Abstract
Proteasome inhibitors are used against human cancer, but their mechanisms of action are not entirely understood. For example, the role of the tumor suppressor p53 is controversial. We reevaluated the role of p53 in proteasome inhibitor-induced apoptosis by using isogenic human cancer cell lines with different p53 status. We found that well-known proteasome inhibitors such as MG132 and bortezomib, as well as the recently discovered proteasome inhibitor thiostrepton, induced p53-independent apoptosis in human cancer cell lines that correlated with p53-independent induction of proapoptotic Noxa but not Puma protein. In addition, these drugs inhibited growth of several cancer cell lines independently of p53 status. Notably, thiostrepton induced more potent apoptosis in HepG2 cells with p53 knockdown than in parental cells with wild-type p53. Our data confirm that proteasome inhibitors generally induce p53-independent apoptosis in human cancer cells.
The proteasome is a multiple-subunit protease complex that targets ubiquitin-tagged proteins for degradation in an ATP-dependent manner. The 20S catalytic proteasome subunit binds to 19S regulatory particles and facilitates the formation of 26S and 30S proteasomes, which recognize and eliminate ubiquitinated proteins.1 Recent progress in the understanding of proteasome function led to the development of proteasome inhibitors as anticancer drugs. Bortezomib (Velcade) was the first proteasome inhibitor approved for the treatment of human cancer (multiple myeloma) in 2003, with probable benefits against other types of cancer.2 Recently, we determined that the thiazole antibiotic thiostrepton, which can induce apoptosis in human cancer cells,3 acts as a proteasome inhibitor (PI).4 The mechanisms of proapoptotic activity of PIs in cancer cells are not well understood, and it is not clear why these drugs selectively kill tumor cells but not normal cells.
p53 is a major tumor suppressor protein that is altered by point mutations in 50% of human cancers, and p53-related pathways are inactivated in the remainder.5 p53 acts as a transcription factor and it executes its tumor suppressor activity mainly via the positive transcriptional regulation of its target genes, such as p21 or Puma, resulting in growth arrest or apoptosis in a context-dependent manner.5 p53 may also induce programmed cell death directly after rapid translocation to the cytosol or mitochondria.6 In addition, p53 negatively regulates a number of transcription factors, such as, FoxM1,7 c-Myc8 or FoxO39 or other genes, such as Plk110 by various mechanisms.
Although p53 expression is strongly induced after treatment of wild-type (wt) p53 cancer cells with PIs, there are opposing views about the role of p53 in PI-induced apoptosis. Some authors suggest that cell death induced by PIs is p53-dependent.11–16 Others, however, point to the p53-independent mechanism of PI-induced apoptosis.17–20 Moreover, it has been shown that one of the p53 targets, Noxa, is induced by proteasome inhibitors in human tumor cells by a p53-independent mechanism21 and is responsible for apoptosis in these cells.18 In the present study, we revisited and reevaluated the role of p53 in PI-induced apoptosis by using isogenic human cancer cell lines that differ only in their p53 status (with wt and inactivated p53).22,23 We found that PIs, MG132, bortezomib, and thiostrepton induce p53-independent expression of proapoptotic Noxa and p53-independent apoptosis in human cancer cells of different origin.
Materials and Methods
Cell Lines and Reagents
Human carcinoma cell lines HCT116 (colon), HepG2 (liver), and MCF-7 (breast) were obtained from the American Type Culture Collection (ATCC, Manassas, VA). Cell lines with stable knockdown of TP53, gene encoding the p53 protein, were generated previously.22 HCT116 cells with deleted p53 were obtained from Dr. Bert Vogelstein.24 HCT116 cells were cultured in Dulbecco's modified Eagle's medium and HepG2 and MCF-7 were cultured in RPMI 1640 supplemented with 10% fetal bovine serum and 1% antibiotics. The cells were maintained under standard cell culture conditions at 37°C and 5% CO2 in a humid environment. Thiostrepton and MG132 were purchased from Sigma-Aldrich (St. Louis, MO); bortezomib (Velcade) was kindly provided by Millennium Pharmaceuticals/Takeda (Cambridge, MA). The p-Babe-bcl-2 vector, described previously,25 was kindly provided by Dr. Nissim Hay. Retrovirus was generated after transfection of retroviral vector p-Babe-bcl-2 in a Phoenix packaging cell line (Orbigen, San Diego, CA). HCT-116 cells were infected with retrovirus carrying bcl-2 for 24 hours followed by treatment with PI.
