Abstract
Bone marrow-derived mononuclear cells (BMMNCs) enhance postischemic neovascularization, and their therapeutic use is currently under clinical investigation. However, cardiovascular risk factors, including diabetes mellitus and hypercholesterolemia, lead to the abrogation of BMMNCs proangiogenic potential. NO has been shown to be critical for the proangiogenic function of BMMNCs, and increased endothelial NO synthase (eNOS) activity promotes vessel growth in ischemic conditions. We therefore hypothesized that eNOS overexpression could restore both the impaired neovascularization response and decreased proangiogenic function of BMMNCs in clinically relevant models of diabetes and hypercholesterolemia. Transgenic eNOS overexpression in diabetic, atherosclerotic, and wild-type mice induced a 1.5- to 2.3-fold increase in postischemic neovascularization compared with control. eNOS overexpression in diabetic or atherosclerotic BMMNCs restored their reduced proangiogenic potential in ischemic hind limb. This effect was associated with an increase in BMMNC ability to differentiate into cells with endothelial phenotype in vitro and in vivo and an increase in BMMNCs paracrine function, including vascular endothelial growth factor A release and NO-dependent vasodilation. Moreover, although wild-type BMMNCs treatment resulted in significant progression of atherosclerotic plaque in ischemic mice, eNOS transgenic atherosclerotic BMMNCs treatment even had antiatherogenic effects. Cell-based eNOS gene therapy has both proangiogenic and antiatherogenic effects and should be further investigated for the development of efficient therapeutic neovascularization designed to treat ischemic cardiovascular disease.
To prevent or treat ischemic diseases, therapeutic neovascularization, the stimulation of tissue vascularization after ischemia, has recently progressed from the bench to the bedside. Strategies include transplantation of angiogenic bone marrow-derived mononuclear cells (BMMNCs) or gene transfer for systemic or local up-regulation of proangiogenic proteins. Clinical studies have demonstrated the safety, feasibility, and efficacy of intracoronary and intramuscular infusion of adult BMMNCs in patients with peripheral arterial disease, acute myocardial infarction, and ischemic cardiomyopathy.1,2
However, despite the excitement surrounding the possible clinical use of BMMNCs, in atherosclerosis, diabetes mellitus, and other risk factors for cardiovascular diseases the availability of bone marrow and progenitor cells is reduced and their function impaired to varying degrees.1,2 Moreover, the safety of BMMNCs treatment has been questioned by studies that found an increase in atherosclerotic plaque size after BMMNCs treatment.3 This potentially hazardous dual effect of therapeutic neovascularization on atherogenesis is explained by the many common pathways of both mechanisms and has been named the Janus phenomenon.4
Impaired bioavailability of NO is a hallmark in patients with cardiovascular disease. Moreover, the enzyme endothelial NO synthase (eNOS) has also been shown to be essential for neovascularization. It has a key regulatory function in endothelial cell growth,5 vascular remodeling,6 angiogenesis,7 and vasodilation8 and plays a crucial role in the functional activity of BMMNCs.9,10 Thus, impaired bioavailability of NO may significantly contribute to the impaired neovascularization response to ischemia in atherosclerosis or diabetes. Therefore, using homebred transgenic mice overexpressing human eNOS,11 the purposes of the present study were to evaluate whether eNOS gene therapy would be able to improve the postischemic neovascularization response in diabetes and atherosclerosis and to restore the impaired proangiogenic potential of BMMNCs without causing simultaneous detrimental proatherogenic effects, overcoming the Janus phenomenon.
Materials and Methods
Mice
The experimental protocol was approved by the Animal Experiments Committee under the national Experiments on Animals Act and adhered to the rules laid down in this national law that serves the implementation of the Guidelines on the Protection of Experimental Animals by the Council of Europe (1986) (directive 86/609/EC). C57BL/6 and apolipoprotein E–deficient (ApoE KO) transgenic mice overexpressing the human eNOS gene under regulation of the human eNOS promoter were obtained, as previously described.11 Mice were backcrossed to C57Bl6 for at least 10 generations (>96% C57Bl6). To induce diabetes, 8-week-old mice were injected intraperitoneally with 40 mg/kg of streptozotocin (Sigma-Aldrich Corp, St. Louis, MO) in 0.05 mol/L sodium citrate, pH 4.5, daily for 5 days.12 Mice were treated with or without NO synthase inhibitor N(G)-nitro-l-arginine methyl ester (10 mg/kg/day in the drinking water; Sigma).
