Abstract
Ocular mucous membrane pemphigoid is an immunobullous disease in which excessive conjunctival fibrosis causes blindness, and the pathogenesis of scarring is incompletely understood. To establish whether profibrotic fibroblasts with an altered phenotype exist in ocular mucous membrane pemphigoid, we compared the functional characteristics of pemphigoid conjunctival fibroblasts to normal conjunctival fibroblasts with respect to cell division; migration; collagen contraction; matrix metalloproteinase, secretion of collagen and chemokines; and myofibroblast differentiation. We found that pemphigoid fibroblasts showed increased cell division (P = 0.01), increased migration in serum-free medium (72 ± 18 migrated cells versus 33 ± 11, P = 0.04), increased collagen contraction in the presence of 10 ng/ml tumor necrosis factor-α, increased collagen type I secretion (P = 0.03), increased secretion of matrix metalloproteinase-3 (P = 0.03), and increased secretion of eotaxin in response to interleukin-13 (P = 0.04). Differences between pemphigoid and normal conjunctival fibroblasts with respect to collagen contraction and MMP secretion in the presence of interleukin-13 were also observed. Together, these findings indicate that pemphigoid conjunctival fibroblasts have a profibrotic phenotype that is maintained in vitro. No differences between pemphigoid fibroblasts obtained from acutely inflamed versus clinically uninflamed conjunctiva were observed. Developing effective antifibrotic therapies will require understanding of the mechanisms that both induce and maintain the profibrotic phenotype.
Ocular mucous membrane pemphigoid (ocular MMP), also known as ocular cicatricial pemphigoid, is a blinding disease characterized by recurrent episodes of inflammation and aggressive conjunctival fibrosis.1,2 It is part of a spectrum of immunobullous disease associated with linear deposition of IgG and IgA autoantibodies directed against epithelial basement membrane zone (BMZ) proteins, in which subepithelial bullae and scarring are the clinical hallmarks.2 Although the mechanisms have not been clearly demonstrated, the autoantibodies are thought to cause subepithelial blisters by disrupting basement membrane adherence via a process involving complement, neutrophils, and proinflammatory cytokines.3 The resulting loss of BMZ adhesion, and inflammation secondary to the BMZ separation process, leads to fibrosis. In the eye, blisters are rarely seen clinically, and the disease usually manifests as a chronic, recurrent cicatricial conjunctivitis. Fibrosis of subepithelial tissues appears as fine, white striae most easily seen surrounding the superficial vessels of substantia propria. Contracture of these striae ultimately leads to bands of abnormal connective tissue which further contract to cause conjunctival “shrinkage.” Extensive subepithelial bands of connective tissue result in symblepharon formation. The functional consequences of scarring in the eye are severe, and patients with advanced conjunctival fibrosis become blind in 30% of cases.4 Developing effective antifibrotic agents is challenging because the cellular and molecular mechanisms of conjunctival scarring are incompletely understood.
Although there is clear evidence that acute inflammation following conjunctival injury in ocular MMP, either iatrogenic due to surgery or due to the autoimmune process, triggers rapid and progressive fibrosis,5,6 it has also been observed that conjunctival fibrosis in ocular MMP can still progress despite apparent clinical control of inflammation by treatment with immunosuppressive therapy.7,8 Histological studies of chronic ocular MMP have shown that even when the conjunctiva appears clinically “white” and uninflamed, there is a significant cellular infiltrate present (“white inflammation”).9,10 Progressive fibrosis, despite immunosuppressive treatment, may be driven by this underlying chronic inflammation and ongoing release of cytokines. Administering higher doses of systemic immunosuppression might possibly control this underlying residual inflammation; however, the majority of mucous membrane pemphigoid patients are elderly and susceptible to toxicity resulting from high-dose immunosuppression.8 Another problem with the administration of immunosuppressive therapy is that there are currently no readily identifiable serological markers11 for disease activity that permit the recognition of an endpoint for the control of subclinical levels of inflammation in clinically controlled disease.
Although inflammation typically precedes the development of fibrosis in most fibrotic disorders, some experimental models suggest that fibrosis is not always characterized by persistent inflammation, and that to a degree, the mechanisms regulating fibrosis are distinct from those controlling inflammation.12 In line with this viewpoint, an alternative theory for the observed progression of fibrosis in ocular MMP is that the fibroblasts have been transformed into an abnormally activated phenotype. Profibrotic phenotypes are observed and maintained in vitro in fibroblasts from patients with scleroderma, pulmonary, renal, and colonic fibrosis.13–15 There is some evidence of phenotypic changes in fibroblasts isolated from mucous membrane pemphigoid patients,16–18 but whether there are pathological alterations in key aspects of fibroblast behavior, such as motility, contractile function, matrix synthesis, and development of myofibroblast characteristics, has not been investigated. Moreover, whether there are differences in fibroblasts isolated from actively inflamed tissue compared with fibroblasts isolated from clinically uninflamed tissue is unknown.
In the present study, we sought to establish whether there are phenotypic differences in fibroblast functional activity between mucous membrane pemphigoid conjunctival fibroblasts and normal conjunctival fibroblasts, and whether fibroblasts isolated from actively inflamed tissue differ from those isolated from clinically uninflamed tissue.
Materials and Methods
Conjunctival Tissues
Bulbar conjunctival biopsies were obtained from patients with ocular MMP, who were classified according to ocular disease activity as having active disease with acute inflammation (n = 10), or chronic disease without clinically apparent inflammation after treatment with immunosuppressive therapy (n = 7). Healthy, normal bulbar conjunctiva from 11 individuals of similar age (50 to 80 years) undergoing routine cataract surgery was used as controls. Details regarding the patients and normal controls are shown in Table 1. Four-millimeter snip biopsies were taken from the superior bulbar conjunctiva as previously described.10
Table 1.
