Abstract
The hypoxic marker carbonic anhydrase (CA) IX has been recognized as a tumor-associated protein and is essential for cancer development. However, because CA IX expression does not always correlate with hypoxia, its regulatory mechanism remains unclear. The objective of the present study was to clarify the role and regulation of CA IX expression in gastric cancer. The immunohistochemical expression of CA IX and hypoxia-inducible factor-1α was assessed in 77 patients with gastric cancer. A methylation-sensitive restriction enzyme method was used to quantify site-specific methylation at −74 bp in the CA9 promoter in tissue from patients with gastric cancer and in corresponding normal tissue. CA9 expression in cell lines was strongly dependent on methylation status but not hypoxic stimuli. In tissue from patients with gastric cancer, the quantity of methylation was significantly correlated with the protein expression (P = 0.003). Moreover, the methylation value was significantly lower in intestinal-type compared with diffuse-type cancer (P = 0.003). Compared with normal mucosa, intestinal-type cancer demonstrated significant hypomethylation, whereas diffuse-type cancer exhibited hypermethylation. In conclusion, expression of CA IX in gastric cancer is predominantly regulated by methylation of a single CpG rather than by hypoxia. Furthermore, epigenetic alterations in CA9 differ between the intestinal and diffuse types of gastric cancer.
Despite its declining incidence, gastric cancer remains a leading cause of cancer-related death worldwide, resulting in approximately 700,000 deaths annually.1 Initiation and progression of gastric cancer is a multistage process. Intestinal-type gastric cancer is believed to arise from a premalignant cascade initiated by Helicobacter pylori infection; however, the molecular mechanism of gastric carcinogenesis is unclear.2,3 It is generally accepted that cancer develops as a result of multiple genetic and epigenetic alterations. Therefore, better understanding of the changes in gene expression that occur during oncogenesis may lead to improvement in cancer diagnosis, treatment, and prevention.
The carbonic anhydrases (CAs) are a family of zinc metalloenzymes that have an important role in cellular pH regulation through reversible hydration of carbon dioxide to carbonic acid.4,5 To date, 16 isozymes have been identified, which differ in tissue distribution, subcellular localization, and catalytic activity.6–8 Two isozymes, CA IX and CA XII, are associated with and overexpressed in many tumors.9–11
CA IX was initially described as a tumor-associated antigen9 and is linked to development of cancer in human beings.12–14 The distribution of CA IX in human tissues exhibits a unique pattern that enables designation of CA IX as a tumor-associated protein. CA IX is present in numerous tumors, predominantly malignant lesions, but is usually absent in the normal tissues from which these tumors originate.6 However, in contrast to other organs, high expression of CA IX is also observed in normal gastric mucosa, whereas its expression is absent or reduced in gastric cancer cell lines and in primary gastric tumors.15,16
Hypoxia up-regulates expression of several genes, including CA9, via hypoxia-inducible factor-1α (HIF-1α) protein binding to the hypoxia-responsive element in the promoter region of various genes.17–19 Expression of CA IX strongly correlates with the level of hypoxia, necrosis, and microvascular density.19,20 On the basis of these findings, CA IX has been recognized as one of the most reliable endogenous markers of cellular hypoxia. However, CA IX expression does not always correlate with pO2 and other hypoxic markers. Several reports previously demonstrated no correlation between the expression of HIF-1α and CA IX in immunohistochemical analysis.21,22 In addition, recent studies have demonstrated that hypomethylation of the CpG site at −74 bp in the CA9 promoter correlated with expression of CA IX in renal cancer and other cancer cell types.23,24 Chen et al25 were the first to propose DNA methylation as a mechanism regulating expression of CA IX in gastric cancer.
The present study quantitatively assessed the site-specific methylation of the CA9 promoter in 8 gastric cancer cell lines and cancer tissues from 77 patients. Correlation of the methylation values with expression of CA IX was examined to clarify the mechanism that regulates CA IX expression. Based on these assessments, a possible role of CA9 promoter methylation during gastric oncogenesis is proposed.
Materials and Methods
Patients
Seventy-seven patients with advanced gastric cancer who underwent curative surgery at our institution between June 2000 and December 2008 were enrolled in the study. None of these patients had hepatic, peritoneal, distant metastasis, and tumor cells in the peritoneal fluid. Stage classification was performed according to the guidelines of the Japanese Gastric Cancer Association.26 The curative potential of resection was classified on the basis of both surgical and histologic observations, as follows: Cur A, no residual disease, with a high probability of cure; Cur B, no residual disease but not fulfilling the criteria for Cur A; and Cur C, definite residual disease. The diagnosis in all 77 patients was Cur B. The 77 patients included 51 men (66.2%) and 26 women (33.8%), who ranged in age from 26 to 88 years [mean (SD), 66.6 (13.0) years]. Fifty-four patients (70.1%) received adjuvant chemotherapy after surgery; the remaining 23 (29.9%) did not receive this treatment because of advanced age or complications. Median (range) duration of follow-up was 23.7 (0.3−102.6) months. Informed consent to use specimens was obtained from all patients, and the study protocol was approved by the Ethics Committee of Saga University Faculty of Medicine (Saga, Japan).
Cell Lines
Eight gastric cancer cell lines (MKN1, MKN7, MKN28, MKN45, MKN74, HSC45, HSC57, and KATO-III) were used for the studies. HSC45 and HSC57 were provided by Dr. K. Yanagihara (Yasuda Women's University, Hiroshima, Japan), and the remaining 6 cell lines were purchased from Cell Bank, RIKEN BioResource Center (Ibaraki, Japan). The cells were cultured in RPMI-1640 medium (Sigma-Aldrich, Inc., St. Louis, MO) and maintained under conditions of either normoxia (20% O2 and 5% CO2 in air) or hypoxia (1% O2, 5% CO2, and 94% N2).