Hind Limb Ischemia Model and Quantification of Neovascularization
Mice underwent surgery to induce unilateral hind limb ischemia, as previously described.13 A total of 1 × 106 freshly isolated BMMNCs were intravenously injected 24 hours after femoral artery ligation. Two weeks after ligation, postischemic neovascularization was evaluated by laser Doppler imaging and microangiography, as previously described.13
Atherosclerosis
Plasma cholesterol levels were measured, and atherosclerotic plaque lesion size and composition in the aortic root were evaluated by immunohistochemistry, as previously described.3
NO and ROS Production
NO production in BMMNCs was assessed by measuring intracellular nitrosation of NO-sensitive fluorochrome 4,5-diaminofluorescein diacetate (Enzo Life Sciences International Inc., Plymouth Meeting, PA). Briefly, BMMNCs were incubated with 10 μmol/L 4,5-diaminofluorescein diacetate for 180 minutes (37°C). Exposure to light was avoided as far as possible throughout experimentation. At 180 minutes, supernatants were removed and cells were washed in fresh 4,5-diaminofluorescein diacetate–free buffer followed by immediate FACS analysis. A FACSCalibur analyzer (BD, Franklin Lakes, NJ) was used to quantify fluorescence (excitation wavelength:, 488 nm; emission wavelength, 530 nm) at the single-cell level, and data were analyzed using Cellquest version 3.3 (BD) software. Cellular reactive oxygen species (ROS) levels, reflecting a balance between oxidant production and removal by endogenous antioxidants, were also quantified using L-012 as described recently.12 BMMNCs were lysed in 50 mmol/L Tris buffer (pH 7.5) containing protease inhibitors (Boehringer Ingelheim GmbH, Ingelheim am Rhein, Germany) and centrifuged at 10,000 × g for 15 minutes at 4°C. Supernatants were then incubated with 100 mmol/L L-012 (Wako Chemical USA Inc., Richmond, VA). Luminescence was counted (Topcount NXT; Perkin Elmer, Waltham, MA) during 20 seconds after a 10-minute interval allowing for the plates to become adapted to the dark.
Growth Factor Assay
After isolation of adherent cells in cell cultures, culture medium was collected and analyzed for vascular endothelial growth factor A (VEGF-A) by enzyme-linked immunosorbent assay (R&D Systems Inc, Minneapolis, MN).
Measurement of Arterial Diameter in Isolated Femoral Arteries
After 24 hours of ischemia, ischemic femoral arteries from control animals were isolated and cannulated at both extremities in a video-monitored perfusion system (arteriograph; Living Systems Instrumentation, Burlington, VT). A total of 0.5 × 106 BMMNCs from wild-type (WT), eNOS transgenic control, and diabetic or hypercholesterolemic mice were then perfused, as earlier described.9
Isolation and Incorporation of BMMNCs
Bone marrow cells were obtained by flushing tibiae and femora of donor mice. Low-density BMMNCs were then isolated by density gradient centrifugation with Ficoll. BMMNCs were plated at a density of 1 × 106 cells/cm2 on 24-well plates (Nalge Nunc International, Rochester, NY) coated with 10 μg/ml of fibronectin (Sigma) and cultured up to 7 days in M199 medium supplemented with 20% fetal bovine serum (Invitrogen), 0.05 mg/ml of bovine pituitary extract (Invitrogen), antibiotics, and 10 U/ml of heparin (Leo Pharma BV, Breda, The Netherlands). Nonadherent cells were then removed and adherent cells were analyzed by immunochemical assay with DiI-labeled acetylated low-density lipoprotein (DiI-LDL; Molecular Probes, Eugene, OR), fluorescein isothiocyanate–labeled Bandeiraea simplicifolia lectin (BS-1 lectin; Sigma), goat anti-eNOS (Santa Cruz Biotechnology Inc., Santa Cruz, CA), and subsequently rabbit anti-goat Alexa fluor 594. Endothelial cell phenotype was revealed by double positive staining for both Dil-LDL and BS-1 lectin and both eNOS and BS-1 lectin. These double-positive cells were negative for the monocytic marker CD45 (data not shown). Cell numbers were counted and expressed in cells per field. Three fields from each culture were counted.
