Abstract
Radiation proctitis is characterized by mucosal inflammation followed by adverse chronic tissue remodeling and is associated with substantial morbidity and mortality. Mast cell hyperplasia has been associated with diseases characterized by pathological tissue remodeling and fibrosis. Rectal tissue from patients treated with radiotherapy shows mast cell hyperplasia and activation, suggesting that these cells play a role in the development of radiation-induced sequelae. To investigate the role of mast cells in radiation damage, experimental radiation proctitis was induced in a mast cell-deficient (Wsh/Wsh) mouse model. The colon and rectum of Wsh/Wsh and wild-type mice were exposed to 27-Gy single-dose irradiation and studied after 2 and 14 weeks. Irradiated rodent rectum showed mast cell hyperplasia. Wsh/Wsh mice developed less acute and chronic rectal radiation damage than their control littermates. Tissue protection was associated with increased tissue neutrophil influx and expression of several inflammatory mediators immediately after radiation exposure. It was further demonstrated that mast cell chymase, tryptase, and histamine could change human muscularis propria smooth muscle cells into a migrating/proliferating and proinflammatory phenotype. These data show that mast cells have deleterious effects on both acute and chronic radiation proctitis, possibly by limiting acute tissue neutrophil influx and by favoring phenotypic orientation of smooth muscle cells, thus making them active participants in the radiation-induced inflammatory process and dystrophy of the rectal wall.
Radiation proctitis is an insidious disease associated with substantial morbidity and mortality. It may result from the treatment of several malignancies in which normal rectal tissue is present in the irradiation field. Clinical expression of bowel complications associated with radiotherapy resembles chronic bowel diseases of other etiologies, such as Crohn's disease and ulcerative colitis, and is generally underdiagnosed, especially when the latent period after radiotherapy exceeds months or years.1–3 Advances in the quality of radiation treatment schedules have improved tumor control, but have also increased the number of cancer survivors subject to treatment-related adverse effects, which are of primary importance in assessing treatment effectiveness.
Acute radiation proctitis affects the majority of irradiated patients and is seen mainly in the mucosal compartment, with superficial mucosal erosion, acute inflammatory infiltrate, and crypt abscesses.4 Although the acute effects can usually be effectively managed and the symptoms are self-limiting in the weeks after treatment, late damage may develop in 5% to 10% of irradiated patients. Chronic complications are associated with damage throughout the bowel wall, with vascular dystrophy and uncontrolled scarring leading to tissue fibrosis.5,6 Only two studies (in human skin and breast tissue) have associated mast cells and fibrosis after radiation therapy,7,8 and there are no data on mast cells and irradiated human gut.
Mast cells are immune effectors involved in allergic and hypersensitivity reactions. They contain numerous mediators (chymase, tryptase, and histamine in particular) that are actively implicated in a number of physiological and pathological situations, varying from normal wound healing and host defense to tissue inflammation and tumor growth.9–11 Although mast cell hyperplasia is not systematically associated with inflammatory bowel disease in humans, there is a consensus that mast cells have a key role in gastrointestinal inflammation.12 Mast cells have also been associated with diseases characterized by pathological tissue remodeling and fibrosis in different organs, including intestinal radiation fibrosis.13–15
Preclinical studies on the role of mast cells in radiation damage show that these cells may have beneficial or detrimental effects, depending on the organ involved. Mast cells are predominantly protective in a model of radiation-induced heart disease in the rat,16 but are associated with areas of collagen deposition and radiation fibrosis in the rat lung and small intestine.15,17,18 There is evidence of mast cell involvement in a preclinical model of small intestinal radiation fibrosis,14 but data are lacking on damage to the rectum, an organ particularly at risk of radiation exposure during radiotherapy of rectal and prostatic tumors. In the present study, mast cell hyperplasia and activation was evidenced in human rectal tissue after preoperative radiotherapy for rectal adenocarcinoma.
To examine the role of mast cells in radiation proctitis, we used a well-established model of mast cell-deficient mice. KitWsh/Wsh mice bear the W-sash (Wsh) spontaneous inversion mutation located proximal to the Kit locus. The Kit W-sh mutation affects Kit expression and mutant mice show severe deficiency in melanoblasts and mast cells. KitW-sh mice have normal levels of all differentiated hematopoietic lineages. Unlike KitW/W-v mice, KitWsh/Wsh mice lack anemia and sterility and are a useful model for mast cell research.19 The lack of anemia implies that mast cell reconstitution in KitWsh/Wsh mice is not necessary to validate the results. We showed that mast cell deficiency protects from both acute and chronic radiation proctitis, suggesting a detrimental role of mast cells in this pathological context. In addition, the present study provides in vitro evidence suggesting a role for mast cells in the development of radiation-induced dystrophy of the muscularis propria (MP), by changing the human primary colonic smooth muscle cell (SMC) phenotype to a migrating/proliferating and proinflammatory phenotype.
Materials and Methods
Human Tissues
Human tissue was obtained in accordance with institutional ethical guidelines (Gustave Roussy Institute J.-C.S.) and with French Medical Research Council guidelines. Tissue was included from 16 patients treated for rectal adenocarcinoma with preoperative radiotherapy (45 Gy, fractions of 2 or 1.8 Gy). Tumors were surgically resected 5 to 7 weeks after treatment. For each patient, specimens of normal tissue were taken from the irradiated field adjacent to the tumor and distant from the tumor, so that patients served as their own controls.20
Sections were stained with 0.1% Sirius Red in a picric acid-saturated aqueous solution for 1.5 hours, then washed with 0.5% glacial acetic acid in distilled water for 5 minutes and counterstained with Mayer's hematoxylin.
For immunostaining, sections were incubated with mouse monoclonal antibodies against human mast cell chymase (NCL-MMC, Novocastra, A; Menarini Diagnostics, Rungis, France) or human mast cell tryptase (Clone AA1; Dako, Trappes, France), and with a biotinylated goat anti-mouse IgG followed by a horseradish peroxidase-avidin-biotin complex (Dako). Detection was achieved using the Vector NovaRED substrate kit for peroxidase (Vector Laboratories, Burlingame, CA). Sections were counterstained with Mayer's hematoxylin.
Animal Tissues
Animals, Irradiation, Radiation Injury Score, and Morphometric Analyses
Experiments were conducted in compliance with French regulations for animal experimentation (Ministry of Agriculture, Act 87-848, 19 October 1987) and approved by the IRSN ethics committee. A total of 120 animals were used. C57BL/6J-kitW-sh/W-sh mice were from the Jackson Laboratory (Bar Harbor, ME). The spontaneous Kit mutation occurred in a C3H/HeH × 101/H mating, and C57Bl/6 background was guaranteed by at least 10 backcrosses to C57Bl/6 mice. Wild-type (Wt) C57Bl/6J mice were thus used as control mice and were purchased from Charles River Laboratories International (Wilmington, MA). Animals were anesthetized and a single 27-Gy (1.4 Gy/min) dose of gamma irradiation was delivered by a cobalt 60 source through a 1 × 0.8-cm window centered on the colorectal region. This model of localized single-dose radiation exposure does not directly simulate fractionation treatment, but generates histopathological lesions similar to those seen clinically (ie, severe acute mucosal ulceration and transmural collagen deposition during the late phase, with 100% rectal obstruction), as shown by Skwarchuk and Travis.21,22
At 2 and 14 weeks after exposure, the colon and rectum were fixed in 4% formaldehyde and embedded in paraffin. Slides were stained with hematoxylin-eosin-saffron. The severity of colorectal damage was assessed using a radiation injury score (RIS) modified23 from the one validated by Hauer-Jensen et al.24 The RIS was designated in a blind fashion by two researchers (K.B. and A.F.). RIS variables consisted of mucosal ulceration, epithelial atypia, thickening of the subserosa, vascular sclerosis, intestinal wall thickening, colitis cystica profunda, and dystrophy of the MP. Radiation injury was graded for each variable as 0 = null, 1 = slight, 2 = moderate, or 3 = severe. Dystrophy of the MP was graded as 0 = none, 1 = dystrophy of a few muscular cell layers in contact with the submucosa, 2 = dystrophy affecting <50% of the MP thickness, and 3 = dystrophy affecting ≥50% of the MP thickness. Morphometric measurements of intestinal wall thickness and thickness of the MP were performed using the VisioL@b 2000 image analysis software package (Biocom, Les Ulis, France). Fifteen measurements per section were obtained all along the injured area.
