SUMMARY
Mammalian brains generate internal activity independent of environmental stimuli. Internally generated states may bring about distinct cortical processing modes. To investigate how brain state impacts cortical circuitry, we recorded intracellularly from the same neurons, under anesthesia and subsequent wakefulness, in rat barrel cortex. In every cell examined throughout layers 2–6, wakefulness produced a temporal pattern of synaptic inputs differing markedly from those under anesthesia. Recurring periods of synaptic quiescence, prominent under anesthesia, were abolished by wakefulness, which produced instead a persistently depolarized state. This switch in dynamics was unaffected by elimination of afferent synaptic input from thalamus, suggesting that arousal alters cortical dynamics by neuromodulators acting directly on cortex. Indeed, blockade of noradrenergic, but not cholinergic, pathways induced synaptic quiescence during wakefulness. We conclude that global brain states can switch local recurrent networks into different regimes via direct neuromodulation. Our results provide a basis for understanding how sleep/wake cycles alter synaptic integration in cortical neurons.
INTRODUCTION
Mammalian brains generate internal activity independent of environmental stimuli. Internally generated activity may reflect arousal, attention, anticipation of reward, or other non-sensory signals related to the behavioral state of an organism. How do global brain states alter activity in local cortical networks, and what are the cellular mechanisms responsible for such changes in cortical processing?
The most overtly observable brain states are perhaps found in the sleep-wake cycle, with substantial behavioral and perceptual differences between sleeping, drowsy, and alert states. Electrical brain potentials measured from the scalp (electroencephalogram; EEG) have long been known to change with such states (Blake, 1937). EEG exhibits prominent slow-wave oscillations (<2 Hz) during natural deep sleep and under anesthesia but not during wakefulness, reflecting marked differences in cortical activity (Steriade et al., 1993b). EEG slow waves derive from relatively synchronous discharges of large populations of neurons (Steriade et al., 1993c; Steriade et al., 2001). These discharges are separated by periods of synaptic quiescence, during which virtually all of the thousands of synapses contacting a neuron are inactive.
Intracellular recording affords a unique view of network activity, reporting a neuron’s synaptic inputs and therefore the net activity of thousands of connected cells. The resulting membrane potential (Vm) modulates the impact of subsequent synaptic inputs. In anesthetized animals, Vm at the time of a sensory stimulus strongly influences the amplitude of post-synaptic potentials as well as the number and relative timing of action potentials evoked (Petersen et al., 2003; Sachdev et al., 2004). Thalamocortical synapses more or less effectively transmit sensory information depending on cortical Vm in slice (Rigas and Castro-Alamancos, 2009; Watson et al., 2008). Therefore, moment-to-moment Vm fluctuations among anatomically connected neurons may influence cells’ actual functional connectivity, perhaps subserving sensory perception, motor integration, working memory, and attention (Haider and McCormick, 2009).
The temporal patterns of synaptic inputs (network dynamics) during wakefulness are less clear. Heroic sharps recordings initially provided several examples of neurons in multi-modal association areas of cat neocortex that exhibit pronounced slow-wave fluctuations during natural sleep but not wakefulness (Steriade et al., 2001). Wakefulness was characterized instead by persistent depolarization and high action potential discharge rates. In contrast, a later whole-cell study described low-frequency fluctuations in layer 2/3 pyramidal neurons in rodent primary somatosensory cortex during “quiet wakefulness” (Petersen et al., 2003; see also Poulet and Petersen, 2008), though these have yet to be directly compared to those during sleep/anesthesia. The earlier cat studies did not observe slow-wave synaptic patterns during wakefulness but did not identify cells. How arousal affects individual neurons of different types has yet to be extensively examined.
The mechanism by which arousal may alter cortical dynamics is also unclear. Electrical stimulation of the brainstem cholinergic center innervating the thalamus enhances thalamic discharge and tonically depolarizes cortical neurons (Steriade et al., 1993a; Steriade et al., 1991; Steriade et al., 1993b). This suggests that arousal influences local cortical networks via long-range afferent synaptic inputs and may differentially affect thalamorecipient and non-thalamorecipient layers. Other studies have, however, shown that stimulation of the basal forebrain, the cortical source of cholinergic innervation, produces awake-like cortical activity in anesthetized animals (Goard and Dan, 2009; Metherate et al., 1992; Steriade et al., 1993a; Steriade et al., 1991; Steriade et al., 1993b). This points towards an alternative mechanism whereby arousal alters cortical activity via direct cholinergic modulation of cortex.
We therefore sought 1) to characterize the impact of arousal on neurons in each cortical layer and 2) to determine the underlying mechanism in awake animals. We recorded whole-cell from the same cortical neurons under both anesthesia and subsequent wakefulness. Neurons were identified morphologically and localized to specific layers of rat barrel cortex. Wakefulness transformed the pattern of background synaptic inputs in every cell examined. Surprisingly, this transformation was not mediated by long-range afferent synapses or cholinergic modulation but rather by direct noradrenergic modulation of local cortical circuits. We conclude that arousal-related brain states force cortical networks into different processing regimes via the locus coeruleus-noradrenergic system.