Immunohistochemistry
Paraffin-embedded sections were incubated using anti–CA IX (R&D Systems, Inc., Minneapolis, MN), 4 μg/ml, and anti-HIF-1α (Novus Biologicals, LLC, Littleton, CO), 1:200, for 2 hours at room temperature, and with the corresponding secondary antibodies for 30 minutes. The slides were washed in PBS, and incubated with a diaminobenzidine substrate kit (Nichirei Corp., Tokyo, Japan). The level of staining for CA IX was scored as high or low, with the percentage of stained cells and the intensity of staining in the membrane.27 Scoring for percentage was as follows: 0, no cells staining positive; 1, <1% positive cells; 2, 1% to 10% positive cells; 3, 10% to 33% positive cells; 4, 33% to 66% positive cells; and 5, >66% positive cells. Scoring for intensity was as follows: 0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining. Tumors with a total score of >6 were considered to have high expression of CA IX. Evaluation of HIF-1α staining was performed as previously reported.28
Total RNA Extraction and Real-Time PCR
Total RNA was extracted from each cell line using an extraction kit (ISOGEN; Nippon Gene, Osaka, Japan). For each cell line, 1 μg of RNA was converted into cDNA using a reverse transcription reaction kit (ReverTra Ace; Toyoba Co., Ltd., Osaka, Japan). The cDNA was used as a template for PCR. RT-PCR was performed using the Light Cycler instrument system (Roche Diagnostics GmbH, Mannheim, Germany). The primers were designed according to cDNA sequences (GenBank, Bethesda, MD) as follows: CA9, 5′-CCGAGCGACGCAGCCTTTGA-3′, 5′-GGCTCCAGTCTCGGCTACCT-3′ (252 bp), and β-actin, 5′-TTAAGGAGAAGCTGTGCTACG-3′, 5′-GTTGAAGGTAGTTTCGTGGAT-3′ (206 bp). A melting curve analysis was used to control for the specificity of the amplification products. The quantitative value was normalized to the β-actin expression, which was used as an internal control. All experiments were performed in triplicate, and mean values were calculated.
5-Aza-2′-Deoxycitidine and Trichostatin A Therapy
Eight gastric cancer cell lines were treated with the demethylating agent 5-Aza-2′-deoxycytidine (5-Aza-dc) (Sigma-Aldrich, Inc.), 5 μmol/L, for 72 hours, with drug replacement every 24 hours. For the last 24 hours, cells were also exposed to the histone deacetylase inhibitor trichostatin A (Sigma-Aldrich, Inc.), 500 nmol/L. Cells were harvested and used for RNA isolation.
Western Blot Analysis
Whole cell lysates from cultured cells were prepared using lysis buffer composed of 150 mmol/L NaCl, 50 mmol/L Tris HCl (pH 7.6), 0.5% Triton X-100, and a protease inhibitor cocktail mix (Roche Diagnostics GmbH). Aliquots containing 10 μg of protein were subjected to 4% to 12% Bis-Tris gel (NuPAGE; Invitrogen Corp., Carlsbad, CA) and electrophoretically transferred onto an Amersham Hybond-ECL membrane (GE Healthcare, Buckinghamshire, UK) in transfer buffer. After blocking with 5% skim milk for 30 minutes, the membrane was incubated with primary antibodies for 2 hours at room temperature. The primary antibodies used for Western blot analyses were anti-CA IX (R&D Systems, Inc.), 1 μg/ml, and anti–β-actin (1:10,000; Sigma-Aldrich, Inc.). After incubation with the corresponding secondary antibodies, the signals were developed using an Amersham ECL Plus Western Blotting Detection System (GE Healthcare).
PCR-Based Assay for Site-Specific Methylation of the CA9 Promoter Region
The genomic DNA was extracted from each cell line or fresh-frozen tissue obtained from patients with gastric cancer using an EZ1 DNA tissue kit (Qiagen GmbH, Hilden, Germany). Site-specific methylation of the CA9 promoter region was quantified using a methylation-sensitive restriction enzyme (MSRE) method according to procedures described previously, with slight modifications.29 In brief, 1 μg of genomic DNA were digested for 2 hours at 37°C with 10 U of the MSRE HhaI (Takara Bio Inc., Shiga, Japan) recognizing the GCGC sequence, which is located at −75 to −72 bp with respect to the transcription start site of the CA9 gene. Twenty nanograms of the digested DNA was used in the subsequent RT-PCR, which was performed using a primer set that brackets the HhaI cleavage site (GCGC), in which the single CpG at −74 bp was included. The quantitative value was normalized to another PCR product using a control primer set. The primers were designed according to the genome sequence (GenBank) as follows: HhaI target region, 5′-GTGAGACTTTGGCTCCATCTCT-3′, 5′-CTGTACGTGCATTGGAAACG-3′ (106 bp), and control region, 5′-TCTGCCCAGTGAAGAGGATT-3′, 5′-GGGAGCCCTCTTCTTCTGATT-3′ (131 bp).
Statistical Analysis
Statistical analysis was performed using commercially available software (SPSS version 15.0J for Windows; SPSS, Inc., Chicago, IL). The Mann-Whitney test was used to compare clinical variables between the two groups, and the χ2 test to compare categorical data. A cutoff value for the CA9 methylation status was determined using a receiver-operator characteristic curve. The survival curves were generated using the Kaplan-Meier method, and statistical differences were compared using the log-rank test. P < 0.05 was considered significant.
Results
Immunohistochemical Staining of CA IX and HIF-1α
CA IX expression was observed in the normal gastric mucosa, in which staining of CA IX was predominantly distributed on the cell membrane, especially on the basolateral side of the gastric glands (Figure 1, A and B). CA IX expression in intestinal metaplastic lesions in the corresponding normal mucosa was not observed in this case (Figure 1A, arrows), whereas expression was occasionally observed in other cases (data not shown). High expression of CA IX was observed in 39 of 77 gastric adenocarcinomas (50.6%). Representative high and low expression levels of CA IX in cancer cells are shown in Figure 1, C and D. Positive staining for HIF-1α was observed in 46 cancer tissue samples (59.7%). Cancer specimens with positive and negative HIF-1α expression are shown in Figure 1, E and F, respectively. In cancer specimens expressing both HIF-1α and CA IX, distribution of the two proteins did not always overlap (Figure 2).
Figure 1.
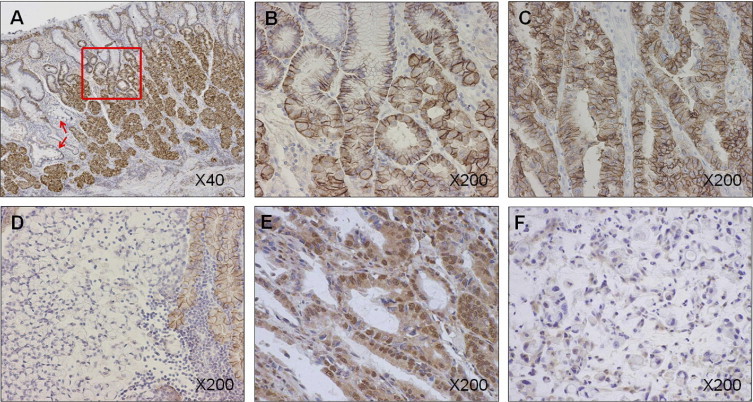
Immunohistochemical analysis of CA IX and HIF-1α expression in advanced gastric cancer. A: Expression of CA IX in normal gastric mucosa adjacent to cancer tissue. High expression of CA IX is observed in normal mucosa. Staining is slightly weaker in the foveolar epithelia than in the fundic glands at the basal part. Arrows indicate intestinal metaplasia. Intestinal metaplasia did not express CA IX in this case. Original magnification, ×40. B: Area of normal gastric mucosa surrounded by the red box in A is expanded. Positive staining for CA IX is observed at the basolateral site of the membrane. Original magnification, ×200. C: Same case as in A. Expression of CA IX in intestinal-type gastric cancer. Strong staining for CA IX is observed diffusely in the membrane and cytoplasm of the cancer cells. Original magnification, ×200. D: Expression of CA IX in diffuse-type gastric cancer. No positive staining for CA IX is observed in cancer cells, whereas adjacent normal mucosa strongly expresses CA IX. Original magnification, ×200. E: Sample positive for HIF-1α. Nuclear staining of HIF-1α is observed. Original magnification, ×200. F: Sample negative for HIF-1α. HIF-1α is undetectable in the nucleus of cancer cells. Original magnification, ×200.