Staining of Mast Cells
Sections were stained with 1% toluidine blue in 0.5 N HCl for 20 minutes followed by 0.7 N HCl for 10 minutes. Mast cells were counted in the whole injured area (×200 magnification) with between 6 and 11 slides per group. Sirius Red staining was performed as described for human tissue sections.
Immunohistology
Sections were incubated with rat monoclonal antibody against F4/80 (Santa Cruz Biotechnologies, Heidelberg, Germany) or rabbit polyclonal antibody against myeloperoxidase (MPO) (Abcam, Paris, France) and immunostaining was performed as described as for human tissue. For each Wt and Wsh/Wsh irradiated tissue, total and MPO-positive cells invading the mucosa were counted in a rectangle of 10,000 μm2 (×400 magnification). The scoring of macrophage invasion was evaluated as follows: 1 = spots restricted to the lamina propria; submucosa and MP negative; 2 = groups of macrophages at the crypt base (<10 cells per crypt) submucosa and MP negative; 3 = numerous macrophages at the crypt base (≥10 cells), strong invasion of the submucosa, presence of macrophages in the MP and subserosa; 4 = strong invasion of all tissue compartments.
Cell Culture
Primary colonic SMCs were isolated from the MP of resected human normal colon (MP-SMCs) and used between P3 and P6.25 Confluent MP-SMCs were irradiated with a 137Cs source (1 Gy/min).
Mast cell leukemia cell line, subline HMC-1560, was kindly provided by Dr. Véronique Machelon (INSERM U764, AP-HP Hôpital Antoine Béclère, Clamart, France). Cells were grown at 37°C with 5% CO2 in modified Eagle's medium (Invitrogen, Cergy Pontoise, France) supplemented with 10% dialyzed fetal calf serum, 7.5% bicarbonate sodium, 1% GlutaMAX and penicillin- streptomycin (all from Gibco BRL; Invitrogen).
RNA Isolation, Reverse Transcription, and Real-Time PCR
Mouse colorectal tissues were frozen in RNAlater RNA stabilization reagent (Qiagen, Valencia, CA) until analysis. Tissue and cell total RNA was prepared with the total RNA isolation kit (RNeasy mini kit, Qiagen). Reverse transcription was performed as described previously.20 PCR was performed using TaqMan gene expression assays (Applied Biosystems, Courtaboeuf, France), with GAPDH and 18S as housekeeping genes for cells and tissues, respectively. Relative mRNA was quantified using the ΔΔCT method.
Protein Measurements
Protein levels were measured using enzyme-linked immunosorbent assay kits (R&D Systems Europe, Lille, France) according to the manufacturer's instructions.
MP-SMC Response to Mast Cell-Conditioned Medium
Conditioned media were prepared by incubating HMC-1 (150 × 103 cells/ml) in modified Eagle's medium (complete medium). After 4 to 5 days of culture, cells were centrifuged and conditioned media were collected. Confluent monolayers of MP-SMCs were exposed to HMC-1 conditioned medium for 30 minutes and then exposed to 20 Gy or sham irradiation. Cells lysis was achieved at 3 hours for gene expression analysis.
Cell Proliferation
MP-SMCs were seeded in 96-well plates. Mast cell tryptase, chymase (R&D Systems Europe), or histamine (Sigma-Aldrich, St Quentin Fallavier, France) was added 48 hours after seeding, and cell viability was determined 48 hours later by measuring luminescence (Mithras LB940; Berthold Technologies, Bad Wildbad, Germany) using the ViaLight Plus kit according to the manufacturer's instructions (Lonza, Emerainville, France).
In Vitro Migration Assay
MP-SMC migration was determined by the scratch injury model in the presence or absence of mast cell tryptase, chymase, or histamine. MP-SMCs were fixed and colored with a methanol solution containing 3% paraformaldehyde and 0.25% crystal violet 72 hours after scratch injury. Migration was measured by counting the number of cells in a 350 × 1000-μm rectangle, representing the size of the initial scratch.
Cell Contraction
MP-SMC contraction was measured using a collagen-based cell contraction assay kit (Euromedex, Mundolsheim, France), 5 × 106 cells/ml, according to the manufacturer's instructions.
MP-SMC mRNA Expression Profiles
MP-SMC mRNA expression profiles were acquired by real-time PCR at 1, 3, and 6 hours after the addition of chymase (5, 10, 20, 40, 80 ng/ml), tryptase (50, 100, 500 ng/ml), histamine (10−6, 10−5, 10−4, 10−3 mol/L), or chymase + tryptase + histamine (20 ng/ml, 100 ng/ml, and 10−4 mol/l, respectively).
Statistical Analyses
Data are reported as means ± SEM. Statistical analyses were performed by analysis of variance, Student's t-test, or the Mann-Whitney rank sum test, as appropriate, with a level of significance of P < 0.05.
Results
Radiation Damage to Human Rectum Is Associated with Mast Cell Hyperplasia
Human rectal radiation damage is characterized by epithelial atypia, mucosal inflammation/ulceration, submucosal edema, inflammation and collagen deposition, vascular dystrophy, and dystrophy of the MP. In the healthy rectum, immunostaining for chymase and tryptase (Figure 1) shows that mast cells are mainly localized in the lamina propria and submucosa (Figure 1, A and G) and are virtually absent from the MP (Figure 1, C and I) and normal vessel wall (Figure 1, E and K). This is consistent with published data on the normal human colon.26 Radiation damage to the rectum shows mast cell hyperplasia in the mucosa and submucosa (Figure 1, B and H), and mast cell invasion in the MP (Figure 1, D and J) and the dystrophic vessel wall [Figure 1, F (arrows) and L]. As revealed by Sirius Red staining, mast cells were present in areas of collagen deposition, namely, in the submucosa, MP, and vessel wall (Figure 1, N, P, and R, respectively) compared with uninjured tissues (Figure 1, M, O, and Q, respectively).
Figure 1.
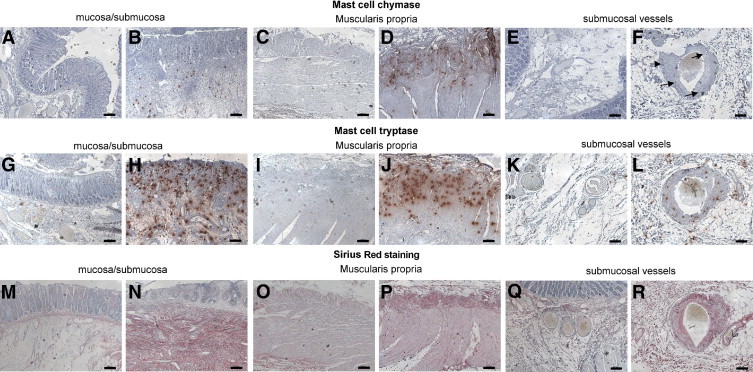
Immunostaining of chymase and tryptase (red spots) in human rectal tissues. In normal tissue, chymase- and/or tryptase-positive mast cells are present in the submucosa and lamina propria (A and G). There are virtually no mast cells in the normal muscularis propria, whereas some spots are visible in the subserosa (C and I). Mast cells are absent from healthy vessel wall (E and K). Radiation injury is associated with activation and increase in mast cell numbers in the ulcerated mucosa and subjacent submucosa (B and H) and with mast cell invasion of the muscularis propria (D and J) and vascular wall [F (arrows) and L]. Sirius Red staining shows dense collagen deposition in pathological tissues in the submucosa (N), muscularis propria (P), and vessel wall (R), compared with corresponding healthy compartments (M, O, and Q, respectively). Note the absence of collagen deposition in the ulcerated mucosa (N). Scale bars = 200 μm.
Experimental Radiation Proctitis in Wt Mice Is Associated with Mast Cell Hyperplasia and Wsh/Wsh Mice Are Protected from Both Acute and Delayed Rectal Damage
In both strains, between 30% (Wsh/Wsh mice) and 40% (Wt mice) of animals died from radiation-induced severe rectal injury between day 20 and the scheduled day of euthanasia, with no difference between strains. The 14-week time point was chosen as showing severe tissue damage before the development of radiation occlusion syndrome, thus excluding the possibility of drawing conclusions about survival rates.
