Abstract
Circulating tumor cell (CTC) number in metastatic cancer patients yields prognostic information consistent with enhanced cell migration and invasion via loss of adhesion, a feature of epithelial-to-mesenchymal transition (EMT). Tumor cells also invade via collective migration with maintained cell-cell contacts and consistent with this is the circulating tumor microemboli (CTM; contiguous groups of tumor cells) that are observed in metastatic cancer patients. Using a blood filtration approach, we examined markers of EMT (cytokeratins, E-cadherin, vimentin, neural cadherin) and prevalence of apoptosis in CTCs and CTM to explore likely mechanism(s) of invasion in lung cancer patients and address the hypothesis that cells within CTM have a survival advantage. Intra-patient and inter-patient heterogeneity was observed for EMT markers in CTCs and CTM. Vimentin was only expressed in some CTCs, but in the majority of cells within CTM; E-cadherin expression was lost, cytoplasmic or nuclear, and rarely expressed at the surface of the cells within CTM. A subpopulation of CTCs was apoptotic, but apoptosis was absent within CTM. This pilot study suggests that EMT is not prosecuted homogeneously in tumor cells within the circulation of lung cancer patients and that collective migration and enhanced survival of cells within CTM might contribute to lung cancer metastasis. Multiplex analysis and further detailed exploration of metastatic potential and EMT in CTCs/CTM is now warranted in a larger patient cohort.
Metastasis usually portends a dismal prognosis for cancer patients and effective therapeutic intervention in the metastatic process remains elusive. This is the case despite decades of research after Paget's “seed and soil” hypothesis in 1889 to explain why primary tumors within one particular organ give rise to secondary tumors at nonrandom sites1 and Ewing's suggestion in 1929 that mechanical factors associated with the anatomy of human vasculature also determine the final destination of metastasizing tumor cells.2 It is now apparent that tumor cell invasion and formation of distant metastasis can progress via three major routes: i) via the bloodstream, ii) via lymphatic vessels, and iii) via transcoelomic spread into the pleural, pericardial, and abdominal cavities.3 The hematogenous system is thought to be the primary and most common route for the formation of distant metastases. Disseminating tumor cells can also circulate to and lie dormant in the bone marrow, potentially for a number of years, and then re-enter the bloodstream en route to secondary metastatic sites.4
According to the widely espoused epithelial-to-mesenchymal transition (EMT) paradigm, suggested by some as essential for metastasis,5,6 invading mesenchymal tumor cells lose cell-cell adhesion. Consistent with this concept, there are increasing reports enumerating individual circulating tumor cells (CTCs) in cancer patients' blood samples. Moreover, using the Food and Drug Administration's approved CellSearch platform, the CTC number is a prognostic biomarker in metastatic breast, prostate, and colorectal cancer patients.7–11 The phenomenon of “partial” or “incomplete” EMT is also purported, in which metastasizing cells adopt some mesenchymal features (eg, expression of vimentin and neural cadherin) but retain some epithelial characteristics (eg, cytokeratin and membrane E-cadherin).12 An alternative model for metastasis involving tumor cell co-operativity has also been postulated based on a rodent model that demonstrated mesenchymal cells provided invasive capability to allow “passenger” noninvasive epithelial cells access to the blood stream where they survived and were responsible for metastasis.13 During collective cell migration, now thought to be an important mechanism of tumor cell invasion,14,15 cancer cells with maintained cell-cell contacts move through tissues in groups. Tumor cell clusters, termed circulating tumor microemboli (CTM) have been reported in the blood stream of colorectal, renal, and prostate cancer patients.16–18 Potentially, CTM could reflect the intravasation of tumor cells that had migrated collectively and entered the blood stream via the “leaky” and chaotic tumor vessels that are a feature of highly angiogenic tumors. This may have important implications; pioneering studies in animal models suggested that i.v. injected CTM have a greater tendency to form metastases than do the equivalent number of injected single tumor cells, and that injection of large clusters of tumor cells produced more metastatic foci than injection of smaller tumor cell clusters.19 Intriguingly, based on these studies in animal models, <0.1% tumor cells in the bloodstream are thought to be capable of secondary tumor formation.14 It has been suggested that this metastatic inefficiency may be due, in part, to the inability of single CTCs to evade anoikis, a form of apoptosis induced by detachment from extracellular matrix and loss of cell-cell contacts20 leading to the hypothesis, as yet untested in cancer patients, that tumor cells within CTM have a survival advantage.