Details of Patients and Controls
| Diagnosis | Case | Age (years) | Sex | DIF | IIF | Disease duration (years) | Bulbar inflammation grade (0 to 4) | Tauber stage44 (upper stage/lower stage) | Topical therapy | Systemic therapy | Other eye pathology |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Active MMP | 1 | 54 | F | IgG, IgA, C3 eye and oral | IgG 1/10 | 0.5 | 3 | IIcIIId/IIcIIId | Hypromellose, acetylcysteine, yellow soft paraffin | None | |
| 2 | 80 | M | IgG, IgA eye | IgG 1/100, IgA 1/10 | 10 | 3 | IIbIIIc(2)/IIdIIId(2) | Carmellose | Mycophenolate + dapsone | ||
| 3 | 55 | M | IgG, IgA eye | Negative | 13 | 3 | IIcIIIc(2)/IIdIIId(1) | Dexamethasone, carmellose | Mycophenolate + dapsone + deflazocort | ||
| 4 | 76 | F | IgG, IgA eye | Negative | 2 | 2.5 | IIc/IIbIIIa(2) | Prednisolone, hypromellose, carmellose | Mycophenolate + dapsone | Sicca, blepharitis | |
| 5 | 81 | M | IgG, IgA eye | IgG 1/10 | 2 | 2.5 | IIaIIIa(1)/IIbIIIb(2) | Carmellose | Mycophenolate + dapsone | ||
| 6 | 83 | M | IgG oral | Negative | 1 | 3 | IIbIIIa(1)/IIcIIIc(2) | Ofloxacin, chloramphenicol | None | ||
| 7 | 62 | F | IgG, IgA, C3 eye and oral | Negative | 18 | 2 | IIcIIIc(2)/IIdIIId(2) | Timolol, carbomer 980 | Mycophenolate | Glaucoma | |
| 8 | 64 | F | IgG, IgA vulva and mouth | ND | 1 | 2 | IIa/IIbIIIc(2) | Prednisolone | None | ||
| 9 | 83 | F | IgG, IgA oral | NC16A ELISA positive | 7 | 3 | IIa/IIbIIIb(2) | Brimonidine, latanoprost, timolol, dorzolamide, carmellose | Mycophenolate + doxycycline | Glaucoma | |
| 10 | 51 | F | IgG, IgA eye | IgG to LAD1 | 0.1 | 3 | I/IIIa(1) | None | None | ||
| Treated | 1 | 59 | F | IgG, IgA eye | Negative | 10 | 1 | IIbIIIb(2)/IIdIIId(2) | Carbomer 980 | Dapsone | |
| uninflamed MMP | 2 | 86 | F | IgG, IgA skin | IgG positive, IgG and IgA to LAD-1 | 15 | 1 | IIb/IIcIIIb(2) | None | None | |
| 3 | 78 | F | IgG, IgA oral | ND | 10 | 1 | IIbIIIb(2)/IIdIIId(2) | None | None | ||
| 4 | 84 | F | IgG, IgA eye | ND | 2 | 0 | IIb/IIcIIIc(2) | None | Mycophenolate | ||
| 5 | 66 | F | IgG, IgA eye | ND | 2 | 0 | IIb/IIcIIIb(2) | None | Dapsone | ||
| 6 | 76 | M | IgG, IgA eye | Negative | 6 | 1.5 | IIdIIId(2)/IIbIIIb(2) | Chloramphenicol, hypromellose, retinoic acid, acetylcysteine | Cyclophosphamide | ||
| 7 | 60 | F | Negative | Positive | 3 | 1 | I/IIaIIIa(1) | Carmellose | Cyclophosphamide + dapsone | ||
| Normal | 1 | 50 | F | — | 0 | — | None | None | Cataract | ||
| control | 2 | 76 | M | — | 0 | — | None | None | Cataract | ||
| 3 | 65 | M | — | 0 | — | None | None | Cataract | |||
| 4 | 70 | F | — | 0 | — | None | None | Cataract | |||
| 5 | 65 | M | — | 0 | — | None | None | Cataract | |||
| 6 | 75 | F | — | 0 | — | None | None | Cataract | |||
| 7 | 73 | F | — | 0 | — | None | None | Cataract | |||
| 8 | 57 | M | — | 0 | — | None | None | Cataract | |||
| 9 | 65 | M | — | 0 | — | None | None | Cataract | |||
| 10 | 79 | F | — | 0 | — | None | None | Cataract | |||
| 11 | 62 | M | — | 0 | — | None | None | Cataract |
DIF, direct immunofluorescence; IIF, indirect immunofluorescence, immunoblotting, and enzyme-linked immunosorbent assay results; MMP, mucous membrane pemphigoid; F, female; M, male; IgG, immunoglobulin G; IgA, immunoglobulin A; ELISA, enzyme-linked immunosorbent assay; LAD-1, Linear IgA Disease-1 protein, which is a cleavage product of bullous pemphigoid BP180 protein; ND, not done.
* Values in the IIF column include the results from immunoblotting and the ELISA.
The diagnosis of MMP was based on clinical presentation with progressive conjunctival cicatrization and positive direct immunofluorescence microscopy of the conjunctiva or other tissues (oral mucosa, genital mucosa, and skin) showing linear deposition of IgG and/or IgA and/or C3 at the BMZ (Figure 1), and/or positive circulating anti-epithelial BMZ autoantibodies.19Serological testing for anti-epithelial BMZ autoantibodies was carried out by i) indirect immunofluorescence microscopy on human salt-split skin (for IgG and IgA), ii) enzyme-linked immunosorbent assay using the immunodominant NC16A domain of BP180 (type XVII collagen) as target antigen (MBL, Nagoya, Japan), iii) immunoblotting with the linear IgA dermatoses antigen 1 (LAD-1) representing the soluble ectodomain of BP180 generated from the concentrated supernatant of cultured human keratinocytes,20 iv) immunoblotting with the extracellular matrix of cultured human keratinocytes for anti-laminin 332 reactivity,21 v) immunblotting with extract from human dermis for antibodies to the p200 antigens and type VII collagen,22 and vi) immunoblotting with the recombinant NC1 domain of type VII collagen, the immunodominant region in epidermolysis bullosa acquisita. Compared with indirect immunofluorescence microscopy, which detects circulating autoantibodies in about half the MMP sera, the combined testing for anti-NC16A and anti–LAD-1 autoantibodies has been shown previously to increase sensitivity to more than 80% in MMP sera.20
Figure 1.
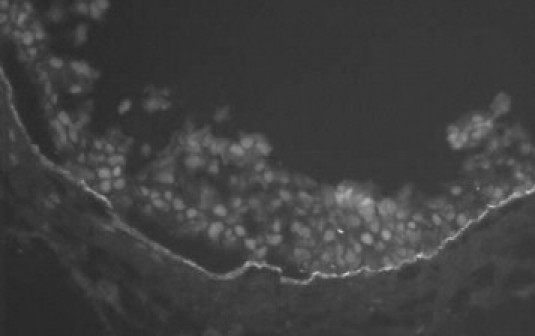
Positive direct immunofluorescence (DIF) microscopy showing linear IgG deposition at the basement membrane zone (BMZ) in a bulbar conjunctival biopsy from patient 2 with active mucous membrane pemphigoid.
No patients with pseudopemphigoid (diagnosed by the presence of conjunctival cicatrization, negative direct immunofluorescence microscopy, negative circulating anti-epithelial autoantibodies, and the presence of an alternative cause for cicatrization, eg, drug-induced conjunctival cicatrization, atopic conjunctivitis, rosacea, lichen planus)19 were included. Drug-induced conjunctival cicatrization was diagnosed if there was a history of use of topical agents previously reported to cause pseudopemphigoid (eg, β-blockers, pilocarpine) and progressive cicatrization in the eye in which the topical agent was used.23 Patients 7 and 9 initially presented with both oral and ocular lesions that were biopsy positive for MMP; they subsequently developed glaucoma, for which they received topical medication. The possibility cannot be excluded that, in these two patients, chronic, low-grade conjunctival inflammation and/or direct tissue damage following chronic administration of topical agents may have added to the conjunctival cicatrization mediated by MMP.23
Indirect immunofluorescence microscopy using human salt-split skin showed the presence of circulating anti-epithelial BMZ antibodies in 5/17 (29%) MMP patients (3/10 active MMP patients, 2/7 uninflamed MMP patients). IgG and/or IgA to LAD-1 was detected in 2/17 (12%) MMP patients, and IgG to BP180 NC16A was present in 1/17 (6%) MMP sera. No circulating autoantibodies against laminin 332 or type VII collagen were detected in any MMP patient.
The immunosuppressive regimen used to control inflammation was that which we have described previously.8 All patients and controls were white. Institutional research and ethics committee approval was granted, and informed consent was obtained from all patients and normal controls participating in the study.
Isolation of Conjunctival Fibroblasts
Fibroblasts were grown from the MMP and normal conjunctival biopsies as previously described.24 The explants were placed into tissue culture wells under a coverslip, and cultured with fibroblast culture medium (FCM) composed of Dulbecco's modified Eagle's medium supplemented with 10% (v/v) heat-inactivated fetal calf serum, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B (all from Gibco Invitrogen Ltd, Paisley, Scotland, UK) at 37°C with 5% (v/v) CO2 in air. The cells were passaged 1:3 with trypsin/EDTA. Cultures were used between passages 3 and 7 for experiments, and cultures were assessed for typical fibroblast morphology by phase contrast microscopy before every experiment. From passage 3 onwards, contaminating epithelial cells had been eliminated in cultures that had been fed only FCM, due to the fastidious nutritional requirements of epithelial cells.
Serum-free medium (SFM) composed of Dulbecco's modified Eagle's medium supplemented with 0.1% bovine serum albumin (Sigma-Aldrich, Gillingham, Dorset, UK) was used where possible in all experiments in which the effect of the addition of a cytokine or growth factor was being evaluated. Testing under serum-free conditions eliminates the possibility that observed responses could be due to the activity of unspecified cytokines and growth factors present in serum-containing medium. 3T3 cells (a fibroblast cell line) were used for initial calibration experiments that compared the negative-control SFM with the positive-control FCM.