Cell Viability Assay
The effect of thiostrepton, MG132, and bortezomib was determined by MTT assay. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was procured from Sigma-Aldrich. Cells were plated at a density of 1 × 104 per well in 200 μL of complete culture medium and were treated with varying concentrations in 96-well microtiter plates. After incubation for 72 hours at 37°C in a humidified incubator, 10 μL MTT (5 mg/ml in PBS) was added to each well, after which the plate was centrifuged briefly. After careful removal of the medium, 0.1 ml buffered dimethyl sulfoxide (DMSO) was added to each well. The absorbance was recorded on a microplate reader at a wavelength of 540 nm.
Detection of Apoptosis
The annexin V-PE staining kit (Enzo Life Sciences, Farmingdale, NY) was used for the detection of apoptotic bodies, following the vendor's protocol. This kit uses a dual-staining protocol in which the cells show fluorescence both of annexin V (apoptotic cells) and of 7-aminoactinomycin D (7AAD) (necrotic cells or late apoptotic cells). Briefly, the tumor cells were grown at a density of 50% confluence in 100-mm culture dishes and were treated with varying concentrations of the drugs for 24 hours. The cells were trypsinized, washed with PBS, and processed for labeling with annexin V-7AAD. The labeled cells were analyzed by flow cytometry.
Immunoblotting
Actively dividing cells were seeded into a 100-mm plate at a density of 7.5 × 105 cells. Cells were treated with thiostrepton, MG132, and bortezomib, after which the cells were lysed. Cells were lysed in immunoprecipitation buffer (20 mmol/L HEPES, 1% Triton X-100, 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 100 mmol/L NaF, 10 mmol/L Na4P2O7, 1 mmol/L sodium orthovanadate, 0.2 mmol/L phenylmethane sulfonylfluoride supplemented with protease inhibitor tablet) (Roche Applied Sciences, Indianapolis, lN) and the protein concentration was determined using the Bio-Rad (Hercules, CA) protein assay reagent. Fifty micrograms of the cell lysates were separated by electrophoresis on SDS-polyacrylamide mini gel and transferred to polyvinylidene difluoride membrane. Immunoblotting was performed with specific antibodies for cleaved caspase-3 and Mcl-1 (9664; Cell Signaling Technology, Danvers, MA), p53-sc-126 horseradish peroxidase (Santa Cruz Biotechnology, Santa Cruz, CA), caspase-7 (9492; Cell Signaling Technology), Puma (4974; Cell Signaling Technology), poly(ADP-ribose) polymerase (PARP) (sc-7150; Santa-Cruz), Noxa (2437; ProSciences, Poway, CA), p21 (556431; BD Biosciences, San Jose, CA), and β-actin (A5441, Sigma-Aldrich).
Results
Antiproliferative Activity of the PIs Was Independent of the p53 Status of the Cells
To determine the effect of PIs on the inhibition of growth of cancer cells differing in p53 status, we performed MTT cell viability assays on a pair of isogenic cell lines with wt p53 (HCT116) and its counterpart differing only in p53 status (HCT116 p53−/−). The wt p53 and p53−/− HCT116 cells were treated with varying concentrations of MG132, bortezomib, and thiostrepton for 72 hours. The PIs potently inhibited the viability of the wt p53 and p53−/− HCT116 cells equally in a dose-dependent manner, with IC50 values of MG132 and thiostrepton in low micromolar and bortezomib in nanomolar concentrations (Figure 1A). The IC50 values were 2.1 ± 0.08 and 1.4 ± 0.06 μmol/L for thiostrepton, 0.51 ± 0.09 and 0.35 ± 0.1 μmol/L for MG132, and 18 ± 0.04 and 15 ± 0.06 nmol/L for bortezomib against the HCT116 wt p53 and p53−/− cells, respectively. Furthermore, the PIs inhibited the viability of breast carcinoma cells MCF-7 (wt p53 and short hairpin RNAs p53 [shp53]) in the same way. The IC50 values were 4.5 ± 0.34 and 4.7 ± 0.2 μmol/L for thiostrepton, 0.34 ± 0.08 and 0.28 ± 0.1 for MG132 and 8 ± 0.04 and 11 ± 0.06 nmol/L for bortezomib against the MCF-7 wt p53 and shp53 cells, respectively (Figure 1B). These data suggest that all used proteasome inhibitors impede cell growth independently of p53 status.