While the preclinical studies3,19 paved the way to understanding the mechanisms of metastasis, one limitation to increased comprehension of human metastasis biology has been the reliance of clinical studies on comparison between primary and secondary tumor biopsies; this has not included a detailed examination of tumor cell phenotype while in transit within the circulation. However, the molecular characterization of circulating tumor cells in cancer patients has been frustrated hitherto by substantial technical hurdles. Using a filter-based size exclusion approach (ISET, Metagenex, Paris, France) alongside immunomagnetic separation based on differential epithelial cell adhesion molecule (EpCam) expression between tumor and blood cells (CellSearch, Veridex, Raritan, NJ), this pilot study begins to explore the viability and phenotype of tumor cells in peripheral blood samples from patients with advanced lung cancer.
Materials and Methods
Patients
All patients gave written, informed consent to donate blood samples for research, according to an ethically approved protocol. Patient characteristics are presented in Table 1. All patients in this study underwent full staging, histopathologic confirmation of the diagnosis, and the patients were treated with palliative intent platinum-based chemotherapy. Parallel blood samples were drawn for storage in CellSave tubes (Veridex) at room temperature for subsequent analysis using the CellSearch platform (Veridex) within 96 hours, or in EDTA tubes stored at 4°C for subsequent evaluation using ISET (Metagenex) within 4 hours.
Table.
Clinical Characteristics of Lung Cancer Patients
| Patient | Age | Sex | Smoking history | Presenting symptoms | PS | Histology | Stage | Site of primary tumor | Others sites of disease | Treatment | Survival |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 63 | F | 80-pack year | Generally unwell, hyponatraemia | 2 | Small cell lung cancer | T4N2M1 extensive | Right middle lobe | Mediastinal nodes, pleura, pericardium, and liver | Carboplatin | Died 51 days |
| 2 | 76 | M | 40-pack year | Breathlessness hyponatraemia | 2 | Small cell lung cancer | T4N2M1 extensive | Right main bronchus | Mediastinal nodes, left adrenal gland, and liver | Carboplatin | Alive 71 days |
| 3 | 73 | M | 58-pack year | Weight loss, anorexia | 2 | Small cell lung cancer | T4N3M1 extensive | Left lower lobe | Mediastinal nodes, pleura, and left adrenal gland | Carboplatin | Died 115 days |
| 4 | 65 | F | 25-pack year | Breathlessness, cough, weight loss | 1 | Nonsmall cell lung cancer (adenocarcinoma) | T4N3M0 stage IIIB | Left lower lobe | Mediastinal nodes | Gemcitabine and carboplatin (sequential XRT) | Alive 108 days |
| 5 | 72 | M | 30-pack year | Cough | 0 | Nonsmall cell lung cancer (bronchioalveolar) | T2N0M1 stage IV | Right lower lobe | Multiple pulmonary nodules | Gemcitabine and carboplatin, erlotinib, XRT to spine | Died 330 days |
| Relapse after 1st-line treatment at 3 months with liver and bone metastases | |||||||||||
| 6 | 77 | M | 80-pack year | Interscapular back pain | 1 | Nonsmall cell lung cancer (squamous cell carcinoma) | T4N1M0 stage IIIB | Right upper lobe | No metastases at presentation | Gemcitabine and carboplatin (sequential XRT lung) | Died 210 days |
| Relapse after 1st-line treatment at 6 months with bone metastases |
F, female; M, male; PS, performance status; XRT, X-ray therapy.
Characterization of Tumor Cells
Tumor cells in 7.5 ml blood samples were assessed using the CellSearch technology according to manufacturer's instructions (Veridex)21 where a tumor cell was defined as that co-expressing EpCam and cytokeratins (CKs, recognizing 8, 18, and 19) without expression of white blood cell (WBC) surface marker CD45 and had a DAPI stained nucleus. Here, cells are presented in a gallery format as mono- and composite pseudo-fluorescent images for manual categorization. Apoptotic cells were identified via characteristic fragmented and condensed DAPI stained nuclear morphology.22 CTM were defined as groups or clusters of tumor cells containing three or more distinct nuclei.
Blood samples were also examined after red blood cell lysis in MetaBuffer (containing 0.8% formaldehyde, Metagenex, Paris, France) and filtration through polycarbonate membranes containing 8 μm pores for the presence of CTCs and CTM using the ISET platform, according to the manufacturer's instructions (Metagenex).23 Filtration of each patient's blood sample (10 ml blood) yielded 10 individual spots on the membrane on which cells or groups of cells >8 μm were deposited. The membrane spots were cut out and subjected to single or dual marker immunohistochemistry (IHC) for CTC/CTM identification and molecular characterization. Tumor cells were identified as those without expression of CD45 (1:40, clone T29/33; Dako, Ely, Cambridgeshire, UK) and by their distinctive nuclear morphology (irregularly shaped and hyperchromatic nuclei).