Toluidine blue staining revealed no mast cells in either unirradiated or irradiated Wsh/Wsh tissues (data not shown). Mast cells were very sparse in Wt control tissues, and were observed mainly in the mucosa and mesentery (Figure 2A, upper panels, arrowheads). Mast cell hyperplasia occurred 14 weeks after exposure in the subserosa underneath ulcerated tissue, underneath the edge of the ulceration, and in the inflamed mucosa and submucosa (Figure 2A, lower panels, arrowheads). Mast cell number decreased 2 weeks after exposure in mucosa, and increased in the serosa/mesenteric border (Figure 2B). At 14 weeks, a global increase in mast cell number occurred in all tissue compartments.
Figure 2.
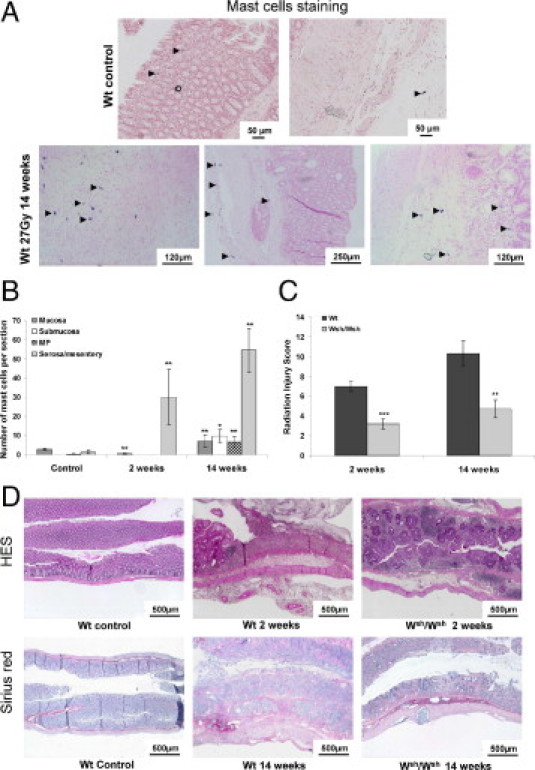
A: Toluidine blue staining of mast cells in Wt tissues. Mast cells are occasionally observed in healthy mucosa and mesentery (upper panels, arrowheads). Mast cell hyperplasia is visible 14 weeks after irradiation in the MP and subserosa, as well as in the mucosa and submucosa (lower panels, arrowheads). B: Number of mast cells per tissue section in each colorectal compartment of Wt unirradiated mice (n = 5) and at 2 weeks (n = 9) and 14 weeks (n = 12) after irradiation, showing significant mast cell hyperplasia at 2 weeks in the serosa/mesentery and in all tissue compartments at 14 weeks after irradiation. *P < 0.05; **P < 0.01. C: RIS in Wt and Wsh/Wsh mice 2 and 14 weeks after exposure (n = 6–15 animals per group). **P < 0.01; ***P < 0.001. Results are presented as means ± SEM. D: Routine tissue staining with hematoxylin-eosin-saffron and collagen staining with Sirius Red. No difference in overall tissue organization was seen between Wt and Wsh/Wsh, so pictures of Wt tissues served as controls. Acute radiation injury is characterized by submucosal edema, transmural inflammation, and total loss of covering epithelium in Wt mice, whereas epithelial lining is preserved and tissue inflammation is reduced in Wsh/Wsh mice. Chronic damage shows transmural collagen deposition, mucosal disorganization, and muscular dystrophy in Wt mice, but reduced damage in Wsh/Wsh mice.
The RIS value during both the acute and late phases of rectal radiation damage in Wsh/Wsh mice was significantly lower than in Wt mice (3.2 ± 0.5 vs. 7.0 ± 0.5 at 2 weeks, P < 0.001, and 4.7 ± 0.9 vs. 10.3 ± 1.3 at 14 weeks, P = 0.001) (Figure 2C). Acute lesions in Wt mice consisted of extensive mucosal ulceration with complete loss of crypt architecture, compared with control tissue (Figure 2D). Conversely, Wsh/Wsh mice showed preserved epithelial lining and a significant number of regenerating crypts. Chronic damage was characterized by strong collagen deposition in Wt mice, compared with controls, as shown by Sirius Red staining. Wsh/Wsh mice showed reduced chronic damage, compared with Wt mice.
Among the seven parameters that make up the RIS, Wsh/Wsh mice showed significantly less epithelial damage and dystrophy of the MP during the acute phase (Figure 3A), and numerous parameters were significantly reduced during the late phase (Figure 3B), together with less intestinal wall thickening and MP smooth muscle mass (Figure 3C).
Figure 3.
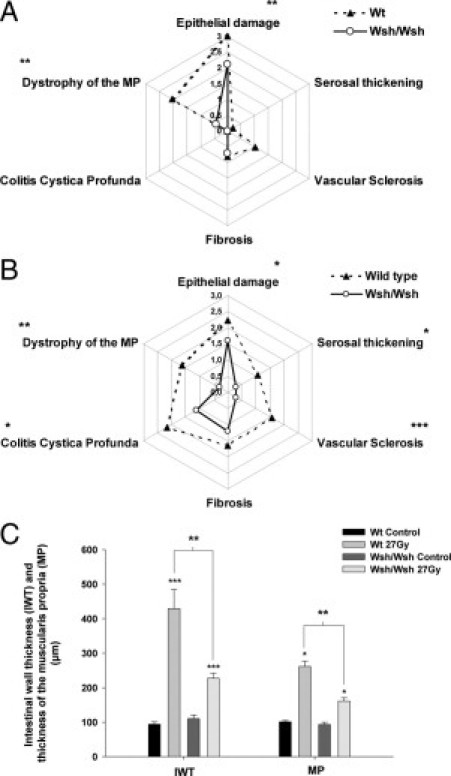
RIS parameters 2 weeks (A) and 14 weeks (B) after irradiation in Wt (dashed line; n = 9) and Wsh/Wsh mice (solid line; n = 15). *P < 0.05; **P < 0.01; ***P < 0.001. C: Intestinal wall thickness and thickness of the MP 14 weeks after injury in unirradiated mice (Wt: n = 7; Wsh/Wsh: n = 7), and in 27-Gy-irradiated Wt (n = 7) and Wsh/Wsh mice (n = 12). *P < 0.05; ***P < 0.001 compared with controls; **P < 0.01 between irradiated groups. Results are presented as means ± SEM.
Gene Expression Profiles of Inflammation Markers in Irradiated Wt and Wsh/Wsh Tissues
In experimental models of radiation gut damage using high single-dose irradiation, the development of chronic injury is known to be governed mainly by the severity of acute damage.27 Wsh/Wsh mice showed reduced acute injury of the epithelium and MP. Based on these observations, we decided to study i) the acute mRNA expression profile of several molecules involved in tissue inflammation and cell recruitment and the effects on invading cells and ii) the influence of three preformed mast cell mediators known to influence vascular and airway SMC phenotype (chymase, tryptase, and histamine) in human primary colonic SMCs.
Two weeks after exposure (Figure 4A), irradiation increased mRNA expression of all tested genes associated with inflammation, with no difference between strains, except for the interleukin-1β (IL-1β) mRNA level, which was significantly higher in Wt than in Wsh/Wsh tissues (P < 0.05). To investigate if differences between strains may be explained by the immediate acute tissue response to irradiation, analyses were performed 3 hours after exposure. The mRNA expression of several cytokines such as RANTES, IL-6, IL-1β, tumor necrosis factor-α (TNFα), and IL-10, but also the strong neutrophil-attracting C-X-C motif chemokines 1 and 2 (CXCL-1 and CXCL-2) were significantly higher in Wsh/Wsh mice than in Wt mice (Figure 4B). These observations were confirmed by the protein levels 7 hours after exposure for CXCL-1 and CXCL-2 (Figure 5, A and B). Both IL-6 and TNFα were undetectable in all cases.
Figure 4.