Figure 1.
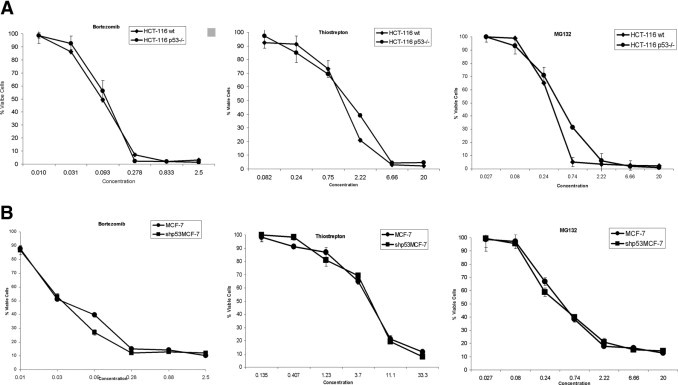
Proteasome inhibitors inhibit the viability of cancer cells in a p53-independent manner. A: Mid-log colon carcinoma HCT 116 (wild type [wt] p53) and p53−/− cells were treated with DMSO (control) or various concentrations of thiostrepton, MG132, and bortezomib for 72 hours and IC50 values were determined by using MTT cell viability assay as described under Materials and Methods. The values shown are mean ± SD for three separate experiments. The IC50 values were 2.1 ± 0.08 and 1.4 ± 0.06 μM/L for thiostrepton, 0.51 ± 0.09 and 0.35 ± 0.1 for MG132, and 18 ± 0.04 and 15 ± 0.07 nM/L for bortezomib against HCT-116 (wt p53 and p53−/−) cells, respectively. B: Mid-log breast carcinoma MCF-7 cells (wt p53 and shp53) were treated with dimethyl sulfoxide or various concentrations of thiostrepton, MG132, and bortezomib for 72 hours; IC50 values were determined by using MTT cell viability assay as described under Materials and Methods. The values shown are mean ± SD for three separate experiments. The IC50 values were 4.5 ± 0.34 and 4.7 ± 0.2 μmol/L for thiostrepton, 0.34 ± 0.08 and 0.28 ± 0.1 for MG132, and 8 ± 0.04 and 11 ± 0.06 nmol/L for bortezomib against MCF-7 wt p53 and shp53 cells, respectively.
PIs Induce p53-Independent Apoptosis in Human Cancer Cells
Actively dividing wt p53 and p53−/− HCT116 cells were treated with ∼50% inhibitory concentrations (IC50) of thiostrepton, MG132, and bortezomib for 24 hours, and cells were stained for annexin V-PE to measure apoptosis. Treatment with 3 μmol/L thiostrepton resulted in 42.8 ± 2.3% and 51.1 ± 5.2% of apoptosis in HCT116 wt p53 and p53−/− cells, respectively; 2 μmol/L MG132 resulted in 38.6 ± 1.3% and 47.5 ± 4.3% programmed cell death, whereas 10 nmol/L bortezomib induced apoptosis in 19.1 ± 0.3% and 24.1 ± 0.8% of HCT116 wt p53 and p53−/− cells, respectively (Figure 2, A and B).
Figure 2.