Immunohistochemistry
Single marker IHC was performed downstream of ISET for each patient. WBC contaminants had a distinctive nuclear morphology, and were rarely found in clusters; four membrane spots were stained for CD45 to confirm WBC exclusion. EMT markers were analyzed one by one using the remaining membrane spots using standard Envision Kits and the Liquid DAB+ Substrate Chromagen System according to manufacturer's instructions (Dako). To investigate EMT, ISET isolated tumor cells were stained for E-cadherin (1:1000, clone 36/E-cadherin; BD Biosciences, San Jose, CA), cytokeratins 4, 5, 6, 8, 10, 13, and 18 (1:100, clone C11; NeoMarkers, Fremont, CA), vimentin (1:100, clone V9; Dako), or neural cadherin (1:100, clone 32/neural cadherin; BD Biosciences). To explore the observed heterogeneity of staining with single EMT markers further, dual-color IHC was performed on a membrane spot from each patient for E-cadherin (1:50, clone 24E10, rabbit mAb; Cell Signaling) and vimentin (1:100, clone V9, mouse; Dako) using EnVision G/2 Doublestain System, rabbit/mouse (DAB+/Permanent Red; Dako). To ascertain whether ISET-identified tumor cells that were unlikely to be captured using the CellSearch system, ISET-isolated CTCs/CTM were examined for EpCam expression (1:100, clone VU-1D9; NeoMarkers). WBCs do not express EpCam, and thus there was no need for dual IHC staining with CD45 to address this question.
SCLC Cell Culture
Cultured small cell lung cancer (SCLC) cell lines were used as positive and negative controls for EMT marker expression. H526 and H524 cells were maintained in RPMI 1640 medium with 10% fetal calf serum, H345 in HITES medium and DMS114 cells in Waymouth medium with 10% fetal calf serum. Cells were cultured in a humidified incubator in 5% CO2 at 37°C, harvested, fixed and stained for EMT markers using the same procedures as for CTM/CTC. WBCs served as negative staining controls for analysis of CKs and EpCam. Images were taken at ×40 magnification using an Olympus BX52 microscope linked with image analysis software (Allegro Plus, Bioview, Rehovot, Israel).
Results
Tumor Cells Within CTM in Lung Cancer Patients May Have a Survival Advantage
The patient who prompted us to conduct this study was a 72-year-old man who was a lifelong smoker, and who presented with a lump in the left axilla, with left-sided chest pain and weight loss. A computed tomographic scan revealed a lung mass, multiple intrapulmonary nodules, and mediastinal lymphadenopathy, in addition to the axillary mass, which was biopsied and was consistent with non small cell lung cancer (NSCLC). There were no contraindications to palliative, platinum-based chemotherapy, and no significant comorbid conditions. Three days after commencing carboplatin and gemcitabine chemotherapy, the patient died of suspected pulmonary embolism. The pathology review reported that the cellular morphology of the CTM in this patient's peripheral blood (identified using ISET) was closely similar to that observed in the tumor biopsy taken from his axillary node (Figure 1, A and B). Altogether the 37 CTM detected in this patient contained 418 distinct nuclei in 3 ml blood. These cells were CD45 negative and had nuclear morphology distinct from both WBC and endothelial cells. The phenotype and viability of CTC and CTM was then explored in six further typical lung cancer patients with various histological subtypes.
Figure 1.
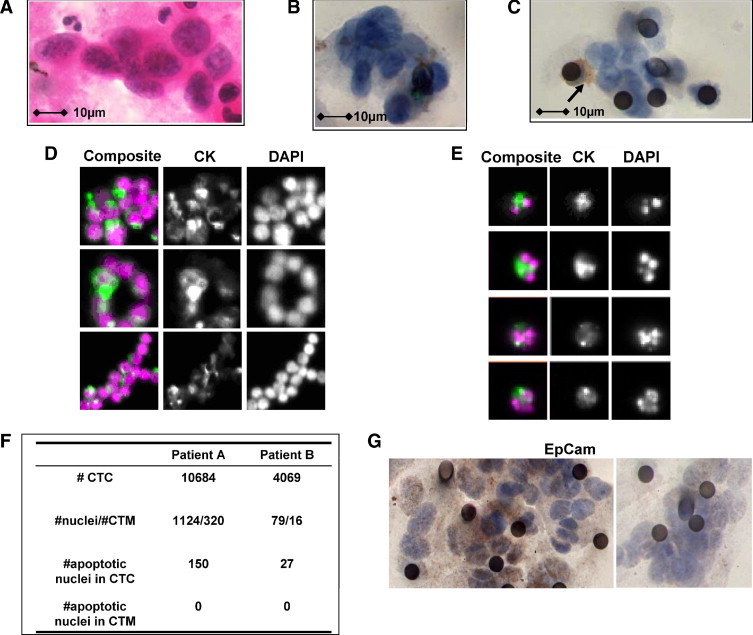
Circulating tumor microemboli (CTM) and circulating tumor cells (CTCs) in lung cancer patients. A: The archival diagnostic cytology specimen from our index patient with non-small cell lung cancer (NSCLC), procured 1 month before recruitment to the study. Cells were obtained by fine needle aspirate from an axillary lymph node metastasis and spread onto a glass slide. Morphological analysis with Papanicolaou's staining confirmed the diagnosis of NSCLC. B: The representative image of CTM isolated by ISET from the same patient as described in A. C: An image of CTM isolated by ISET from patient 1 with extensive stage small cell lung cancer (SCLC). The black circles are the 8 μm pores in the ISET membranes. Membranes were stained for the leukocyte marker CD45 and contaminant white blood cells (WBCs) were