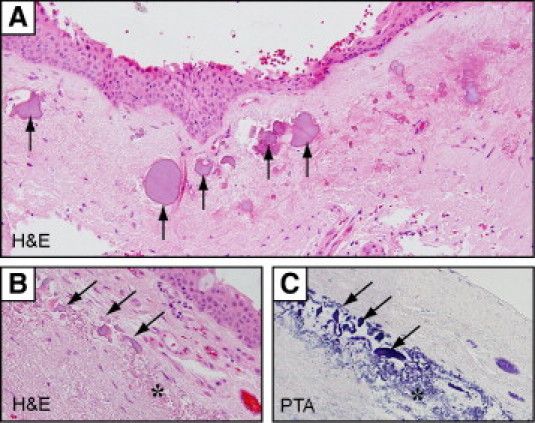
Stromal plaques in pterygia stained with H&E (A and B) or phosphotungstic acid (PTA) (C). Irregular-shaped stromal plaques (arrows) with an amorphous appearance were frequently associated with elastotic changes (asterisk). Plaques appear lilac in H&E-stained sections and deep blue in PTA-stained sections. Elastin fibers also stained blue with PTA. Original magnification: ×200 (A); ×600 (B and C).
Small clusters of basal cells were observed in 18% of our pterygium specimens. Cells within these aggregates were smaller, had increased nuclear-to-cytoplasm ratio, consisted of 8 to 15 cells anchored to the basement membrane, and were invariably associated with corneal-like epithelium near the head of the pterygium (Figure 4A, ovals). Morphologically, these cells appeared primitive and less differentiated than their suprabasal counterparts, suggesting that they may be stem cell like. Such observations prompted us to partially phenotype these cells by immunostaining, using well-accepted markers of LSCs. Indeed, cells within these microclusters demonstrated immunoreactivity to CK-15, CK-19, p63 (pan), and p63α (Figure 4, C, D, F, and G, respectively) and were double-immunoreactive to CK-15/p63α (Figure 4H) and CK-19/p63α (not shown), but lacked immunoreactivity to Ki-67 (proliferation marker) (Figure 4E) or Cx43 (gap junction protein) (data not shown). Our findings suggest that, although these cells are not proliferating (lacked Ki-67 expression), they retain proliferative potential (strong p63α expression) and may become activated when appropriate signaling mechanisms are initiated during pterygium development. These cell clusters were documented by Ernst Fuchs36 more than a century ago in his seminal article “Ueber das Pterygium” at both a microscopic (Figure 4B) and macroscopic level (Figure 5A) as small spots or flecks at the head of pterygia. Commonly known as Fuchs' flecks (or Fuchs' patches, Fuchs' islets), these pterygium cell clusters could be visualized in our patients by slit-lamp examination (Figure 5, B and C) and by confocal microscopy (Figure 5, D and E).
Figure 4.
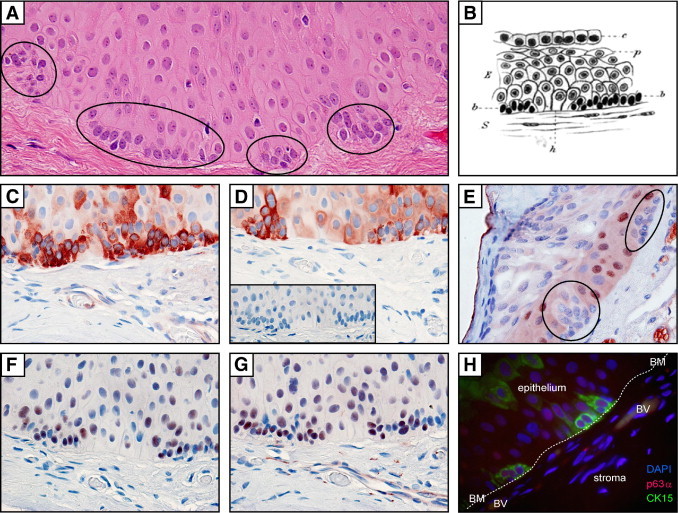
Stem cell microclusters in pterygium tissue. A: H&E-stained section of a pterygium illustrating multiple mini-aggregates of basal epithelial cells (ovals). B: Pterygium epithelium as illustrated by Ernst Fuchs36 in 1892. C–G: Pterygium immunolabeled with CK-15, CK-19, Ki-67, p63 (pan), and p63α (C–G, respectively), using an indirect immunoperoxidase method with 3-amino-9-ethylcarbazole chromogen; red denotes positive labeling. Staining is absent in sections incubated with control IgG (D, inset). H: Indirect immunofluorescent double-labeling of pterygium epithelial cell clusters with CK-15 (green), p63α (red), and counterstained with DAPI (blue); note basement membrane (BM, indicated by dotted line) and blood vessels (BV). Original magnification, ×1000 oil emersion (all photomicrographs). Image B is reproduced with permission from Springer (original publication: Fuchs E. Ueber der Pterygium. Graefes Archiv Ophthalmol 1892, 38:1–89).
Figure 5.