To demonstrate incorporation of BMMNC-derived endothelial cells into ischemic muscles, green Alexa fluor 546-labeled BMMNCs (1 × 106 cells/100 μL of PBS) were intravenously administered 24 hours after induction of hind limb ischemia. The gastrocnemius muscles were harvested 4 days after injection of BMMNCs. Incorporated BMMNCs were detected by immunostaining with a biotinylated isolectin B4 (Sigma) followed by incubation with rhodamine red streptavidin (Jackson ImmunoResearch Laboratories Inc., West Grove, PA). The number of infiltrating cells was also evaluated using green Alexa fluor 546-labeled BMMNCs isolated from WT or eNOS transgenic mice. Two days after BMMNCs injection, the ischemic gastrocnemius muscles were harvested, weighed, minced, and digested in 450 U/ml of collagenase I, 125 U/ml of collagenase XI, 60 U/ml of DNAseI, and 60 U/ml of hyaluronidase (Sigma-Aldrich) for 1 hour at 37°C. Cell suspensions were layered on Histopaque 1083 (Sigma-Aldrich) for gradient density centrifugation. In the mononuclear cells fraction, the number of cells being green Alexa fluor 546/DAPI+ was then evaluated on a LSRII Flow Cytometer (BD) with the FACSDiva software (BD).
Statistical Analysis
Statistical analysis was performed using Student's t-test or 1-way analysis of variance, followed by post hoc analysis, as appropriate. Data are reported as mean ± SEM. Statistical significance was accepted when P < 0.05.
Results
eNOS Overexpression Is Proangiogenic
Postischemic hind limb neovascularization was impaired in diabetic mice and ApoE KO mice compared with WT mice, demonstrated by a 1.3-fold decreased foot perfusion and vessel density (P < 0.05, Figure 1A). eNOS overexpression in the endothelium of diabetic mice and ApoE KO mice resulted in an increase in foot perfusion and vessel density (P < 0.001), reaching above WT levels. Endothelial eNOS overexpression in WT mice resulted in the strongest neovascularization response (1.5- to 1.7-fold increase compared with WT controls; P < 0.001). Of interest, treatment with the NOS inhibitor N(G)-nitro-l-arginine methyl ester abrogated postischemic vessel growth in mice overexpressing eNOS, underscoring the role of NO in the observed protective effects.
Figure 1.
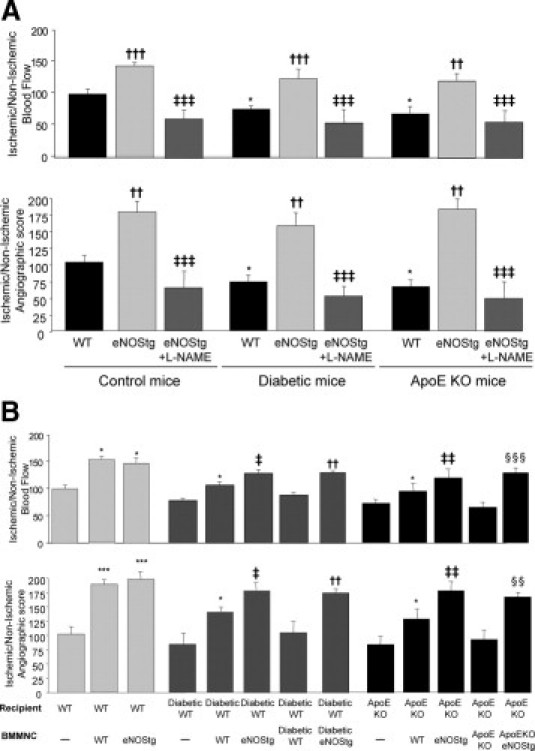
A: eNOS overexpression increases postischemic neovascularization. Quantification of foot perfusion (upper) measurements by laser Doppler imaging and vessel density measurements by microangiography (lower). Values are mean ± SEM; n = 10. *P < 0.05 versus WT control mice, ††P < 0.01 and †††P < 0.001 versus WT mice, and ‡‡‡P < 0.001 versus eNOS transgenic mice. B: Bone marrow eNOS overexpression restores the proangiogenic effect on postischemic neovascularization. Quantitative evaluation (upper) of foot perfusion by laser Doppler imaging and vessel density measurements by microangiography (lower). Values are ± SEM; n = 10. *P < 0.05 and ***P < 0.001 versus PBS-treated mice, ‡P < 0.05 and ‡‡P < 0.01 versus mice receiving WT BMMNCs, ††P < 0.01 versus diabetic mice receiving diabetic BMMNC, and §§P < 0.01 and §§§P < 0.001 versus ApoE KO mice receiving ApoEKO BMMNCs.