Figure 2.
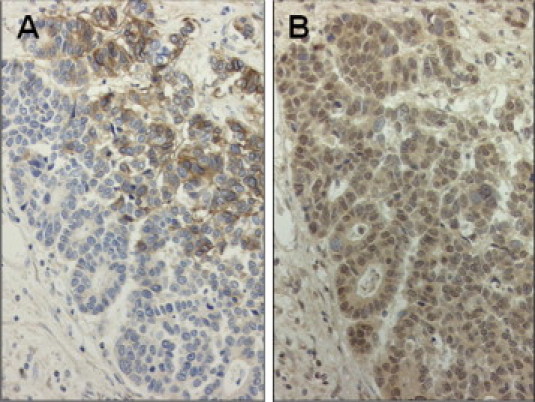
Immunohistochemical expression of CA IX (A) and HIF-1α (B) in serial tissue sections that express both HIF-1α and CA IX. Distribution patterns of these two proteins did not always overlap. Original magnification, ×200.
Expression of CA IX was significantly higher in intestinal-type cancers [22 of 30 (73.3%)] compared with diffuse-type cancers [17 of 47 (36.2%)] (P = 0.002) (Table 1). Furthermore, CA IX was more strongly expressed in tumors with intestinal metaplasia compared with those without intestinal metaplasia (P = 0.005) (Table 1). However, CA IX expression demonstrated no significant correlation with HIF-1α expression. In addition, no statistically significant correlations were observed between CA IX expression and other factors including age, sex, depth of cancer invasion (T), lymph node metastasis (N), lymphatic invasion (ly), vascular invasion (v), or tumor stage (Table 1).
Table 1.
Correlation Between CA IX Expression and Clinicopathologic Features
| Variable | CA IX expression |
P value | |
|---|---|---|---|
| High (n = 39) | Low (n = 38) | ||
| Age, mean (SD), years | 57.8 (13.6) | 65.5 (12.5) | 0.433 |
| Sex, M/F | 23/16 | 28/10 | 0.230 |
| Histologic type, intestinal/diffuse | 22/17 | 8/30 | 0.002 |
| Intestinal metaplasia, no/yes | 9/30 | 21/17 | 0.005 |
| Depth of cancer invasion, T1,2/T3,4 | 9/30 | 13/25 | 0.321 |
| Lymph node metastasis, no/yes | 3/36 | 5/33 | 0.481 |
| Lymphatic invasion, no/yes | 2/37 | 2/36 | 1.000 |
| Vascular invasion, no/yes | 14/25 | 16/22 | 0.644 |
| Stage, II/III–IV | 4/35 | 3/35 | 1.000 |
| Adjuvant chemotherapy, no/yes | 10/29 | 13/25 | 0.462 |
| HIF-1α, positive/negative | 23/16 | 23/15 | 1.000 |
Unless otherwise indicated, data are given as number of patients.
M, male; F, female.
CA IX Expression and Patient Survival
The relationship between patient outcome and CA IX expression was statistically analyzed in 77 patients with advanced gastric cancer. The level of CA IX expression was not associated with patient survival (Figure 3)A, whereas expression of HIF-1α was significantly associated with poor prognosis. Disease-specific survival in patients postive for HIF-1α (n = 46) was significantly shorter than in those negative for HIF-1α (n = 31) (P = 0.013) (Figure 3B).
Figure 3.
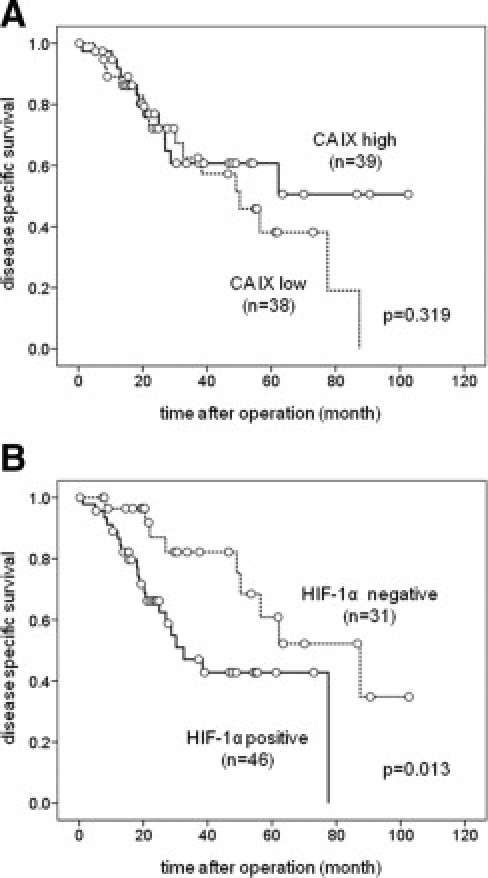
Kaplan-Meier estimates of disease-specific survival in 77 patients with gastric cancer. A: Patient survival according to expression of CA IX. CA IX expression did not correlate with survival (P = 0.319). B: Patient survival according to expression of HIF-1α. HIF-1α–negative patients demonstrate significantly longer survival compared with HIF-1α–positive cases (P = 0.013).