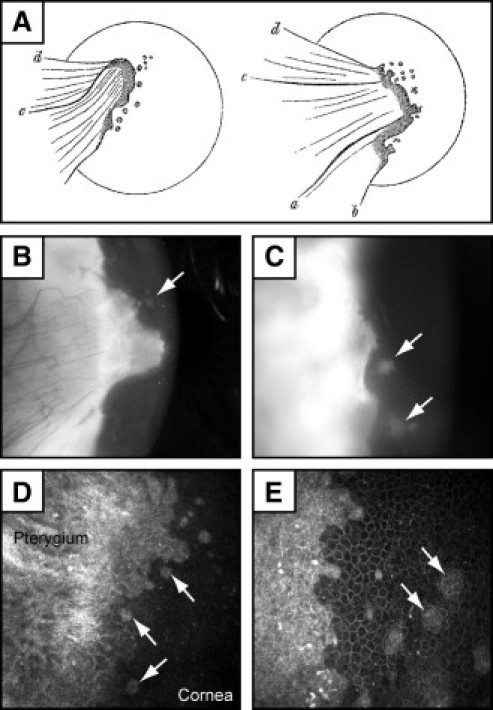
Clinical appearance of Fuchs' flecks in pterygia. A: Original illustrations by Ernst Fuchs 36 show small spots or fleckchen in the cap region at the head of pterygia. B–E: Pterygia with Fuchs' flecks (arrows) under slit lamp (B and C) and in vivo confocal microscopy (D and E). Slit lamp photographs were taken with an iPIX camera (Designs For Vision, Ronkonkoma, NY) and confocal micrographs with an HRT 3 Rostock cornea module (Heidelberg Engineering, Heidelberg, Germany). Confocal images were taken with a 63× objective. The field of view is 400µm × 400µm for (D) and 300µm × 300µm for (E). Image A is reproduced with permission from Springer (original publication: Fuchs E. Ueber der Pterygium. Graefes Archiv Ophthalmol 1892, 38:1–89).
Atypical Histopathological Findings in Pterygia
Atypical epithelial and melanocytic lesions were identified in 12% of pterygia (Figure 6 and Table 4). Rete ridge-like down-growths were occasionally noted in the hyperplastic epithelium (Figure 6A). Foci of epithelial dysplasia ranging from mild to moderate to severe (Figure 6, B, C, and D, respectively) were observed in pterygium epithelium, where cells displayed increased nuclear-to-cytoplasmic ratio and loss of polarity affecting the basal (Figure 6B), the suprabasal (Figure 6C), or the entire epithelium (Figure 6D). Nonetheless, the metastatic potential of these lesions is low, given that they do not breach the basement membrane (Figure 6, A–D).
Figure 6.
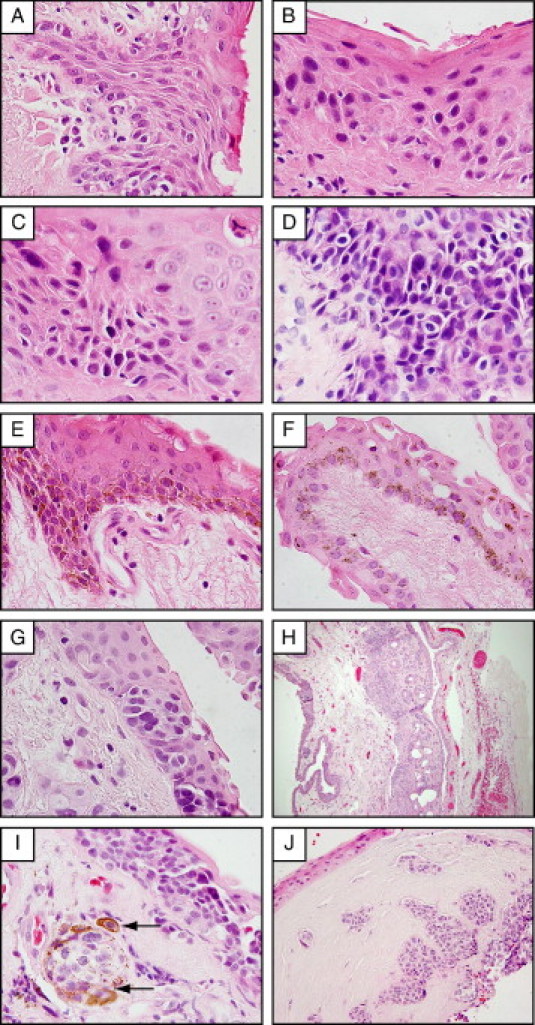
Atypical histological features in pterygia included pterygium epithelium demonstrating papillary folding (A) and dysplastic changes ranging from mild (B) to moderate (C) to severe (D). Melanocytic lesions included racial pigmentation (note layers of pigmented basal melanocytes) (E), PAM without atypia (F), PAM with atypia (G), subconjunctival nonpigmented compound nevus (H), and a nonpigmented nevus (I) from a patient with epidermolysis bullosa that invaded the corneal stroma (J). Arrows in I point to melanophages that have acquied pigmented granules and surround a melanocytic nest. Sections were assessed after H&E staining. Original magnification: ×1000 oil emersion (A–G and I); ×100 (H); ×400 (J).
Melanocytic lesions were identified in 7% of our samples, which included racial pigmentation (7%) (Figure 6E), primary acquired melanosis (PAM) without (5%) and with atypia (1%) (Figure 6, F and G, respectively), and two cases of conjunctival nevi (Figure 6, H–J). One nevus extended from the pterygium body into the corneal stroma, with well-circumscribed nonpigmented melanocytic nests found at the surgical margin (Figure 6, I and H). Nonpigmented nevi may be difficult to detect clinically, especially if masked by an inflamed pterygium. CK-19 staining was therefore used to demarcate pterygium epithelium, distinct from the underlying symmetrical nevus (Figure 7A). Under higher magnification, CK-19-negative melanocytes were identified as uniform, rounded, occasionally displaying hyperchromatic nuclei, and generally confined to cohesive epithelioid nevocytic nests without pagetoid spread (Figure 7C). When samples were labeled with melanocytic marker S100B, stromal-epithelial junctional involvement became more obvious (Figure 7, B and D). Similar staining patterns were observed with other melanocytic markers, such as melan A and HMB-45 (data not shown). S100B-positive cells were also detected within the pterygium epithelium; however, in contrast to the epithelioid melanocytes within the nevus, these intraepithelial cells were dendritiform in morphology (Figure 7E). The second nevus displayed cell invasion of the epithelium (pagetoid spread; not shown) with prominent and lateral nonpigmented melanocytic spread. This specimen, although diagnosed as a benign lesion, was viewed as a suspect invasive melanoma, because of the spread of S100B-positive melanocytic nests from the main disease foci within the conjunctiva into the corneal stroma (Figure 7F).
Figure 7.