Proliferation
Fibroblast cell division was analyzed by flow cytometry using the fluorescent probe CFSE (carboxyfluorescein diacetate succinimidyl ester) (Vybrant CFDA SE Cell Tracer Kit, Invitrogen). Fibroblasts deprived of serum for 24 hours were detached with trypsin, and the cell pellet was incubated with 1 μmol/L CFSE in serum-free medium at 37°C for 10 minutes, then ice-cold FCM was added to stop the reaction. The cell pellet was resuspended in ice-cold FCM for 30 minutes to allow free CFSE to come out of the cells, then the cells were resuspended at 2 × 105 cells/ml in FCM. At time 0, one quarter of these cells were immediately acquired for flow cytometry (10,000 events…etc) these cells (time 0) were immediately acquired for flow cytometry (10,000 events; FACSCalibur; BD Biosciences, Oxford, UK). The remaining cells were seeded into six-well culture plates at a density of 4 × 105 cells per well in FCM for 72 hours (time 96 hours). After 96 hours, the cells were detached with trypsin and 10,000 events acquired for flow cytometry. Winlist software (Verity Software House, Topsham, ME) was used to analyze the number of divided cells per 5000 undivided cells.
Migration
Cell culture inserts incorporating polyethylene terephthalate membranes with a pore size of 8 μm (BD Falcon; VWR International, Lutterworth, Leicestershire, UK), which fit into wells in a tissue culture plate, were used to assess fibroblast migration. Cells were seeded into the inserts at a density of 8000 per insert in 200 μl of SFM and allowed to attach to the upper surface of the membrane for 4 hours. A volume of 700 μl of test medium, consisting of either i) SFM or ii) 10% serum-containing FCM, was added to the well in the tissue culture plate, so that the test medium was in contact with the undersurface of the membrane in the culture insert. The cells were incubated for 16 hours to permit migration, through the pores, to the undersurface of the membrane. The culture inserts were then washed with PBS to remove excess protein, fixed in 70% (v/v) methanol for 5 minutes, stained with Mayer's hematoxylin (Dako, Ely, Cambridgeshire, UK) for 30 minutes, and then rinsed in tap water. Settled cells on the upper surface of the membrane in the culture inserts were removed using cotton swabs. The number of migrated cells on the membrane per 10× objective field (average of five fields) was counted using an inverted phase contrast microscope and cell counting software (Image J public domain Java image processing program; http://rsb.info.nih.gov/ij/, last accessed July 22, 2006).
Collagen Contraction
To assess matrix contraction, free-floating, relaxed collagen gel lattice models were used. Three-dimensional, fibroblast-populated, type I rat tail collagen (5 mg/ml; Sigma-Aldrich) lattices were prepared with 16.7 × 105 cells/ml of lattice mixture as previously described.25 The collagen lattices were incubated for 2 hours at 37°C to set, in the culture wells, then the lattices were detached immediately after feeding with either i) SFM, ii) SFM containing 10 ng/ml interleukin-13 (recombinant human IL-13; R&D Systems, Abingdon, UK), iii) SFM containing 10 ng/ml tumor necrosis factor-α (recombinant human TNFα; R&D Systems), or iv) FCM containing 10% gelatinase-free fetal calf serum (Gibco, Invitrogen, Paisley, UK). The optimal concentration of IL-13 and TNFα used was determined from previous results.26,27 Gelatin Sepharose beads (Gelatin Sepharose 4B; Amersham Biosciences, Little Chalfont, UK) were incubated in serum at a concentration of 10% with gentle rocking for 2 hours to produce gelatinase-free serum. Reduction in lattice area at days 1, 3, and 7 due to contraction was digitally photographed, and the gel areas calculated using image analysis software (Image J).
Matrix Metalloproteinase Protein Levels
Conditioned medium collected from contracting lattices was analyzed for matrix metalloproteinase-1 (MMP-1), MMP-2, MMP-3, MMP-8, MMP-10, MMP-13, tissue inhibitor of matrix metalloproteinase-1 (TIMP-1), TIMP-2, and TIMP-4 protein levels by using an antibody-coated membrane array (Raybiotech Inc, Norcross, GA) in accordance with the manufacturer's instructions. MMP and TIMP levels in baseline SFM and FCM were subtracted from the conditioned medium results.
Collagen Type I Secretion
Conditioned medium collected from contracting lattices was also analyzed for secretion of the C-terminal propeptide of type I collagen using an enzyme-linked immunosorbent assay (Quidel Corp, San Diego, CA) carried out according to the manufacturer's instructions.
Chemokine Secretion
Confluent fibroblasts in six-well culture plates were serum starved for 24 hours, then stimulated for 72 hours with a panel of cytokines in 10% heat-inactivated serum-containing FCM. The cytokines selected were those previously reported to be present in ocular MMP, including transforming growth factor-β (TGFβ) 50 ng/ml,16 IL-4 20 ng/ml,28 interferon-γ (IFNγ) 200 ng/ml,28 TNFα 10 ng/ml,27 and IL-13 100 ng/ml.26 The cell-free culture supernatants were analyzed for presence of the chemokines IL-6, CXCL-8 (IL-8), CXCL-10 [IP-10 (human interferon-inducible protein 10)], CCL-11 (eotaxin-1), and CXCL-9 [MIG (monokine induced by IFNγ)] using a cytometric bead array and FCAP Array Software (BD Biosciences) and flow cytometer (FACSCalibur; BD Biosciences).
Immunohistochemistry for α-Smooth Muscle Actin
Immunohistochemistry was carried out on glycol methacrylate resin–embedded sections of active pemphigoid conjunctiva, treated pemphigoid and normal conjunctiva, prepared as described in a previous publication.9 Endogenous peroxidase was inhibited using 0.3% hydrogen peroxide in 0.1% sodium azide, followed by incubation with 10% fetal calf serum to block nonspecific binding. The sections were incubated overnight at room temperature with monoclonal mouse antibody against human α-smooth muscle actin (α-SMA; M0851 Dako; Dako), 1:10 dilution. After washing with PBS, biotinylated rabbit anti-mouse immunoglobulin (Dako) was applied to the sections, followed by incubation with streptavidin-peroxidase (Dako), and finally amino-ethyl carbazole (AEC; Dako), forming a red AEC reaction product. The specimens were counterstained with Mayer's hematoxylin (Dako) and mounted with Glycergel Mounting Medium (Dako). Vascular smooth muscle in the sections was used as a positive control; and absence of the primary antibody and an isotype-matched, irrelevant monoclonal antibody (mouse IgG2a; Dako) were used as two negative controls. The number of stromal cells stained with α-smooth muscle actin were counted in a masked fashion in five representative high-power fields per patient, using an Olympus (Southend-on-Sea, Essex, UK) BX51 microscope with image analysis software.
Immunodetection of Myofibroblast Differentiation in Mechanically Stressed, Fibroblast-Seeded Collagen Lattices
Mechanically stressed, fibroblast-seeded collagen lattices were prepared to assess for myofibroblast differentiation.29 To generate mechanical stress in the collagen matrix, after polymerization of the lattice mixture, the lattices remained attached to the culture well and were cultured in FCM for 24 hours to allow the development of mechanical tension. At 24 hours, the lattice was fixed with 4% paraformaldehyde, then completely detached with a pipette tip. Triplicate samples per donor were tested, and at least six donors were tested in each of the groups: active pemphigoid fibroblasts, treated pemphigoid fibroblasts, and normal conjunctival fibroblasts. The fixed collagen lattices were permeabilized for 15 minutes with 1% Triton X-100 (Sigma-Aldrich) at room temperature. For immunostaining of α-SMA, monoclonal anti–α-SMA antibody conjugated with Cy3 (Clone 1A4; Sigma-Aldrich) was added to the lattices (1:200 in blocking buffer) for 3 hours at room temperature. After the lattices were rinsed with PBS and exposed for 1 minute to 4′,6-diaminidino-2-phenylindole (DAPI) dye (1:5000 in PBS; Sigma-Aldrich), they were rinsed and mounted on a 35-mm MatTek glass-bottomed dish (MatTek Corporation, Ashland, MA) with a fluorescent mountant and covered with a coverslip. Cells were viewed with a laser scanning confocal microscope (Leica TCS SP2; Leica, Milton Keynes, UK). The proportion of myofibroblasts and fibroblasts was determined by counting the number of cells containing assembled α-SMA stress fibers as a proportion of the total number of cells visualized by DAPI-stained nuclei. Five random fields of view at ×40 final magnification were analyzed with Leica image analysis software.