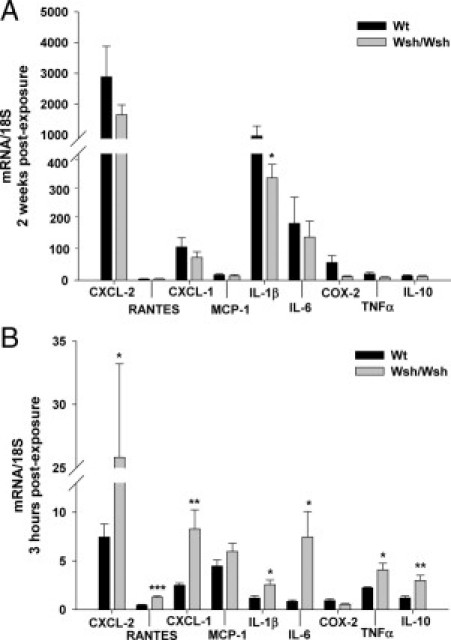
mRNA expression profiles of colorectal tissues from Wt (n = 7) and Wsh/Wsh mice (n = 7) compared with respective control levels (standardized to 1) 2 weeks (A) and 3 hours (B) after irradiation. *P < 0.05; **P < 0.01; ***P < 0.001 between irradiated groups. Results are presented as means ± SEM.
Figure 5.
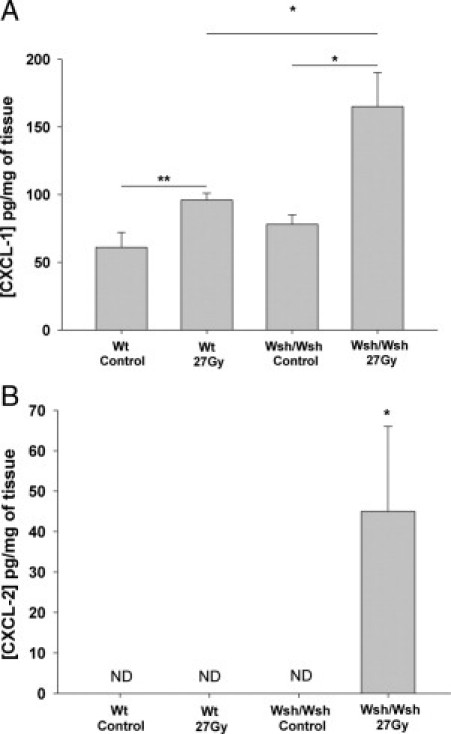
Concentration of CXCL-1 (A) and CXCL-2 (B) in picograms per milligram of wet tissue in Wt and Wsh/Wsh mice 7 hours after sham (n = 6) or 27-Gy irradiation (n = 9). *P < 0.05; **P < 0.01. Results are presented as means ± SEM. ND, not detectable.
Neutrophil and Macrophage Patterns 2 and 14 Weeks Postexposure
We next investigated the effects on tissue neutrophil influx. We also looked at macrophages, which are known regulators of tissue neutrophil recruitment. In healthy Wt and Wsh/Wsh tissues (Figure 6A), neutrophils were located in the lamina propria and the submucosa. Irradiation induced neutrophil influx in all compartments in Wt as well as in Wsh/Wsh mice. Two weeks after exposure, the MPO-positive cell count revealed a higher proportion of neutrophils in ulcerated mucosa of Wsh/Wsh mice (51.4% of total cells were MPO-positive, vs. 25.5% in Wt mice; Figure 6B). The number of circulating neutrophils, measured 10 days after exposure, significantly increased in Wsh/Wsh mice compared with Wt mice, in which neutrophil numbers remained at the level of unirradiated animals (1.14 ± 0.21 vs. 0.38 ± 0.09 106 cells/ml, respectively; P < 0.05, data not shown). No significant difference in the percentage of MPO-positive cells was seen in injured tissues of Wt and Wsh/Wsh mice 14 weeks after irradiation (Figure 6C).
Figure 6.
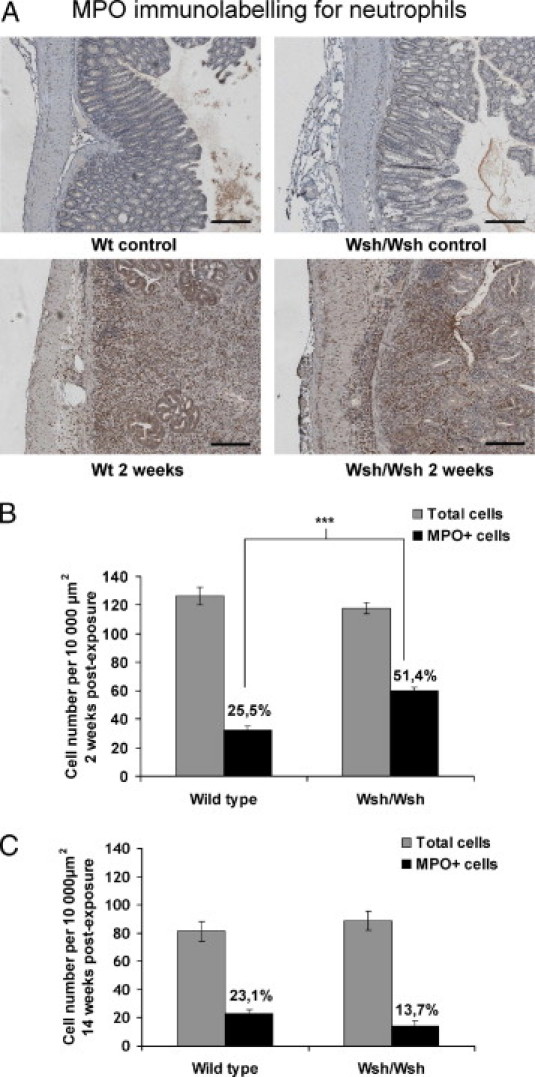
A: Immunostaining for neutrophils (in red). In unirradiated tissues, neutrophils are present in the submucosa and lamina propria in Wt, as well as in Wsh/Wsh tissues (upper panels). Irradiation induces neutrophil recruitment in all compartments in both strains (lower panels). B: Acute mucosal infiltrate in Wsh/Wsh mice (n = 8) shows a higher proportion of MPO-positive cells, compared with Wt (n = 8). ***P < 0.001. C: Late mucosal infiltrate showing no significant difference in the proportion of MPO-positive cells between Wt and Wsh/Wsh mice. Results are presented as means ± SEM.
In healthy Wt and Wsh/Wsh tissues, macrophages were localized in the lamina propria (Figure 7A). They were rarely observed in the submucosa, except around vessels and occasionally in the serosa. Two weeks after exposure, macrophages had infiltrated all compartments, including the MP, in both mouse strains. Immunostaining scores for macrophage invasion were similar in tissues of both Wt and Wsh/Wsh strains 2 and 14 weeks after exposure (Figure 7B). F4/80 gene expression measured by real time PCR in irradiated tissues did not differ between strains (data not shown).
Figure 7.
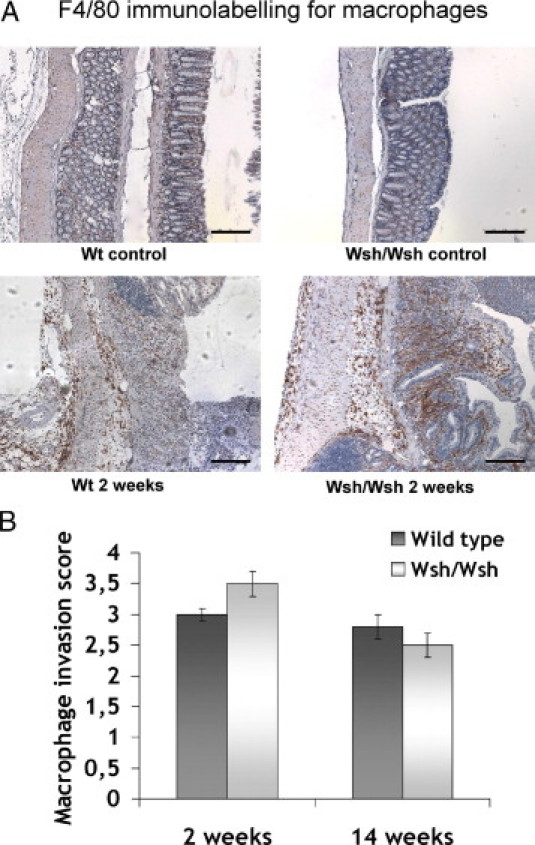
A: Macrophages immunostaining. In unirradiated tissues, macrophages are present in the lamina propria and submucosa in Wt, as well as in Wsh/Wsh tissues (upper panels). Irradiation induces macrophage infiltration in all tissue compartments in both strains (lower panels). Scale bars = 200 μm. B: Acute and late macrophage invasion scores do not differ between strains Results are presented as means ± SEM for n = 6 (Wt) and n = 10 animals (Wsh/Wsh).