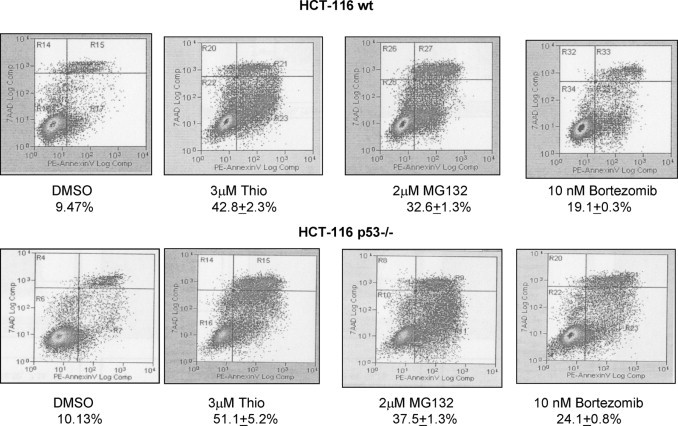
p53-independent induction of apoptosis by proteasome inhibitors in colon cancer cells. Colon carcinoma HCT 116 wild type (wt) p53 cells were treated with 3 μmol/L thiostrepton (Thio), 2 μmol/L MG132, and 10 nmol/L bortezomib for 24 hours, then stained with annexin V-PE and 7AAD and analyzed by flow cytometry. Colon carcinoma cells with knockout of p53 (HCT 116 p53−/−) were treated with 3 mmol/L thiostrepton, 2 μmol/L MG132, and 10 nmol/L bortezomib for 24 hours, then stained with annexin V-PE and 7AAD and analyzed by flow cytometry. The values shown are mean ± SD for three separate experiments.
To further demonstrate the p53-independent apoptotic activity of proteasome inhibitors, we used isogenic cell lines HCT116, MCF-7, and HepG2 with wt and inactivated p53 (HCT116 p53−/−, shp53MCF-7, and shp53HepG2).22,24 The wt p53 and the knockdown p53 cells were treated with thiostrepton, MG132, and bortezomib and then were immunoblotted for PARP, cleaved caspase-3, p53, and β-actin. Additionally, HCT116 wt p53 and p53−/− cells were treated with a known p53-dependent inducer of apoptosis, 5-fluorouracil (5-FU) as a positive control for p53-dependent apoptosis.24 As expected, PIs demonstrated induction/stabilization of p53 in the wt p53 cells, but the knockdown cells exhibited little to no levels of p53. The induction of apoptosis as demonstrated by induction of caspase-3 and PARP cleavage by the PIs was very similar in the wt p53 cells (HCT116, MCF-7, and HepG2) and their counterparts with inactivated p53 (HCT116-p53−/−, shp53-MCF-7, and shp53-HepG2), confirming that PIs induce p53-independent apoptosis (Figure 3, A–C). Notably, thiostrepton did not induce caspase-3 or PARP cleavage in the wt p53-HepG2 cells, but shp53-HepG2 cells underwent potent apoptosis after treatment with thiostrepton (Figure 3C). These data suggest that in some cases p53 may protect cells against drug-induced apoptosis.26 In contrast, in control experiments 5-FU induced strictly p53-dependent apoptosis in wt p53-HCT116, but not in p53−/− HCT11624 cells, as demonstrated by PARP and caspase-3 cleavage (Figure 3A). Overexpression of Bcl-2 inhibits apoptosis induced by proteasome inhibitors (Figure 3A), suggesting that the apoptosis is mediated through the mitochondrial pathway.27
Figure 3.
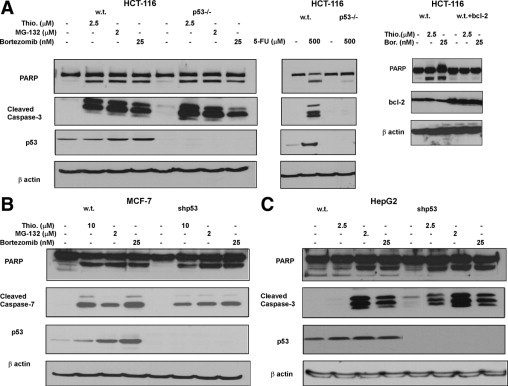
Caspase-3 and poly(ADP-ribose) polymerase (PARP) cleavage is p53-independent after treatment with proteasome inhibitor. A: Colon carcinoma HCT 116 wild type (w.t.) p53 and p53−/− cells were treated with 2.5 μmol/L thiostrepton, 2 μmol/L MG132, 25 nmol/L bortezomib, and 500 μmol/L 5-FU for 24 hours, then lysed and subjected to immunoblot analysis with antibodies against PARP, caspase-3 (cleaved), p53, and β-actin. HCT116 cells and HCT-116 cells overexpressing bcl-2 were treated with 2.5 μmol/L and 25 nmol/L bortezomib for 24 hours, then lysed and immunoblotted for PARP, bcl-2, and β-actin. B: Breast carcinoma MCF-7 w.t. p53 and shp53 cells were treated with 10 μmol/L thiostrepton, 2 μmol/L MG132, and 25 nmol/L bortezomib for 48 hours, then lysed and subjected to immunoblot analysis with antibodies against PARP, caspase-3 (cleaved), p53, and β-actin. C: Liver carcinoma HepG2 w.t. p53 and shp53 cells were treated with 2.5 μmol/L thiostrepton, 2 μmol/L MG132, and 25 nmol/L bortezomib for 24 hours, then lysed and subjected to immunoblot analysis with antibodies against PARP, caspase-3 (cleaved), p53, and β-actin.