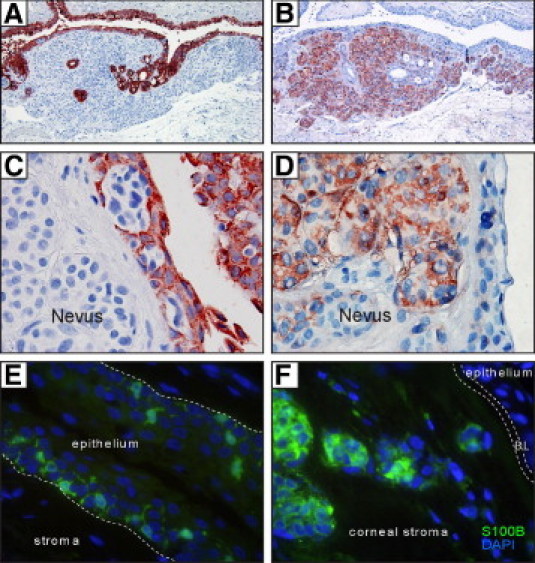
Cytokeratin and S100B staining of nevi in pterygia. Indirect immunoperoxidase (A–D) and immunofluorescence (E and F) techniques demonstrate CK-19 immunoreactivity in pterygium epithelium (A and C) or S100B immunoreactivity in nevus melanocytes (B, D, and F) and in dendritiform cells scattered within the epithelium (E). In panels A–D, positive labeling is denoted by red color (from 3-amino-9-ethylcarbazole chromogen) and nuclei counterstaining in blue (hematoxylin). In panels E and F, S100B expression is denoted by green immunofluorescence and DAPI counterstaining in blue. In E, the hatched line indicates the pterygium basement membrane; in F, it indicates Bowman's layer (BL). Original magnification: ×100 (A and B); ×1000 oil emersion (C–F).
Last, we identified cutaneous features within a recurrent pterygium from a 67-year-old man (Figure 8). This unusual wing-shaped lesion had the macroscopic appearance of a typical pterygium, except that hairs were visibly growing out of the body (Figure 8A). On microscopy, we observed a nonkeratinizing squamous epithelium with goblet cells adjacent to well-differentiated cutaneous elements such as hair follicles and sebaceous and sweat glands (Figure 8, B–D). These features are reminiscent of limbal dermoids,37 but this patient had no history of such lesions before he developed a pterygium.
Figure 8.
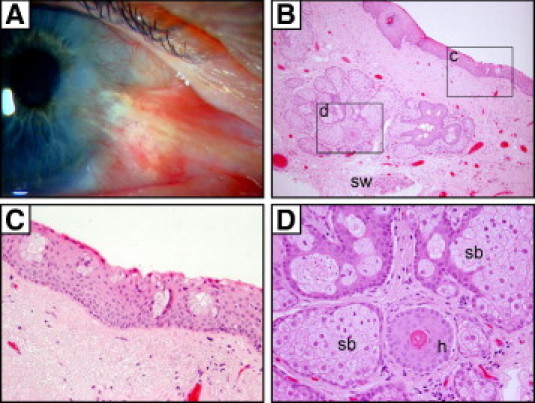
Cutaneous elements in a recurrent pterygium. Clinical image of a pterygium with hairs growing from the body (A) and its corresponding H&E-stained paraffin sections (B–D). The regions encompassed by the rectangles (c and d) in panel B are magnified in panels C and D, respectively. Goblet cell clusters within the conjunctival portion of the pterygium (C) overlie cutaneous elements (D) such as sebaceous glands (sb), hair follicles (h), and sweat glands (sw). Original magnification: ×100 (B); ×400 (C and D).
Discussion
By standardizing the orientation of our specimens (Figure 1D), we identified several previously documented common, uncommon, and novel histopathological features in pterygia. Common findings included a prominent migratory front of actively proliferating and locally invasive epithelium with evidence of Bowman's layer dissolution (Figure 2), which we have previously shown to be mediated by the activity of UV-induced matrix metalloproteinase.3,6 Other features included a reactive fibrovascular stroma with evidence of epithelial-mesenchymal transition,38 elastosis, and intravascular, subepithelial, and intraepithelial leukocyte infiltration (Figure 2), likely mediated through UV-induced cytokines and growth factors.2,9 As in previous studies, leukocyte infiltrates in our pterygium specimens were neutrophils, T cells, plasma cells, macrophages, and mast cells.39,40 The stroma comprising an altered extracellular matrix has also been studied and shown to consist of excess deposits of collagens, heparin sulfates, versican, laminin, and fibronectin.41,42 In addition, we also observed foci of amorphous basophilic material in some pterygia, which were frequently associated with elastotic changes within the stroma. Plaques and associated elastotic changes appeared blue after treatment with phosphotungstic acid, suggesting that they may have a similar chemical composition (Figure 3C). Although these deposits have been documented by others,43,44 their composition and precisely how they form are still unknown. Stromal plaques have also been described in climatic droplet keratopathy,45 and it has been hypothesized that these plaques may be derived from UV-denatured plasma constituents.46 Proteomic analysis of stromal plaques found in climatic droplet keratopathy revealed that they were rich in annexin A2 and glyceraldehyde 3-dehydrogenase.47 We can only speculate that the stromal plaques in pterygia may be similar in composition to those reported in climatic droplet keratopathy, given that both diseases are strongly associated with UV exposure.16 One reason for their presence in only a proportion of specimens might be related to the stage of pterygium development and amount of cumulative UV exposure.
Controversy also surrounds the origins of the osmophilic elastoid bands within the pterygium substantia propria. Although it was originally described as actinic elastosis (elastotic degeneration of collagen) through the actions of UV radiation, because similar changes have been described in skin exposed to solar radiation,48 Austin et al44 now claim that elastodysplasia plays a role through excess production of elastin derived from UV-activated or injured fibroblasts. Evidence to support this theory was presented by Wang et al,49 who detected mutations in the 3′-untranslated region of tropoelastin in pingueculae and UV-irradiated conjunctival fibroblasts.