Quantification of CA9 Methylation in Gastric Cancer Cell Lines
A diagram of the promoter region of the CA9 gene is shown in Figure 4. There are 10 CpG sites between −200 and +300 bp that do not constitute a CpG island. The present study focused on the CpG at −74 bp, which is harbored within the HhaI cleavage site GCGC at −75 to −72 bp. There is a single HhaI site in the promoter region. Figure 4B shows the methylation status of CpG at −74 bp in the eight gastric cancer cell lines. Undigested DNA was universally amplified with a primer targeting the HhaI site. The PCR product of the digested DNA was decreased in MKN74, MKN45, HSC45, HSC57, and KATO-III cells, showing unmethylation of the CpG at −74 bp. In particular, the HSC57 and KATO-III cells demonstrated no signal, indicating complete digestion by HhaI. In contrast, the PCR signals were not altered by HhaI digestion in MKN1, MNK7, or MKN28 cells, indicating that the gene is methylated at this site in these cell lines. In contrast, the digested DNA was equally amplified with a primer set targeting the control region, which does not contain the HhaI cleavage site. Quantitative methylation values of the CA9 promoter, which were normalized to the PCR product of the control region, are shown in Figure 4C.
Figure 4.
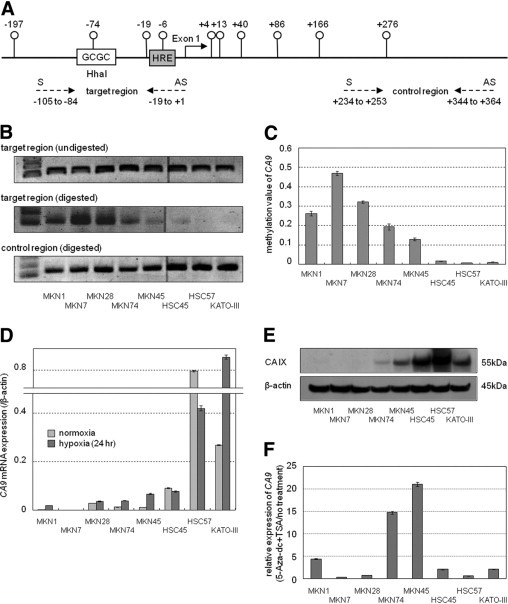
A: Diagram of promoter region of the CA9 gene. There are 10 CpG dinucleotides (°) between −200 and +300 that do not constitute a CpG island. There is only one HhaI site in the promoter region. HRE, hypoxia-responsive element: 5′-TGCACGTA-3′). Dashed arrows, Location of PCR primers. S, Sense primer; AS, antisense primer. B: Extent of PCR product with or without HhaI digestion was visualized on agarose gels. Upper lane, Single bands are equally detectable after PCR of target region without HhaI digestion. Middle lane, HhaI digestion followed by PCR of target region. A decrease in PCR product is observed in several cell lines, indicating that an unmethylated CpG site was digested by HhaI. Lower lane, PCR product of control region, in which no HhaI site exists. Each DNA sample was pretreated with HhaI. C: Methylation value of CA9, which was normalized to PCR product of control region, in the eight gastric cancer cell lines. Data are given as mean (SD) of triplicate experiments. D: Real-time quantitative RT-PCR of CA9 under conditions of normoxia and hypoxia. Expression under hypoxia was investigated after exposure to hypoxia for 24 hours. Hypoxic induction of CA9 was observed in MKN1, MKN74, MKN45, and KATO-III cells. E: Western blot analysis of CA IX. The CA IX protein was undetectable in MKN1, MKN7, and MKN28 cells. Ten micrograms of protein per sample were loaded, and equal loading was confirmed using β-actin as a control. F:CA9 expression was restored using 5-Aza-dc and trichostatin A treatment in MKN1, MKN74, and MKN45 cells. β-actin was used as internal control. Data [mean (SD)] are expressed relative to untreated cells.
Correlation Between Promoter Methylation and Expression of CA IX
Figure 4D shows the mRNA expression of CA9 under conditions of normoxia and hypoxia. The level of expression of CA9 mRNA under normoxia was detected in the order HSC57 > KATO-III > HSC45 cells. Little mRNA expression was detected in the other five cell lines. The hypoxic induction of mRNA was observed in four cell lines: MKN-1, MKN74, MKN45, and KATO-III. Figure 4E shows the expression of the CA IX protein under normoxia. CA IX was undetectable in MKN1, MKN7, and MKN28 cells. The expression pattern of the CA IX protein was similar to that of the mRNA under normoxia.
Restoration of CA9 Expression after Treatment With 5-Aza-dc and Trichostatin A
Figure 4F shows mRNA expression of CA9 after treatment with 5-Aza-dc and trichostatin A in the eight gastric cancer cells. Expression of CA9 was elevated in three cell lines, MKN1, MKN74, and MKN45, all of which carried hypermethylation of CpG at −74 bp, after drug treatment. In contrast, CA9 expression was not restored in the remaining two cell lines, MKN7 and MKN28, with CA9 hypermethylation.
Methylation of CA9 in Patients with Gastric Cancer
The methylation status of gastric cancer tissue specimens and the corresponding normal mucosa was assessed using an MSRE method. DNA from HSC57 cells was analyzed in parallel with each reaction to confirm the complete digestion by HhaI. Figure 5A shows the comparative analysis of the methylation values between the 77 cancer and corresponding normal tissues. In normal tissues, the methylation values ranged from 0.142 to 0.461 (median, 0.301). In contrast, the values in cancer tissues varied widely, ranging from 0.068 to 0.552 (median, 0.309). There were no statistically significant differences between the two groups. In the analysis of the 77 cancer tissue specimens, the methylation value of CA9 was significantly lower in patients with high CA IX expression (n = 39) than in those with low expression of CA IX (n = 38) (P = 0.003) (Figure 5B). The methylation value was significantly lower in intestinal-type cancers (n = 30) compared with diffuse-type cancers (n = 47) (P = 0.003) (Figure 5C).
Figure 5.
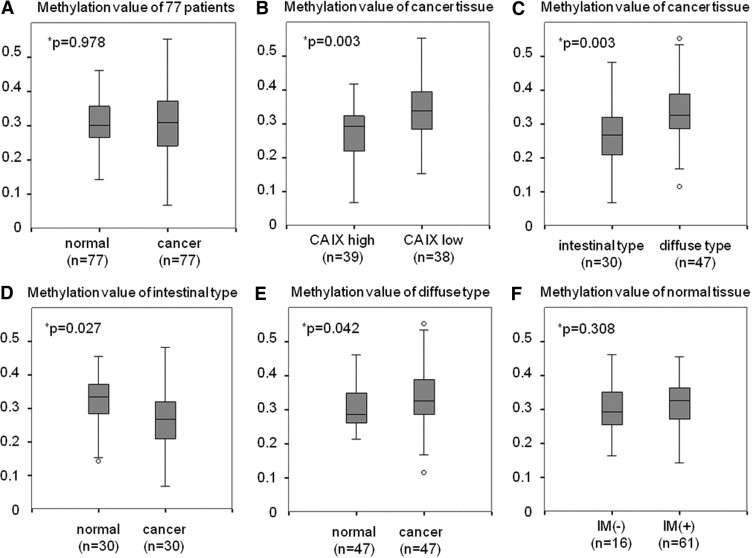
Comparative analysis of the methylation value using the 77 cancer and corresponding normal tissue samples. P values calculated with the Mann-Whitney test. IM, intestinal metaplasia. A: Methylation values were more variable in cancer tissues than in normal mucosa. B: Methylation value of CA9 was significantly lower in the case with high CA IX expression compared with those with low CA IX expression (P = 0.003). C: Diffuse-type gastric cancer (n = 47) is highly methylated compared with intestinal-type cancer (n = 30) (P = 0.003). D: Methylation value of intestinal-type cancer is significantly lower than in normal tissue (P = 0.027). E: Methylation value of diffuse-type cancer is significantly higher than in normal tissue (P = 0.042). F: Presence of intestinal metaplasia had no effect on methylation value in normal mucosa (P = 0.308).