identified by positive brown CD45 staining (shown with arrow). D: Typical images (composite and single pseudo-color fluorescence) for CD45 negative, cytokeratin positive, and DAPI stained SCLC CTM identified by CellSearch technology (see Materials and Methods), with the intact and homogenous DAPI stained nuclei of viable CTCs compared to the nuclei of apoptotic CTCs in Figure 1E. E: Similar CellSearch gallery presentations of apoptotic single CTCs with condensed or fragmented DAPI stained nuclei. F: Tabulation of the prevalence and apoptotic profile of the CellSearch isolated CTCs and CTM from SCLC patients I and II. G: Displays EpCam positive stained CTM (left panel) and EpCam negative CTM (right panel) isolated by ISET from the same patient with NSCLC.
In patients with SCLC, CTM were identified by both ISET and CellSearch methodologies (eg, Figure 1, C and D). Using CellSearch, which defines tumor cells as both EpCam and cytokeratin positive, SCLC CTM were composed of clusters, rings, and elongated strands (Figure 1D) consistent with some of the morphologies described for collective migration.15 Blood samples from SCLC patients I and II had high CTC counts of >1000 cells in 7.5 ml blood consistent with our previous study24 and contained numerous CTM allowing a comparative appraisal of the prevalence of nuclei that exhibited the classical condensed and fragmented nuclear morphologies of apoptosis (Figure 1E).22 While numerous apoptotic CTCs were detected in both of these patients, no cells with nuclear apoptotic morphology (DAPI stained) were observed in any of the cells forming CTM (summarized in Figure 1F). Similarly using the ISET approach, no cells were identified with apoptotic nuclei (H&E stained) within CTM of SCLC or NSCLC patients.
Epithelial Markers Are Heterogeneously Expressed in CTM and CTCs of Lung Cancer Patients
In contrast to the detection of CTM in SCLC patients by both techniques, although identified by ISET, NSCLC CTM were not detected by CellSearch, which relies on EpCam expression for isolation of CTC/CTM. IHC analysis of EpCam in ISET isolated CTM from NSCLC patients, which demonstrated a heterogeneous expression profile. Figure 1G exemplifies EpCam-positive (left panel) and EpCam-negative CTM (right panel) from the same patient with NSCLC.
CTM and CTCs isolated by ISET from patients with SCLC (I-III) and NSCLC (IV-VI) were examined by IHC for EMT markers. Representative images are shown in Figure 2 for single staining of epithelial markers E-cadherin or CKs. The expression of E-Cadherin in epithelial cells is often described as linear and plasma membrane localized as was observed in cytospins of H526 SCLC cells that grow as clusters (Figure 2A, right panel). Figure 2A (left and middle panel) shows examples of E-cadherin expression in CTM and CTCs isolated from SCLC patients. There was no evidence of plasma membrane E-cadherin staining in solitary CTCs, as predicted by the EMT paradigm, but counterintuitively, loss of plasma membrane located E-cadherin was observed in the majority of CTM. However, considerable interpatient and intrapatient heterogeneity was observed regarding loss or cytoplasmic and/or nuclear re-localization of E-cadherin between CTCs and CTM, and even within a CTM (Figure 2A, left panel). Similarly, heterogeneity of E-cadherin expression and subcellular location was noted in CTM of NSCLC patients (not shown).
Figure 2.
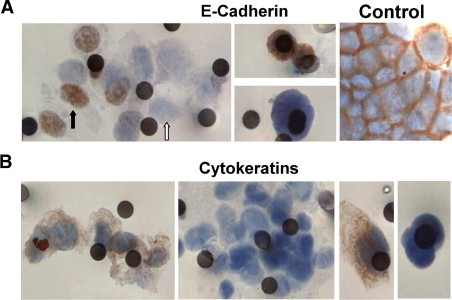
Profile of epithelial markers in circulating tumor microemboli (CTM) and circulating tumor cells (CTCs) from lung cancer patients. Blood samples from lung cancer patients were subjected to ISET filtration and CTM/CTCs were profiled for E-cadherin or CKs (see Materials and Methods). A: E-cadherin expression profiles in CTM (left panel), CTCs (middle panels) from patients with SCLC, and in cultured SCLC H526 cells that served as positive control for normal staining profile of E-cadherin. Black and white arrows indicate nuclear staining of E-cadherin and loss of E-cadherin, respectively. B: The heterogeneous expression of CKs in CTM (left and middle left panel) and CTCs (middle right and right panel) from patients with lung cancer. Images shown are representative from the patient cohort (see Table 1).
