Abstract
UV radiation indirectly regulates melanogenesis in melanocytes through a paracrine regulatory mechanism involving keratinocytes. Protease-activated receptor (PAR)-2 activation induces melanosome transfer by increasing phagocytosis of melanosomes by keratinocytes. This study demonstrated that macrophage migration inhibitory factor (MIF) stimulated PAR-2 expression in human keratinocytes. In addition, we showed that MIF stimulated stem cell factor (SCF) release in keratinocytes; however, MIF had no effect on the release of endothelin-1 or prostaglandin E2 in keratinocytes. In addition, MIF had no direct effect on melanin and tyrosinase synthesis in cultured human melanocytes. The effect of MIF on melanogenesis was also examined using a three-dimensional reconstituted human epidermal culture model, which is a novel, commercially available, cultured human epidermis containing functional melanocytes. Migration inhibitory factor induced an increase in melanin content in the epidermis after a 9-day culture period. Moreover, melanin synthesis induced by UV-B stimulation was significantly down-regulated by anti-MIF antibody treatment. An in vivo study showed that the back skin of MIF transgenic mice had a higher melanin content than that of wild-type mice after 12 weeks of UV-B exposure. Therefore, MIF-mediated melanogenesis occurs mainly through the activation of PAR-2 and SCF expression in keratinocytes after exposure to UV-B radiation.
Exposure to UV radiation leads to various short-term deleterious cutaneous effects, including sunburn and immunosuppression, and long-term consequences that lead to premature aging, including hyperpigmentation.1 UV radiation indirectly regulates melanogenesis in melanocytes through a paracrine regulatory mechanism involving keratinocytes. UV-B–induced pigmentation occurs when human keratinocytes exposed to UV-B are stimulated to produce and secrete several mediators that trigger the activation of melanocytes and act as potent mitogens and melanogens for human melanocytes.2–4 The two main paracrine melanogenic cytokines, stem cell factor (SCF) and endothelin (ET)-1, have been demonstrated to play pivotal roles in skin pigmentation, including UV-B–induced pigmentation.5 In addition, prostaglandins (PGs) are key mediators of diverse functions in the skin; and several reports6,7 have suggested that PGs mediate postinflammatory pigmentary changes by modulating melanin synthesis and melanocyte dendricity.
Protease-activated receptor (PAR)-2 is a member of a novel G-protein–coupled seven-transmembrane receptor family.8 These receptors are irreversibly activated through proteolytic cleavage of their amino termini. Subsequent to proteolytic cleavage, the newly exposed NH2 terminus acts as a tethered peptide ligand, which binds and activates the receptor. Protease-activated receptor-2 is involved in skin pigmentation because it increases the phagocytosis of melanosomes by keratinocytes.9 UV irradiation is a potent stimulus for melanosome transfer. The PAR-2 expression in human skin was previously up-regulated by UV irradiation.10
There is emerging evidence that melanocyte function is regulated by several cytokines that are secreted by surrounding keratinocytes in a paracrine fashion. IL-1α plays an autocrine role in enhancing the secretion of ET-1 in UV-B–exposed human keratinocytes.4 The production and secretion of SCF and ET-1 by keratinocytes are generally augmented by several cytokines, such as IL-1α and tumor necrosis factor (TNF)-α.5 The exogenous addition of TNF-α to human keratinocytes in culture stimulates the secretion of ET-1 because of increased transcription.11
The cytokine macrophage migration inhibitory factor (MIF) was first discovered 50 years ago as a T-cell–derived factor that inhibits the random migration of macrophages.12,13 Recently, MIF was reevaluated as a proinflammatory cytokine and pituitary-derived hormone that potentiates endotoxemia.14 Subsequent work15 showed that T cells and macrophages secrete MIF in response to glucocorticoids and on activation by various proinflammatory stimuli. Migration inhibitory factor is expressed primarily in T cells and macrophages; however, recent studies16–19 have revealed that this protein is ubiquitously expressed by various types of cells. Skin keratinocytes are capable of producing a variety of cytokines and are thought to be the principal source of cytokines from the epidermis after UV irradiation. Enhanced MIF production is observed in the skin after UV-B irradiation.20,21 A recent study22 suggested a potentially broader role for MIF in skin inflammation because of its ability to enhance PAR-2 expression. Therefore, MIF may play a pathophysiological role in inflammatory reactions in the skin.
This study investigated the role of MIF in UV-B–induced melanogenesis using cultured human keratinocytes and melanocytes. Furthermore, the long-term UV-B effect in skin melanogenesis was examined in vivo using MIF transgenic (Tg) mice.
Materials and Methods
Materials
The following materials were obtained from commercial sources: an RNA extraction kit (Isogen; Nippon Gene, Tokyo, Japan); a synthesis kit [First-Strand cDNA Synthesis Kit; GE Health Care, Buckinghamshire, UK; an assay kit Methyl thiazolyl tetrazorium (MTT)] (CellTiter 96 AQ; Promega, Madison, WI); medium (Dulbecco's modified Eagle's minimal medium; Gibco, Grand Island, NY); and recombinant human MIF, expressed in Escherichia coli BL21/DE3 (Novagen, Madison, WI) and purified as previously described.23 This MIF contained less than 1 pg of endotoxin/μg protein, as determined by the chromogenic amebocyte assay (Lumulus; BioWhittaker, Walkerville, MD). The anti–PAR-2 polyclonal antibody was obtained from Santa Cruz Biotechnology, Santa Cruz, CA; and soybean trypsin inhibitor (STI), CD117/c-kit/SCF-receptor antibody-2 (clone K44.2, mouse monoclonal antibody) and anti–β-actin antibodies were obtained from Sigma-Aldrich Co, St Louis, MO. The Western blot detection system was obtained from Cell Signaling Technology, Beverly, MA. The neutralizing anti-MIF polyclonal antibody was prepared as previously described.24 All other reagents were of analytical grade.