The role of eNOS overexpression on cell-based therapeutic neovascularization was evaluated by intravenous transplantation of different BMMNCs after induction of ischemia in WT, diabetic, and ApoE KO recipient mice. In WT recipient mice, transplantation of WT BMMNCs and eNOS transgenic BMMNCs resulted in a comparable 1.3- to 1.9-fold increase in foot perfusion and vessel density compared with saline (P < 0.01, Figure 1B). In diabetic mice, diabetic BMMNC transplantation did not improve neovascularization response. Conversely, administration of WT BMMNCs increased vessel growth in the diabetic ischemic area but to a lesser extent than that of eNOS transgenic BMMNCs. Interestingly, eNOS transgenic diabetic BMMNC transplantation resulted in a significant 1.3- to 1.5-fold increase in postischemic neovascularization and reached WT BMMNCs levels compared with saline and diabetic BMMNCs (P < 0.01). Similarly, eNOS overexpression restored the therapeutic potential of ApoE KO BMMNCs.
Differentiation of eNOS Overexpressing BMMNCs
Western blot analysis of BMMNCs confirmed an increased presence of eNOS protein in eNOS transgenic BMMNCs (P < 0.05, Figure 2A). Concordantly, NO production by eNOS transgenic BMMNCs was significantly elevated compared with control BMMNCs (P < 0.05, Figure 2B). We also showed that BMMNC-derived ROS were up-regulated in diabetic and ApoE KO BMMNCs, as previously described.12 However, eNOS overexpression did not affect ROS levels (Figure 2B).
Figure 2.
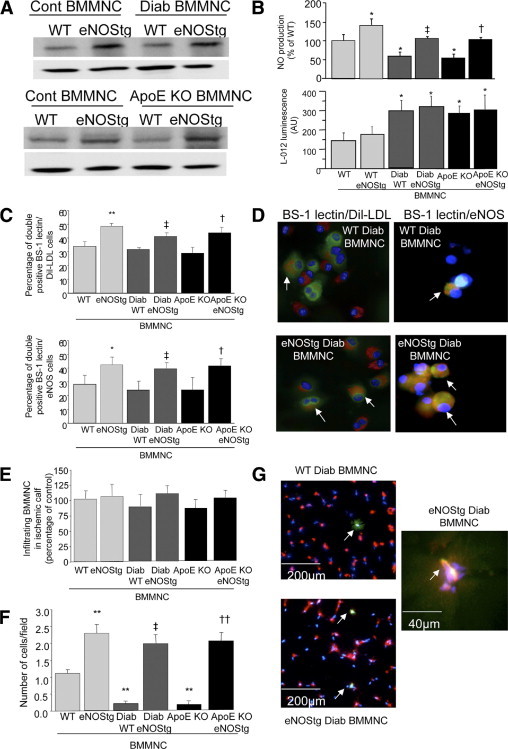
eNOS overexpression increases differentiation of BMMNCs. Representative quantitative Western blot (A) and NO and ROS production (B) in BMMNCs isolated from WT or eNOS transgenic control, diabetic, and ApoE KO mice. C: Quantification of percentage of BMMNCs that double positively stained for BS1 lectin and DiI-LDL (upper) or BS-1 lectin and eNOS (lower), regarding the cells with the endothelial phenotype. D: Representative images of BMMNC cultures. Arrows indicate double positive cells with endothelial phenotype for Dil-LDL (red) and BS-1 lectin (green) or eNOS (red) and BS-1 lectin, respectively. Nuclei were stained with DAPI (blue). E: Quantitative evaluation of the number of infiltrating BMMNCs in the ischemic area. BMMNCs were isolated from WT or eNOS transgenic control, diabetic, and ApoE KO mice. Two days after BMMNCs injection in WT mice with hind limb ischemia, the gastrocnemius muscles were digested and the number of cells that were green Alexa fluor 546 hi/DAPI+ was then evaluated in the mononuclear cells fraction. Quantitative analysis (F) and representative photomicrographs (G) of incorporated BMMNC-derived endothelial cells in histological sections from ischemic skeletal muscles. BMMNCs were stained using Alexa fluor 546 cell tracker (green). Mouse vasculature was identified by isolectin B4 staining (red). Nuclei were stained with DAPI (blue). Arrows indicate incorporated BMMNC-derived endothelial cells. Values are mean ± SEM; n = 4. *P < 0.05 and **P < 0.01 versus WT BMMNCs, ‡P < 0.05 versus WT diabetic BMMNCs, and †P < 0.05 and ††P < 0.01 versus ApoE KO BMMNCs.