Cytokeratins are major intermediate filament proteins and epithelial cell markers and their loss is an attribute of EMT by the strict definition. However, Figure 2B shows intrapatient heterogeneity in expression of CKs in CTM [CK-positive CTM (left panel), CK-negative CTM (left middle panel)] and CTCs [CK-positive CTC (right middle panel), CK-negative CTC (right panel)], which is more consistent with incomplete EMT in some tumor cells within the circulation. Similarly, SCLC CTM detected by CellSearch also demonstrated inter-patient heterogeneity and even within CTM heterogeneity in expression of CK (Figure 1D).
Mesenchymal Markers Are Heterogeneously Expressed in CTM and CTCs of Lung Cancer Patients
CTM and CTCs isolated by ISET from patients with SCLC (I-III) and NSCLC (IV-VI) were examined by IHC for mesenchymal markers vimentin or neural cadherin with representative images displayed in Figure 3. The majority of CTM identified in patients I-VI exhibited readily detectable or strongly expressed vimentin consistent with a mesenchymal phenotype, but at odds with the concept of upregulated vimentin expression being tightly linked to loss of cell-cell adhesion during EMT. Figure 3A shows examples of heterogeneous vimentin expression in CTM (left and middle panel) and CTCs (upper and lower right panel) from patients with lung cancer.
Figure 3.
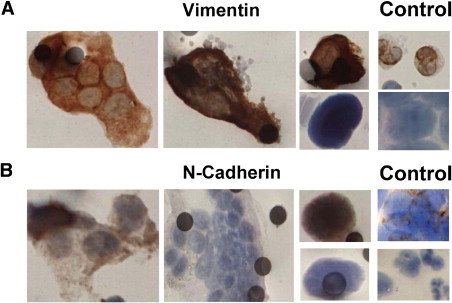
Profile of mesenchymal markers in circulating tumor microemboli (CTM) and circulating tumor cells (CTCs) from lung cancer patients. Blood samples from lung cancer patients were subjected to ISET filtration and CTM/CTCs were profiled for vimentin or neural cadherin (see Materials and Methods). A: Vimentin staining in lung cancer patients' CTM (left and middle panel), CTCs (upper and lower right panel), with white blood cells and cultured DMS114, used as positive and negative staining controls. White blood cells are discriminated from CTM/CTCs by virtue of their smaller size and distinct nuclear morphology. B: Heterogeneous staining for neural cadherin in both CTM (left and middle panel) and CTCs (upper and lower right panel). Cultured H524 SCLC cells were used as the positive control and white blood cells were negatively stained and distinguished from tumor cells by virtue of their smaller size and distinct nuclear morphology. Images shown are representative from the patient cohort (see Table 1).
A second mesenchymal marker, neural cadherin was examined on separate membrane spots, to further explore the EMT profile of tumor cells in the circulation. Neural cadherin is categorized as an invasive cadherin and its expression is associated with increased cell motility and promotion of tumor invasion, and it is frequently up-regulated in cancers in tandem with loss of plasma membrane located E-cadherin.25 Heterogeneous expression of neural cadherin was observed in CTC and CTM within and between patients, again consistent with a partial EMT phenotype. Figure 3B shows examples of positive neural cadherin staining in CTM (left panel), negative staining of a CTM (middle panel), and examples of a positively and a negatively stained CTC (upper and lower right panel).
The results using single marker staining for E-cadherin, CKs, vimentin, or neural cadherin, all showed considerable cell heterogeneity within and between patients, and in some instances even between cells within a CTM. These data, however, could not address the question of whether CTCs or cells within CTM express markers of both mesenchymal and epithelial phenotypes. To approach this technically more challenging objective, a two-color IHC method was adapted to stain E-cadherin and vimentin in ISET isolated CTCs and CTM simultaneously from blood samples of all of the patients in this pilot study. Figure 4 shows examples of this dual staining: all vimentin positive (red) CTM observed were negative for E-cadherin (brown), (panel A); panel B shows a rare example of a CTM with membraneous E-cadherin staining, which did not express vimentin; Figure 4C shows a CTC with cytoplasmic E-cadherin that did not express vimentin, and on the same membrane spot, a small cell, most likely a WBC that is vimentin positive and E-cadherin negative; Figure 4D shows vimentin positive, E-cadherin negative WBCs with typical neutrophil nuclear morphology. The dual staining further exemplified the heterogeneity of CTM and CTCs in lung cancer patients, but so far has not revealed cells that express both vimentin and E-cadherin (whether located at the plasma membrane or in the cytosol or nucleus). Further development of multi-staining methodology will be required to explore EMT in tumor cells circulating in lung cancer patients in more detail.