The results suggest that mast cells may influence the acute mucosal radiation response in mice. Mast cells also invaded the external muscular layers of the human rectum after 45-Gy radiotherapy (Figure 8A).
Figure 8.

A: Activated mast cells in irradiated human rectal muscularis propria, as evidenced by chymase and tryptase immunostaining. B: Influence of chymase, tryptase, and histamine on MP-SMC proliferation. Luminescent signals corresponding to MP-SMC numbers in control conditions or 48 hours after addition of chymase, tryptase, or histamine. C: Influence of chymase, tryptase, and histamine on MP-SMC migration. Number of migrating MP-SMCs in the wound 72 hours after scratch injury, in control conditions or after addition of chymase, tryptase, or histamine. **P < 0.01; ***P < 0.001. Results are presented as means ± SEM for three independent experiments.
Influence of Mast Cell-Conditioned Medium on MP-SMC Gene Expression Profile
The neutrophil and macrophage patterns prompted us to analyze the role of mast cell mediators in the SMC phenotype. As a proof of principle of the possible influence of mast cells on SMC phenotype, experiments were first performed with mast cell-conditioned medium. Mast cell-conditioned medium induced a shift of SMC phenotype to a proinflammatory secretory state, increasing mRNA expression of cyclooxygenase-2 (COX-2), CXCL-2, and IL-6. This was true for unirradiated as well as for 20-Gy-irradiated SMCs. Moreover, previous irradiation significantly exacerbated SMC response to mast cell-conditioned medium (Table 1).
Table 1.
mRNA Expression Levels of PTGS2 (gene product : COX-2), IL-6, and CXCL-2 in Smooth Muscle Cells 3 Hours after Exposure to 20 Gy Alone, HMC-1 Conditioned Medium Alone, or 20 Gy + HMC-1 Conditioned Medium
| Gene | 20 Gy (± SEM) | HMC-1 conditioned medium | 20 Gy + HMC-1 conditioned medium |
|---|---|---|---|
| PTGS2 | 1.95 ± 0.24⁎ | 2.17 ± 0.29⁎ | 4.87 ± 0.83⁎⁎† |
| IL-6 | 3.09 ± 0.88⁎ | 6.38 ± 0.90⁎⁎ | 33.20 ± 5.11⁎⁎†† |
| CXCL-2 | 3.39 ± 0.24⁎⁎ | 1.35 ± 0.06⁎ | 4.27 ± 0.85⁎⁎† |
Gene expression is compared with expression level of control SMCs, standardized to 1. Data are reported as means ± SEM for three independent experiments.
P < 0.05;
P < 0.01 compared with control.
P < 0.01;
P < 0.001 between HMC-1 conditioned medium and 20Gy + HMC-1 conditioned medium.
Influence of Mast Cell Mediators Chymase, Tryptase, and Histamine on MP-SMC Phenotype
All further experiments were performed on unirradiated SMCs, to avoid radiation-induced cell cycle arrest, which may compromise studies on cell migration and proliferation. Chymase, tryptase, and histamine were chosen as the main preformed mast-cell mediators likely to influence MP-SMC phenotype.
Mast cell chymase and tryptase significantly increased both MP-SMC proliferation and migration, whereas histamine had the opposite effect in vitro (Figure 8, B and C). No significant modification of cell contraction was noted in the presence of chymase, tryptase, or histamine (data not shown).
Neither chymase nor tryptase modified MP-SMC mRNA expression profiles of a set of proteins that characterize tissue inflammation (data not shown). In contrast, histamine influenced mRNA expression of several of these proteins (Figure 9A). One hour after histamine addition, only CXCL-2 and TGFbeta expressions were increased, compared with untreated MP-SMC cultures. At 3 hours, histamine significantly increased COX-2 and reduced stem cell factor (SCF), intercellular adhesion molecule 1, connective tissue growth factor, and α-2 type I collagen. At 6 hours, histamine increased IL-6, IL-8, COX-2, and CXCL-2 and reduced SCF, intercellular adhesion molecule 1, matrix metalloproteinase 2, and COL3A1. This was verified by the protein levels in cell supernatants at 8, 16, and 24 hours for IL-6 and IL-8 (Figure 9, B and C). In all cases, the changes in mRNA expression in the presence of histamine + chymase + tryptase did not show any additive or synergistic effect compared with the addition of histamine alone. Notably, expression of stem cell factor, which was reduced by histamine alone, increased 6 hours after the simultaneous addition of chymase, tryptase, and histamine (fold change 2.10 ± 0.14 compared with control expression, P < 0.01) (data not shown).
Figure 9.

A: mRNA expression profile of MP-SMCs 1, 3, and 6 hours after addition of 10−5 mol/L histamine. B: IL-6 concentration (pg/ml) in MP-SMC supernatants in control conditions or 8, 16, and 24 hours after addition of 10−5 mol/L histamine. C: IL-8 concentration (pg/ml) in MP-SMC supernatants in control conditions or 8, 16, and 24 hours after addition of 10−5 mol/L histamine. *P < 0.05; **P < 0.01; ***P < 0.001. Results are presented as means ± SEM.
Similar mRNA expression profiles in response to histamine addition were obtained on previously 20-Gy-irradiated SMCs (data not shown).
Discussion
The present findings show that human radiation proctitis is associated with hyperplasia and activation of chymase-positive and/or tryptase-positive mast cells. We confirmed the occurrence of mast cell hyperplasia in a model of radiation proctitis in mice and showed that mast cell-deficient mice are protected from both acute and late damage, strongly supporting a detrimental role for mast cells in radiation proctitis.
Experimental models using high single-dose radiation exposure of the gut are known to have a strong consequential component.27 Therapeutic strategies or genetic mutations reducing acute damage in these models often reduce late damage in rats and mice.28–32 With the present study, we have confirmed the link between acute and late damage in single high-dose irradiation models by demonstrating that the absence of mast cells reduces both acute and late colorectal damage in mice. Conversely, Zheng et al14 showed that mast cell deficiency in rats exposed to a high single dose exacerbated acute lesions of the small intestine but protected from late damage. Discrepancies may be explained by differences in species, irradiation dose, and radiation response of the small intestine versus colon-rectum. Mast cells comprise a heterogeneous population of cells that are conditioned by their environment in both normal and pathological situations.10 Mast cells in mice may have different functions than in rats. This may be illustrated by models of bleomycin-induced lung damage, in which mast cells contribute to lung injury and fibrosis in mice but not in rats.33,34 Moreover, the exact effects of mast cell deficiency on gut physiology in rats and mice are unknown, and may result in different responses to tissue trauma, including radiation damage. Finally, and considering that Wt and Wsh/Wsh mice came from different facilities and were not cohoused, one cannot exclude the possibility that differences in luminal microbiota may affect tissue response to irradiation and may have a role in the differences in tissue damage observed between strains.35
Even though mast cell numbers are very different in healthy and irradiated human and mouse tissues, mast cell balance is tipped toward mast cell hyperplasia and/or recruitment in both cases by ionizing radiation. The detrimental role of mast cells in mice seems to fit with what happens in human rectal tissues, in which mast cell hyperplasia is associated with tissue injury and collagen deposition. Further investigations are necessary, however. In particular, more observations of human tissue after radiation damage are needed, as well as kinetic studies including the late phase of radiation damage.
The expression profile of genes involved in inflammation and fibrosis obtained 3 hours after exposure reflects the direct cellular response to ionizing radiation, and may be determinant in the initiation and progression of future tissue damage. We show that Wsh/Wsh mice exhibit a stronger acute response to ionizing radiation than Wt mice. The absence of mast cells results in increased mRNA and protein levels of CXCL-1 and CXCL-2, two chemokines strongly involved in leukocyte and in particular neutrophil recruitment. This is followed at 2 weeks by a higher proportion of neutrophils in ulcerated areas of irradiated tissues of Wsh/Wsh mice, compared with Wt mice, with no difference in macrophage influx.