Induction of Pro- and Antiapoptotic Factors after PI Treatment
After the demonstration of p53-independent apoptosis in isogenic human cancer cell lines, we investigated the levels of various pro- and antiapoptotic factors after treatment with PIs (Figure 4, A–C). Among the proapoptotic regulators studied, the level of Noxa (a direct p53 target) was consistently up-regulated after treatment with PIs (Figure 4, A and B) in a p53-independent manner.18 After treatment with PIs, however, HepG2 cells (wt p53 and shp53) did not demonstrate any expression of Noxa (data not shown), suggesting that Noxa may be dispensable for PI-induced apoptosis in some human cancer cells. On the other hand, after treatment with the PIs, HCT116, MCF-7, and HepG2 demonstrated a p53-dependent induction of Puma (another direct p53 target gene) that did not correlate with degree of apoptosis.
Figure 4.
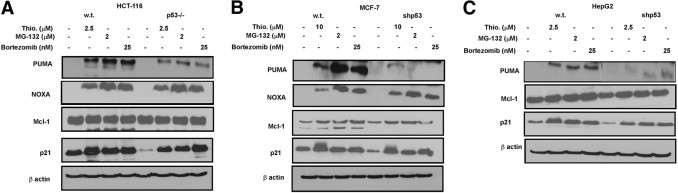
Effect of PI on p53-regulated pro- and antiapoptotic proteins. A: Colon carcinoma HCT 116 wild-type (wt) p53 and p53−/− cells were treated with 2.5 μmol/L thiostrepton, 2 μmol/L MG132, and 25 nmol/L bortezomib for 24 hours, then lysed and subjected to immunoblot analysis with antibodies against Puma, Noxa, Mcl-1, p21, and β-actin. B: Breast carcinoma MCF-7 w.t. p53 and shp53 cells were treated with 10 μmol/L thiostrepton, 2 μmol/L MG132, and 25 nmol/L bortezomib for 48 hours, then lysed and subjected to immunoblot analysis with antibodies against Puma, Noxa, Mcl-1, p21, and β-actin C: Liver carcinoma HepG2 w.t. p53 and shp53 cells were treated with 2.5 μmol/L thiostrepton, 2 μmol/L MG132, and 25 nmol/L bortezomib for 24 hours, then lysed and subjected to immunoblot analysis with antibodies against Puma, Noxa, Mcl-1, p21, and β-actin.
Treatment with PIs resulted in stabilization of the antiapoptotic p21 protein in wt p53 cells and in the cells with inactivated p53, but p21 expression was significantly lower in p53-compromised cells than in the isogenic wt p53 cell lines (Figure 4). Higher levels of antiapoptotic p2128 in wt p53 HepG2 cells treated with thiostrepton (Figure 4C) may explain protection of wt p53 cells from thiostrepton-induced apoptosis (Figure 3C).26 In contrast, stabilization of antiapoptotic short-lived Mcl-1 protein after treatment with PIs was p53-independent.
Discussion
Proteasome inhibitors are currently used for cancer treatment, but the precise mechanisms of their anticancer activity are still poorly understood. Moreover, some important questions are yet to be resolved, such as the role of p53 in PI-induced apoptosis. Numerous reports have addressed p53-dependent apoptosis induced by PIs. For example, Lopes et al11 showed that PC12 and Rat-1 cells expressing dominant–negative p53 became resistant to MG115-induced apoptosis. Similarly, human Burkitt lymphomas with wt p53 and overexpression of Hdm2 were sensitive to bortezomib, unless p53 activity was compromised with HPV-E6.14 It has been suggested that the PIs epoxomicin15 and MG13213 induce p53- and Puma-dependent apoptosis in HCT116 cells. Moreover, MacLaren et al12 demonstrated that PI-induced apoptosis in mammary epithelial cells is also p53-dependent.