Figure 4.
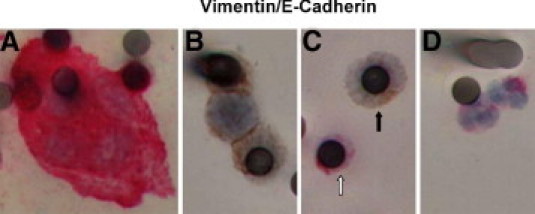
Epithelial (E-cadherin) and mesenchymal (vimentin) markers in circulating tumor microemboli (CTM) and circulating tumor cells (CTCs) profiled by dual-color IHC. Blood samples from lung cancer patients were subjected to ISET filtration and CTM/CTCs were profiled for both epithelial (E-cadherin) and mesenchymal (vimentin) markers by dual-color IHC (see Materials and Methods). A: Vimentin-positive CTM (cytoplasm stained in red) with negative staining for E-cadherin (brown). B: E-cadherin positive CTM (brown) without vimentin expression. C: Displays CTC (black arrow), which is positively stained for E-cadherin in cytosol and negative for vimentin. White blood cells (white arrow in C and cells in D) serve as control and demonstrate positive staining for vimentin, negative staining for E-cadherin, and typical neutrophil nuclear morphology.
Discussion
It is widely accepted that neoplasms are heterogeneous. However, evidence for this is mainly derived from preclinical studies and/or histopathology of primary compared to metastatic tumors.3 The present study, to our knowledge, for the first time has revealed the heterogeneity of cancer cells in the circulation by beginning to profile the phenotypes of CTM and CTCs from lung cancer patients.
The observation that there was no evidence of apoptosis within CTM in contrast to single CTCs is consistent with the hypothesis that tumor cells in CTM (with assumed maintenance of cell-to-cell survival signals) are more likely to suppress anoikis than single CTCs, as predicted by the earlier preclinical studies.19 An enhanced survival advantage of CTM also might be afforded by continued production of autocrine pro-migratory factors, matrix proteases (required for collective migration),26 and protection of the innermost cells within CTM from immunological assault by lymphocytes and natural-killer cells.
This exploratory study revealed considerable heterogeneity within and between lung cancer patients regarding epithelial and mesenchymal markers in CTM and CTCs. Intuitively, based on the widely reported premise of EMT that invasion is accomplished by mesenchymal tumor cells,5 and that reversion to the epithelial phenotype occurs at the site of metastasis,5 one might expect tumor cells in the circulation to have undergone EMT and circulating mesenchymal tumor cells to have lost cell-cell contacts. A predominant characteristic of EMT is loss or subcellular redistribution of the epithelial adhesion molecule E-cadherin, a homophilic transmembrane glycoprotein that constructs a tight junction connection to adjacent epithelial cells.27 Our study demonstrated a heterogeneous expression profile of E-cadherin in CTM and CTCs. The mechanism for nuclear translocation of E-cadherin is not precisely known, but is thought to occur similarly to that for β-catenin, accumulation of which is either in the cytoplasm or in the nucleus correlates with susceptibility to enter into an EMT and acquisition of an invasive phenotype.5 Nuclear accumulation of E-cadherin in neuroendocrine tumors of the pancreas has been correlated to more aggressive disease.28 The significance of nuclear E-cadherin in SCLC (also a neuroendocrine-derived tumor) is not known but can now be investigated in CTM/CTCs without the need for invasive tumor biopsies. In addition to the modulation of markers of cell-cell contact, EMT is also described as involving cytoskeletal remodeling with altered intermediate filament usage from a cytokeratin-rich, more rigid structure to a vimentin-rich, more flexible structure that allows greater cell mobility.5 Increased expression of vimentin, particularly when associated with a loss or non-plasma membrane localized E-cadherin,5 is a cardinal feature of EMT that has been observed in the majority of CTCs and CTM from our study.