Surprisingly, increased neutrophil chemoattractant expression and subsequently higher neutrophil numbers were associated with less tissue damage in Wsh/Wsh mice. This contradicts current understanding of the role of neutrophils in tissue damage, namely, that neutrophils are generally considered deleterious in intestinal inflammation. Tissue protection in Wsh/Wsh is also visible at days 3 and 7 (data not shown), excluding the possibility that higher neutrophil numbers at 2 weeks may result from earlier stronger tissue damage. Several studies have reported a potential role of acute neutrophil influx in tissue protection. In Smad3 knockout mice, 30-Gy irradiation of flank skin resulted in enhancement of migrating neutrophils at 6–8 hours, compared with Wt skin, and was correlated with radioprotection 6 weeks later.36 Moreover, mast cell reconstitution in W/Wv mice by Smad3−/− bone marrow mast cells offered protection against septic shock induced by cecal ligation and puncture and was associated with increased neutrophil numbers in peritoneal exudates.37 Finally, in the same model of experimental sepsis in mice, Alves-Filho et al38 observed that IL-33 favors neutrophil chemotaxis to the site of infection and reduces animal mortality after cecal ligation and puncture; the IL-33-treated animals exhibited increased neutrophil influx in the peritoneal cavity and a better bacterial clearance.
It can be hypothesized that neutrophils, which are known to be recruited acutely after irradiation and are recognized to be important in bacterial defense, may have a protective role in mouse acute radiation proctitis, which is characterized by complete loss of intestinal barrier, allowing bacterial transmigration. The late phase of radiation tissue damage is characterized by diminished reactive inflammation in favor of chronic scarring and is associated with an overall reduction in tissue neutrophil influx in both strains. Finally, further studies are needed to understand how neutrophils protect tissue from radiation proctitis and to clarify the role of mast cells in the regulation of neutrophil influx in this particular pathological situation.
Several studies have demonstrated that SMCs may be active participants in inflammatory processes such as atherosclerosis and asthma.39–41 SMCs may undergo a shift to a proliferating/migrating/secreting phenotype, thus contributing to the promotion of persistent tissue inflammation. SMCs secrete numerous inflammatory mediators, including after radiation exposure, and are known as the major source of collagen in fibrosing gut.42–44 In human rectal tissues, we show here that mast cell hyperplasia and activation occurs in the dystrophic MP after 45-Gy preoperative radiotherapy for rectal adenocarcinoma. This is consistent with a report of accumulation of mast cells in the thickened MP of fibrotic strictures in Crohn's disease.26 Mast cells are known to influence barrier properties45 and visceral sensitivity46 in the rat gut and migrating motor complexes in rats after Nippostrongylus brasiliensis infection.47 There are no data on the putative influence of mast cells on SMC phenotype of the human gut or on their role in MP inflammation and dystrophy.
Using a pertinent model of primary SMCs isolated from human colonic MP, the present study shows that HMC-1-conditioned medium increases SMC mRNA expression of different inflammatory mediators. This is true for unirradiated as well as for 20-Gy-irradiated SMCs. One interesting observation is the sensitization of irradiated SMCs to HMC-1 conditioned medium, compared with unirradiated cells. For example, several studies showed a synergism between TNFα and interferon-γ on CXCL-10 release in airway SMCs. Recently, Clarke et al48 demonstrated that TNFα and interferon-γ synergistically recruited the transcriptional coactivator CREB-binding protein to the CXCL-10 promoter along with heightened recruitment of RNA polymerase II to the promoter. Further studies will be necessary under our experimental conditions to specify the mechanisms by which irradiated cells are sensitized to the mediators contained in the mast cell-conditioned medium. Mast cell mediators and irradiation may severally activate numerous transcription factors involved in the transcriptional regulation of COX-2, CXCL-2, and IL-6, and may also synergistically recruit coactivators. Such a sensitization of irradiated cells to inflammatory mediators may have important repercussions in the development of tissue damage in cases of radiation exposure in the context of pre-existing inflammation.
Mimicking the effect of mast cells by tryptase, chymase, or histamine addition influences SMC phenotype. Mast cell tryptase and chymase treatment stimulates both proliferation and migration of colonic SMCs, two phenotypic features which may actively participate in dystrophy of the MP. These in vitro data are supported by the fact that an absence of mast cells in vivo reduces radiation-induced SMC proliferation in the rat small intestine,14 and we show here that Wsh/Wsh mice have reduced MP thickness, revealing diminished SMC hyperplasia as well. Finally, several studies have demonstrated that mast cell tryptase, chymase, and/or histamine may influence vascular SMC phenotype in human abdominal aortic aneurysms and favor atherosclerosis and plaque growth in mice.49,50 Mast cells invade the submucosal vessel wall in rectal tissues after radiotherapy, and may be involved in radiation-induced vascular dystrophy. This is part of our future investigations.
Histamine is known to stimulate both the migration and proliferation of vascular SMCs,51 which participate in intimal thickening. We show here that histamine, although it reduces both migration and proliferation of MP-SMCs, shifts their expression toward a proinflammatory rather than a profibrosing profile. Histamine increases MP-SMC mRNA levels of several molecules that may contribute to immune cell invasion, promote inflammation, and favor mast cell persistence in the tissue. For example, IL-8 and COX-2, both pivotal mediators of intestinal inflammation, are implicated in vascular SMC migration and inflammation of the vessel wall in atherosclerosis.39,52–54 TGFβ shows strong mast cell chemoattractant properties in vitro,55 and human airways SMCs promote mast cell survival and proliferation in part by the secretion of IL-6 and SCF.56 In our model we show that (unlike histamine alone, which decreases SCF expression) the simultaneous presence of histamine, chymase, and tryptase increases MP-SMC expression of SCF, a mast cell growth and surviving factor. In our model, in which histamine treatment mimics mast cell activation, MP-SMCs may actively participate in radiation-induced MP dystrophy by favoring mast cell recruitment, proliferation, and/or survival.
Finally, studies could be performed with mast cells stabilizers such as cromoglycate or doxantrazole, which may allow discriminating between mechanisms dependent and independent of degranulation. Moreover, an intriguing perspective on the present work could come from the use of mast cell inhibitors such as imatinib mesylate, which reduces radiation-induced pulmonary mast cell influx and lung damage in mice.57
In conclusion, mast cell hyperplasia and activation is associated with human radiation proctitis, and the presence of mast cells is deleterious in both the acute and chronic phases of experimental radiation proctitis. Mast cells may reduce the acute neutrophil influx necessary for tissue protection and thus favor a shift of MP-SMCs toward a proliferating/migrating/secreting phenotype, thereby perpetuating colonic wall inflammation. The present data highlight the complexity of the roles played by mast cells in different pathophysiological processes and the need for extensive studies in each specific context, but support the value of developing strategies targeting mast cell mediators to ameliorate or prevent radiation proctitis.
Footnotes
Supported by the Institute for Radiological Protection and Nuclear Safety (IRSN), Fontenay-aux-Roses Cedex, France.