Cell Culture
The primary cultured normal human keratinocytes were obtained from Kurabo Co, Tokyo. The keratinocytes were grown in a keratinocyte growth medium (Lonza Walkerville, Inc., Walkersville, MD) containing 0.1 ng/ml epidermal growth factor, 0.5 μg/ml insulin, 0.5 μg/ml hydrocortisone, 50 μg/ml gentamicin, 50 ng/ml amphotericin B, and 0.4% (v/v) bovine pituitary extract under 95% air and 5% CO2 at 37°C, according to the manufacturer's protocol. The cells were plated in 75-cm2 flasks, grown to near confluence, and the complete keratinocyte growth medium was removed. Primary cultured normal human melanocytes were obtained from Kurabo Co. The melanocytes were grown in medium 254S (Invitrogen, Carlsbad, CA) containing 1% Human Melanocyte Growth Supplement (HMGS, Invitrogen) under 95% air and 5% CO2 at 37°C, according to the manufacturer's protocol. The cells were plated in 75-cm2 flasks and grown to near confluence, and the complete melanocyte growth medium was removed. The medium was replaced with minimum growth medium consisting of MCDB 153 containing 2% fetal calf serum and 30 mg/ml bovine pituitary extract. The MelanoDerm kit, which is a viable reconstituted three-dimensional human epidermis containing melanocytes and keratinocytes containing 24 U of 8 mm-diameter tissue samples, was obtained from MatTek Corp, Ashland, MA. This study used the L-NMM medium supplied by the manufacturer, and cultures were maintained at the air–liquid interface, according to the manufacturer's instructions. In brief, pigmented epidermal equivalents were removed from the agarose-containing 24-well plates and were equilibrated at 37°C in 5% CO2 for 1 hour with the prewarmed maintenance medium supplied with the kit. The pigmented epidermal tissues were then placed under sterile conditions on top of the culture stand and immersed in 5 ml of prewarmed fresh maintenance medium in 6-well plates for subsequent treatment with compounds. A 100 μL aliquot of each compound in vehicle was applied directly to the cultured skin surface three times each week, and the tissue samples were kept in an incubator at 37°C in 5% CO2 throughout the experiments. The media were changed every other day with 5 ml of fresh medium, and each compound was applied to the tissue surface at the various concentrations as appropriate. The tissue specimens were harvested at 7 and 14 days after treatment.
Mice
The MIF-overexpressing Tg mice were established after cDNA microinjection, and their physical and biochemical characteristics, including body weight, blood pressure, and serum levels of cholesterol and blood glucose, were normal, as previously reported.25 The expression of the transgene was regulated by a hybrid promoter composed of the cytomegalovirus enhancer and the β-actin or β-globin promoter, as previously reported.26 The strain of the original MIF Tg mice was ICR, and the mice were backcrossed with C57BL/6 for at least 10 generations. The Tg mice were maintained by heterozygous sibling mating. The MIF Tg and wild-type (WT) mice were maintained under specific pathogen-free conditions at the Institute for Animal Experiments of the University of Toyama, Toyoma, Japan. Experiments using mice were conducted according to the guidelines set by the University of Toyama Institutional Animal Care and Use Committee under an approved protocol. All experiments were performed on 8-week-old female adult mice.
UV-B Irradiation
The UV-B light source was a fluorescent lamp (GL40SE; Sankyo Denki Co, Kanagawa, Japan) that emits 0.1 mW/cm2 of UV light between 280 and 315 nm (peak, 306 nm) at a distance of 40 cm, as measured by a UV radiometer (EKO Instruments Co, Tokyo, Japan). Normal human keratinocytes were inoculated at a density of 1.0 × 104 cells in 35 mm φ dishes. After 24 hours of cultivation, cells were irradiated with 5 mJ/cm2 UV-B in HANKS buffer. After UV-B irradiation, cells were cultured in keratinocyte growth medium for 1, 3, 6, and 24 hours. In some experiments, cultures (MelanoDerm) were irradiated with 100 mJ/cm2 UV-B on days 1, 3, and 5, in combination with anti-MIF antibody treatment. The MIF Tg and WT mice had their backs shaved with electric clippers and were exposed to 300 mJ/cm2 UV-B. The UV-B radiation was administered three times weekly (on days 1, 3, and 5), and skin was obtained at 2, 4, and 12 weeks. After UV-B irradiation, the mice were euthanized at the indicated points. The mice were macroscopically observed, and skin sections were excised from the dorsal surface and then used for either Western blot analyses or immunohistochemical staining. In some experiments, skin sections were obtained on day 14 for the Western blot analyses.
Cell Stimulation
Cultured keratinocytes or melanocytes were incubated in various concentrations of recombinant MIF. After treatment, the total RNA was isolated using reagent (Trizol; Invitrogen) for quantitative real-time PCR. The conditioned medium and cell lysates were collected. The concentration of cytokines in the culture supernatants was measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits, including kits for SCF, PGE2 (R&D Systems, MN), and ET-1 (IBL, Gunma, Japan). The cell lysates were used for the melanin content assay and Western blot analyses. In some experiments, cultures (MelanoDerm) were incubated with recombinant human MIF in combination with UV-B, STI (a PAR-2 antagonist), or an anti-human c-kit/SCF-receptor antibody. The cell lysates of these cultures were used for the melanin content assay.
Cell Viability Assay
Normal human melanocytes were placed in 96-well plates at a density of 3 × 104 cells/well. After 1 day of cultivation, cells were incubated in recombinant MIF (30, 100, and 300 ng/ml) for 24 hours. The viability of melanocytes was estimated using the MTT assay. The MTT assay was performed according to the manufacturer's protocol. In brief, 200 μL of 2.5 mg/ml MTT solution was added to cultures, and cells were incubated for 2 hours at 37°C. The absorbance was determined on a plate reader at 570 nm.
Melanin Content Assay
The melanin content was determined as previously described.27 After centrifugation, the cell pellets were dissolved in 200 μL of 1 N NaOH; and aliquots (100 μL) were transferred to 96-well microtiter plates. Melanin concentrations were determined by measuring the optical density at 405 nm, using a standard curve generated from synthetic melanin (Sigma-Aldrich). The melanin content is expressed in μg/ml of dissolved tissue.
Melanogenic Activity Assays
Cellular tyrosinase activity using l-dopa as the substrate was determined as described.28 The cells were washed with PBS and lysed with 50 μL of 1% Triton28 X-100/PBS. After sonication, 10 μL of each cell lysate was added to 100 μL of 1% l-dopa–PBS and incubated at 37°C for 60 minutes. The absorbance was measured at 405 nm in the plate reader (Spectra Max; Molecular Devices, Toronto, ON, Canada). The cell lysates were measured for protein content using a protein assay standard (Bio-Rad Laboratory, Philadelphia, PA). The relative tyrosinase activity of the treated cells compared with the control cells was expressed as percentage activity, using the following formula: % Activity = [A405 of Treated Cells/Protein (mg)] × 100%/[A405 of Control Cells/Protein (mg)].