Immunohistochemical analysis of BMMNCs cultured in endothelial-specific medium demonstrated a significant increase in BMMNCs differentiation into cells with endothelial phenotype of eNOS transgenic BMMNCs compared with BMMNCs, revealed by a greater percentage of BS-1 lectin/Dil-LDL and BS-1 lectin/eNOS double positive cells (P < 0.01, Figure 2, C and D). Similarly, eNOS overexpression increased the number of incorporated BMMNCs-derived endothelial cells into capillaries of ischemic muscle (Figure 2, F and G). Conversely, administration of WT or eNOS transgenic BMMNCs did not affect the number of infiltrating BMMNCs in the ischemic area (Figure 2E).
Paracrine Effects of eNOS Overexpressing BMMNCs
BMMNCs are also capable of stimulating neovascularization by secretion of proangiogenic factors. Culture medium was collected from BMMNCs differentiation cell cultures. Using enzyme-linked immunosorbent assay, quantitative measurements of VEGF-A demonstrated an increase in VEGF-A secretion in culture medium from eNOS transgenic BMMNCs compared with WT BMMNCs (Figure 3A). Finally, because the proangiogenic effects of BMMNCs also rely on the cells' ability to modulate vascular function, we assessed BMMNC effects on vascular diameter in isolated perfused mouse femoral arteries (238 ± 5 μm, internal diameter). Intraluminal administration of BMMNCs induced a rapid vasodilation (internal diameter increased by 27 ± 2 μm for 5 × 105 cells/ml). eNOS transgenic BMMNCs increased vasodilation by 1.7-fold over WT BMMNCs (P < 0.01). Interestingly, WT diabetic BMMNCs and ApoE KO BMMNCs only slightly affected vessel diameter, whereas eNOS overexpression fully restored the vasodilatory potential of diabetic and ApoE KO BMMNCs (Figure 3B).
Figure 3.
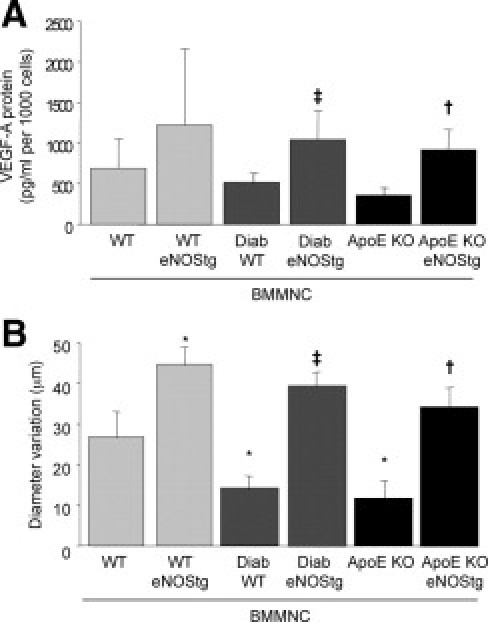
eNOS overexpression increases the paracrine potential of BMMNCs. A: Quantification by enzyme-linked immunosorbent assays of secretion of VEGF-A in cell culture medium. Values are mean ± SEM; n = 4. **P < 0.01 versus WT BMMNCs, ‡P < 0.05 versus WT diabetic BMMNC, and †P < 0.01 versus ApoE KO BMMNCs. ND indicates not detected. B: Quantitative evaluation of ischemic femoral artery diameter isolated from control mice after intraluminal injection of BMMNCs isolated from WT, eNOS transgenic control, diabetic, or ApoE KO mice. Values are mean ± SEM; n = 10. *P < 0.05 versus WT, ‡P < 0.05 versus WT diabetic BMMNCs, and †P < 0.05 versus ApoE KO BMMNCs.