Statistical Analysis
Differences between two groups were examined for statistical significance using the Mann-Whitney U-test, and one-way analysis of variance was used to compare the means of three or more unmatched groups. When used, box-whisker plots show median, upper and lower quartiles, and maximum and minimum. P < 0.05 was considered significant.
Results
Pemphigoid and Normal Conjunctival Fibroblasts Appear to Have Similar Morphology
No gross differences in the morphology of pemphigoid fibroblasts compared with normal conjunctival fibroblasts were detected (Figure 2), nor between pemphigoid fibroblasts obtained from acutely inflamed tissue and chronic uninflamed tissue.
Figure 2.
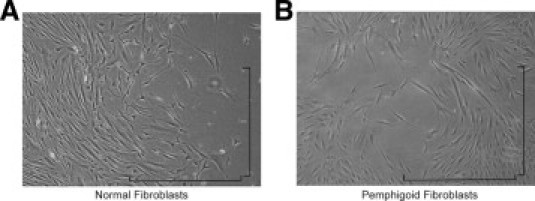
Morphology of normal and pemphigoid fibroblasts in vitro. Inverted phase contrast microscopy of (A) normal conjunctival fibroblasts 6 days after the fourth subpassage and (B) pemphigoid conjunctival fibroblasts from acutely inflamed tissue 5 days after the fourth subpassage. Pemphigoid fibroblasts were similar in appearance to normal conjunctival fibroblasts. Magnification ×100, bar = 1 mm.
Comparison of Early Passage versus Late Passage Pemphigoid Fibroblasts
Comparison of early versus late passage normal control conjunctival fibroblasts and pemphigoid conjunctival fibroblasts showed no significant differences in all assays, including collagen contraction (Figure 3), proliferation (not shown), and migration (not shown).
Figure 3.
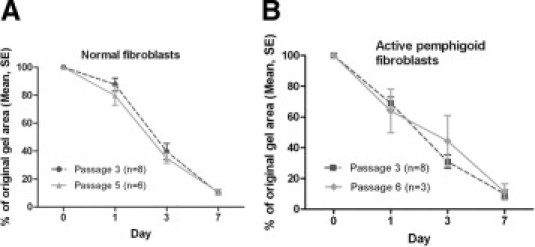
Comparison of collagen contraction by early passage versus late passage fibroblasts. A: No difference in collagen contraction over 7 days in FCM between passage 3 versus passage 5 normal conjunctival fibroblasts. B: No difference in collagen contraction over 7 days in FCM between passage 3 and passage 6 actively inflamed pemphigoid conjunctival fibroblasts. n = number of individual experiments.
Comparison of Actively Inflamed versus Uninflamed Pemphigoid Biopsy Fibroblasts
Comparison of actively inflamed versus uninflamed pemphigoid conjunctival fibroblasts in all assays did not show any clinically or statistically significant differences, even at early passage. Figure 4 shows the similarity between actively inflamed and uninflamed pemphigoid conjunctival fibroblasts in cell division and collagen contraction experiments. Migration assays were also similar (not shown).
Figure 4.
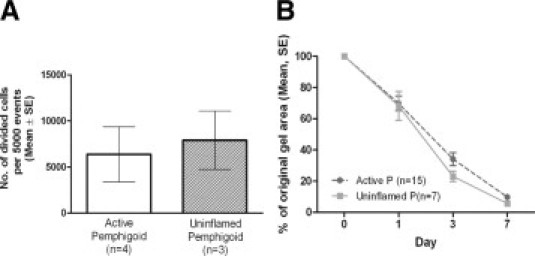
Comparison of actively inflamed versus uninflamed pemphigoid conjunctival fibroblasts. A: No difference in proliferation between actively inflamed versus uninflamed pemphigoid fibroblasts (passage 3 cells in both groups) after 96 hours' culture in FCM. n = number of individual donors. B: No clinically significant difference in collagen contraction over 7 days in FCM, between actively inflamed versus uninflamed pemphigoid fibroblasts. Although there appears to be a difference in the gel areas at day 3, the magnitude of this difference is small (34% − 23% = 11%) and not considered clinically significant. P = pemphigoid, n = number of individual experiments.
Pemphigoid Fibroblasts Have a Greater Number of Cell Divisions Than Normal Conjunctival Fibroblasts
Pemphigoid conjunctival fibroblasts showed significantly increased numbers of divided cells compared with normal conjunctival fibroblasts after 96 hours in culture (average 7026 ± 2020 vs. 1216 ± 558 divided cells per 5000 events, P = 0.01) (Figure 5).
Figure 5.
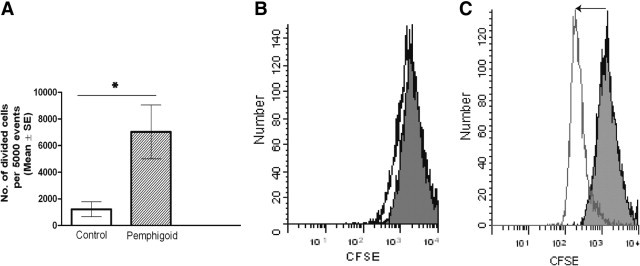
Fibroblast cell division assessed by flow cytometric measurement of dilution of the fluorescent label CFSE (carboxyfluorescein diacetate succinimidyl ester). A: After a 96-hour culture period in 10% serum-containing medium, pemphigoid fibroblasts had significantly increased numbers of divided cells compared with normal control conjunctival fibroblasts (*P = 0.01). Results are the mean and SE from five or more individuals in each group. B: Representative histogram of number of events (y axis) versus CFSE fluorescence (x axis) in a normal fibroblast line. Cell division is indicated by decreased fluorescence to the left of the time 0 peak. Gray shaded peak is the fluorescence of CFSE in the cells at time 0. Black line is the fluorescence at 96 hours. C: Histogram of number of events versus CFSE fluorescence in a pemphigoid fibroblast line. Light gray shaded peak is the fluorescence of CFSE in the cells at time 0. Gray line is the fluorescence at 96 hours.
Increased Migration by Pemphigoid Conjunctival Fibroblasts under Serum-Free Conditions
The number of pemphigoid fibroblasts that migrated through the porous membranes toward the SFM was significantly higher than the number of normal control fibroblasts that migrated toward SFM (72 ± 18 vs. 33 ± 11, P = 0.04) (Figure 6). No significant difference between pemphigoid and normal fibroblasts in the number of cells migrating toward 10% serum-containing FCM was detected (data not shown).
Figure 6.
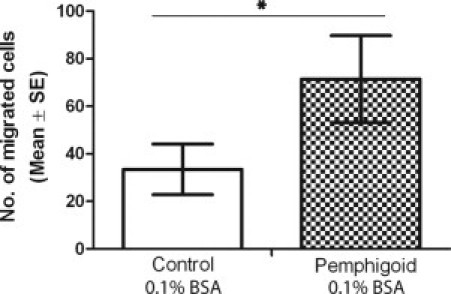
Comparison of conjunctival fibroblast migration toward 0.1% bovine serum albumin (BSA) serum-free medium (SFM). Pemphigoid fibroblasts migrated more than normal control fibroblasts under serum-free conditions. Results are the mean and SE of eight individual experiments. *P = 0.04.