Further comparative analysis between normal and cancer tissues was performed. When the comparison was restricted to intestinal-type cancer, the methylation value was significantly higher in normal tissues compared with cancer tissues (P = 0.027) (Figure 5D). In contrast, the value was significantly lower in normal tissues compared with cancer tissues in diffuse-type cancer (P = 0.042) (Figure 5E). The methylation value of CA9 in normal tissues did not change regardless of the presence or absence of intestinal metaplasia (Figure 5F).
Definition of Cutoff Value for CA9 Methylation Status
The methylation status of CA9 was divided into methylation and unmethylation groups according to the appropriate cutoff value determined using a receiver-operator characteristic curve. The cutoff value was determined to be 0.3165, with 63.2% sensitivity and 69.2% specificity. Thirty-six patients were included in the methylation group, and the remaining 41 patients were included in the unmethylation group.
Correlation Between Methylation Status of CA9 and Patient Characteristics
Patient clinicopathologic features were compared between the methylated (n = 36) and unmethylated (n = 41) groups (Table 2). Methylation status was significantly correlated with expression of CA IX (P = 0.006). Furthermore, methylation status was significantly correlated with histologic type (P = 0.006). Intestinal metaplasia and lymph node metastasis were also associated with methylation status (P = 0.009 and P = 0.022, respectively).
Table 2.
Correlation Between Methylation Status of CA9 and Clinicopathologic Features
| Variable | Methylation status of CA9 |
P value | |
|---|---|---|---|
| Methylation (n = 36) | Unmethylation (n = 41) | ||
| Age, mean (SD), years | 65.6 (11.4) | 67.6 (14.4) | 0.510 |
| Sex, M/F | 25/11 | 26/15 | 0.635 |
| Histologic type, intestinal/diffuse | 8/28 | 22/19 | 0.006 |
| Intestinal metaplasia, no/yes | 20/16 | 10/31 | 0.009 |
| Depth of cancer invasion, T1,2/T3,4 | 7/29 | 15/26 | 0.131 |
| Lymph node metastasis, no/yes | 7/29 | 1/40 | 0.022 |
| Lymphatic invasion, no/yes | 2/34 | 2/39 | 1.000 |
| Vascular invasion, no/yes | 14/22 | 16/25 | 1.000 |
| Stage, II/III–IV | 5/31 | 2/39 | 0.242 |
| Adjuvant chemotherapy, no/yes | 11/25 | 12/29 | 1.000 |
| CA IX, high/low | 12/24 | 27/14 | 0.006 |
Unless otherwise indicated, data are given as number of patients.
M, male; F, female.
Hypomethylation of CA9 in Intestinal-Type Gastric Cancer and Hypermethylation in Diffuse-Type Cancer
The ratio of the methylation value (cancer-to-normal) was calculated for each of the 77 paired samples, and patients were classified into hypermethylation (>1) and hypomethylation (<1) groups. Table 3 gives the relationship between tumor histologic type and the ratio of the methylation value. Twenty-two of 30 intestinal cancers (73.3%) were hypomethylated, and 31 of 47 diffuse-type cancers (66.0%) were hypermethylated. The χ2 test demonstrated a significant correlation between the methylation ratio and histologic type (P = 0.001).
Table 3.
Correlation Between Ratio of Methylation Value (Cancer/Normal) and Histologic Type
| Histologic type | Ratio of methylation value (cancer/normal) |
P value | ||
|---|---|---|---|---|
| >1 (n = 39) | <1 (n = 38) | Total | ||
| Intestinal/diffuse | 8/31 | 22/16 | 28/49 | 0.001 |
Discussion
Adaptation of cancer cells to hypoxia and acidosis is a crucial process in cancer progression.30,31 Cancer cells produce a large amount of lactic acid, which is generated through anaerobic metabolism and insufficient vascular clearing, resulting in acidification of the tumor microenvironment.32 Carbon dioxide and lactic acid are important sources of acidity in tumors, and these metabolites promote tumor growth and metastasis.33–36
CA IX is induced by hypoxia via the HIF-1α pathway,19 and the protein has an essential role in tumor acidification through hydration of carbon dioxide.37 CA IX is highly expressed in various cancers including lung,38 esophageal,39 breast,40 and renal41 malignant lesions, whereas expression is usually absent in normal tissues.8 Based on these findings, CA IX has been recognized as a hypoxic marker and a tumor-associated protein linked to cancer development.12–14 In addition, previous reports have demonstrated that overexpression of CA IX and HIF-1α is associated with poor prognosis in several cancers including gastric carcinoma.42–46 However, other reports have shown no correlation or discordant expression between these two proteins.21,22
The present study initially assessed the immunohistochemical expression of CA IX and HIF-1α in resected gastric cancer tissue specimens to investigate the hypoxia-dependent regulation of CA IX. CA IX was significantly correlated with tumor histologic type (P = 0.002) (Table 1), however, its expression was not correlated with HIF-1α. Furthermore, HIF-1α, but not CA IX, was associated with survival in the 77 patients with gastric cancer with a similar pathologic background (Figure 3). These results suggest the hypoxia-independent regulation of CA IX in gastric cancer. Recent studies in renal cancer demonstrated striking evidence that expression of CA9 is, at least in part, regulated by site-specific hypomethylation at −74 bp in the CA9 promoter.23,24 In addition, one study showed that the loss of CA9 expression in gastric cancer cells was restored after treatment with 5-Aza-dc, indicating that the expression was silenced by DNA methylation.25 These studies further prompted investigation of CA9 promoter methylation to explain the HIF-1α-independent regulation of this gene.