The concept that pterygia arise from LSCs has been a topic of discussion for decades. In a seminal article in 1971, Davanger and Evensen23 concluded that “a failure in the limbal structure may be the cause of pterygia.” Some 20 years later, Tseng et al26 speculated that pterygia represent a focal zone of LSC dysfunction (Figure 1, A and B). Our model of chronic focal UV radiation to the limbal margin50 provides an explanation for how LSCs and their niche might be damaged, and Dushku et al51 postulated that pterygia arise from altered limbal basal epithelial cells that took on a migratory phenotype.
More than a century ago, Fuchs36 identified small clusters of conjunctival-like basal cells dispersed among the corneal-like epithelium in pterygia (Figure 4B). Such islands of pterygium epithelial cells were also reported in our previous work.9 In the present study, we further characterized these small, morphologically primitive cells as resembling LSCs through their coexpression of CK-15, CK-19, and p63α (Figure 4H) and localized them to the advancing head of the pterygium. In location and size, they are similar to Fuchs' flecks,36 which can be visualized by slit lamp and by in vivo confocal imaging (Figure 5). Our observations support the notion that pterygium is a disease of LSCs, and the presence of these cell clusters may account for the clinical behavior of pterygium. We hypothesize that these primitive cell clusters may be activated to proliferate, forming an invasive pterygium (Figure 1A).
It is unclear why pterygium epithelial cell clusters are present in only ∼20% of our specimens, but we suspect that i) some may have escaped detection, because their small size makes them difficult to identify without serial sectioning, or that ii) their presence may be associated with actively growing pterygia, but it is our normal practice to resect lesions when they are quiescent. Further studies are also required to establish if pterygium epithelial cell clusters are functionally, phenotypically, or genetically altered as a result of UV radiation.
Finally, within a clinically apparent pterygium, we found cutaneous elements (hair follicles, sebaceous glands, and sweat glands) adjacent to goblet cells in pterygium epithelium (Figure 7). This unusual finding supports the notion that stem cells within the limbus might retain the capacity to differentiate into skin-like tissue. The precise mechanism for this reprogramming is unclear, but we speculate that UV radiation may be the trigger, because we have recently noted the development of cutaneous elements in corneas of UV-irradiated mice (unpublished observation). Alternatively, these cutaneous elements might have originated from the caruncle, which is known to contain hairs and sebaceous and sweat glands, as well as lacrimal glands and goblet cells.52
Ocular surface squamous neoplasia (OSSN) is a spectrum of diseases that ranges from epithelial dysplasia to carcinoma in situ to squamous cell carcinoma.53,54 It is commonly found in elderly men with a history of chronic UV exposure.55 Increased incidence of OSSN has also been reported in HIV-positive individuals, with OSSN showing a female predominance and sometimes presenting at a younger age.56,57 As with pterygia, UV exposure is thought to contribute to the pathogenesis of OSSN.55,58 We observed mild to severe epithelial dysplasia in 5% of our pterygia, but no carcinoma in situ or squamous cell carcinoma. This is in contrast to studies from Malawi and South Africa,17,31 where >29% of pterygia had concurrent OSSN (Table 1), leading to suggestions that tumors may arise from preexisting lesions (such as pterygia). Recently, Hirst et al32 noted the full spectrum of OSSN in ∼10% of their pterygia from Brisbane, Australia (approximately double the rate of our Sydney cohort). One possible explanation for this disparity within an Australian population is greater UV exposure in Brisbane, compared with Sydney, because Brisbane is closer to the equator (latitude 27°28′ S versus 33°51′ S). Increased UV exposure may also explain higher OSSN rates in pterygia in African populations, but racial factors (continental origin) and concurrent HIV infections might also play a role. The relationship between pterygia and neoplasms is not completely understood, but UV-induced mutations in tumor suppressor genes may play a role.19 The tumor suppressor protein p53 is responsible for regulating cell-cycle arrest59 and is hypothesized to be mutated in pterygia, based on its overexpression in pterygium tissues.60–62 In OSSN, p53 is similarly overexpressed,63 and signature UV-induced mutations (CC to TT transitions) have been detected in these lesions.64 Whether p53 mutations are present within our newly identified pterygium cell clusters remains to be investigated. In one study, no TP53 mutations were observed in pterygium specimens after laser-capture microdissection of the entire epithelium.65
Primary acquired melanosis of the conjunctiva is a clinical classification of melanocytic lesions that falls between nevus and invasive melanoma. PAM occurs predominantly in middle-aged persons of European continental origin, but can be present in patients as young as 15 years of age.66 PAM is further grouped into lesions with and without cytological atypia, and it may account for 11% of all conjunctival tumors.67,68 Histological grading of PAM is important in its management, because PAM with atypia progresses to melanoma,66 and lesions with epithelioid features have been associated with invasion and metastasis,68 but PAM without atypia do not progress to melanoma.66 In the present series of 100 pterygia, we observed seven cases of racial pigmentation, six cases of PAM (with and without atypia), and two cases of nevi (one patient had a history of cutaneous melanoma; the other had epidermolysis bullosa). These frequencies are comparable to those in a study by Perra et al,33 who reported seven cases of PAM and two cases of nevi in a series of 80 pterygia. These numbers, although small, are of concern for the ophthalmologist, because PAM with atypia is considered a premalignant condition and 13% to 50% of PAM cases may progress to melanoma,66,69 a potentially fatal disease if undetected. Shields et al67 reported that 13% of patients with PAM had bilateral disease. In PAM with atypia, the mean interval for melanoma development is 39 months.66 Therefore, close monitoring by clinicians with longer follow-up periods are required in patients with pterygia in which PAM with atypia has been identified.