Pemphigoid Fibroblasts Show Increased Collagen Contraction in Response to TNF-α, and Reduced Contraction in Response to IL-13, Compared with Normal Conjunctival Fibroblasts
We next assessed collagen contraction by the fibroblasts. Although there was no detectable difference in the contraction of relaxed collagen gel lattices by pemphigoid and normal conjunctival fibroblasts in either SFM (Figure 7A) or 10% serum-containing FCM (Figure 7B), the pemphigoid fibroblasts did contract collagen in response to TNFα (Figure 7C). We have previously observed that normal conjunctival fibroblasts do not contract collagen in response to TNFα.22 In contrast, the addition of IL-13 to the collagen lattice conditioned medium resulted in less contraction by pemphigoid fibroblasts compared with normal fibroblasts, relative to the amount of contraction in the SFM negative control (Figure 7D).
Figure 7.
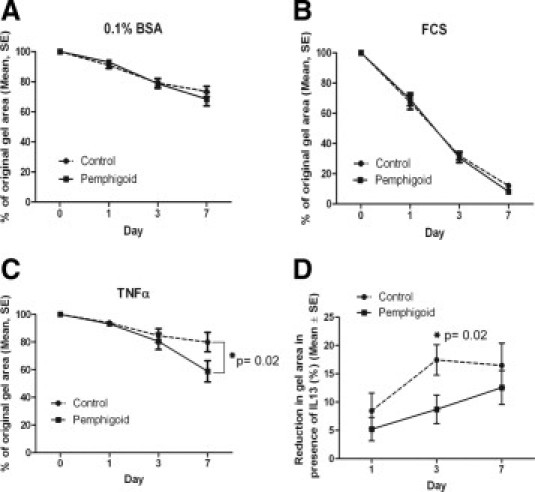
Fibroblast-populated, collagen gel lattice contraction over 7 days by pemphigoid versus normal conjunctival fibroblasts showing (A) no difference in contraction in 0.1% bovine serum albumin (BSA) serum-free medium and (B) no difference in contraction in 10% fetal calf serum (FCS)-containing medium. C: In the presence of 10 ng/ml tumor necrosis factor-α (TNFα), pemphigoid fibroblasts contract collagen more than normal fibroblasts by day 7 (P = 0.02). D: On the other hand, in the presence of interleukin-13 (IL-13) at day 3, pemphigoid fibroblasts contract collagen less vigorously than normal fibroblasts do at day 3 (P = 0.02), but by day 7, there is no significant difference. The reduction in gel area in response to IL-13 at day 3 is less in pemphigoid fibroblasts than normal fibroblasts (relative to the serum-free medium negative control). Results of all graphs are the mean and SE from six or more donors per group.
Pemphigoid Fibroblasts Secrete More Matrix Metalloproteinase-3 and Less Matrix Metalloproteinase-13 in the Presence of Serum
During days 0 to 3 of collagen lattice contraction in 10% serum-containing medium, pemphigoid fibroblasts secrete more MMP-3 (P = 0.04) and less MMP-13 (P = 0.02) compared with normal conjunctival fibroblasts (Figure 8A). During days 3 to 7 of collagen lattice contraction in 10% serum-containing medium, there was no difference in MMP and TIMP secretion between pemphigoid and normal fibroblasts (data not shown). An increase in MMP-3 secretion by pemphigoid fibroblasts compared with normal fibroblasts was also observed during collagen contraction in serum-free medium from day 0 to 7 (data not shown).
Figure 8.
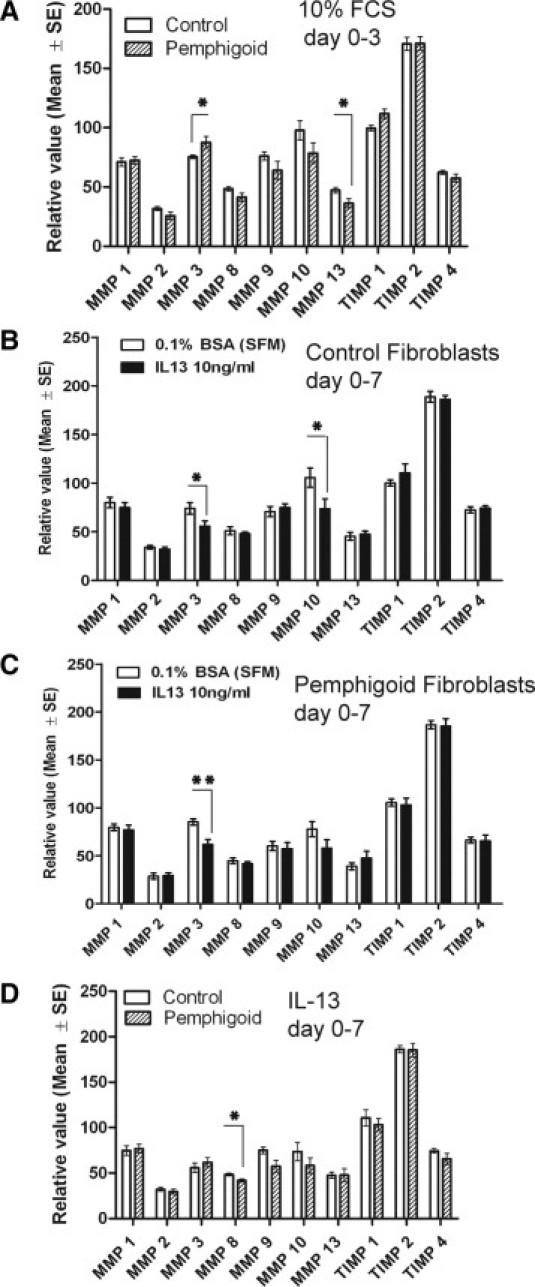
Matrix metalloproteinase (MMP) and tissue inhibitor of matrix metalloproteinase (TIMP) levels in conditioned medium of conjunctival fibroblast-populated collagen gels. Data are relative values compared with the membrane array negative control. A: During days 0 to 3 of collagen lattice contraction in 10% fetal calf serum (FCS)-containing medium, pemphigoid fibroblasts secreted higher levels of MMP-3 (P = 0.04) and lower levels of MMP-13 (P = 0.02) compared with normal fibroblasts. B: During days 0 to 7 of collagen lattice contraction in the presence of IL-13 10 ng/ml in SFM, normal conjunctival fibroblasts secrete lower levels of MMP-3 (P = 0.04) and MMP-10 (P = 0.01), and (C) pemphigoid fibroblasts similarly secrete lower levels of MMP-3 (P = 0.004). D: When compared directly in the presence of IL-13, pemphigoid fibroblasts secrete less MMP-8 (P = 0.02) compared with normal fibroblasts. Results are the mean and SE from six individual donors per graph. *P < 0.05; **P < 0.01.
In the presence of IL-13 over days 0 to 7, both normal and pemphigoid fibroblasts secrete less MMP-3 (Figure 8, B and C), and when pemphigoid fibroblasts and normal fibroblasts are compared directly, pemphigoid fibroblasts secrete less MMP-8 than normal fibroblasts in the presence of IL-13 (Figure 8D).
Pemphigoid Fibroblasts Secrete More Type I Collagen
During days 3 to 7 of collagen lattice contraction in 10% serum-containing medium, pemphigoid fibroblasts secrete more C-terminal propeptide of type I collagen compared with normal conjunctival fibroblasts (median 62.2 vs. 31.4 ng/ml, P = 0.03) (Figure 9). During collagen lattice contraction in the presence of IL-13 in serum-containing medium, there was no significant difference in type I collagen secretion by pemphigoid versus normal conjunctival fibroblasts (data not shown).
Figure 9.
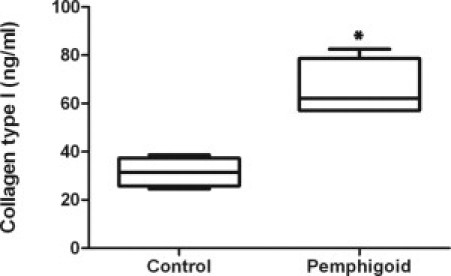
Comparison of C-terminal propeptide of type I collagen (CICP) secretion by pemphigoid fibroblasts versus normal conjunctival fibroblasts during days 3 to 7 of collagen lattice contraction in 10% serum-containing medium. Pemphigoid fibroblasts secrete more type I collagen than normal fibroblasts (*P = 0.03). Results are from four individual donors in box-whisker plots.