Quantitative Real-Time PCR
Total cellular RNA was isolated from keratinocytes using a reagent (Trizol; Invitrogen), according to the manufacturer's protocol. cDNA was generated from 3 μg of total RNA using a kit (First Strand cDNA Synthesis kit; GE Health Care), according to the manufacturer's protocol, primed with an NotI-d(T)18 bifunctional primer. The PCR reactions were performed in a total volume of 20 μL using an instrument (LightCycler FastStart DNA MasterPLUS SYBR Green I; Roche Diagnostics, Basel, Switzerland), using 9 μL of PCR-grade water, 1 μL of each primer (10 μmol/L), 5 μL (10 ng) of cDNA template, and 4 μL of mix (Master Mix) at a ×5 concentration. The following PCR program was used: denaturation (10 minutes at 95°C), an amplification and quantification program repeated 45 times (10 seconds at 95°C), 1 minute at the appropriate annealing temperature for the gene-specific primers (PAR-2,29 5′-CCGAACTAAGAAGAAGCACC-3′ and 5′-AGAAAAAGCCAATAAGCACACAT-3′; SCF,5 5′-ACTGACTCTGGAATCTTTCTCAGG-3′ and 5′-GATGTTTTGCCAAGTCATTGTTGG-3′; and ribosomal 28S,30 5′-CCCAGTGCTCTGAATGTCAA-3′ and 5′-AGTGGGAATCTCGTTCATCC-3′), 10 seconds at 72°C with a single fluorescence measurement, a melting program (58°C to 95°C with a heating rate 0.5°C/s), and cooling down to 40°C. To determine the crossing point, the “second derivative maximum increase rate” of the newly synthesized DNA per cycle was used on the basis of computer software (Light Cycler Software package, Version 3.5.3; Roche Diagonostics). To confirm the amplification specificity, the PCR products from each primer pair were subjected to melting curve analysis and manual inspection of PCR products after each run by agarose gel electrophoresis. The expression of the target genes (ie, PAR-2 and SCF) was presented as a ratio and normalized to an endogenous reference (ribosomal 28S),31 relative to the control.
Western Blot Analysis
The cultured keratinocytes or epidermis sample of each mouse was homogenized using a homogenizer (Polytron). The protein concentrations of the cell homogenates were quantified using a protein assay reagent kit (Micro BCA). Equal amounts of homogenates were dissolved in 20 μL of Tris-HCL, 50 mmol/L (pH 6.8), containing 1% 2-mercaptoethanol, 2% SDS, 20% glycerol, and 0.04% bromophenol blue; and the samples were heated to 100°C for 5 minutes. The samples were then subjected to SDS–polyacrylamide gel electrophoresis and electrophoretically transferred onto a nitrocellulose membrane. The membranes were blocked with 1% nonfat dry milk powder in PBS; probed with antibodies against PAR-2, SCF, and MIF; and subsequently reacted with secondary IgG antibodies coupled with horseradish peroxidase. The resultant complexes were processed for the detection system according to the manufacturer's protocol. The relative amounts of proteins associated with specific antibodies were normalized according to the intensities of β-actin.
Fontana-Massson Silver Stain
The dorsal skin was surgically excised from mice sacrificed under ether euthanasia. The tissue specimens were fixed in 10% buffered formalin and embedded in paraffin. Next, the tissues were cut into 5-μm sections, deparaffinized through a series of xylol-alcohol to absolute alcohol and through a series of alcohol-water to water, and prepared for routine Fontana-Masson silver staining. In brief, the sections were transferred to Fontana silver solution and stored in a covered dish in the dark for 18 hours. After washing in distilled water twice, the sections were fixed in 5% sodium thiosulphate for 5 minutes. After washing in tap water for 5 minutes, the sections were counterstained in 0.1% nuclear fast red for 5 minutes. After rinsing with distilled water quickly, the sections were dehydrated and mounted in medium (Permount). The image acquisition and analyses were performed as previously described for quantitative studies on the skin samples to estimate melanin density,32 with some modifications using an instrument (Win Roof; Mitani Corp, Tokyo). The thresholding function of this instrument was used to select the stained melanin pigment. The melanin density was calculated as a proportion of the total area from the basement membrane to the upper layers of the dermis at the ×40 objective. Five random fields were analyzed per section (one section per mouse, with five mice per group).
Statistical Analysis
All values are expressed as the mean ± SD of the respective test or control group. Statistical significance between the control and test groups was evaluated by either the Student's t-test or one-way analysis of variance. P < 0.05 was considered significant.
Results
MIF Enhances the Expression of PAR-2 in Keratinocytes
This study first examined the effects of MIF on the expression of PAR-2 in human keratinocytes. A real-time quantitative RT-PCR analysis revealed that PAR-2 mRNA expression in keratinocytes significantly increased in response to recombinant MIF (50 or 100 ng/ml) at 1 hour after stimulation (versus 0 ng/ml). Similarly, UV-B (50 mJ/cm2) stimulated the PAR-2 mRNA expression in keratinocytes at 1 hour after stimulation, which reached a maximum at 3 hours (Figure 1A). A Western blot analysis revealed that the PAR-2 protein levels were dose-dependently up-regulated at 50 and 100 ng/ml MIF (Figure 1B).
Figure 1.
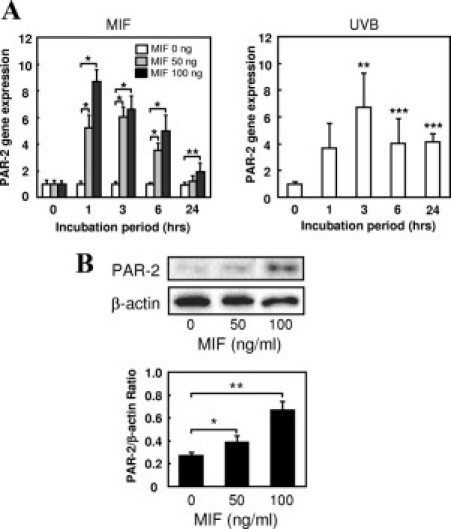
Enhancement of PAR-2 by UV-B and MIF in keratinocytes. A: Cultured human keratinocytes were treated with MIF (0, 50, or 100 ng/ml) or UV-B (50 mJ/cm2) for 1, 3, 6, or 24 hours. Total RNA was isolated, and PAR-2 mRNA expression was examined by a quantitative RT-PCR assay using ribosomal 28S as an internal control. The MIF and UV-B both increased PAR-2 mRNA expression in a dose-dependent manner. The data are plotted as the mean ± SD of the relative ratio (n = 5, *P < 0.001, **P < 0.01 and ***P < 0.05 versus 0 ng/ml). The data shown are representative of three independent experiments. B: A Western blot analysis of PAR-2 protein expression in cultured human keratinocytes incubated with MIF (0, 50, or 100 ng/ml) for 24 hours. The relative amounts of protein associated with specific antibodies were normalized to the intensity of β-actin (n = 3, *P < 0.05 and **P < 0.005). The data shown are representative of three independent experiments.