Amelanotic melanoma and PAM of the conjunctiva are rare, but cases have been reported.70,71 The nevi in our case series were not pigmented. These lesions are clinically and histologically difficult to diagnose. Immunohistochemical markers such as S100B, HMB-45, and melan A may help identify and differentiate between benign and malignant melanocytic lesions. In particular, HMB-45-positive staining is reported to be associated with atypia and is recommended for differentiating between PAM with and without atypia.72,73 The Wilms tumor gene74 and Ki-67 staining73 may be used to distinguish between benign and malignant nevi. In our series, all melanocytes labeled intensely with S100B, whereas the other melanocytic markers, HMB-45 and melan A, had a similar distribution but with less intense staining. The nevus associated with epidermolysis bullosa was faintly immunoreactive to HMB-45, but did not stain for Ki-67 (data not shown), suggesting that it might be a benign nevus. Nonetheless, long-term follow-up is required given its location at the surgical margin within the corneal stroma.
In summary, we have provided evidence that pterygium is a disease of stem cells in which clusters of pterygium epithelial cells expressing putative limbal stem cell markers correspond to Fuchs' flecks at the head of the pterygium. Our study also showed that preneoplastic diseases (such as PAM with atypia and OSSN) may coexist with pterygia, and we speculate that cumulative genetic damage from chronic UV exposure may be a shared etiology between these conditions. These preneoplastic conditions could remain undiagnosed if the excised pterygium is discarded, and we recommend that all pterygia be subjected to thorough histological evaluation. Incomplete excision of PAM with atypia or OSSN is of concern, given the potential for transformation and recurrence.66,75 In such cases, use of topical chemotherapeutic agents,76 such as interferon α-2b alone77 or in combination with retinoic acid,78 is proving to be highly efficacious and may be useful in the treatment of residual disease. Annual follow-up for the remainder of the patient's life is advisable.66,75
Footnotes
Supported by a Career Development award (no. 455358, to N.D.) from the National Health and Medical Research Council of Australia.
References
- 1.Duke-Elder S., editor. Diseases of the Outer Eye Part 1: System of Ophthalmology 8. Kimpton; London: 1965. pp. 569–585. [Google Scholar]
- 2.Di Girolamo N., Chui J., Coroneo M.T., Wakefield D. Pathogenesis of pterygia: role of cytokines, growth factors, and matrix metalloproteinases. Prog Retin Eye Res. 2004;23:195–228. doi: 10.1016/j.preteyeres.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Di Girolamo N., McCluskey P., Lloyd A., Coroneo M.T., Wakefield D. Expression of MMPs and TIMPs in human pterygia and cultured pterygium epithelial cells. Invest Ophthalmol Vis Sci. 2000;41:671–679. [PubMed] [Google Scholar]
- 4.Di Girolamo N., Coroneo M.T., Wakefield D. Active matrilysin (MMP-7) in human pterygia: potential role in angiogenesis. Invest Ophthalmol Vis Sci. 2001;42:1963–1968. [PubMed] [Google Scholar]
- 5.Di Girolamo N., Kumar R.K., Coroneo M.T., Wakefield D. UVB-mediated induction of interleukin-6 and -8 in pterygia and cultured human pterygium epithelial cells. Invest Ophthalmol Vis Sci. 2002;43:3430–3437. [PubMed] [Google Scholar]
- 6.Di Girolamo N., Coroneo M.T., Wakefield D. UVB-elicited induction of MMP-1 expression in human ocular surface epithelial cells is mediated through the ERK1/2 MAPK-dependent pathway. Invest Ophthalmol Vis Sci. 2003;44:4705–4714. doi: 10.1167/iovs.03-0356. [DOI] [PubMed] [Google Scholar]
- 7.Di Girolamo N., Coroneo M., Wakefield D. Epidermal growth factor receptor signaling is partially responsible for the increased matrix metalloproteinase-1 expression in ocular epithelial cells after UVB radiation. Am J Pathol. 2005;167:489–503. doi: 10.1016/S0002-9440(10)62992-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Girolamo N., Wakefield D., Coroneo M.T. UVB-mediated induction of cytokines and growth factors in pterygium epithelial cells involves cell surface receptors and intracellular signaling. Invest Ophthalmol Vis Sci. 2006;47:2430–2437. doi: 10.1167/iovs.05-1130. [DOI] [PubMed] [Google Scholar]
- 9.Chui J., Di Girolamo N., Wakefield D., Coroneo M.T. The pathogenesis of pterygium: current concepts and their therapeutic implications. Ocul Surf. 2008;6:24–43. doi: 10.1016/s1542-0124(12)70103-9. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi H., Kohshima S. Unique morphology of the human eye. Nature. 1997;387:767–768. doi: 10.1038/42842. [DOI] [PubMed] [Google Scholar]
- 11.Moran D.J., Hollows F.C. Pterygium and ultraviolet radiation: a positive correlation. Br J Ophthalmol. 1984;68:343–346. doi: 10.1136/bjo.68.5.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Threlfall T.J., English D.R. Sun exposure and pterygium of the eye: a dose-response curve. Am J Ophthalmol. 1999;128:280–287. doi: 10.1016/s0002-9394(99)00161-0. [DOI] [PubMed] [Google Scholar]