References
- 1.Bentzen S.M., Dörr W., Anscher M.S., Denham J.W., Hauer-Jensen M., Marks L.B., Williams J. Normal tissue effects: reporting and analysis. Semin Radiat Oncol. 2003;13:189–202. doi: 10.1016/S1053-4296(03)00036-5. [DOI] [PubMed] [Google Scholar]
- 2.Andreyev H.J. Gastrointestinal problems after pelvic radiotherapy: the past, the present and the future. Clin Oncol (R Coll Radiol) 2007;19:790–799. doi: 10.1016/j.clon.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Andreyev J. Gastrointestinal symptoms after pelvic radiotherapy: a new understanding to improve management of symptomatic patients. Lancet Oncol. 2007;8:1007–1017. doi: 10.1016/S1470-2045(07)70341-8. [DOI] [PubMed] [Google Scholar]
- 4.Hovdenak N., Fajardo L.F., Hauer-Jensen M. Acute radiation proctitis: a sequential clinicopathologic study during pelvic radiotherapy. Int J Radiat Oncol Biol Phys. 2000;48:1111–1117. doi: 10.1016/s0360-3016(00)00744-6. [DOI] [PubMed] [Google Scholar]
- 5.Garg A.K., Mai W.Y., McGary J.E., Grant W.H., 3rd, Butler E.B., Teh B.S. Radiation proctopathy in the treatment of prostate cancer. Int J Radiat Oncol Biol Phys. 2006;66:1294–1305. doi: 10.1016/j.ijrobp.2006.07.1386. [DOI] [PubMed] [Google Scholar]
- 6.Denham J.W., O'Brien P.C., Dunstan R.H., Johansen J., See A., Hamilton C.S., Bydder S., Wright S. Is there more than one late radiation proctitis syndrome? Radiother Oncol. 1999;51:43–53. doi: 10.1016/s0167-8140(99)00027-4. [DOI] [PubMed] [Google Scholar]
- 7.Riekki R., Harvima I.T., Jukkola A., Risteli J., Oikarinen A. The production of collagen and the activity of mast-cell chymase increase in human skin after irradiation therapy. Exp Dermatol. 2004;13:364–371. doi: 10.1111/j.0906-6705.2004.00164.x. [DOI] [PubMed] [Google Scholar]
- 8.Westbury C.B., Reis-Filho J.S., Dexter T., Mahler-Araujo B., Fenwick K., Iravani M., Grigoriadis A., Parry S., Robertson D., Mackay A., Ashworth A., Yarnold J.R., Isacke C.M. Genome-wide transcriptomic profiling of microdissected human breast tissue reveals differential expression of KIT (c-Kit, CD117) and oestrogen receptor-alpha (ERalpha) in response to therapeutic radiation. J Pathol. 2009;219:131–140. doi: 10.1002/path.2581. [DOI] [PubMed] [Google Scholar]
- 9.Weller K., Foitzik K., Paus R., Syska W., Maurer M. Mast cells are required for normal healing of skin wounds in mice. FASEB J. 2006;20:2366–2368. doi: 10.1096/fj.06-5837fje. [DOI] [PubMed] [Google Scholar]
- 10.Galli S.J., Tsai M. Mast cells: versatile regulators of inflammation, tissue remodeling, host defense and homeostasis. J Dermatol Sci. 2008;49:7–19. doi: 10.1016/j.jdermsci.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maltby S., Khazaie K., McNagny K.M. Mast cells in tumor growth: angiogenesis, tissue remodelling and immune-modulation. Biochim Biophys Acta. 2009;1796:19–26. doi: 10.1016/j.bbcan.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He S.H. Key role of mast cells and their major secretory products in inflammatory bowel disease. World J Gastroenterol. 2004;10:309–318. doi: 10.3748/wjg.v10.i3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crivellato E., Beltrami C.A., Mallardi F., Ribatti D. The mast cell: an active participant or an innocent bystander? Histol Histopathol. 2004;19:259–270. doi: 10.14670/HH-19.259. [DOI] [PubMed] [Google Scholar]
- 14.Zheng H., Wang J., Hauer-Jensen M. Role of mast cells in early and delayed radiation injury in rat intestine. Radiat Res. 2000;153:533–539. doi: 10.1667/0033-7587(2000)153[0533:romcie]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Richter K.K., Langberg C.W., Sung C.C., Hauer-Jensen M. Increased transforming growth factor beta (TGF-beta) immunoreactivity is independently associated with chronic injury in both consequential and primary radiation enteropathy. Int J Radiat Oncol Biol Phys. 1997;39:187–195. doi: 10.1016/s0360-3016(97)00290-3. [DOI] [PubMed] [Google Scholar]
- 16.Boerma M., Wang J., Wondergem J., Joseph J., Qiu X., Kennedy R.H., Hauer-Jensen M. Influence of mast cells on structural and functional manifestations of radiation-induced heart disease. Cancer Res. 2005;65:3100–3107. doi: 10.1158/0008-5472.CAN-04-4333. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe S., Watanabe K., Oishi T., Aiba M., Kageyama K. Mast cells in the rat alveolar septa undergoing fibrosis after ionizing irradiation: Ultrastructural and histochemical studies. Lab Invest. 1974;31:555–567. [PubMed] [Google Scholar]
- 18.Ward W.F., Molteni A., Ts'ao C.H., Hinz J.M. Captopril reduces collagen and mast cell accumulation in irradiated rat lung. Int J Radiat Oncol Biol Phys. 1990;19:1405–1409. doi: 10.1016/0360-3016(90)90351-j. [DOI] [PubMed] [Google Scholar]
- 19.Grimbaldeston M.A., Chen C.C., Piliponsky A.M., Tsai M., Tam S.Y., Galli S.J. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–848. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milliat F., François A., Isoir M., Deutsch E., Tamarat R., Tarlet G., Atfi A., Validire P., Bourhis J., Sabourin J.C., Benderitter M. Influence of endothelial cells on vascular smooth muscle cells phenotype after irradiation: implication in radiation-induced vascular damages. Am J Pathol. 2006;169:1484–1495. doi: 10.2353/ajpath.2006.060116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skwarchuk M.W., Travis E.L. Volume effects and epithelial regeneration in irradiated mouse colorectum. Radiat Res. 1998;149:1–10. [PubMed] [Google Scholar]
- 22.Skwarchuk M.W., Travis E.L. Changes in histology and fibrogenic cytokines in irradiated colorectum of two murine strains. Int J Radiat Oncol Biol Phys. 1998;42:169–178. doi: 10.1016/s0360-3016(98)00201-6. [DOI] [PubMed] [Google Scholar]
- 23.Jullien N., Blirando K., Milliat F., Sabourin J.C., Benderitter M., François A. Up-regulation of endothelin type A receptor in human and rat radiation proctitis: preclinical therapeutic approach with endothelin receptor blockade. Int J Radiat Oncol Biol Phys. 2009;74:528–538. doi: 10.1016/j.ijrobp.2008.12.086. [DOI] [PubMed] [Google Scholar]
- 24.Hauer Jensen M., Sauer T., Devik F., Nygaard K. Effects of dose fractionation on late roentgen radiation damage of rat small intestine. Acta Radiol Oncol. 1983;22:381–384. doi: 10.3109/02841868309134056. [DOI] [PubMed] [Google Scholar]
- 25.Bourgier C., Haydont V., Milliat F., François A., Holler V., Lasser P., Bourhis J., Mathé D., Vozenin-Brotons M.C. Inhibition of Rho kinase modulates radiation induced fibrogenic phenotype in intestinal smooth muscle cells through alteration of the cytoskeleton and connective tissue growth factor expression. Gut. 2005;54:336–343. doi: 10.1136/gut.2004.051169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gelbmann C.M., Mestermann S., Gross V., Köllinger M., Schölmerich J., Falk W. Strictures in Crohn's disease are characterised by an accumulation of mast cells colocalised with laminin but not with fibronectin or vitronectin. Gut. 1999;45:210–217. doi: 10.1136/gut.45.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denham J.W., Hauer-Jensen M., Kron T., Langberg C.W. Treatment-time-dependence models of early and delayed radiation injury in rat small intestine. Int J Radiat Oncol Biol Phys. 2000;48:871–887. doi: 10.1016/s0360-3016(00)00708-2. [DOI] [PubMed] [Google Scholar]
- 28.Zheng H., Wang J., Koteliansky V.E., Gotwals P.J., Hauer-Jensen M. Recombinant soluble transforming growth factor beta type II receptor ameliorates radiation enteropathy in mice. Gastroenterology. 2000;119:1286–1296. doi: 10.1053/gast.2000.19282. [DOI] [PubMed] [Google Scholar]
- 29.Wang J., Albertson C.M., Zheng H., Fink L.M., Herbert J.M., Hauer-Jensen M. Short-term inhibition of ADP-induced platelet aggregation by clopidogrel ameliorates radiation-induced toxicity in rat small intestine. Thromb Haemost. 2002;87:122–128. [PubMed] [Google Scholar]