Pemphigoid Fibroblasts Secrete More Eotaxin in Response to IL-13
To investigate whether pemphigoid fibroblasts are more secretory than normal fibroblasts, and have the potential to draw in high numbers of inflammatory cells and thus cause a greater degree of conjunctival inflammation by secreting chemoattractant molecules called chemokines, we evaluated secretion of chemokines following 72 hours' stimulation with key ocular MMP cytokines, and found that pemphigoid fibroblasts secreted more CCL-11 (eotaxin-1) in response to IL-13 (Figure 10). No significant differences between pemphigoid and normal fibroblasts with respect to secretion of IL-6, CXCL-8 (IL-8), CXCL-10 (IP-10), or CXCL-9 (MIG) were detected, either in the absence of cytokines or in response to stimulation by IL-4, IFNγ, or TGFβ.
Figure 10.
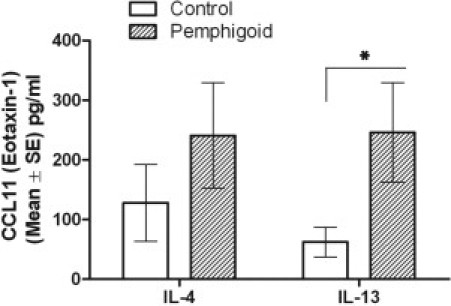
Comparison of eotaxin secretion by pemphigoid fibroblasts versus normal fibroblasts in response to IL-4 and IL-13. Pemphigoid fibroblasts secrete more eotaxin in response to IL-13 than normal fibroblasts. (*P = 0.04). Results are the mean and SE from at least six individual donors.
Myofibroblast Differentiation
There was very little stromal α-SMA staining in both pemphigoid and normal bulbar conjunctiva, and no differences were detected between pemphigoid and normal conjunctiva. We also found no significant differences between pemphigoid fibroblasts and normals in the expression of assembled α-SMA filaments in collagen lattices after 24 hours of mechanical stress (data not shown).
Discussion
In this study, we have shown that conjunctival fibroblasts from ocular MMP patients display a distinct fibrotic phenotype characterized by increased cell division, chemotaxis, collagen, and matrix metalloproteinase synthesis that is maintained over multiple passages in vitro. BMZ separation in MMP, and inflammation secondary to the BMZ separation process, induces fibrosis in subepithelial tissues and may be responsible for these observed changes in fibroblast function. It is probable that these changes reflect alterations in gene expression pathways, deregulation of transcription factors such as SP1 and SMAD3, altered surface receptor expression, and/or altered protein kinase (ERK, FAK) signaling pathways.30 New therapies targeting the fibrotic process in MMP would need to address the factors that induce and maintain these alterations. In the presence of the profibrotic type 2 helper T-cell mediator IL-13, which we have previously shown to be expressed by conjunctival stromal cells in ocular MMP,26 pemphigoid fibroblasts appear to respond with less contraction and reduced matrix metalloproteinase production, but with increased secretion of the chemokine CCL-11 (eotaxin-1). The reduced contraction activity by pemphigoid fibroblasts in response to IL-13 could be a consequence of the cell's increased protein synthetic activity (increased secretion of chemokine CCL-11) diverting cellular activity away from contraction. It is also possible that IL-13 receptor expression on pemphigoid fibroblasts has been down-regulated as a consequence of previous chronic IL-13 exposure in vivo, thus decreasing the contraction response because fewer receptors are available for stimulation. In contrast, we found that pemphigoid fibroblasts showed a late contraction response to TNFα whereas normal conjunctival fibroblasts did not. This could perhaps be due to TNFα stimulating expression of TGFβ31 by the pemphigoid fibroblasts. This requires further investigation.
We did not detect any differences between pemphigoid fibroblasts isolated from acutely inflamed versus uninflamed tissue. This may be because the phenotypic/genetic transformation into profibrotic fibroblasts has been induced by the acute inflammatory insult, and this transformation persists even after the inflammation has subsided.
Increased proliferation of ocular MMP conjunctival fibroblasts has previously been observed,18 as has increased expression of macrophage migration inhibitory factor, macrophage-colony stimulating factor, connective tissue growth factor, and heat shock protein 47 by ocular MMP fibroblasts.16,17 Preliminary studies have also suggested that ocular MMP fibroblasts express the proto-oncogene c-myc (Hunt LE et al IOVS 1991;32:ARVO Abstract 938) and show ultrastructural changes indicating increased protein synthesis that persist in culture (Biesman BS et al IOVS 1994;Suppl 35(4):ARVO Abstract 170). Our findings of increased proliferative, secretory, and matrix synthetic activity by pemphigoid fibroblasts are in agreement with these previous reports.
We were not able to detect myofibroblast characteristics in pemphigoid fibroblasts. Simple cytoplasmic expression of unassembled actin is not an indicator of myofibroblasts; morphological detection of α-SMA filaments assembled as stress fibers by confocal microscopy is necessary.32 It is possible that 24 hours of mechanical stress was too short to enable differences between pemphigoid and normal fibroblasts to emerge, and perhaps 48 hours might have allowed differences to have been observed. Adding TGFβ, which promotes myofibroblast differentiation, particularly in the presence of mechanical tension,33 may also have been helpful. Furthermore, use of an alternative α-SMA antibody might have produced different findings. Finally, it is also possible that cell types other than the resident tissue fibroblasts are the source of myofibroblasts in ocular MMP. There is emerging evidence from studies of pulmonary fibrosis and other disorders that epithelial cells could be an important source of myofibroblasts, via the process of epithelial–mesenchymal transdifferentiation.34
We observed increased MMP-3 and decreased MMP-13 secretion by pemphigoid conjunctival fibroblasts during collagen lattice contraction. MMP-3, also known as stromelysin-1, degrades pro-MMPs-1, -7, -8, -9, -13, proteoglycans, laminin, and fibronectin. It has been observed to play a role in skin and corneal wound healing and pterygia,35–37 and is produced during collagen lattice contraction by human Tenon's capsule fibroblasts.35 Broad-spectrum inhibition of MMP activity has reduced matrix contraction and collagen production in vitro35 and in an animal model of glaucoma surgery.38 On the other hand, the profibrotic mediator IL-13, which is present in the stromal tissues of ocular MMP,26 causes a reduction in MMP-3 synthesis by both normal and pemphigoid conjunctival fibroblasts, so whether the effect of IL-13 overrides the inherent behavior of pemphigoid fibroblasts with regard to MMP-3 synthesis in vivo is yet to be determined. MMP-13 (collagenase-3) digests collagens I, II, III, IV, gelatin, fibronectin, and proteoglycans. Reduced MMP-13 could either result in a net increase in matrix due to reduced matrix degradation, or could be associated with reduced fibroblast locomotion through extracellular matrix and subsequent matrix contraction, which has been observed to involve MMPs.35 Which of these two alternatives is the dominant process in vivo warrants investigation.
Characteristics of fibrotic fibroblasts are probably unique to each disease, given that, for example, migration can be increased or decreased, depending on the disease from which the fibroblasts have been isolated.39 There is also evidence that the phenotype of fibrotic fibroblasts varies according to disease duration and severity.40
Developing effective antifibrotic therapies will require understanding of both the cellular sources of the profibrotic fibroblasts, and the mechanisms that activate and recruit these cells to sites of scarring. To elucidate what happens in ocular MMP, knowledge of what happens in a disease such as scleroderma provides framework from which to work, with the understanding that although the processes occurring in the two diseases are clearly not identical, there are some similarities. In scleroderma, there appears to be a process of selection leading to the propagation of certain apoptosis-resistant profibrotic subpopulations of fibroblasts, which are preferentially expanded or selectively activated within lesional tissue.30 Moreover, it appears that both resident and circulating cell types can contribute to fibroblast differentiation, thus in part accounting for the observed heterogeneity of fibroblasts both derived from within and between tissues. Potential sources of profibrotic fibroblasts include resident mesenchymal cells, bone marrow–derived mesenchymal precursors,41 circulating fibroblast progenitors including peripheral blood mononuclear cells,30 epithelial-mesenchymal transition, and pericytes.