The CA9 promoter region contains only 10 CpG dinucleotides, which do not constitute a CpG island (Figure 4A). Using an MSRE method, site-specific methylation at −74 bp in the CA9 promoter was quantified in eight gastric cancer cell lines. Methylation of the single CpG showed an inverse correlation with expression of the CA IX protein (Figure 4, C and E). In contrast, hypoxic induction of CA9 mRNA was observed in only four of eight gastric cancer cell lines (Figure 4D). Furthermore, in one of the four cell lines with hypoxic induction of CA9 mRNA, the CA IX protein could not be detected even in cells cultured under hypoxia (data not shown). In addition, when gastric cancer cell lines were treated with 5-Aza-dc and trichostatin A, CA9 expression was restored in three of five cell lines that demonstrated CA9 methylation (Figure 4F). These findings indicate that expression of CA9 is predominantly regulated by promoter methylation rather than the hypoxic pathway in gastric cancer cells.
Methylation of CA9 in the 77 paired gastric cancer tissues and the normal mucosa was also quantified using an MSRE system. Methylation values were more variable in cancers (Figure 5A), which suggests that cancers are more heterogeneous at the CpG site at −74 bp in comparison with normal mucosa. In a methylation analysis restricted to cancer tissues, the methylation value significantly correlated with CA IX expression and tumor histologic type (Figure 5, B and C). The cutoff value differentiating the methylation status of the CA9 promoter also revealed a significant correlation between CA9 methylation and protein expression, and histologic type (Table 2). These results indicated that site-specific methylation at −74 bp is crucial for determining CA IX expression. Unmethylation at −74 bp in the CA9 promoter, which leads to expression of CA IX, is frequently observed in intestinal-type cancer. Conversely, methylation at −74 bp, which silences CA IX expression, predominantly occurs in diffuse-type cancer.
CA IX is usually absent in normal epithelium.15 In contrast to other organs, high expression of CA IX in normal gastric mucosa has been reported.15,16 In the present study, the methylation value of CA9 in normal mucosa was also estimated using an MSRE method (Figure 5A). The methylation value in cancer tissues was compared with the baseline methylation value in the matched normal mucosa specimens. The results showed that the methylation value in intestinal-type cancer was significantly lower than in normal tissue (P = 0.027) (Figure 5D). In contrast, the methylation value in diffuse-type cancer was significantly higher than in normal tissue (P = 0.042) (Figure 5E). In calculation of the ratio of the methylation value in cancer and normal tissues (Table 3), it was observed that intestinal-type cancers exhibited hypomethylation (ratio, <1) more frequently than hypermethylation (ratio, >1). Conversely, the diffuse-type cancers exhibited more frequent hypermethylation. These results suggest that the epigenetic alterations of CA9 differ between the intestinal and diffuse types of gastric cancer, which lead to differences in CA IX expression.
Previous studies have demonstrated conflicting features insofar as the biological behavior of CA IX. NIH3T3 fibroblasts and AGS gastric cancer cells transfected with CA9 exhibited significantly increased cell proliferation.10,25 These reports indicated an oncogenic role of CA9. In sharp contrast, CA IX–deficient mice develop gastric hyperplasia, which is associated with increased proliferation.47 It is possible that the former studies might support the oncogenic model for intestinal-type cancer. Site-specific hypomethylation at −74 bp, leading to CA9 expression, possibly contributes to development of this type of cancer. In contrast, the latter model might explain the possibility that site-specific methylation leads to silencing of CA9 expression, accelerating development of diffuse-type cancer.
Intestinal metaplasia is believed to be an origin of intestinal-type cancer.3 In agreement with this theory, compared with diffuse-type cancers, intestinal-type cancers more frequently contained intestinal metaplasia surrounding cancer foci (data not shown). The significant correlation between CA9 methylation and the presence of intestinal metaplasia in cancer tissues (Table 2) indicated that intestinal metaplastic lesions more frequently coexist with intestinal-type cancers than with diffuse-type cancers. This raises the question as to whether hypomethylation of CA9 might occur in the intestinal metaplastic state, which is believed to be a precancerous lesion of intestinal-type cancer. To investigate this, the methylation value of CA9 in normal tissues was compared in mucosa samples positive or negative for intestinal metaplasia. However, the results demonstrated that the presence of intestinal metaplasia did not affect the methylation value at −74 bp in normal mucosa (Figure 5F). At immunohistochemical analysis, CA IX expression in intestinal metaplasia varied widely between the 77 samples of normal mucosa (data not shown). This indicates that CA9 hypomethylation might not occur in the intestinal metaplastic state during the oncogenic process of intestinal-type cancers.
In conclusion, the present study demonstrates for the first time, to our knowledge, that expression of CA IX in gastric cancer is predominantly regulated by methylation of a single CpG site at the −74 bp position rather than by hypoxia. Furthermore, epigenetic alterations of CA9 differ in accordance with tumor histologic type, with the gene being significantly unmethylated in intestinal-type cancers and methylated in diffuse-type cancers.
Acknowledgment
We thank Mr. Fumihiro Mutoh for helpful contributions to the immunohistochemical studies.
Footnotes
Supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (Y.K.).