MIF Enhances the Expression of SCF by Keratinocytes
Several articles have reported that UV light stimulates the secretion of SCF, ET-1, and PGE2 from keratinocytes, which induces melanogenesis. Therefore, the effect of MIF on the release of these mediators was examined in the cultured human keratinocytes by ELISA. The results showed that UV-B irradiation increased the secretion of SCF from keratinocytes. Similarly, MIF (100 ng/ml) caused a significant release of SCF from cultured keratinocytes compared with control cultures (without MIF stimulation, Figure 2A). On the other hand, MIF had no significant effect on the secretion of ET-1 or PGE2 by the keratinocytes. Real-time quantitative RT-PCR of cultured keratinocytes was performed to determine the comparative levels of SCF mRNA transcripts in response to various concentrations of MIF. The results showed that MIF (50 and 100 ng/ml) caused a statistically significant enhancement in the expression of the SCF mRNA transcript in keratinocytes, especially at 3 hours after MIF stimulation (Figure 2B).
Figure 2.
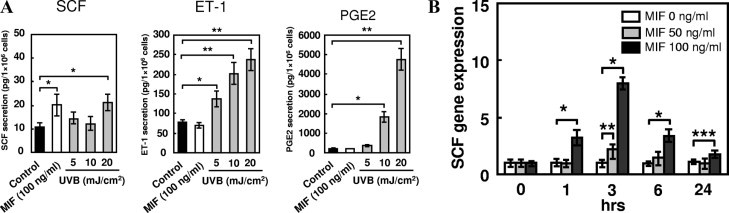
Effects of MIF on the production of SCF, ET-1, and PGE2 by keratinocytes. A: Cultured keratinocytes were treated with 100 ng/ml MIF or various doses of UV-B for 24 hours. The concentrations of SCF, ET-1, and PGE2 protein in the culture media were determined by ELISA. The results are expressed as the mean ± SD of five experiments (*P < 0.005 and **P < 0.001). B: Keratinocytes were stimulated with 0, 50, or 100 ng/ml MIF, and the expression of SCF mRNA was evaluated by real-time RT-PCR (n = 5; *P < 0.001, **P < 0.01, and ***P < 0.05 versus 0 ng/ml). The data shown are representative of three independent experiments.
Effects of MIF on Melanin Synthesis and Tyrosinase Activity in Cultured Melanocytes
Human cultured melanocytes were incubated with various concentrations of recombinant MIF for 24 hours. The effect of MIF on cell viability was examined by an MTT assay. The viability of melanocytes was not changed by a high MIF concentration (300 ng/ml, Figure 3A). Similarly, MIF had no direct effect on melanin synthesis or the tyrosinase activities of the cells (Figure 3, B and C). This indicated that MIF has no direct regulatory effect on melanocytes.
Figure 3.

Effects of MIF on cell viability, melanin synthesis, and tyrosinase activity in cultured melanocytes. Cultured human melanocytes were treated with human recombinant MIF at the indicated concentrations for 24 hours. A: The cell viability was measured using the MTT assay. B: The cells were lysed with 1 N NaOH, and absorbance at 405 nm was examined. The melanin content was estimated using a standard curve of synthetic melanin. C: Cells were lysed with 1% Triton X-100/PBS(−). The tyrosinase activities were measured using 1% l-dopa as the substrate. The results are expressed as the percentage of 0 ng/ml treated groups. The results are expressed as the mean ± SD of five experiments. (These experiments were repeated three times, with similar results.)
Effect of MIF on Melanogenesis Determined Using a Three-Dimensional Reconstituted Human Epidermal Culture Model
MelanoDerm, which is a novel commercially available cultured human epidermis containing functional melanocytes, was used to clarify whether MIF secreted by keratinocytes is required for the synthesis of melanin in melanocytes. The results revealed that 300 ng/ml MIF induced an increase in the melanin content in the human epidermis–MelanoDerm cultures compared with cultures grown without MIF stimulation after a 9-day culture period (P < 0.01, Figure 4A). In addition, UV-B irradiation (100 mJ/cm2) in combination with 300 ng/ml MIF induced a further increase in melanin content. We also found that the melanin synthesis induced by 100 mJ/cm2 UV-B stimulation was significantly down-regulated by anti-MIF antibody (30 μg/ml) treatment (P < 0.005, Figure 4B). Furthermore, MIF-induced melanin synthesis was significantly suppressed by treatment with STI (0.5% PAR-2 antagonist) or a c-kit/SCF-receptor antibody (40 μg/ml) (P < 0.001 for STI and P < 0.01 for SCF, Figure 4C).
Figure 4.
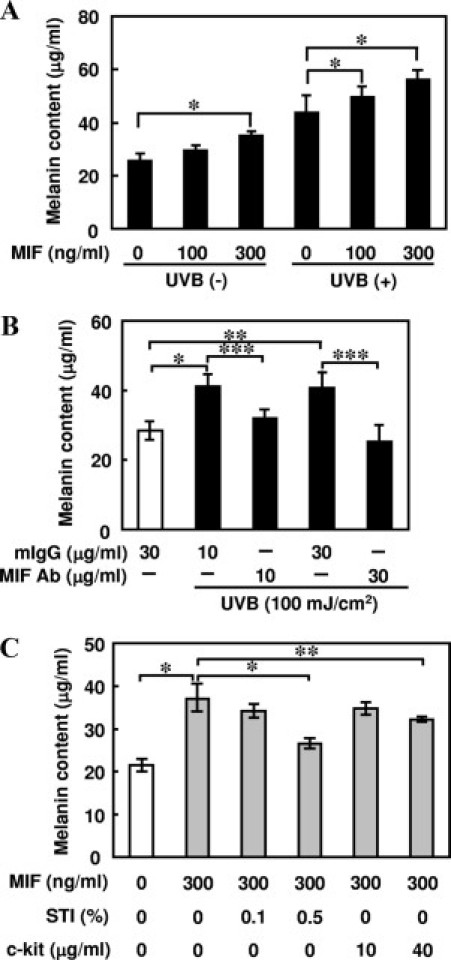
Effects of MIF on melanogenesis in reconstituted three-dimensional human epidermis (MelanoDerm) cultures. A: The cultures (MelanoDerm) were treated with various concentrations of MIF for 9 days, with or without UV-B irradiation on days 1, 3, and 5. Equivalent numbers of cells were lysed with 1 N NaOH and measured for absorbance at 405 nm. The melanin content was estimated using a standard curve of synthetic melanin. The values are the mean ± SD derived from five equivalents (*P < 0.01). B: The cultures (MelanoDerm) were treated with neutralizing anti-MIF IgG antibody (10 or 30 μg/ml) or mouse IgG for 11 days with 100 mJ/cm2 UV-B exposure, and the melanin content was estimated. The results are expressed as the mean ± SD of five experiments (*P < 0.0005, **P < 0.001, and ***P < 0.005). C: The cultures (MelanoDerm) were treated with 300 ng/ml MIF in combination with various concentrations of STI (a PAR-2 antagonist) or a c-kit/SCF-receptor antibody for 9 days, and the melanin content was estimated. The results are expressed as the mean ± SD of five experiments (*P < 0.001 and **P < 0.01). The data shown are representative of three independent experiments.