- 13.McCarty C.A., Fu C.L., Taylor H.R. Epidemiology of pterygium in Victoria, Australia. Br J Ophthalmol. 2000;84:289–292. doi: 10.1136/bjo.84.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan C.S., Lim T.H., Koh W.P., Liew G.C., Hoh S.T., Tan C.C., Au Eong K.G. Epidemiology of pterygium on a tropical island in the Riau Archipelago. Eye. 2006;20:908–912. doi: 10.1038/sj.eye.6702046. [DOI] [PubMed] [Google Scholar]
- 15.Lim R., Mitchell P., Cumming R.G. Cataract associations with pinguecula and pterygium: the Blue Mountains Eye Study. Am J Ophthalmol. 1998;126:717–719. doi: 10.1016/s0002-9394(98)00140-8. [DOI] [PubMed] [Google Scholar]
- 16.Taylor H.R., West S.K., Rosenthal F.S., Munoz B., Newland H.S., Emmett E.A. Corneal changes associated with chronic UV irradiation. Arch Ophthalmol. 1989;107:1481–1484. doi: 10.1001/archopht.1989.01070020555039. [DOI] [PubMed] [Google Scholar]
- 17.Clear A.S., Chirambo M.C., Hutt M.S. Solar keratosis, pterygium, and squamous cell carcinoma of the conjunctiva in Malawi. Br J Ophthalmol. 1979;63:102–109. doi: 10.1136/bjo.63.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerkenezov N. A pterygium survey of the far north coast of New South Wales. Trans Ophthalmol Soc Aust. 1956;16:110–119. [PubMed] [Google Scholar]
- 19.Di Girolamo N. Signalling pathways activated by ultraviolet radiation: role in ocular and cutaneous health. Curr Pharm Des. 2010;16:1358–1375. doi: 10.2174/138161210791033923. [DOI] [PubMed] [Google Scholar]
- 20.Coroneo M.T., Muller-Stolzenburg N.W., Ho A. Peripheral light focusing by the anterior eye and the ophthalmohelioses. Ophthalmic Surg. 1991;22:705–711. [PubMed] [Google Scholar]
- 21.Di Girolamo N., Bosch M., Zamora K., Coroneo M.T., Wakefield D., Watson S.L. A contact lens-based technique for expansion and transplantation of autologous epithelial progenitors for ocular surface reconstruction. Transplantation. 2009;87:1571–1578. doi: 10.1097/TP.0b013e3181a4bbf2. [DOI] [PubMed] [Google Scholar]
- 22.Figueira E.C., Di Girolamo N., Coroneo M.T., Wakefield D. The phenotype of limbal epithelial stem cells. Invest Ophthalmol Vis Sci. 2007;48:144–156. doi: 10.1167/iovs.06-0346. [DOI] [PubMed] [Google Scholar]
- 23.Davanger M., Evensen A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature. 1971;229:560–561. doi: 10.1038/229560a0. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg M.F., Bron A.J. Limbal palisades of Vogt. Trans Am Ophthalmol Soc. 1982;80:155–171. [PMC free article] [PubMed] [Google Scholar]
- 25.Shortt A.J., Secker G.A., Munro P.M., Khaw P.T., Tuft S.J., Daniels J.T. Characterization of the limbal epithelial stem cell niche: novel imaging techniques permit in vivo observation and targeted biopsy of limbal epithelial stem cells. Stem Cells. 2007;25:1402–1409. doi: 10.1634/stemcells.2006-0580. [DOI] [PubMed] [Google Scholar]
- 26.Tseng S.C.G., Chen J.J.Y., Huang A.J.W., Kruse F.E., Maskin S.L., Tsai R.J.F. Classification of conjunctival surgeries for corneal diseases based on stem cell concept. Ophthalmol Clin North Am. 1990;3:595–610. [Google Scholar]
- 27.Huang A.J., Tseng S.C. Corneal epithelial wound healing in the absence of limbal epithelium. Invest Ophthalmol Vis Sci. 1991;32:96–105. [PubMed] [Google Scholar]
- 28.Mourits M.P., Wyrdeman H.K., Jurgenliemk-Schulz I.M., Bidlot E. Favorable long-term results of primary pterygium removal by bare sclera extirpation followed by a single 90Strontium application. Eur J Ophthalmol. 2008;18:327–331. doi: 10.1177/112067210801800301. [DOI] [PubMed] [Google Scholar]
- 29.Hirst L.W. The treatment of pterygium. Surv Ophthalmol. 2003;48:145–180. doi: 10.1016/s0039-6257(02)00463-0. [DOI] [PubMed] [Google Scholar]
- 30.Ang L.P., Chua J.L., Tan D.T. Current concepts and techniques in pterygium treatment. Curr Opin Ophthalmol. 2007;18:308–313. doi: 10.1097/ICU.0b013e3281a7ecbb. [DOI] [PubMed] [Google Scholar]
- 31.Sevel D., Sealy R. Pterygia and carcinoma of the conjunctiva. Trans Ophthalmol Soc UK. 1969;88:567–578. [PubMed] [Google Scholar]
- 32.Hirst L.W., Axelsen R.A., Schwab I. Pterygium and associated ocular surface squamous neoplasia. Arch Ophthalmol. 2009;127:31–32. doi: 10.1001/archophthalmol.2008.531. [DOI] [PubMed] [Google Scholar]
- 33.Perra M.T., Colombari R., Maxia C., Zucca I., Piras F., Corbu A., Bravo S., Scarpa A., Sirigu P. Finding of conjunctival melanocytic pigmented lesions within pterygium. Histopathology. 2006;48:387–393. doi: 10.1111/j.1365-2559.2006.02346.x. [DOI] [PubMed] [Google Scholar]
- 34.Erie J.C., Campbell R.J., Liesegang T.J. Conjunctival and corneal intraepithelial and invasive neoplasia. Ophthalmology. 1986;93:176–183. doi: 10.1016/s0161-6420(86)33764-3. [DOI] [PubMed] [Google Scholar]
- 35.Coroneo M.T. Beheading the pterygium. Ophthalmic Surg. 1992;23:691–692. [PubMed] [Google Scholar]
- 36.Fuchs E. Ueber das Pterygium [Concerning the pterygium]: German. Graefes Arch Ophthalmol. 1892;38:1–89. [Google Scholar]