Apart from selective amplification of subpopulations of activated phenotype fibroblasts derived from various sources, induction of the profibrotic fibroblast phenotype is also thought to be influenced by profibrotic mediators in the inflammatory milieu,30,42 and sometimes cell–cell contact between immune cells and fibroblasts.15 The phenotypic changes have been shown, in pulmonary fibrosis fibroblasts, to reflect genome-wide derangements of the gene expression pathway.43
In ocular MMP, there is significant inflammation induced by the autoimmune process that could selectively amplify resident, bone marrow–derived or circulating mesenchymal cells influenced by the inflammatory milieu.9 TGFβ-driven epithelial-mesenchymal transition could also play a role, given that when conjunctival epithelial injury is incurred in the absence of treatment with systemic immunosuppression, rapid worsening of ocular MMP is observed.5 Developing effective antifibrotic therapies will require understanding of the mechanisms that both induce and maintain the profibrotic phenotype.
Footnotes
Supported by an Action Medical Research UK Fellowship (RTF1226) (V.P.J.S.) during the conduct of this research; in part by the UK National Institute for Health Research Biomedical Research Centre in Ophthalmology at Moorfields Eye Hospital and UCL Institute of Ophthalmology (V.P.J.S. and J.K.G.D.); and in part by the Schleswig-Holstein Cluster of Excellence in Inflammation Research (DFG EXC 306/1) (E.S. and D.Z.).
References
- 1.Ahmed M., Zein G., Khawaja F., Foster C.S. Ocular cicatricial pemphigoid: pathogenesis, diagnosis and treatment. Prog Retin Eye Res. 2004;23:579–592. doi: 10.1016/j.preteyeres.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Chan L.S., Ahmed A.R., Anhalt G.J., Bernauer W., Cooper K.D., Elder M.J., Fine J.D., Foster C.S., Ghohestani R., Hashimoto T., Hoang-Xuan T., Kirtschig G., Korman N.J., Lightman S., Lozada-Nur F., Marinkovich M.P., Mondino B.J., Prost-Squarcioni C., Rogers R.S., III, Setterfield J.F., West D.P., Wojnarowska F., Woodley D.T., Yancey K.B., Zillikens D., Zone J.J. The first international consensus on mucous membrane pemphigoid: definition, diagnostic criteria, pathogenic factors, medical treatment, and prognostic indicators. Arch Dermatol. 2002;138:370–379. doi: 10.1001/archderm.138.3.370. [DOI] [PubMed] [Google Scholar]
- 3.Black A.P., Wojnarowska F., Ogg G.S. Role of T cells in the pathogenesis of mucous membrane pemphigoid. Expert Rev Dermatol. 2006;1:25–30. [Google Scholar]
- 4.Hardy K.M., Perry H.O., Pingree G.C., Kirby T.J., Jr Benign mucous membrane pemphigoid. Arch Dermatol. 1971;104:467–475. [PubMed] [Google Scholar]
- 5.Mondino B.J., Brown S.I., Lempert S., Jenkins M.S. The acute manifestations of ocular cicatricial pemphigoid: diagnosis and treatment. Ophthalmology. 1979;86:543–555. doi: 10.1016/s0161-6420(79)35486-0. [DOI] [PubMed] [Google Scholar]
- 6.Mondino B.J., Brown S.I. Ocular cicatricial pemphigoid. Ophthalmology. 1981;88:95–100. doi: 10.1016/s0161-6420(81)35069-6. [DOI] [PubMed] [Google Scholar]
- 7.Miserocchi E., Baltatzis S., Roque M.R., Ahmed A.R., Foster C.S. The effect of treatment and its related side effects in patients with severe ocular cicatricial pemphigoid. Ophthalmology. 2002;109:111–118. doi: 10.1016/s0161-6420(01)00863-6. [DOI] [PubMed] [Google Scholar]
- 8.Saw V.P., Dart J.K., Rauz S., Ramsay A., Bunce C., Xing W., Maddison P.G., Phillips M. Immunosuppressive therapy for ocular mucous membrane pemphigoid strategies and outcomes. Ophthalmology. 2008;115:253–261. doi: 10.1016/j.ophtha.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 9.Bernauer W., Wright P., Dart J.K., Leonard J.N., Lightman S. Cytokines in the conjunctiva of acute and chronic mucous membrane pemphigoid: an immunohistochemical analysis. Graefes Arch Clin Exp Ophthalmol. 1993;231:563–570. doi: 10.1007/BF00936519. [DOI] [PubMed] [Google Scholar]
- 10.Elder M.J. The role of cytokines in chronic progressive conjunctival cicatrisation. Dev Ophthalmol. 1997;28:159–175. doi: 10.1159/000060714. [DOI] [PubMed] [Google Scholar]
- 11.Setterfield J., Shirlaw P.J., Bhogal B.S., Tilling K., Challacombe S.J., Black M.M. Cicatricial pemphigoid: serial titres of circulating IgG and IgA antibasement membrane antibodies correlate with disease activity. Br J Dermatol. 1999;140:645–650. doi: 10.1046/j.1365-2133.1999.02763.x. [DOI] [PubMed] [Google Scholar]
- 12.Stramer B.M., Mori R., Martin P. The inflammation-fibrosis link: A Jekyll and Hyde role for blood cells during wound repair. J Invest Dermatol. 2007;127:1009–1017. doi: 10.1038/sj.jid.5700811. [DOI] [PubMed] [Google Scholar]
- 13.Ramos C., Montano M., Garcia-Alvarez J., Ruiz V., Uhal B.D., Selman M., Pardo A. Fibroblasts from idiopathic pulmonary fibrosis and normal lungs differ in growth rate, apoptosis, and tissue inhibitor of metalloproteinases expression. Am J Respir Cell Mol Biol. 2001;24:591–598. doi: 10.1165/ajrcmb.24.5.4333. [DOI] [PubMed] [Google Scholar]
- 14.Schuttert J.B., Liu M.H., Gliem N., Fiedler G.M., Zopf S., Mayer C., Muller G.A., Grunewald R.W. Human renal fibroblasts derived from normal and fibrotic kidneys show differences in increase of extracellular matrix synthesis and cell proliferation upon angiotensin II exposure. Pflugers Arch. 2003;446:387–393. doi: 10.1007/s00424-003-1026-y. [DOI] [PubMed] [Google Scholar]
- 15.Trojanowska M. What did we learn by studying scleroderma fibroblasts. Clin Exp Rheumatol. 2004;22:S59-–S63. [PubMed] [Google Scholar]
- 16.Razzaque M.S., Foster C.S., Ahmed A.R. Role of collagen-binding heat shock protein 47 and transforming growth factor-beta1 in conjunctival scarring in ocular cicatricial pemphigoid. Invest Ophthalmol Vis Sci. 2003;44:1616–1621. doi: 10.1167/iovs.02-0644. [DOI] [PubMed] [Google Scholar]
- 17.Razzaque M.S., Foster C.S., Ahmed A.R. Role of macrophage migration inhibitory factor in conjunctival pathology in ocular cicatricial pemphigoid. Invest Ophthalmol Vis Sci. 2004;45:1174–1181. doi: 10.1167/iovs.03-1138. [DOI] [PubMed] [Google Scholar]
- 18.Roat M.I., Sossi G., Lo C.Y., Thoft R.A. Hyperproliferation of conjunctival fibroblasts from patients with cicatricial pemphigoid. Arch Ophthalmol. 1989;107:1064–1067. doi: 10.1001/archopht.1989.01070020126045. [DOI] [PubMed] [Google Scholar]