eNOS Overexpression Is Antiatherogenic
Atherosclerotic lesions were analyzed in ApoE KO mice after induction of hind limb ischemia and subsequent BMMNCs treatment. Plasma cholesterol levels were similar in all groups. Treatment with ApoE KO BMMNCs did not affect plaque size or composition in contrast with treatment with WT BMMNCs (60% increase in lesion size, without differences in composition, P < 0.001; Table 1). Remarkably, treatment with eNOS transgenic ApoE KO BMMNCs had an inhibitory effect on plaque progression (44% decrease in lesion size; P < 0.05). Furthermore, plaque composition changed due to eNOS transgenic ApoE KO BMMNC treatment into a more stable phenotype (40% decrease in macrophages, P < 0.05).
Table 1.
Bone Marrow eNOS Overexpression Has Inhibitory Effects on Atherosclerotic Lesion Size Progression in the Aortic Sinus of ApoE KO Mice⁎
| PBS | WT BMMNCs | ApoE KO BMMNCs | eNOS transgenic ApoE KO BMMNCs | |
|---|---|---|---|---|
| Total cholesterol, mM | 13.2 ± 1.6 | 12.8 ± 1.2 | 11.9 ± 2.4 | 11.4 ± 3.5 |
| Lesion size, μm2 | 108,838 ± 14,257 | 178,257 ± 16,687† | 110,654 ± 13,458 | 75,125 ± 9458†‡ |
| MOMA2-positive staining, % | 28.3 ± 2.4 | 34.2 ± 3.1 | 30.4 ± 2.7 | 20.1 ± 1.9†‡ |
| α-Actin-positive staining, % | 9.4 ± 0.3 | 10.6 ± 1.1 | 11.1 ± 2.1 | 10.2 ± 1.5 |
| Sirius red-positive staining, % | 23.1 ± 2.6 | 20.2 ± 1.9 | 24.3 ± 2.7 | 25.4 ± 2.5 |
MOMA2, monocyte/macrophage marker 2.
Values are expressed as mean ± SEM; n = 10.
P < 0.05 versus PBS.
P < 0.05 versus ApoE KO BMMNCs.
Discussion
In the present study, we showed that eNOS overexpression improved the postischemic neovascularization response in healthy, diabetic, and atherosclerotic mice. Subsequently, eNOS overexpression in bone marrow repaired the decreased proangiogenic functional activity of diabetic and atherosclerotic BMMNCs. Finally, the results of the present study demonstrated a unique combined proangiogenic and antiatherogenic effect of cell-based eNOS gene therapy in atherosclerotic mice.
The critical role of eNOS in postischemic neovascularization has been well established. In eNOS-deficient mice neovascularization is decreased, resulting in severe limb loss14; in parallel, up-regulation of eNOS activity by eNOS gene delivery15 or bovine eNOS overexpression16 enhances postischemic blood flow recovery and limb function. In our study we confirmed these findings in healthy mice and extended them to a more clinically relevant model, the diabetic and atherosclerotic mouse. eNOS overexpression in diabetic and atherosclerotic mice induced a strong postischemic neovascularization response comparable with WT mice. Mechanisms of the positive neovascularization effects of eNOS up-regulation included increase in vasodilation,8 increase in vessel density as shown by microangiographic measurements, and increase in vasculogenesis as a result of restoration of pathological bone marrow proangiogenic function. Indeed, the enhanced postischemic neovascularization response after treatment of ischemic mice with eNOS transgenic diabetic or eNOS transgenic ApoE KO BMMNCs revealed that up-regulation of eNOS repaired the reduced proangiogenic function of pathological BMMNCs. This was further explained by an increased differentiation into cells with endothelial phenotype and an increase in their paracrine potential, including VEGF-A release and NO-dependent vessel dilation.9 It is noteworthy that, although no changes in the amount of total eNOS protein were observed, NO release was decreased in diabetic BMMNCs compared with nondiabetic control BMMNCs. Our findings are consistent with prior reports showing that hyperglycemia and diabetes are associated with impaired eNOS functions, at least in part, through inhibition of eNOS phosphorylation.17,18