The detection of CTM from patients with NSCLC by ISET, but not CellSearch technology, is intriguing and could be explained by physical failure of larger NSCLC CTM to be moved within the magnetic field to allow imaging, and/or because cells in NSCLC CTM have insufficient expression of EpCam for immunomagnetic isolation and/or CKs for identification. Although EpCam (a type I transmembrane glycoprotein) is widely expressed in most epithelial tumors, including SCLC and NSCLC,29 it was expressed heterogeneously in NSCLC CTM (exemplified for NSCLC patient VI) (Figure 1G). Similarly CKs demonstrated expression heterogeneity in CTM from patients with lung cancer. These data suggest potential for underestimation of tumor cell burden in the circulation of lung cancer patients using the CellSearch technology and other platforms that use epithelial marker positive selection, and argue instead for combinations of marker independent and dependent methods to fully describe the tumor cell burden in the circulation.
Although a small cohort of patients has been evaluated in this report, and expansion of the study is now warranted, CTM were found in all 7 patients studied, and thus seem to be common in patients with both small cell and non-small cell histological subtypes of lung cancer with metastatic disease. The phenotypic characteristics of these CTM and CTCs are most consistent with an emerging model of metastasis as a process requiring both epithelial and mesenchymal properties, and the absence of apoptotic nuclei in CTM in all patients studied so far suggests a survival advantage.
There are clear advantages and obvious limitations with the characterization of ISET isolated CTCs and CTM. On the one hand, the high-resolution morphology of cells deposited on the filter allows identification of typical nuclear morphologies associated with WBCs, which can be confirmed by CD45 staining. This approach also allows the morphology of cells assigned as cancer cells to be compared with that of a diagnostic biopsy (Figure 1). However, multiple staining of cells deposited on the membrane spots remains technically challenging; therefore, a detailed assessment of phenotype has not been possible to date. We used 10 spots cut from the polycarbonate membranes on which cells were deposited by filtration, and we divided their use for IHC staining for each of the following markers: CD45, EpCam, vimentin, neural cadherin, and E-cadherin, or CKs. Although obvious heterogeneity was observed for every marker assessed individually, the mutual relationships between epithelial and mesenchymal markers are largely, unanswered questions. To begin to address this issue, dual-color IHC for E-cadherin and vimentin was developed to reveal a lack (so far) in dual-stained CTCs or CTM (Figure 4). Further method development is now needed to pursue this approach in further detail.
In addition, a prospective study is warranted to understand the clinical significance of the presence and prevalence of CTM in lung cancer patients. Further detailed analysis of CTCs and CTM will allow a better understanding of the molecular mechanisms driving their behavior and may provide new insights for therapeutic control.
Acknowledgments
The authors thank Helen Doran (Consultant Lung Cancer Pathologist, Wythenshawe Hospital, Manchester, UK) for her expert review of pathological specimens.
Footnotes
This study was supported by Cancer Research UK grant C147/a12328. J-M.H. was supported by a Cancer Research UK China FellowshipC480/A7421. M.K. was supported by a Cancer Research UK/AstraZeneca Clinical Pharmacology Fellowship. L.P. was supported by European Union CHEMORES FP6 contract LSHC-CT-2007-037665.
J.-M.H. and M.K. contributed equally to this work.
Contributor Information
Fiona Blackhall, Email: fiona.blackhall@christie.nhs.uk.
Caroline Dive, Email: cdive@picr.man.ac.uk.
References
- 1.Paget S. The distribution of secondary growths in cancer of the breast. The Lancet. 1889;133:571–573. [PubMed] [Google Scholar]
- 2.Ewing J. Lymphoepithelioma. Am J Pathol. 1929;5:99–108. [PMC free article] [PubMed] [Google Scholar]
- 3.Fidler I.J. Tumor heterogeneity and the biology of cancer invasion and metastasis. Cancer Res. 1978;38:2651–2660. [PubMed] [Google Scholar]
- 4.Riethdorf S., Wikman H., Pantel K. Review: biological relevance of disseminated tumor cells in cancer patients. Int J Cancer. 2008;123:1991–2006. doi: 10.1002/ijc.23825. [DOI] [PubMed] [Google Scholar]