- 19.Thorne J.E., Anhalt G.J., Jabs D.A. Mucous membrane pemphigoid and pseudopemphigoid. Ophthalmology. 2004;111:45–52. doi: 10.1016/j.ophtha.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt E., Skrobek C., Kromminga A., Hashimoto T., Messer G., Brocker E.B., Yancey K.B., Zillikens D. Cicatricial pemphigoid: igA and IgG autoantibodies target epitopes on both intra- and extracellular domains of bullous pemphigoid antigen 180. Br J Dermatol. 2001;145:778–783. doi: 10.1046/j.1365-2133.2001.04471.x. [DOI] [PubMed] [Google Scholar]
- 21.Lazarova Z., Sitaru C., Zillikens D., Yancey K.B. Comparative analysis of methods for detection of anti-laminin 5 autoantibodies in patients with anti-epiligrin cicatricial pemphigoid. J Am Acad Dermatol. 2004;51:886–892. doi: 10.1016/j.jaad.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Zillikens D., Kawahara Y., Ishiko A., Shimizu H., Mayer J., Rank C.V., Liu Z., Giudice G.J., Tran H.H., Marinkovich M.P., Brocker E.B., Hashimoto T. A novel subepidermal blistering disease with autoantibodies to a 200-kDa antigen of the basement membrane zone. J Invest Dermatol. 1996;106:1333–1338. doi: 10.1111/1523-1747.ep12349283. [DOI] [PubMed] [Google Scholar]
- 23.Broadway D. Drug-induced conjunctival cicatrisation. Dev Ophthalmol. 1997;28:86–101. doi: 10.1159/000060707. [DOI] [PubMed] [Google Scholar]
- 24.Khaw P.T., Ward S., Porter A., Grierson I., Hitchings R.A., Rice N.S. The long-term effects of 5-fluorouracil and sodium butyrate on human Tenon's fibroblasts. Invest Ophthalmol Vis Sci. 1992;33:2043–2052. [PubMed] [Google Scholar]
- 25.Mazure A., Grierson I. In vitro studies of the contractility of cell types involved in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 1992;33:3407–3416. [PubMed] [Google Scholar]
- 26.Saw V.P., Offiah I., Dart R.J., Galatowicz G., Dart J.K., Daniels J.T., Calder V.L. Conjunctival interleukin-13 expression in mucous membrane pemphigoid and functional effects of interleukin-13 on conjunctival fibroblasts in vitro. Am J Pathol. 2009;175:2406–2415. doi: 10.2353/ajpath.2009.090579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saw V.P., Dart R.J., Galatowicz G., Daniels J.T., Dart J.K., Calder V.L. Tumor necrosis factor-alpha in ocular mucous membrane pemphigoid and its effect on conjunctival fibroblasts. Invest Ophthalmol Vis Sci. 2009;50:5310–5317. doi: 10.1167/iovs.08-3345. [DOI] [PubMed] [Google Scholar]
- 28.Caproni M., Calzolari A., Giomi B., Santucci M., Ficarra G., Fabbri P. IL-4. IL-5, TGF-beta1 and IFN-gamma mRNAs detected by a new in situ amplification system in cicatricial pemphigoid. Exp Dermatol. 2002;11:421–427. doi: 10.1034/j.1600-0625.2002.110505.x. [DOI] [PubMed] [Google Scholar]
- 29.Garrett Q., Khaw P.T., Blalock T.D., Schultz G.S., Grotendorst G.R., Daniels J.T. Involvement of CTGF in TGF-beta1-stimulation of myofibroblast differentiation and collagen matrix contraction in the presence of mechanical stress. Invest Ophthalmol Vis Sci. 2004;45:1109–1116. doi: 10.1167/iovs.03-0660. [DOI] [PubMed] [Google Scholar]
- 30.Abraham D.J., Eckes B., Rajkumar V., Krieg T. New developments in fibroblast and myofibroblast biology: implications for fibrosis and scleroderma. Curr Rheumatol Rep. 2007;9:136–143. doi: 10.1007/s11926-007-0008-z. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan D.E., Ferris M., Pociask D., Brody A.R. Tumor necrosis factor-alpha induces transforming growth factor-beta1 expression in lung fibroblasts through the extracellular signal-regulated kinase pathway. Am J Respir Cell Mol Biol. 2005;32:342–349. doi: 10.1165/rcmb.2004-0288OC. [DOI] [PubMed] [Google Scholar]
- 32.Tomasek J.J., Gabbiani G., Hinz B., Chaponnier C., Brown R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 33.Vaughan M.B., Howard E.W., Tomasek J.J. Transforming growth factor-beta1 promotes the morphological and functional differentiation of the myofibroblast. Exp Cell Res. 2000;257:180–189. doi: 10.1006/excr.2000.4869. [DOI] [PubMed] [Google Scholar]
- 34.Radisky D.C., LaBarge M.A. Epithelial-mesenchymal transition and the stem cell phenotype. Cell Stem Cell. 2008;2:511–512. doi: 10.1016/j.stem.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Daniels J.T., Cambrey A.D., Occleston N.L., Garrett Q., Tarnuzzer R.W., Schultz G.S., Khaw P.T. Matrix metalloproteinase inhibition modulates fibroblast-mediated matrix contraction and collagen production in vitro. Invest Ophthalmol Vis Sci. 2003;44:1104–1110. doi: 10.1167/iovs.02-0412. [DOI] [PubMed] [Google Scholar]
- 36.Li D.Q., Lee S.B., Gunja-Smith Z., Liu Y., Solomon A., Meller D., Tseng S.C. Overexpression of collagenase (MMP-1) and stromelysin (MMP-3) by pterygium head fibroblasts. Arch Ophthalmol. 2001;119:71–80. [PubMed] [Google Scholar]
- 37.Wong T.T., Sethi C., Daniels J.T., Limb G.A., Murphy G., Khaw P.T. Matrix metalloproteinases in disease and repair processes in the anterior segment. Surv Ophthalmol. 2002;47:239–256. doi: 10.1016/s0039-6257(02)00287-4. [DOI] [PubMed] [Google Scholar]
- 38.Wong T.T., Mead A.L., Khaw P.T. Matrix metalloproteinase inhibition modulates postoperative scarring after experimental glaucoma filtration surgery. Invest Ophthalmol Vis Sci. 2003;44:1097–1103. doi: 10.1167/iovs.02-0366. [DOI] [PubMed] [Google Scholar]
- 39.Rieder F., Brenmoehl J., Leeb S., Scholmerich J., Rogler G. Wound healing and fibrosis in intestinal disease. Gut. 2007;56:130–139. doi: 10.1136/gut.2006.090456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corriveau M.P., Boufaied I., Lessard J., Chabaud S., Senecal J.L., Grodzicky T., Chartier S., Raymond Y., Moulin V.J. The fibrotic phenotype of systemic sclerosis fibroblasts varies with disease duration and severity of skin involvement: reconstitution of skin fibrosis development using a tissue engineering approach. J Pathol. 2009;217:534–542. doi: 10.1002/path.2482. [DOI] [PubMed] [Google Scholar]
- 41.Hashimoto N., Jin H., Liu T., Chensue S.W., Phan S.H. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karlson T.D., Whiting C.V., Bland P.W. Proinflammatory cytokine synthesis by mucosal fibroblasts from mouse colitis is enhanced by interferon-γ-mediated up-regulation of CD40 signalling. Clin Exp Immunol. 2007;147:313–323. doi: 10.1111/j.1365-2249.2006.03267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Larsson O., Diebold D., Fan D., Peterson M., Nho R.S., Bitterman P.B., Henke C.A. Fibrotic myofibroblasts manifest genome-wide derangements of translational control. PLoS ONE. 2008;3:e3220. doi: 10.1371/journal.pone.0003220. [DOI] [PMC free article] [PubMed] [Google Scholar]