- 37.Garner A. The pathology of tumours at the limbus. Eye (Lond) 1989;3:210–217. doi: 10.1038/eye.1989.30. [DOI] [PubMed] [Google Scholar]
- 38.Kato N., Shimmura S., Kawakita T., Miyashita H., Ogawa Y., Yoshida S., Higa K., Okano H., Tsubota K. Beta-catenin activation and epithelial-mesenchymal transition in the pathogenesis of pterygium. Invest Ophthalmol Vis Sci. 2007;48:1511–1517. doi: 10.1167/iovs.06-1060. [DOI] [PubMed] [Google Scholar]
- 39.Perra M.T., Maxia C., Zucca I., Piras F., Sirigu P. Immunohistochemical study of human pterygium. Histol Histopathol. 2002;17:139–149. doi: 10.14670/HH-17.139. [DOI] [PubMed] [Google Scholar]
- 40.Ribatti D., Nico B., Maxia C., Longo V., Murtas D., Mangieri D., Perra M.T., De Giorgis M., Piras F., Crivellato E., Sirigu P. Neovascularization and mast cells with tryptase activity increase simultaneously in human pterygium. J Cell Mol Med. 2007;11:585–589. doi: 10.1111/j.1582-4934.2007.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.John-Aryankalayil M., Dushku N., Jaworski C.J., Cox C.A., Schultz G., Smith J.A., Ramsey K.E., Stephan D.A., Freedman K.A., Reid T.W., Carper D.A. Microarray and protein analysis of human pterygium. Mol Vis. 2006;12:55–64. [PubMed] [Google Scholar]
- 42.Naib-Majani W., Eltohami I., Wernert N., Watts W., Tschesche H., Pleyer U., Breipohl W. Distribution of extracellular matrix proteins in pterygia: an immunohistochemical study. Graefes Arch Clin Exp Ophthalmol. 2004;242:332–338. doi: 10.1007/s00417-003-0846-y. [DOI] [PubMed] [Google Scholar]
- 43.Vojniković B., Njirić S., Zamolo G., Toth I., Apanjol J., Coklo M. Histopathology of the pterygium in population on Croatian Island Rab. Coll Antropol. 2007;31(Suppl 1):39–41. [PubMed] [Google Scholar]
- 44.Austin P., Jakobiec F.A., Iwamoto T. Elastodysplasia and elastodystrophy as the pathologic bases of ocular pterygia and pinguecula. Ophthalmology. 1983;90:96–109. doi: 10.1016/s0161-6420(83)34594-2. [DOI] [PubMed] [Google Scholar]
- 45.D'Alena P., Wood I.S. Labrador keratopathy: a microscopic study. Am J Ophthalmol. 1972;74:430–435. doi: 10.1016/0002-9394(72)90902-6. [DOI] [PubMed] [Google Scholar]
- 46.Johnson G.J., Overall M. Histology of spheroidal degeneration of the cornea in Labrador. Br J Ophthalmol. 1978;62:53–61. doi: 10.1136/bjo.62.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menegay M., Lee D., Tabbara K.F., Cafaro T.A., Urrets-Zavalia J.A., Serra H.M., Bhattacharya S.K. Proteomic analysis of climatic keratopathy droplets. Invest Ophthalmol Vis Sci. 2008;49:2829–2837. doi: 10.1167/iovs.07-1438. [DOI] [PubMed] [Google Scholar]
- 48.Ansari M.W., Rahi A.H., Shukla B.R. Pseudoelastic nature of pterygium. Br J Ophthalmol. 1970;54:473–476. doi: 10.1136/bjo.54.7.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang I.J., Hu F.R., Chen P.J., Lin C.T. Mechanism of abnormal elastin gene expression in the pinguecular part of pterygia. Am J Pathol. 2000;157:1269–1276. doi: 10.1016/S0002-9440(10)64642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coroneo M.T. Albedo concentration in the anterior eye: a phenomenon that locates some solar diseases. Ophthalmic Surg. 1990;21:60–66. [PubMed] [Google Scholar]
- 51.Dushku N., Reid T.W. Immunohistochemical evidence that human pterygia originate from an invasion of vimentin-expressing altered limbal epithelial basal cells. Curr Eye Res. 1994;13:473–481. doi: 10.3109/02713689408999878. [DOI] [PubMed] [Google Scholar]
- 52.Duke-Elder S., Wybar K.C., editors. The Anatomy of the Visual System: System of Ophthalmology 2. Kimpton; London: 1961. p. 557. [Google Scholar]
- 53.Grossniklaus H.E., Green W.R., Luckenbach M., Chan C.C. Conjunctival lesions in adults: a clinical and histopathologic review. Cornea. 1987;6:78–116. doi: 10.1097/00003226-198706020-00002. [DOI] [PubMed] [Google Scholar]
- 54.Basti S., Macsai M.S. Ocular surface squamous neoplasia: a review. Cornea. 2003;22:687–704. doi: 10.1097/00003226-200310000-00015. [DOI] [PubMed] [Google Scholar]
- 55.Lee G.A., Hirst L.W. Ocular surface squamous neoplasia. Surv Ophthalmol. 1995;39:429–450. doi: 10.1016/s0039-6257(05)80054-2. [DOI] [PubMed] [Google Scholar]
- 56.Pola E.C., Masanganise R., Rusakaniko S. The trend of ocular surface squamous neoplasia among ocular surface tumour biopsies submitted for histology from Sekuru Kaguvi Eye Unit, Harare between 1996 and 2000. Cent Afr J Med. 2003;49:1–4. [PubMed] [Google Scholar]
- 57.Nkomazana O., Tshitswana D. Ocular complications of HIV infection in sub-Sahara Africa. Curr HIV/AIDS Rep. 2008;5:120–125. doi: 10.1007/s11904-008-0019-z. [DOI] [PubMed] [Google Scholar]
- 58.Ng J., Coroneo M.T., Wakefield D., Di Girolamo N. Ultraviolet radiation and the role of matrix metalloproteinases in the pathogenesis of ocular surface squamous neoplasia. Invest Ophthalmol Vis Sci. 2008;49:5295–5306. doi: 10.1167/iovs.08-1988. [DOI] [PubMed] [Google Scholar]