Sensitivity of MIF Tg Mice to the Development of Skin Pigmentation by Long-Term Exposure to UV-B
To clarify the roles of MIF in skin melanogenesis, MIF-overexpressing Tg and WT mice were subjected to long-term UV-B exposure, as described in the UV-B Irradiation section of Materials and Methods; and mice were observed for the formation of melanin pigment in the skin at 2, 4, and 12 weeks. The production of MIF, PAR-2, and SCF in the epidermis was determined by Western blot analysis after UV-B irradiation. Skin was obtained 2 weeks after UV-B exposure, and the epidermis of MIF Tg mice showed intense expression of MIF, PAR-2, and SCF compared with the epidermis of WT mice (Figure 5A). The MIF Tg and WT mice exposed to long-term UV-B began to develop melanin pigment after 4 weeks. The density of the melanin content in MIF Tg mouse skin at 12 weeks was significantly increased compared with that of WT mouse skin (P < 0.01; Figure 5, B and C). The MIF Tg mice showed strong pigmentation on their back skin at 12 weeks after UV-B exposure. In contrast, WT mice show moderate pigmentation (Figure 5D).
Figure 5.
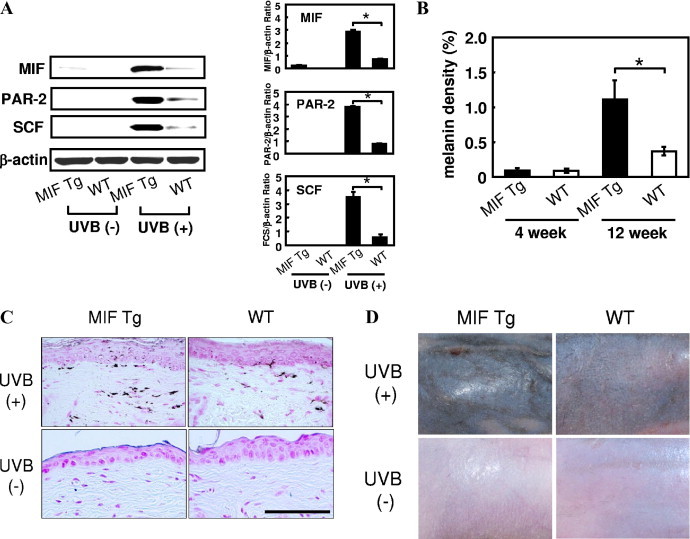
UV-B–induced skin pigmentation in MIF Tg and WT mice. A: After three courses of UV-B exposure, MIF Tg and WT mouse skin samples were obtained on day 14, and a Western blot analysis was performed using MIF, PAR-2, and SCF antibodies. The relative amounts of protein associated with specific antibodies were normalized to the intensity of β-actin (n = 5, *P < 0.0001). FCS indicates fetal calf serum. B: The MIF Tg and WT mice were subjected to long-term UV-B exposure. The details of the protocols are described in the UV-B Irradiation section of Materials and Methods. The skin melanin density was determined at 4 and 12 weeks. The results are expressed as the mean ± SD (*P < 0.01). C: Representative examples of micrographs of Fontana-Masson staining are shown from experiments conducted using skin samples obtained at 12 weeks from the MIF Tg and WT mice. Increased pigmentation was observed in the skin of MIF Tg mice compared with WT mouse skin. Nonirradiated MIF Tg mice and nonirradiated WT mice did not develop any significant skin pigmentation. Scale bar = 100 μm. D: Clinical manifestations at 12 weeks after UV-B exposure. The MIF Tg mice showed strong pigmentation on their back skin. In contrast, the WT mice showed moderate pigmentation. Nonirradiated MIF Tg mice and nonirradiated WT mice showed no pigmentation. (These experiments were repeated two times, with similar results.)
Discussion
Long-term UV radiation is the primary physiological stimulus that causes pigmentation in human skin. Melanogenesis is initiated on exposure of skin to UV radiation, with the first step being amino acid tyrosine oxidation by tyrosinase, the rate-limiting enzyme.33 Melanin-containing melanosomes are transferred to neighboring keratinocytes, providing protection against UV radiation, a well-characterized physiological stimulator of melanogenesis.34,35 The present study demonstrated that G-protein–coupled receptor PAR-2 mRNA and protein expression were enhanced in vitro in keratinocytes by MIF treatment. The PAR-2 mRNA expression started to increase 1 hour after stimulation, peaked at 3 hours, and then was sustained for up to 6 hours after exposure. Increased PAR-2 protein expression was also detected at 24 hours in the keratinocytes after MIF stimulation. The key mechanism underlying melanosome transfer to keratinocytes involves the process of phagocytosis. The PAR-2 receptor is important in skin pigmentation because PAR-2 activation results in increased uptake of melanosomes by keratinocytes through phagocytosis.9,36 In keratinocytes, PAR-2 is activated by trypsin, mast cell tryptase, factor VIIa, or factor Xa, and by synthetic peptides that mimic the amino terminal portion of the receptor.37–40 The mechanism of UV-induced PAR-2 up-regulation remains to be determined, although it is well documented that UVR induces keratinocytes to produce and secrete cytokines, including IL-1, IL-6, IL-8, and TNF-α. Studies41 have shown that PAR-2 mRNA is up-regulated by TNF-α and IL-1 in vascular endothelial cells. The PAR-2 mRNA and protein levels are elevated by MIF in human skin endothelial cells.22 UV-B irradiation also leads to increased MIF expression in keratinocytes.20
In support of these observations, the current study revealed that MIF is highly effective in stimulating the secretion of SCF from cultured keratinocytes. The paracrine linkage between keratinocytes and melanocytes within the epidermis secreting and responding to cytokines plays an important role in increasing melanization in UV light–induced cutaneous pigmentation. A few of these cytokines are known mitogens for human melanocytes, including basic fibroblast growth factor, SCF, granulocyte-macrophage colony-stimulating factor, hepatocyte growth factor, and ET-1.42–46 Several cytokines, such as IL-1α and TNF-α, significantly increase the expression of SCF protein by cultured human keratinocytes.5 Accordingly, MIF may induce PAR-2 to mediate cutaneous pigmentation by increasing both the uptake of melanosomes by keratinocytes and the release of SCF, which stimulates melanogenesis.