References
- 1.Kamangar F., Dores G.M., Anderson W.F. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;10:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Yuasa Y. Control of gut differentiation and intestinal-type gastric carcinogenesis. Nat Rev Cancer. 2003;3:592–600. doi: 10.1038/nrc1141. [DOI] [PubMed] [Google Scholar]
- 3.Busuttil R.A., Boussioutas A. Intestinal metaplasia: a premalignant lesion involved in gastric carcinogenesis. J Gastroenterol Hepatol. 2009;24:193–201. doi: 10.1111/j.1440-1746.2008.05774.x. [DOI] [PubMed] [Google Scholar]
- 4.Sly W.S., Hu P.Y. Human carbonic anhydrases and carbonic anhydrase deficiencies. Annu Rev Biochem. 1995;64:375–401. doi: 10.1146/annurev.bi.64.070195.002111. [DOI] [PubMed] [Google Scholar]
- 5.Lindskog S. Structure and mechanism of carbonic anhydrase. Pharmacol Ther. 1997;74:1–20. doi: 10.1016/s0163-7258(96)00198-2. [DOI] [PubMed] [Google Scholar]
- 6.Thiry A., Dogné J.M., Masereel B., Supuran C.T. Targeting tumor-associated carbonic anhydrase IX in cancer therapy. Trends Pharmacol Sci. 2006;27:566–573. doi: 10.1016/j.tips.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Parkkila S., Parkkila A.K. Carbonic anhydrase in the alimentary tract: roles of the different isozymes and salivary factors in the maintenance of optimal conditions in the gastrointestinal canal. Scand J Gastroenterol. 1996;31:305–317. doi: 10.3109/00365529609006403. [DOI] [PubMed] [Google Scholar]
- 8.Potter C.P., Harris A.L. Diagnostic, prognostic and therapeutic implications of carbonic anhydrases in cancer. Br J Cancer. 2003;89:2–7. doi: 10.1038/sj.bjc.6600936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pastoreková S., Závadová Z., Kostál M., Babusíková O., Závada J. A novel quasi-viral agent, MaTu, is a two-component system. Virology. 1992;187:620–626. doi: 10.1016/0042-6822(92)90464-z. [DOI] [PubMed] [Google Scholar]
- 10.Pastorek J., Pastoreková S., Callebaut I., Mornon J.P., Zelník V., Opavský R., Zat'ovicová M., Liao S., Portetelle D., Stanbridge E.J. Cloning and characterization of MN, a human tumor-associated protein with a domain homologous to carbonic anhydrase and a putative helix-loop-helix DNA binding segment. Oncogene. 1994;9:2877–2888. [PubMed] [Google Scholar]
- 11.Türeci O., Sahin U., Vollmar E., Siemer S., Göttert E., Seitz G., Parkkila A.K., Shah G.N., Grubb J.H., Pfreundschuh M., Sly W.S. Human carbonic anhydrase XII: cDNA cloning, expression, and chromosomal localization of a carbonic anhydrase gene that is overexpressed in some renal cell cancers. Proc Natl Acad Sci USA. 1998;95:7608–7613. doi: 10.1073/pnas.95.13.7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Závada J., Závadová Z., Pastoreková S., Ciampor F., Pastorek J., Zelník V. Expression of MaTu-MN protein in human tumor cultures and in clinical specimens. Int J Cancer. 1993;54:268–274. doi: 10.1002/ijc.2910540218. [DOI] [PubMed] [Google Scholar]
- 13.Liao S.Y., Brewer C., Závada J., Pastorek J., Pastorekova S., Manetta A., Berman M.L., DiSaia P.J., Stanbridge E.J. Identification of the MN antigen as a diagnostic biomarker of cervical intraepithelial squamous and glandular neoplasia and cervical carcinomas. Am J Pathol. 1994;145:598–609. [PMC free article] [PubMed] [Google Scholar]
- 14.Saarnio J., Parkkila S., Parkkila A.K., Haukipuro K., Pastoreková S., Pastorek J., Kairaluoma M.I., Karttunen T.J. Immunohistochemical study of colorectal tumors for expression of a novel transmembrane carbonic anhydrase, MN/CA IX, with potential value as a marker of cell proliferation. Am J Pathol. 1998;153:279–285. doi: 10.1016/S0002-9440(10)65569-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pastoreková S., Parkkila S., Parkkila A.K., Opavský R., Zelník V., Saarnio J., Pastorek J. Carbonic anhydrase IX. MN/CA IX: analysis of stomach complementary DNA sequence and expression in human and rat alimentary tracts. Gastroenterology. 1997;112:398–408. doi: 10.1053/gast.1997.v112.pm9024293. [DOI] [PubMed] [Google Scholar]
- 16.Kivelä A.J., Kivelä J., Saarnio J., Parkkila S. Carbonic anhydrases in normal gastrointestinal tract and gastrointestinal tumours. World J Gastroenterol. 2005;11:155–163. doi: 10.3748/wjg.v11.i2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semenza G.L. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002;8:s62–s67. doi: 10.1016/s1471-4914(02)02317-1. [DOI] [PubMed] [Google Scholar]
- 18.Semenza G.L. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 19.Wykoff C.C., Beasley N.J., Watson P.H., Turner K.J., Pastorek J., Sibtain A., Wilson G.D., Turley H., Talks K.L., Maxwell P.H., Pugh C.W., Ratcliffe P.J., Harris A.L. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60:7075–7083. [PubMed] [Google Scholar]
- 20.Loncaster J.A., Harris A.L., Davidson S.E., Logue J.P., Hunter R.D., Wycoff C.C., Pastorek J., Ratcliffe P.J., Stratford I.J., West C.M. Carbonic anhydrase (CA IX) expression, a potential new intrinsic marker of hypoxia: correlations with tumor oxygen measurements and prognosis in locally advanced carcinoma of the cervix. Cancer Res. 2001;61:6394–6399. [PubMed] [Google Scholar]
- 21.Tomes L., Emberley E., Niu Y., Troup S., Pastorek J., Strange K., Harris A., Watson P.H. Necrosis and hypoxia in invasive breast carcinoma. Breast Cancer Res Treat. 2003;81:61–69. doi: 10.1023/A:1025476722493. [DOI] [PubMed] [Google Scholar]
- 22.Tan E.Y., Yan M., Campo L., Han C., Takano E., Turley H., Candiloro I., Pezzella F., Gatter K.C., Millar E.K., O'Toole S.A., McNeil C.M., Crea P., Segara D., Sutherland R.L., Harris A.L., Fox S.B. The key hypoxia regulated gene CA IX is upregulated in basal-like breast tumours and is associated with resistance to chemotherapy. Br J Cancer. 2009;100:405–411. doi: 10.1038/sj.bjc.6604844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho M., Uemura H., Kim S.C., Kawada Y., Yoshida K., Hirao Y., Konishi N., Saga S., Yoshikawa K. Hypomethylation of the MN/CA9 promoter and upregulated MN/CA9 expression in human renal cell carcinoma. Br J Cancer. 2001;85:563–567. doi: 10.1054/bjoc.2001.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jakubicková L., Biesová Z., Pastoreková S., Kettmann R., Pastorek J. Methylation of the CA9 promoter can modulate expression of the tumor-associated carbonic anhydrase IX in dense carcinoma cell lines. Int J Oncol. 2005;26:1121–1127. [PubMed] [Google Scholar]