- 5.Kalluri R., Weinberg R.A. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thiery J.P. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 7.Cohen S.J., Punt C.J., Iannotti N., Saidman B.H., Sabbath K.D., Gabrail N.Y., Picus J., Morse M., Mitchell E., Miller M.C., Doyle G.V., Tissing H., Terstappen L.W., Meropol N.J. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 8.Cristofanilli M., Budd G.T., Ellis M.J., Stopeck A., Matera J., Miller M.C., Reuben J.M., Doyle G.V., Allard W.J., Terstappen L.W., Hayes D.F. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 9.de Bono J.S., Scher H.I., Montgomery R.B., Parker C., Miller M.C., Tissing H., Doyle G.V., Terstappen L.W., Pienta K.J., Raghavan D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 10.Hou J.M., Krebs M., Ward T., Morris K., Sloane R., Blackhall F., Dive C. Circulating tumor cells, enumeration and beyond. Cancers. 2010;2:1236–1250. doi: 10.3390/cancers2021236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krebs M.G., Hou J.M., Ward T., Blackhall F.H., Dive C. Circulating tumour cells: their utility in cancer management and predicting outcomes. Ther Adv Med Oncol. 2010;2:351–365. doi: 10.1177/1758834010378414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christiansen J.J., Rajasekaran A.K. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–8326. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- 13.Tsuji T., Ibaragi S., Shima K., Hu M.G., Katsurano M., Sasaki A., Hu G.F. Epithelial-mesenchymal transition induced by growth suppressor p12CDK2-AP1 promotes tumor cell local invasion but suppresses distant colony growth. Cancer Res. 2008;68:10377–10386. doi: 10.1158/0008-5472.CAN-08-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedl P., Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 15.Ilina O., Friedl P. Mechanisms of collective cell migration at a glance. J Cell Sci. 2009;122:3203–3208. doi: 10.1242/jcs.036525. [DOI] [PubMed] [Google Scholar]
- 16.Brandt B., Junker R., Griwatz C., Heidl S., Brinkmann O., Semjonow A., Assmann G., Zanker K.S. Isolation of prostate-derived single cells and cell clusters from human peripheral blood. Cancer Res. 1996;56:4556–4561. [PubMed] [Google Scholar]
- 17.Kats-Ugurlu G., Roodink I., de Weijert M., Tiemessen D., Maass C., Verrijp K., van der Laak J., de Waal R., Mulders P., Oosterwijk E., Leenders W. Circulating tumour tissue fragments in patients with pulmonary metastasis of clear cell renal cell carcinoma. J Pathol. 2009;219:287–293. doi: 10.1002/path.2613. [DOI] [PubMed] [Google Scholar]
- 18.Molnar B., Ladanyi A., Tanko L., Sreter L., Tulassay Z. Circulating tumor cell clusters in the peripheral blood of colorectal cancer patients. Clin Cancer Res. 2001;7:4080–4085. [PubMed] [Google Scholar]
- 19.Fidler I.J., Gersten D.M., Riggs C.W. Relationship of host immune status to tumor cell arrest, distribution, and survival in experimental metastasis. Cancer. 1977;40:46–55. doi: 10.1002/1097-0142(197707)40:1<46::aid-cncr2820400110>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 20.Frisch S.M., Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riethdorf S., Fritsche H., Muller V., Rau T., Schindlbeck C., Rack B., Janni W., Coith C., Beck K., Janicke F., Jackson S., Gornet T., Cristofanilli M., Pantel K. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res. 2007;13:920–928. doi: 10.1158/1078-0432.CCR-06-1695. [DOI] [PubMed] [Google Scholar]
- 22.Kerr J.F., Wyllie A.H., Currie A.R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vona G., Sabile A., Louha M., Sitruk V., Romana S., Schutze K., Capron F., Franco D., Pazzagli M., Vekemans M., Lacour B., Brechot C., Paterlini-Brechot P. Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulating tumor cells. Am J Pathol. 2000;156:57–63. doi: 10.1016/S0002-9440(10)64706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou J.M., Greystoke A., Lancashire L., Cummings J., Ward T., Board R., Amir E., Hughes S., Krebs M., Hughes A., Ranson M., Lorigan P., Dive C., Blackhall F.H. Evaluation of circulating tumor cells and serological cell death biomarkers in small cell lung cancer patients undergoing chemotherapy. Am J Pathol. 2009;175:808–816. doi: 10.2353/ajpath.2009.090078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agiostratidou G., Hulit J., Phillips G.R., Hazan R.B. Differential cadherin expression: potential markers for epithelial to mesenchymal transformation during tumor progression. J Mammary Gland Biol Neoplasia. 2007;12:127–133. doi: 10.1007/s10911-007-9044-6. [DOI] [PubMed] [Google Scholar]
- 26.Liotta L.A., Saidel M.G., Kleinerman J. The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res. 1976;36:889–894. [PubMed] [Google Scholar]
- 27.van Roy F., Berx G. The cell-cell adhesion molecule E-cadherin. Cell Mol Life Sci. 2008;65:3756–3788. doi: 10.1007/s00018-008-8281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serra S., Salahshor S., Fagih M., Niakosari F., Radhi J.M., Chetty R. Nuclear expression of E-cadherin in solid pseudopapillary tumors of the pancreas. JOP. 2007;8:296–303. [PubMed] [Google Scholar]
- 29.Went P.T., Lugli A., Meier S., Bundi M., Mirlacher M., Sauter G., Dirnhofer S. Frequent EpCam protein expression in human carcinomas. Hum Pathol. 2004;35:122–128. doi: 10.1016/j.humpath.2003.08.026. [DOI] [PubMed] [Google Scholar]