The term Janus phenomenon has been invented for the dual effect of protein (FGF, MCP-1)–, gene (VEGF, TNF)–, or cell (BMMNCs, EPC)–based therapeutic angiogenesis on progression and destabilization of atherogenesis.4 NO appears to be a possible exception to the Janus phenomenon because it has established proneovascularization and antiatherogenic effects. However, these effects have never been investigated at the same time in the same model. Our study is the first study using eNOS cell-based gene therapy for the stimulation of postischemic neovascularization and simultaneously focusing on prevention of supplemental proatherogenic effects. Interestingly, WT BMMNC treatment led to increased plaque size in ischemic atherosclerotic mice, whereas ApoE KO BMMNC treatment had no effect on lesion size, as previously described.3 However, at the same time, we could indeed demonstrate an antiatherogenic effect of eNOS transgenic atherosclerotic BMMNCs, thus blunting the Janus phenomenon. In our studies, only occasional donor BMMNCs were identified in the atherosclerotic lesions, making a physical or local contribution of transplanted BMMNCs to plaque growth less likely. Another possible explanation could be the production of chemokines by transplanted BMMNCs; however, we could not detect any differences in monocyte chemotactic protein-1 and VEGF blood levels in the different BMMNCs-treated mice (data not shown). It is likely that the potent anti-inflammatory effect of eNOS overexpression is the main responsible factor in the antiatherogenic effect of eNOS transgenic ApoE KO BMMNC treatment, as described previously.11 Definitely, further studies will be necessary to investigate in more detail not only our interesting observation of the combined proangiogenic and antiatherogenic potential of eNOS cell-based therapy but also the complete concept of BMMNCs treatment–induced atherogenesis. Nevertheless, these results imply that eNOS up-regulation is a promising target for local or stem cell-based therapeutic neovascularization in patients with ischemic (cardio)vascular disease.
Acknowledgments
We thank Prof. Hero van Urk (Erasmus Medical Center Rotterdam) and Prof. Jaap Hamming (Leiden University Medical Center) for their continuous scientific support.
Footnotes
Supported by grants from Stichting Lijf en Leven (R.d.C), Breedtestrategie Erasmus MC (R.d.C.); by The Netherlands Organization for Health Research and Development (AGIKO stipend 920-0-291) (B.M.); by Prof. Michaël van Vloten Fund (B.M.); by Leducq Foundation network of excellence; and by Fondation de France (J.-S.S.).
References
- 1.Segers V.F., Lee R.T. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 2.Aranguren X.L., Verfaillie C.M., Luttun A. Emerging hurdles in stem cell therapy for peripheral vascular disease. J Mol Med. 2009;87:3–16. doi: 10.1007/s00109-008-0394-3. [DOI] [PubMed] [Google Scholar]
- 3.Silvestre J.S., Gojova A., Brun V., Potteaux S., Esposito B., Duriez M., Clergue M., Le Ricousse-Roussanne S., Barateau V., Merval R., Groux H., Tobelem G., Levy B., Tedgui A., Mallat Z. Transplantation of bone marrow-derived mononuclear cells in ischemic apolipoprotein E-knockout mice accelerates atherosclerosis without altering plaque composition. Circulation. 2003;108:2839–2842. doi: 10.1161/01.CIR.0000106161.43954.DF. [DOI] [PubMed] [Google Scholar]
- 4.Epstein S.E., Stabile E., Kinnaird T., Lee C.W., Clavijo L., Burnett M.S. Janus phenomenon: the interrelated tradeoffs inherent in therapies designed to enhance collateral formation and those designed to inhibit atherogenesis. Circulation. 2004;109:2826–2831. doi: 10.1161/01.CIR.0000132468.82942.F5. [DOI] [PubMed] [Google Scholar]
- 5.Ziche M., Morbidelli L., Masini E., Amerini S., Granger H.J., Maggi C.A., Geppetti P., Ledda F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest. 1994;94:2036–2044. doi: 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudic R.D., Shesely E.G., Maeda N., Smithies O., Segal S.S., Sessa W.C. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest. 1998;101:731–736. doi: 10.1172/JCI1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murohara T., Witzenbichler B., Spyridopoulos I., Asahara T., Ding B., Sullivan A., Losordo D.W., Isner J.M. Role of endothelial nitric oxide synthase in endothelial cell migration. Arterioscler Thromb Vasc Biol. 1999;19:1156–1161. doi: 10.1161/01.atv.19.5.1156. [DOI] [PubMed] [Google Scholar]