In contrast, in the present study we showed that, in isogenic human cancer cell lines of different origin (namely, HCT116, colon; MCF-7, breast; and HepG2, liver) that differ only in their p53 status, the proteasome inhibitors MG-132, bortezomib, and thiostrepton induce p53-independent apoptosis (Figures 2 and 3). Cell death induced by these drugs was correlated with p53-independent induction of proapoptotic protein Noxa, but not with p53-dependent up-regulation of proapoptotic Puma (Figure 4). These data support previous reports by An et al,17 Ri et al,20 Perez-Galan et al,19 and Fernandez et al,18 who showed that bortezomib induced p53-independent apoptosis in human lymphoma and melanoma cells via the up-regulation of Noxa. However, we also found that PIs induced p53-independent apoptosis in HepG2 cells (Figure 3C) without expression of Noxa (data not shown). In addition, we found that PIs inhibit growth of human cancer cell line in MTT assay independently of p53 status (Figure 1). Notably, we also demonstrated that inactivation of p53 in HepG2 cells makes these cells more sensitive to thiostrepton (Figure 3C), suggesting that in some cases p53 may protect against thiostrepton-induced apoptosis.26
Anticancer activity of PIs was explained in part by stabilization of p53, IκB (NF-κB inhibition),29 Noxa18 or the CDK inhibitor p27,30 by activation of JNK and Fas,31 by ROS induction,32 and by suppression of FoxM1.4 Overall, the present data confirm that PI-induced apoptosis generally is p53-independent, because we studied this process in isogenic human cancer cell lines differing only in their p53 status and we did not see any change in apoptosis or inhibition of cell growth based on p53 status of the cells. It is possible, however, that stabilization of p53 induced by low concentrations of PIs in wt p53 cells may result in more potent apoptosis than in the cells with deleted p53 or that, in some special cases, stabilization of p53 by PIs is favorable for PI-induced apoptosis. On the other hand, in some instances p53 protects cells from PI- induced apoptosis. Additional experiments are needed to explain the possible discrepancies of the p53 role in PI-induced apoptosis.
Acknowledgments
We thank Dr. Bert Vogelstein (Johns Hopkins University) for the kind gift of HCT116-p53−/− cell line. We thank Dr. Nissim Hay (University of Illinois at Chicago) for the p-Babe-bcl2 vector. We thank Dr. Angela Tyner (University of Illinois at Chicago) for her valuable comments.
Footnotes
This work was supported by NIH grants 1R01CA1294414 and 1R21CA134615 to ALG.
References
- 1.Rubin D.M., Finley D. Proteolysis. The proteasome: a protein-degrading organelle. Curr Biol. 1995;5:854–858. doi: 10.1016/s0960-9822(95)00172-2. [DOI] [PubMed] [Google Scholar]
- 2.Lenz H.J. Clinical update: proteasome inhibitors in solid tumors. Cancer Treat Rev. 2003;29(Suppl 1):41–48. doi: 10.1016/s0305-7372(03)00082-3. [DOI] [PubMed] [Google Scholar]
- 3.Bhat U.G., Halasi M., Gartel A.L. Thiazole antibiotics target FoxM1 and induce apoptosis in human cancer cells. PLoS One. 2009;4:e5592. doi: 10.1371/journal.pone.0005592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhat U.G., Halasi M., Gartel A.L. FoxM1 is a general target for proteasome inhibitors. PLoS One. 2009;4:e6593. doi: 10.1371/journal.pone.0006593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vousden K.H., Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 6.Mihara M., Erster S., Zaika A., Petrenko O., Chittenden T., Pancoska P., Moll U.M. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11:577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 7.Pandit B., Halasi M., Gartel A.L. p53 negatively regulates expression of FoxM1. Cell Cycle. 2009;8:3425–3427. doi: 10.4161/cc.8.20.9628. [DOI] [PubMed] [Google Scholar]