The MelanoDerm is a three-dimensional reconstituted human epidermal culture model using melanocytes and keratinocytes in the context of their responses to melanogenic regulators. This model provides substantial improvement for final testing of compounds that might affect melanocyte and/or keratinocyte function.47 This study demonstrated that MIF induced an increase in the melanin content in the MelanoDerm experiments after a 9-day culture period. Moreover, melanin synthesis induced by UV-B stimulation was significantly down-regulated by anti-MIF antibody treatment. UV radiation influences the melanogenesis of melanocytes indirectly through a paracrine regulation process involving keratinocytes.48,49 An anti-MIF antibody effectively reduces tumor growth and neovascularization in lymphoma cells and vascular endothelial cells in vivo.20 Consistent with this finding, anti-MIF antibodies were effective in reducing melanogenesis in the human epidermis.
Migration inhibitory factor is a cytokine that plays a critical role in several inflammatory conditions. High levels of MIF expression have been found in a variety of inflammatory conditions, such as atopic dermatitis in the epidermal layer of inflammatory skin lesions.20 In addition to its inflammatory properties, MIF may have d-dopachrome tautomerase activity. Rosengren et al50 reported that MIF has catalyzed the tautomerization of d-dopachrome to 5,6-dihydroxyindole-2-carboxylic acid, and MIF may play a role in regulating the biosynthesis of neuromelanin.51 However, the physiological significance of this catalytic function in the skin is not clear. The current study found that MIF has no direct effect on tyrosinase and melanin synthesis in cultured human melanocytes. In addition, the proliferation rate of melanocytes was unchanged, even by a high MIF concentration. Several cytokines in the epidermis affect skin melanogenesis. A hypopigmenting effect has been demonstrated for transforming growth factor-α1 using B16/F10 mouse melanoma cells as a model.52 Interleukin-1α is involved in inhibiting the proliferation of neonatal murine epidermal melanoblasts and in stimulating the differentiation, melanogenesis, and dendritogenesis of melanocytes.53 Tumor necrosis factor-α is also a potent inhibitor of tyrosinase activity in normal human melanocytes.54 Current evidence suggests that MIF has no direct effect on melanogenesis in normal melanocytes.
Finally, we also found that long-term UV-B exposure in MIF Tg mice resulted in a greater melanin content in their skin than in the skin of UV-B–irradiated WT mice. In addition, UV-B stimulated the expression of MIF, PAR-2, and SCF in the epidermis of MIF Tg mice more strongly than in the epidermis of WT mice. These results confirmed the in vitro observation that MIF enhances melanogenesis. Moreover, MIF-induced melanogenesis was significantly suppressed by treatment with a PAR-2 antagonist or a c-kit/SCF-receptor antibody in the MelanoDerm cultures. More tumors were also observed in the MIF Tg mice compared with the WT mice after long-term UV-B irradiation.55 Migration inhibitory factor has a direct proinflammatory role and has been implicated in tumorigenesis.56 Moreover, intense inflammation in MIF Tg mice in response to UV-B irradiation correlated with the early onset of carcinogenesis and the higher incidence of tumors after long-term UV-B irradiation.55 In this context, it is believed that MIF could play a critical role in UV-B-induced photoaging and skin carcinogenesis.
In conclusion, the current study provided new evidence that MIF mediates melanogenesis primarily through the activation of PAR-2 and SCF expression in keratinocytes after exposure to UV-B radiation. Consequently, this newly identified mechanism may contribute to the overall understanding of photoaging, including UV-B–induced melanogenesis. Therefore, these findings are promising for the potential development of MIF inhibitors for the prevention or treatment of photodamage in skin.
Footnotes
Supported by Grant-in-Aid for Scientific Research 20591337 from the Japan Society for the Promotion of Science.
A.E. and Y.Y. contributed equally to this work.
References
- 1.Young A.R. Cumulative effects of ultraviolet radiation on the skin: cancer and photoaging. Semin Dermatol. 1990;9:25–31. [PubMed] [Google Scholar]
- 2.Yada Y., Higuchi K., Imokawa G. Effects of endothelins on signal transduction and proliferation in human melanocytes. J Biol Chem. 1991;266:18352–18357. [PubMed] [Google Scholar]
- 3.Imokawa G., Yada Y., Miyagishi M. Endothelins secreted from human keratinocytes are intrinsic mitogens for human melanocytes. J Biol Chem. 1992;267:24675–24680. [PubMed] [Google Scholar]
- 4.Imokawa G., Kobayashi T., Miyagishi M., Higashi K., Yada Y. The role of endothelin-1 in epidermal hyperpigmentation and signaling mechanisms of mitogenesis and melanogenesis. Pigment Cell Res. 1997;10:218–228. doi: 10.1111/j.1600-0749.1997.tb00488.x. [DOI] [PubMed] [Google Scholar]
- 5.Hachiya A., Kobayashi A., Yoshida Y., Kitahara T., Takema Y., Imokawa G. Biphasic expression of two paracrine melanogenic cytokines, stem cell factor and endothelin-1, in ultraviolet B-induced human melanogenesis. Am J Pathol. 2004;165:2099–2109. doi: 10.1016/S0002-9440(10)63260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott G., Jacobs S., Leopardi S., Anthony F.A., Learn D., Malaviya R., Pentland A. Effects of PGF2alpha on human melanocytes and regulation of the FP receptor by ultraviolet radiation. Exp Cell Res. 2005;304:407–416. doi: 10.1016/j.yexcr.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 7.Scott G., Fricke A., Fender A., McClelland L., Jacobs S. Prostaglandin E2 regulates melanocyte dendrite formation through activation of PKCzeta. Exp Cell Res. 2007;313:3840–3850. doi: 10.1016/j.yexcr.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Déry O., Corvera C.U., Steinhoff M., Bunnett N.W. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am J Physiol. 1998;274(pt 1):C1429–C1452. doi: 10.1152/ajpcell.1998.274.6.C1429. [DOI] [PubMed] [Google Scholar]