- 30.Wang J., Zheng H., Ou X., Albertson C.M., Fink L.M., Herbert J.M., Hauer-Jensen M. Hirudin ameliorates intestinal radiation toxicity in the rat: support for thrombin inhibition as strategy to minimize side-effects after radiation therapy and as countermeasure against radiation exposure. J Thromb Haemost. 2004;2:2027–2035. doi: 10.1111/j.1538-7836.2004.00960.x. [DOI] [PubMed] [Google Scholar]
- 31.Torres S., Thim L., Milliat F., Vozenin-Brotons M.C., Olsen U.B., Ahnfelt-Rønne I., Bourhis J., Benderitter M., François A. Glucagon-like peptide-2 improves both acute and late experimental radiation enteritis in the rat. Int J Radiat Oncol Biol Phys. 2007;69:1563–1571. doi: 10.1016/j.ijrobp.2007.08.051. [DOI] [PubMed] [Google Scholar]
- 32.Milliat F., Sabourin J.C., Tarlet G., Holler V., Deutsch E., Buard V., Tamarat R., Atfi A., Benderitter M., François A. Essential role of plasminogen activator inhibitor type-1 in radiation enteropathy. Am J Pathol. 2008;172:691–701. doi: 10.2353/ajpath.2008.070930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Brien-Ladner A.R., Wesselius L.J., Stechschulte D.J. Bleomycin injury of the lung in a mast-cell-deficient model. Agents Actions. 1993;39:20–24. doi: 10.1007/BF01975709. [DOI] [PubMed] [Google Scholar]
- 34.Okazaki T., Hirota S., Xu Z.D., Maeyama K., Nakama A., Kawano S., Hori M., Kitamura Y. Increase of mast cells in the liver and lung may be associated with but not a cause of fibrosis: demonstration using mast cell-deficient Ws/Ws rats. Lab Invest. 1998;78:1431–1438. [PubMed] [Google Scholar]
- 35.Crawford P.A., Gordon J.I. Microbial regulation of intestinal radiosensitivity. Proc Natl Acad Sci USA. 2005;102:13254–13259. doi: 10.1073/pnas.0504830102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flanders K.C., Ho B.M., Arany P.R., Stuelten C., Mamura M., Paterniti M.O., Sowers A., Mitchell J.B., Roberts A.B. Absence of Smad3 induces neutrophil migration after cutaneous irradiation: possible contribution to subsequent radioprotection. Am J Pathol. 2008;173:68–76. doi: 10.2353/ajpath.2008.070937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanamaru Y., Sumiyoshi K., Ushio H., Ogawa H., Okumura K., Nakao A. Smad3 deficiency in mast cells provides efficient host protection against acute septic peritonitis. J Immunol. 2005;174:4193–4197. doi: 10.4049/jimmunol.174.7.4193. [DOI] [PubMed] [Google Scholar]
- 38.Alves-Filho J.C., Sônego F., Souto F.O., Freitas A., Verri W.A., Jr, Auxiliadora-Martins M., Basile-Filho A., McKenzie A.N., Xu D., Cunha F.Q., Liew F.Y. Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat Med. 2010;16:708–712. doi: 10.1038/nm.2156. [DOI] [PubMed] [Google Scholar]
- 39.Orr A.W., Hastings N.E., Blackman B.R., Wamhoff B.R. Complex regulation and function of the inflammatory smooth muscle cell phenotype in atherosclerosis. J Vasc Res. 2009;47:168–180. doi: 10.1159/000250095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woodman L., Siddiqui S., Cruse G., Sutcliffe A., Saunders R., Kaur D., Bradding P., Brightling C. Mast cells promote airway smooth muscle cell differentiation via autocrine up-regulation of TGF-beta 1. J Immunol. 2008;181:5001–5007. doi: 10.4049/jimmunol.181.7.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bradding P. Mast cell regulation of airway smooth muscle function in asthma. Eur Respir J. 2007;29:827–830. doi: 10.1183/09031936.00017707. [DOI] [PubMed] [Google Scholar]
- 42.Salinthone S., Singer C.A., Gerthoffer W.T. Inflammatory gene expression by human colonic smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G627–G637. doi: 10.1152/ajpgi.00462.2003. [DOI] [PubMed] [Google Scholar]
- 43.Linard C., Ropenga A., Vozenin-Brotons M.C., Chapel A., Mathe D. Abdominal irradiation increases inflammatory cytokine expression and activates NF-kappaB in rat ileal muscularis layer. Am J Physiol Gastrointest Liver Physiol. 2003;285:G556–G565. doi: 10.1152/ajpgi.00094.2003. [DOI] [PubMed] [Google Scholar]
- 44.Wang J., Zheng H., Sung C.C., Richter K.K., Hauer-Jensen M. Cellular sources of transforming growth factor-beta isoforms in early and chronic radiation enteropathy. Am J Pathol. 1998;153:1531–1540. doi: 10.1016/s0002-9440(10)65741-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moriez R., Leveque M., Salvador-Cartier C., Barreau F., Theodorou V., Fioramonti J., Bueno L., Eutamene H. Mucosal mast cell proteases are involved in colonic permeability alterations and subsequent bacterial translocation in endotoxemic rats. Shock. 2007;28:118–124. doi: 10.1097/SHK.0b013e3180315ba9. [DOI] [PubMed] [Google Scholar]
- 46.Bueno L., Fioramonti J. Effects of inflammatory mediators on gut sensitivity. Can J Gastroenterol. 1999;(Suppl 13 A):42A–46A. doi: 10.1155/1999/846809. [DOI] [PubMed] [Google Scholar]
- 47.Gay J., Fioramonti J., Garcia-Villar R., Buéno L. Alterations of intestinal motor responses to various stimuli after Nippostrongylus brasiliensis infection in rats: role of mast cells. Neurogastroenterol Motil. 2000;12:207–214. doi: 10.1046/j.1365-2982.2000.00201.x. [DOI] [PubMed] [Google Scholar]
- 48.Clarke D.L., Clifford R.L., Jindarat S., Proud D., Pang L., Belvisi M., Knox A.J. TNFalpha and IFNgamma synergistically enhance transcriptional activation of CXCL10 in human airway smooth muscle cells via STAT-1, NF-kappaB, and the transcriptional coactivator CREB-binding protein. J Biol Chem. 2010;285:29101–29110. doi: 10.1074/jbc.M109.0999952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mäyränpää M.I., Trosien J.A., Fontaine V., Folkesson M., Kazi M., Eriksson P., Swedenborg J., Hedin U. Mast cells associate with neovessels in the media and adventitia of abdominal aortic aneurysms. J Vasc Surg. 2009;50:388–395. doi: 10.1016/j.jvs.2009.03.055. discussion 395–386. [DOI] [PubMed] [Google Scholar]
- 50.Sun J., Sukhova G.K., Wolters P.J., Yang M., Kitamoto S., Libby P., MacFarlane L.A., Mallen-St Clair J., Shi G.P. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007;13:719–724. doi: 10.1038/nm1601. [DOI] [PubMed] [Google Scholar]
- 51.Miyazawa N., Watanabe S., Matsuda A., Kondo K., Hashimoto H., Umemura K., Nakashima M. Role of histamine H1 and H2 receptor antagonists in the prevention of intimal thickening. Eur J Pharmacol. 1998;362:53–59. doi: 10.1016/s0014-2999(98)00716-x. [DOI] [PubMed] [Google Scholar]
- 52.Grimm M.C., Elsbury S.K., Pavli P., Doe W.F. Interleukin 8: cells of origin in inflammatory bowel disease. Gut. 1996;38:90–98. doi: 10.1136/gut.38.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang D., Dubois R.N. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene. 2010;29:781–788. doi: 10.1038/onc.2009.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vardeh D., Wang D., Costigan M., Lazarus M., Saper C.B., Woolf C.J., Fitzgerald G.A., Samad T.A. COX2 in CNS neural cells mediates mechanical inflammatory pain hypersensitivity in mice. J Clin Invest. 2009;119:287–294. doi: 10.1172/JCI37098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gruber B.L., Marchese M.J., Kew R.R. Transforming growth factor-beta 1 mediates mast cell chemotaxis. J Immunol. 1994;152:5860–5867. [PubMed] [Google Scholar]
- 56.Hollins F., Kaur D., Yang W., Cruse G., Saunders R., Sutcliffe A., Berger P., Ito A., Brightling C.E., Bradding P. Human airway smooth muscle promotes human lung mast cell survival, proliferation, and constitutive activation: cooperative roles for CADM1, stem cell factor, and IL-6. J Immunol. 2008;181:2772–2780. doi: 10.4049/jimmunol.181.4.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas D.M., Fox J., Haston C.K. Imatinib therapy reduces radiation-induced pulmonary mast cell influx and delays lung disease in the mouse. Int J Radiat Biol. 2010;86:436–444. doi: 10.3109/09553001003674863. [DOI] [PubMed] [Google Scholar]