- 8.Sachdeva M., Zhu S., Wu F., Wu H., Walia V., Kumar S., Elble R., Watabe K., Mo Y.Y. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc Natl Acad Sci USA. 2009;106:3207–3212. doi: 10.1073/pnas.0808042106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.You H., Yamamoto K., Mak T.W. Regulation of transactivation-independent proapoptotic activity of p53 by FOXO3a. Proc Natl Acad Sci USA. 2006;103:9051–9056. doi: 10.1073/pnas.0600889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sur S., Pagliarini R., Bunz F., Rago C., Diaz L.A., Jr, Kinzler K.W., Vogelstein B., Papadopoulos N. A panel of isogenic human cancer cells suggests a therapeutic approach for cancers with inactivated p53. Proc Natl Acad Sci USA. 2009;106:3964–3969. doi: 10.1073/pnas.0813333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopes U.G., Erhardt P., Yao R., Cooper G.M. p53-dependent induction of apoptosis by proteasome inhibitors. J Biol Chem. 1997;272:12893–12896. doi: 10.1074/jbc.272.20.12893. [DOI] [PubMed] [Google Scholar]
- 12.MacLaren A.P., Chapman R.S., Wyllie A.H., Watson C.J. p53-dependent apoptosis induced by proteasome inhibition in mammary epithelial cells. Cell Death Differ. 2001;8:210–218. doi: 10.1038/sj.cdd.4400801. [DOI] [PubMed] [Google Scholar]
- 13.Ding W.X., Ni H.M., Chen X., Yu J., Zhang L., Yin X.M. A coordinated action of Bax: PUMA, and p53 promotes MG132-induced mitochondria activation and apoptosis in colon cancer cells. Mol Cancer Ther. 2007;6:1062–1069. doi: 10.1158/1535-7163.MCT-06-0541. [DOI] [PubMed] [Google Scholar]
- 14.Yu D., Carroll M., Thomas-Tikhonenko A. p53 status dictates responses of B lymphomas to monotherapy with proteasome inhibitors. Blood. 2007;109:4936–4943. doi: 10.1182/blood-2006-10-050294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Concannon C.G., Koehler B.F., Reimertz C., Murphy B.M., Bonner C., Thurow N., Ward M.W., Villunger A., Strasser A., Kogel D., Prehn J.H. Apoptosis induced by proteasome inhibition in cancer cells: predominant role of the p53/PUMA pathway. Oncogene. 2007;26:1681–1692. doi: 10.1038/sj.onc.1209974. [DOI] [PubMed] [Google Scholar]
- 16.Vaziri S.A., Grabowski D.R., Hill J., Rybicki L.R., Burk R., Bukowski R.M., Ganapathi M.K., Ganapathi R. Inhibition of proteasome activity by bortezomib in renal cancer cells is p53 dependent and VHL independent. Anticancer Res. 2009;29:2961–2969. [PMC free article] [PubMed] [Google Scholar]
- 17.An W.G., Hwang S.G., Trepel J.B., Blagosklonny M.V. Protease inhibitor-induced apoptosis: accumulation of wt p53, p21WAF1/CIP1, and induction of apoptosis are independent markers of proteasome inhibition. Leukemia. 2000;14:1276–1283. doi: 10.1038/sj.leu.2401812. [DOI] [PubMed] [Google Scholar]
- 18.Fernández Y., Verhaegen M., Miller T.P., Rush J.L., Steiner P., Opipari A.W., Jr, Lowe S.W., Soengas M.S. Differential regulation of Noxa in normal melanocytes and melanoma cells by proteasome inhibition: therapeutic implications. Cancer Res. 2005;65:6294–6304. doi: 10.1158/0008-5472.CAN-05-0686. [DOI] [PubMed] [Google Scholar]
- 19.Pérez-Galán P., Roué G., Villamor N., Montserrat E., Campo E., Colomer D. The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and Noxa activation independent of p53 status. Blood. 2006;107:257–264. doi: 10.1182/blood-2005-05-2091. [DOI] [PubMed] [Google Scholar]