- 9.Seiberg M. Keratinocyte-melanocyte interactions during melanosome transfer. Pigment Cell Res. 2001;14:236–242. doi: 10.1034/j.1600-0749.2001.140402.x. [DOI] [PubMed] [Google Scholar]
- 10.Scott G., Deng A., Rodriguez-Burford C., Seiberg M., Han R., Babiarz L., Grizzle W., Bell W., Pentland A. Protease-activated receptor 2, a receptor involved in melanosome transfer, is upregulated in human skin by ultraviolet irradiation. J Invest Dermatol. 2001;117:1412–1420. doi: 10.1046/j.0022-202x.2001.01575.x. [DOI] [PubMed] [Google Scholar]
- 11.Tsuboi R., Sato C., Shi C.M., Nakamura T., Sakurai T., Ogawa H. Endothelin-1 acts as an autocrine growth factor for normal human keratinocytes. J Cell Physiol. 1994;159:213–220. doi: 10.1002/jcp.1041590204. [DOI] [PubMed] [Google Scholar]
- 12.Bloom B.R., Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966;153:80–82. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- 13.David J.R. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci U S A. 1966;56:72–77. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernhagen J., Calandra T., Mitchell R.A., Martin S.B., Tracey K.J., Voelter W., Manogue K.R., Cerami A., Bucala R. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365:756–759. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- 15.Calandra T., Bernhagen J., Mitchell R.A., Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994;179:1895–1902. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lanahan A., Williams J.B., Sanders L.K., Nathans D. Growth factor-induced delayed early response genes. Mol Cell Biol. 1992;12:3919–3929. doi: 10.1128/mcb.12.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wistow G.J., Shaughnessy M.P., Lee D.C., Hodin J., Zelenka P.S. A macrophage migration inhibitory factor is expressed in the differentiating cells of the eye lens. Proc Natl Acad Sci U S A. 1993;90:1272–1275. doi: 10.1073/pnas.90.4.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bacher M., Meinhardt A., Lan H.Y., Mu W., Metz C.N., Chesney J.A., Calandra T., Gemsa D., Donnelly T., Atkins R.C., Bucala R. Migration inhibitory factor expression in experimentally induced endotoxemia. Am J Pathol. 1997;150:235–246. [PMC free article] [PubMed] [Google Scholar]
- 19.Nishihira J. Macrophage migration inhibitory factor (MIF): its essential role in the immune system and cell growth. J Interferon Cytokine Res. 2000;20:751–762. doi: 10.1089/10799900050151012. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu T., Abe R., Ohkawara A., Nishihira J. Ultraviolet B radiation upregulates the production of macrophage migration inhibitory factor (MIF) in human epidermal keratinocytes. J Invest Dermatol. 1999;112:210–215. doi: 10.1046/j.1523-1747.1999.00486.x. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu T. Role of macrophage migration inhibitory factor (MIF) in the skin. J Dermatol Sci. 2005;37:65–73. doi: 10.1016/j.jdermsci.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Shimizu T., Nishihira J., Watanabe H., Abe R., Honda A., Ishibashi T., Shimizu H. Macrophage migration inhibitory factor (MIF) is induced by thrombin and factor Xa in endothelial cells. J Biol Chem. 2004;279:13729–13737. doi: 10.1074/jbc.M400150200. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe H., Shimizu T., Nishihira J., Abe R., Nakayama T., Taniguchi M., Sabe H., Ishibashi T., Shimizu H. Ultraviolet A-induced production of matrix metalloproteinase-1 is mediated by macrophage migration inhibitory factor (MIF) in human dermal fibroblasts. J Biol Chem. 2004;279:1676–1683. doi: 10.1074/jbc.M303650200. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu T., Abe R., Ohkawara A., Mizue Y., Nishihira J. Macrophage migration inhibitory factor is an essential immunoregulatory cytokine in atopic dermatitis. Biochem Biophys Res Commun. 1997;240:173–178. doi: 10.1006/bbrc.1997.7633. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki S., Nishihira J., Ishibashi T., Yamasaki Y., Obikane K., Echigoya M., Sado Y., Ninomiya Y., Kobayashi K. Transgene of MIF induces podocyte injury and progressive mesangial sclerosis in the mouse kidney. Kidney Int. 2004;65:469–481. doi: 10.1111/j.1523-1755.2004.00394.x. [DOI] [PubMed] [Google Scholar]
- 26.Akagi Y., Isaka Y., Akagi A., Ikawa M., Takenaka M., Moriyama T., Yamauchi A., Horio M., Ueda N., Okabe M., Imai E. Transcriptional activation of a hybrid promoter composed of cytomegalovirus enhancer and beta-actin/beta-globin gene in glomerular epithelial cells in vivo. Kidney Int. 1997;51:1265–1269. doi: 10.1038/ki.1997.172. [DOI] [PubMed] [Google Scholar]
- 27.Virador V., Kobayashi N., Matsunaga J., Hearing V.J. A standardized protocol for assessing regulators of pigmentation. Anal Biochem. 1999;270:207–219. doi: 10.1006/abio.1999.4090. [DOI] [PubMed] [Google Scholar]
- 28.Maeda K., Fukuda M. Atbutin: mechanism of its depigmentating action in human melanocyte culture. J Pharmacol Exp Ther. 1996;276:765–769. [PubMed] [Google Scholar]
- 29.Trian T., Girodet P.O., Ousova O., Marthan R., Tunon-de-Lara J.M., Berger P. RNA interference decreases PAR-2 expression and function in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2006;34:49–55. doi: 10.1165/rcmb.2005-0187OC. [DOI] [PubMed] [Google Scholar]
- 30.Mahoney D.J., Carey K., Fu M.H., Snow R., Cameron-Smith D., Parise G., Tarnopolsky M.A. Real-time RT-PCR analysis of housekeeping genes in human skeletal muscle following acute exercise. Physiol Genomics. 2004;18:226–231. doi: 10.1152/physiolgenomics.00067.2004. [DOI] [PubMed] [Google Scholar]
- 31.Steele B.K., Meyers C., Ozbun M.A. Variable expression of some “housekeeping” genes during human keratinocyte differentiation. Anal Biochem. 2002;307:341–347. doi: 10.1016/s0003-2697(02)00045-3. [DOI] [PubMed] [Google Scholar]
- 32.Dwyer T., Muller H.K., Blizzard L., Ashbolt R., Phillips G. The use of spectrophotometry to estimate melanin density in Caucasians. Cancer Epidermiol Biomarkers Prev. 1998;7:203–206. [PubMed] [Google Scholar]
- 33.Duchon J., Matous B., Pechan Z. In: On the chemical nature of urinary melanogens: Structure and Control of the Melanocyte. Prota G., Muhlbock O., editors. Springer-Verlag; Berlin: 1996. pp. 175–184. [Google Scholar]