- 8.Mees B., Wagner S., Ninci E., Tribulova S., Martin S., van Haperen R., Kostin S., Heil M., de Crom R., Schaper W. Endothelial nitric oxide synthase activity is essential for vasodilation during blood flow recovery but not for arteriogenesis. Arterioscler Thromb Vasc Biol. 2007;27:1926–1933. doi: 10.1161/ATVBAHA.107.145375. [DOI] [PubMed] [Google Scholar]
- 9.You D., Waeckel L., Ebrahimian T.G., Blanc-Brude O., Foubert P., Barateau V., Duriez M., Lericousse-Roussanne S., Vilar J., Dejana E., Tobelem G., Levy B.I., Silvestre J.S. Increase in vascular permeability and vasodilation are critical for proangiogenic effects of stem cell therapy. Circulation. 2006;114:328–338. doi: 10.1161/CIRCULATIONAHA.105.589937. [DOI] [PubMed] [Google Scholar]
- 10.Landmesser U., Engberding N., Bahlmann F.H., Schaefer A., Wiencke A., Heineke A., Spiekermann S., Hilfiker-Kleiner D., Templin C., Kotlarz D., Mueller M., Fuchs M., Hornig B., Haller H., Drexler H. Statin-induced improvement of endothelial progenitor cell mobilization, myocardial neovascularization, left ventricular function, and survival after experimental myocardial infarction requires endothelial nitric oxide synthase. Circulation. 2004;110:1933–1939. doi: 10.1161/01.CIR.0000143232.67642.7A. [DOI] [PubMed] [Google Scholar]
- 11.van Haperen R., de Waard M., van Deel E., Mees B., Kutryk M., van Aken T., Hamming J., Grosveld F., Duncker D.J., de Crom R. Reduction of blood pressure, plasma cholesterol, and atherosclerosis by elevated endothelial nitric oxide. J Biol Chem. 2002;277:48803–48807. doi: 10.1074/jbc.M209477200. [DOI] [PubMed] [Google Scholar]
- 12.Ebrahimian T.G., Heymes C., You D., Blanc-Brude O., Mees B., Waeckel L., Duriez M., Vilar J., Brandes R.P., Levy B.I., Shah A.M., Silvestre J.S. NADPH oxidase-derived overproduction of reactive oxygen species impairs postischemic neovascularization in mice with type 1 diabetes. Am J Pathol. 2006;169:719–728. doi: 10.2353/ajpath.2006.060042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silvestre J.S., Thery C., Hamard G., Boddaert J., Aguilar B., Delcayre A., Houbron C., Tamarat R., Blanc-Brude O., Heeneman S., Clergue M., Duriez M., Merval R., Levy B., Tedgui A., Amigorena S., Mallat Z. Lactadherin promotes VEGF-dependent neovascularization. Nat Med. 2005;11:499–506. doi: 10.1038/nm1233. [DOI] [PubMed] [Google Scholar]
- 14.Murohara T., Asahara T., Silver M., Bauters C., Masuda H., Kalka C., Kearney M., Chen D., Symes J.F., Fishman M.C., Huang P.L., Isner J.M. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith R.S., Jr, Lin K.F., Agata J., Chao L., Chao J. Human endothelial nitric oxide synthase gene delivery promotes angiogenesis in a rat model of hindlimb ischemia. Arterioscler Thromb Vasc Biol. 2002;22:1279–1285. doi: 10.1161/01.atv.0000026613.18742.67. [DOI] [PubMed] [Google Scholar]
- 16.Amano K., Matsubara H., Iba O., Okigaki M., Fujiyama S., Imada T., Kojima H., Nozawa Y., Kawashima S., Yokoyama M., Iwasaka T. Enhancement of ischemia-induced angiogenesis by eNOS overexpression. Hypertension. 2003;41:156–162. doi: 10.1161/01.hyp.0000053552.86367.12. [DOI] [PubMed] [Google Scholar]
- 17.Gallagher K.A., Liu Z.J., Xiao M., Chen H., Goldstein L.J., Buerk D.G., Nedeau A., Thom S.R., Velazquez O.C. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest. 2007;117:1249–1259. doi: 10.1172/JCI29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du X.L., Edelstein D., Dimmeler S., Ju Q., Sui C., Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest. 2001;108:1341–1348. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]