- 20.Ri M., Iida S., Ishida T., Ito A., Yano H., Inagaki A., Ding J., Kusumoto S., Komatsu H., Utsunomiya A., Ueda R. Bortezomib-induced apoptosis in mature T-cell lymphoma cells partially depends on upregulation of Noxa and functional repression of Mcl-1. Cancer Sci. 2009;100:341–348. doi: 10.1111/j.1349-7006.2008.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikiforov M.A., Riblett M., Tang W.H., Gratchouck V., Zhuang D., Fernandez Y., Verhaegen M., Varambally S., Chinnaiyan A.M., Jakubowiak A.J., Soengas M.S. Tumor cell-selective regulation of NOXA by c-MYC in response to proteasome inhibition. Proc Natl Acad Sci USA. 2007;104:19488–19493. doi: 10.1073/pnas.0708380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radhakrishnan S.K., Gierut J., Gartel A.L. Multiple alternate p21 transcripts are regulated by p53 in human cells. Oncogene. 2006;25:1812–1815. doi: 10.1038/sj.onc.1209195. [DOI] [PubMed] [Google Scholar]
- 23.Bunz F., Dutriaux A., Lengauer C., Waldman T., Zhou S., Brown J.P., Sedivy J.M., Kinzler K.W., Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 24.Bunz F., Hwang P.M., Torrance C., Waldman T., Zhang Y., Dillehay L., Williams J., Lengauer C., Kinzler K.W., Vogelstein B. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J Clin Invest. 1999;104:263–269. doi: 10.1172/JCI6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kennedy S.G., Kandel E.S., Cross T.K., Hay N. Akt/Protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol Cell Biol. 1999;19:5800–5810. doi: 10.1128/mcb.19.8.5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halasi M., Schraufnagel D., Gartel A.L. Wild-type p53 protects normal cells against apoptosis induced by thiostrepton. Cell Cycle. 2009;8:2850–2851. doi: 10.4161/cc.8.17.9414. [DOI] [PubMed] [Google Scholar]
- 27.Voortman J., Checinska A., Giaccone G., Rodriguez J.A., Kruyt F.A. Bortezomib, but not cisplatin, induces mitochondria-dependent apoptosis accompanied by up-regulation of noxa in the non-small cell lung cancer cell line NCI-H460. Mol Cancer Ther. 2007;6:1046–1053. doi: 10.1158/1535-7163.MCT-06-0577. [DOI] [PubMed] [Google Scholar]
- 28.Gartel A.L., Tyner A.L. The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther. 2002;1:639–649. [PubMed] [Google Scholar]
- 29.Mitsiades N., Mitsiades C.S., Richardson P.G., Poulaki V., Tai Y.T., Chauhan D., Fanourakis G., Gu X., Bailey C., Joseph M., Libermann T.A., Schlossman R., Munshi N.C., Hideshima T., Anderson K.C. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood. 2003;101:2377–2380. doi: 10.1182/blood-2002-06-1768. [DOI] [PubMed] [Google Scholar]
- 30.Nickeleit I., Zender S., Sasse F., Geffers R., Brandes G., Sorensen I., Steinmetz H., Kubicka S., Carlomagno T., Menche D., Gutgemann I., Buer J., Gossler A., Manns M.P., Kalesse M., Frank R., Malek N.P. Argyrin a reveals a critical role for the tumor suppressor protein p27(kip1) in mediating antitumor activities in response to proteasome inhibition. Cancer Cell. 2008;14:23–35. doi: 10.1016/j.ccr.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 31.Mitsiades N., Mitsiades C.S., Poulaki V., Chauhan D., Fanourakis G., Gu X., Bailey C., Joseph M., Libermann T.A., Treon S.P., Munshi N.C., Richardson P.G., Hideshima T., Anderson K.C. Molecular sequelae of proteasome inhibition in human multiple myeloma cells. Proc Natl Acad Sci USA. 2002;99:14374–14379. doi: 10.1073/pnas.202445099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pei X.Y., Dai Y., Grant S. The proteasome inhibitor bortezomib promotes mitochondrial injury and apoptosis induced by the small molecule Bcl-2 inhibitor HA14-1 in multiple myeloma cells. Leukemia. 2003;17:2036–2045. doi: 10.1038/sj.leu.2403109. [DOI] [PubMed] [Google Scholar]