- 34.Gordon P.R., Mansur C.P., Gilchrest B.A. Regulation of human melanocyte growth, dendricity, and melanization by keratinocyte derived factors. J Invest Dermatol. 1989;92:565–572. doi: 10.1111/1523-1747.ep12709595. [DOI] [PubMed] [Google Scholar]
- 35.Romero-Graillet C., Aberdam E., Clement M., Ortonne J.P., Ballotti R. Nitric oxide produced by ultraviolet-irradiated keratinocytes stimulates melanogenesis. J Clin Invest. 1997;99:1–8. doi: 10.1172/JCI119206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharlow E.R., Paine C.S., Babiarz L., Eisinger M., Shapiro S., Seiberg M. The protease activated receptor-2 upregulates keratinocyte phagocytosis. J Cell Sci. 2000;113:3093–3101. doi: 10.1242/jcs.113.17.3093. [DOI] [PubMed] [Google Scholar]
- 37.Santulli R.J., Derian C.K., Darrow A.L., Tomko K.A., Eckardt A.J., Seiberg M., Scarborough R.M., Andrade-Gordon P. Evidence for the presence of a protease activated receptor distinct from the thrombin receptor in human keratinocytes. Proc Natl Acad Sci U S A. 1995;92:9151–9155. doi: 10.1073/pnas.92.20.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schechter N.M., Brass L.F., Lavker R.M., Jensen P.J. Reaction of mast cell proteases tryptase and chymase with protease activated receptors (PARs) on keratinocytes and fibroblasts. J Cell Physiol. 1998;176:365–373. doi: 10.1002/(SICI)1097-4652(199808)176:2<365::AID-JCP15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 39.Steinhoff M., Corveram C.U., Thoma M.S., Kong W., McAlpine B.E., Caughey G.H., Ansel J.C., Bunnett N.W. Proteinase-activated receptor-2 in human skin: tissue distribution and activation of keratinocytes by mast cell tryptase. Exp Dermatol. 1999;8:282–294. doi: 10.1111/j.1600-0625.1999.tb00383.x. [DOI] [PubMed] [Google Scholar]
- 40.Camerer E., Huang W., Coughlin S.R. Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc Natl Acad Sci U S A. 2000;97:5255–5260. doi: 10.1073/pnas.97.10.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nystedt S., Ramakrishnan V., Sundelin J. The proteinase-activated receptor 2 is induced by inflammatory mediators in human endothelial cells. J Biol Chem. 1996;271:14910–14915. doi: 10.1074/jbc.271.25.14910. [DOI] [PubMed] [Google Scholar]
- 42.Halaban R., Langdon R., Birchall N., Cuono C., Baurd A., Scott G., Moellmann G., McGuire J. Basic fibroblast growth factor from human keratinocytes is a natural mitogen for melanocytes. J Cell Biol. 1988;107:1611–1619. doi: 10.1083/jcb.107.4.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imokawa G., Yada Y., Morisaki N., Kimura M. Biological characterization of human fibroblast-derived mitogenic factors for human melanocytes. Biochem J. 1998;330(pt 3):1235–1239. doi: 10.1042/bj3301235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imokawa G., Yada Y., Kimura M., Morisaki N. Granulocyte/macrophage colony-stimulating factor is an intrinsic keratinocyte-derived growth factor for human melanocytes in UVA-induced melanosis. Biochem J. 1996;313(pt 2):625–631. doi: 10.1042/bj3130625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsumoto K., Tajima H., Nakamura T. Hepatocyte growth factor is a potent stimulator of human melanocyte DNA synthesis and growth. Biochem Biophys Res Commun. 1991;176:45–51. doi: 10.1016/0006-291x(91)90887-d. [DOI] [PubMed] [Google Scholar]
- 46.Imokawa G., Miyagishi M., Yada Y. Endothelin-1 as a new melanogen: coordinated expression of its gene and the tyrosinase gene in UVB-exposed human epidermis. J Invest Dermatol. 1995;105:32–37. doi: 10.1111/1523-1747.ep12312500. [DOI] [PubMed] [Google Scholar]
- 47.Yoon T.J., Lei T.C., Yamaguchi Y., Batzer J., Wolber R., Hearing V.J. Reconstituted 3-dimensional human skin of various ethnic origins as an in vitro model for studies of pigmentation. Anal Biochem. 2003;318:260–269. doi: 10.1016/s0003-2697(03)00172-6. [DOI] [PubMed] [Google Scholar]
- 48.Hara M., Yaar M., Gilchrest B.A. Endothelin-1 of keratinocytes origin is a mediator of melanocyte dendricity. J Invest Dermatol. 1995;105:744–748. doi: 10.1111/1523-1747.ep12325522. [DOI] [PubMed] [Google Scholar]
- 49.Hunt G., Todd C., Cresswell J.E., Thody A.J. α-MSH and its analog Nle4Dphe7α-MSH affect morphology, tyrosinase activity and melanogenesis in cultured human melanocytes. J Cell Sci. 1994;107:205–211. doi: 10.1242/jcs.107.1.205. [DOI] [PubMed] [Google Scholar]
- 50.Rosengren E., Bucala R., Aman P., Jacobsson L., Odh G., Metz C.N., Rorsman H. The immunoregulatory mediator macrophage migration inhibitory factor (MIF) catalyzes a tautomerization reaction. Mol Med. 1996;2:143–149. [PMC free article] [PubMed] [Google Scholar]
- 51.Matsunaga J., Sinha D., Solano F., Santis C., Wistow G., Hearing V. Macrophage migration inhibitory factor (MIF): its role in catecholamine metabolism. Cell Mol Biol. 1999;45:1035–1040. [PubMed] [Google Scholar]
- 52.Martínez-Esparza M., Jiménez-Cervantes C., Solano F., Lozano J.A., García-Borrón J.C. Mechanisms of melanogenesis inhibition by tumor necrosis factor-alpha in B16/F10 mouse melanoma cells. Eur J Biochem. 1998;255:139–146. doi: 10.1046/j.1432-1327.1998.2550139.x. [DOI] [PubMed] [Google Scholar]
- 53.Hirobe T., Ootaka H. Interleukin-1alpha stimulates the differentiation of melanocytes but inhibits the proliferation of melanoblasts from neonatal mouse epidermis. Zoolog Sci. 2007;24:959–970. doi: 10.2108/zsj.24.959. [DOI] [PubMed] [Google Scholar]
- 54.Swope V., Abdel-Malek Z., Kassem L., Norlund J. Interleukins-1alpha and 6 and tumor necrosis factor-A are paracrine inhibitors of human melanocyte proliferation and melanogenesis. J Invest Dermatol. 1991;96:180–185. doi: 10.1111/1523-1747.ep12460991. [DOI] [PubMed] [Google Scholar]
- 55.Honda A., Abe R., Yoshihisa Y., Makino T., Matsunaga K., Nishihira J., Shimizu H., Shimizu T. Deficient deletion of apoptotic cells by macrophage migration inhibitory factor (MIF) overexpression accelerates photocarcinogenesis. Carcinogenesis. 2009;30:1597–1605. doi: 10.1093/carcin/bgp160. [DOI] [PubMed] [Google Scholar]
- 56.Bach J.P., Rinn B., Meyer B., Dodel R., Bacher M. Role of MIF in inflammation and tumorigenesis. Oncology. 2008;75:127–133. doi: 10.1159/000155223. [DOI] [PubMed] [Google Scholar]