- 25.Chen J., Röcken C., Hoffmann J., Krüger S., Lendeckel U., Rocco A., Pastoreková S., Malfertheiner P., Ebert M.P. Expression of carbonic anhydrase 9 at the invasion front of gastric cancers. Gut. 2005;54:920–927. doi: 10.1136/gut.2004.047340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Japanese Gastric Cancer Association: Japanese classification of gastric carcinoma, 2nd English edition. Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 27.Allred D.C., Harvey J.M., Berardo M., Clark G.M. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- 28.Nakamura J., Kitajima Y., Kai K., Hashiguchi K., Hiraki M., Noshiro H., Miyazaki K. HIF-1alpha is an unfavorable determinant of relapse in gastric cancer patients who underwent curative surgery followed by adjuvant 5-FU chemotherapy. Int J Cancer. 2009;127:1158–1171. doi: 10.1002/ijc.25129. [DOI] [PubMed] [Google Scholar]
- 29.Pogribny I.P., Pogribna M., Christman J.K., James S.J. Single-site methylation within the p53 promoter region reduces gene expression in a reporter gene construct: possible in vivo relevance during tumorigenesis. Cancer Res. 2000;60:588–594. [PubMed] [Google Scholar]
- 30.Pouysségur J., Dayan F., Mazure N.M. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 31.Brahimi-Horn M.C., Chiche J., Pouysségur J. Hypoxia signalling controls metabolic demand. Curr Opin Cell Biol. 2007;19:223–229. doi: 10.1016/j.ceb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Gatenby R.A., Gillies R.J. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 33.Saarnio J., Parkkila S., Parkkila A.K., Waheed A., Casey M.C., Zhou X.Y., Pastoreková S., Pastorek J., Karttunen T., Haukipuro K., Kairaluoma M.I., Sly W.S. Immunohistochemistry of carbonic anhydrase isozyme IX (MN/CA IX) in human gut reveals polarized expression in the epithelial cells with the highest proliferative capacity. J Histochem Cytochem. 1998;46:497–504. doi: 10.1177/002215549804600409. [DOI] [PubMed] [Google Scholar]
- 34.Závada J., Závadová Z., Pastorek J., Biesová Z., Jezek J., Velek J. Human tumour-associated cell adhesion protein MN/CA IX: identification of M75 epitope and of the region mediating cell adhesion. Br J Cancer. 2000;82:1808–1813. doi: 10.1054/bjoc.2000.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helmlinger G., Sckell A., Dellian M., Forbes N.S., Jain R.K. Acid production in glycolysis-impaired tumors provides new insights into tumor metabolism. Clin Cancer Res. 2002;8:1284–1291. [PubMed] [Google Scholar]
- 36.Stubbs M., McSheehy P.M., Griffiths J.R., Bashford C.L. Causes and consequences of tumour acidity and implications for treatment. Mol Med Today. 2000;6:15–19. doi: 10.1016/s1357-4310(99)01615-9. [DOI] [PubMed] [Google Scholar]
- 37.Svastová E., Hulíková A., Rafajová M., Zat'ovicová M., Gibadulinová A., Casini A., Cecchi A., Scozzafava A., Supuran C.T., Pastorek J., Pastoreková S. Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett. 2004;577:439–445. doi: 10.1016/j.febslet.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 38.Vermylen P., Roufosse C., Burny A., Verhest A., Bosschaerts T., Pastoreková S., Ninane V., Sculier J.P. Carbonic anhydrase IX antigen differentiates between preneoplastic malignant lesions in non–small cell lung carcinoma. Eur Respir J. 1999;14:806–811. doi: 10.1034/j.1399-3003.1999.14d14.x. [DOI] [PubMed] [Google Scholar]
- 39.Turner J.R., Odze R.D., Crum C.P., Resnick M.B. MN antigen expression in normal, preneoplastic, and neoplastic esophagus: a clinicopathological study of a new cancer-associated biomarker. Hum Pathol. 1997;28:740–744. doi: 10.1016/s0046-8177(97)90185-4. [DOI] [PubMed] [Google Scholar]
- 40.Bartosová M., Parkkila S., Pohlodek K., Karttunen T.J., Galbavý S., Mucha V., Harris A.L., Pastorek J., Pastoreková S. Expression of carbonic anhydrase IX in breast is associated with malignant tissues and is related to overexpression of c-erbB2. J Pathol. 2002;197:314–321. doi: 10.1002/path.1120. [DOI] [PubMed] [Google Scholar]
- 41.Liao S.Y., Aurelio O.N., Jan K., Závada J., Stanbridge E.J. Identification of the MN/CA9 protein as a reliable diagnostic biomarker of clear cell carcinoma of the kidney. Cancer Res. 1997;57:2827–2831. [PubMed] [Google Scholar]
- 42.Swinson D.E., Jones J.L., Richardson D., Wykoff C., Turley H., Pastorek J., Taub N., Harris A.L., O'Byrne K.J. Carbonic anhydrase IX expression, a novel surrogate marker of tumor hypoxia, is associated with a poor prognosis in non–small-cell lung cancer. J Clin Oncol. 2003;21:473–482. doi: 10.1200/JCO.2003.11.132. [DOI] [PubMed] [Google Scholar]
- 43.Chia S.K., Wykoff C.C., Watson P.H., Han C., Leek R.D., Pastorek J., Gatter K.C., Ratcliffe P., Harris A.L. Prognostic significance of a novel hypoxia-regulated marker, carbonic anhydrase IX, in invasive breast carcinoma. J Clin Oncol. 2001;19:3660–3668. doi: 10.1200/JCO.2001.19.16.3660. [DOI] [PubMed] [Google Scholar]
- 44.Korkeila E., Talvinen K., Jaakkola P.M., Minn H., Syrjänen K., Sundström J., Pyrhönen S. Expression of carbonic anhydrase IX suggests poor outcome in rectal cancer. Br J Cancer. 2009;100:874–880. doi: 10.1038/sj.bjc.6604949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koukourakis M.I., Bentzen S.M., Giatromanolaki A., Wilson G.D., Daley F.M., Saunders M.I., Dische S., Sivridis E., Harris A.L. Endogenous markers of two separate hypoxia response pathways (hypoxia inducible factor 2 alpha and carbonic anhydrase 9) are associated with radiotherapy failure in head and neck cancer patients recruited in the CHART randomized trial. J Clin Oncol. 2006;24:727–735. doi: 10.1200/JCO.2005.02.7474. [DOI] [PubMed] [Google Scholar]
- 46.Driessen A., Landuyt W., Pastoreková S., Moons J., Goethals L., Haustermans K., Nafteux P., Penninckx F., Geboes K., Lerut T., Ectors N. Expression of carbonic anhydrase IX (CA IX), a hypoxia-related protein, rather than vascular-endothelial growth factor (VEGF), a pro-angiogenic factor, correlates with an extremely poor prognosis in esophageal and gastric adenocarcinomas. Ann Surg. 2006;243:334–340. doi: 10.1097/01.sla.0000201452.09591.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gut M.O., Parkkila S., Vernerova Z., Rohde E., Závada J., Höcker M., Pastorek J., Karttunen T., Gibadulinová A., Závadová Z., Knobeloch K.P., Wiedenmann B., Svoboda J., Horak I., Pastoreková S. Gastric hyperplasia in mice with targeted disruption of the carbonic anhydrase gene Car9. Gastroenterology. 2002;123:1889–1903. doi: 10.1053/gast.2002.37052. [DOI] [PubMed] [Google Scholar]