- 59.Oren M. Decision making by p53: life, death and cancer. Cell Death Differ. 2003;10:431–442. doi: 10.1038/sj.cdd.4401183. [DOI] [PubMed] [Google Scholar]
- 60.Dushku N., Reid T.W. P53 expression in altered limbal basal cells of pingueculae, pterygia, and limbal tumors. Curr Eye Res. 1997;16:1179–1192. doi: 10.1076/ceyr.16.12.1179.5036. [DOI] [PubMed] [Google Scholar]
- 61.Dushku N., Hatcher S.L., Albert D.M., Reid T.W. p53 expression and relation to human papillomavirus infection in pingueculae, pterygia, and limbal tumors. Arch Ophthalmol. 1999;117:1593–1599. doi: 10.1001/archopht.117.12.1593. [DOI] [PubMed] [Google Scholar]
- 62.Tan D.T., Lim A.S., Goh H.S., Smith D.R. Abnormal expression of the p53 tumor suppressor gene in the conjunctiva of patients with pterygium. Am J Ophthalmol. 1997;123:404–405. doi: 10.1016/s0002-9394(14)70141-2. [DOI] [PubMed] [Google Scholar]
- 63.Guthoff R., Marx A., Stroebel P. No evidence for a pathogenic role of human papillomavirus infection in ocular surface squamous neoplasia in Germany. Curr Eye Res. 2009;34:666–671. doi: 10.1080/02713680903007162. [DOI] [PubMed] [Google Scholar]
- 64.Ateenyi-Agaba C., Dai M., Le Calvez F., Katongole-Mbidde E., Smet A., Tommasino M., Franceschi S., Hainaut P., Weiderpass E. TP53 mutations in squamous-cell carcinomas of the conjunctiva: evidence for UV-induced mutagenesis. Mutagenesis. 2004;19:399–401. doi: 10.1093/mutage/geh048. [DOI] [PubMed] [Google Scholar]
- 65.Schneider B.G., John-Aryankalayil M., Rowsey J.J., Dushku N., Reid T.W. Accumulation of p53 protein in pterygia is not accompanied by TP53 gene mutation. Exp Eye Res. 2006;82:91–98. doi: 10.1016/j.exer.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 66.Shields J.A., Shields C.L., Mashayekhi A., Marr B.P., Benavides R., Thangappan A., Phan L., Eagle R.C., Jr Primary acquired melanosis of the conjunctiva: risks for progression to melanoma in 311 eyes: The 2006 Lorenz E. Zimmerman lecture. Ophthalmology. 2008;115 doi: 10.1016/j.ophtha.2007.07.003. 511–519.e2. [DOI] [PubMed] [Google Scholar]
- 67.Shields C.L., Demirci H., Karatza E., Shields J.A. Clinical survey of 1643 melanocytic and nonmelanocytic conjunctival tumors. Ophthalmology. 2004;111:1747–1754. doi: 10.1016/j.ophtha.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 68.Sugiura M., Colby K.A., Mihm M.C., Jr, Zembowicz A. Low-risk and high-risk histologic features in conjunctival primary acquired melanosis with atypia: clinicopathologic analysis of 29 cases. Am J Surg Pathol. 2007;31:185–192. doi: 10.1097/01.pas.0000213339.32734.64. [DOI] [PubMed] [Google Scholar]
- 69.Folberg R., McLean I.W., Zimmerman L.E. Primary acquired melanosis of the conjunctiva. Hum Pathol. 1985;16:129–135. doi: 10.1016/s0046-8177(85)80061-7. [DOI] [PubMed] [Google Scholar]
- 70.Bongiorno M.R., Lodato G., Affronti A., Aragona F., Arico M. Amelanotic conjunctival melanoma. Cutis. 2006;77:377–381. [PubMed] [Google Scholar]
- 71.Tatla T., Hungerford J., Plowman N., Ghufoor K., Keene M. Conjunctival melanoma: the role of conservative surgery and radiotherapy in regional metastatic disease. Laryngoscope. 2005;115:817–822. doi: 10.1097/01.MLG.0000157327.10597.86. [DOI] [PubMed] [Google Scholar]
- 72.Sharara N.A., Alexander R.A., Luthert P.J., Hungerford J.L., Cree I.A. Differential immunoreactivity of melanocytic lesions of the conjunctiva. Histopathology. 2001;39:426–431. doi: 10.1046/j.1365-2559.2001.01168.x. [DOI] [PubMed] [Google Scholar]
- 73.Jakobiec F.A., Bhat P., Colby K.A. Immunohistochemical studies of conjunctival nevi and melanomas. Arch Ophthalmol. 2010;128:174–183. doi: 10.1001/archophthalmol.2009.394. [DOI] [PubMed] [Google Scholar]
- 74.Furusato E., Hidayat A.A., Man Y.G., Auerbach A., Furusato B., Rushing E.J. WT1 and Bcl2 expression in melanocytic lesions of the conjunctiva: an immunohistochemical study of 123 cases. Arch Ophthalmol. 2009;127:964–969. doi: 10.1001/archophthalmol.2009.183. [DOI] [PubMed] [Google Scholar]
- 75.Tabin G., Levin S., Snibson G., Loughnan M., Taylor H. Late recurrences and the necessity for long-term follow-up in corneal and conjunctival intraepithelial neoplasia. Ophthalmology. 1997;104:485–492. doi: 10.1016/s0161-6420(97)30287-5. [DOI] [PubMed] [Google Scholar]
- 76.Kim J.W., Abramson D.H. Topical treatment options for conjunctival neoplasms. Clin Ophthalmol. 2008;2:503–515. doi: 10.2147/opth.s1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schechter B.A., Koreishi A.F., Karp C.L., Feuer W. Long-term follow-up of conjunctival and corneal intraepithelial neoplasia treated with topical interferon alfa-2b. Ophthalmology. 2008;115:1291–1296. doi: 10.1016/j.ophtha.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 78.Skippen B., Tsang H.H., Assaad N.N., Coroneo M.T. Rapid response of refractory ocular surface dysplasia to combination treatment with topical all-trans retinoic acid and interferon alfa-2b. Arch Ophthalmol. 2010;128:1368–1369. doi: 10.1001/archophthalmol.2010.241. [DOI] [PubMed] [Google Scholar]