RESULTS
Wakefulness alters cortical dynamics
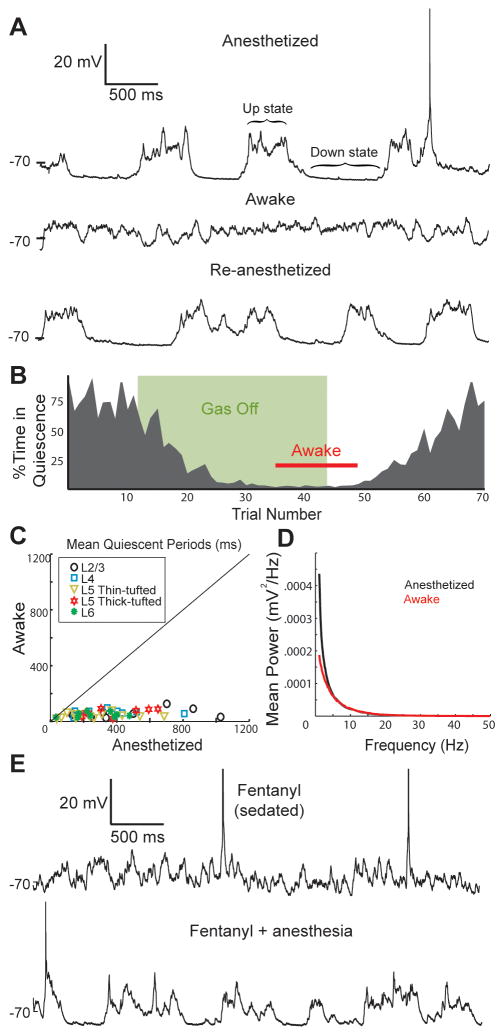
In head-fixed rats, we recorded whole-cell from 105 neurons in layers 2–6 (L2–6) of rat barrel cortex. Slow-wave fluctuations were prominent in a representative L2/3 pyramidal neuron during administration of gaseous isoflurane anesthesia (Figure 1A, upper). In the same cell, prolonged periods of synaptic quiescence disappeared during wakefulness, which was defined by overt jaw/face/whisker/paw movements and desynchronized EEG following termination of gas flow (middle; Movie S1). Pronounced slow-wave fluctuations were restored when the animal was re-anesthetized (lower), confirming that the effect of wakefulness on Vm was not artifact due to rupturing of the cell membrane by animal movement. To quantify Vm changes, we algorithmically detected periods of synaptic quiescence (Figure S1A). Sustained synaptic quiescence decreased after the anesthetic was switched off (Figure 1B). This coordinated synaptic inactivity virtually disappeared before the animal awoke and remained absent until the anesthetic resumed.
Figure 1. Wakefulness abolishes periods of synaptic quiescence in all cortical layers.
(A) In vivo whole-cell recording of a L2/3 pyramidal neuron while a rat was anesthetized (top), awake (middle) and re-anesthetized (bottom).
(B) Percentage of each 5-second trial that the cell spent during periods of synaptic quiescence.
(C) Scatterplot of the mean lengths of quiescent periods during wakefulness and anesthesia.
(D) Average Vm power spectra for all neurons during anesthesia and wakefulness.
(E) A L4 neuron under fentanyl sedation and subsequent administration of anesthetic.
We analyzed 52 anatomically identified cortical neurons (9–13 in each layer; 3 smooth inhibitory and 49 spiny excitatory cells). Recordings were maintained during anesthetized, awake and re-anesthetized phases. In every cell examined, wakefulness dramatically reduced mean quiescent periods (Figure 1C). Our algorithm is generous, classifying some epochs with minimal synaptic input as periods of quiescence (Figure S1B). Including such false positives, nominal periods of quiescence accounted for only 1.1 ± 0.5% of the awake period (mean ± SD). Thus, wakefulness lacks periods during which the entire cortical network is inactive. Increased tonic input could conceivably mask slow alternation between periods of high and low synaptic activity. To test this, we calculated Vm power spectra. Power at low frequencies was reduced across layers (Figure 1D) and within layers (Figure S1C) when animals awoke, pointing to a true reduction in slow waves. These results indicate that arousal alters the temporal patterns of synaptic inputs.
An alternative preparation, used for both rodents and primates, is to combine opioid sedation and local anesthesia to avoid confounds of general anesthetics on neural activity (Bruno and Sakmann, 2006; Disney et al., 2007). Vm in a fentanyl-sedated rat resembled awake Vm and lacked quiescent states (Figure 1E, upper). Subsequent administration of general anesthetic introduced long stretches of synaptic quiescence in the same cell (lower; n = 3). Sedation produced quiescent periods and power spectra that closely approximated that of awake animals (Figures S1D,E). Our sedated data and anesthetized/awake data together demonstrate that cortical networks possess different dynamics during wakefulness and anesthesia/sleep.
The awake state is a persistent up-like state
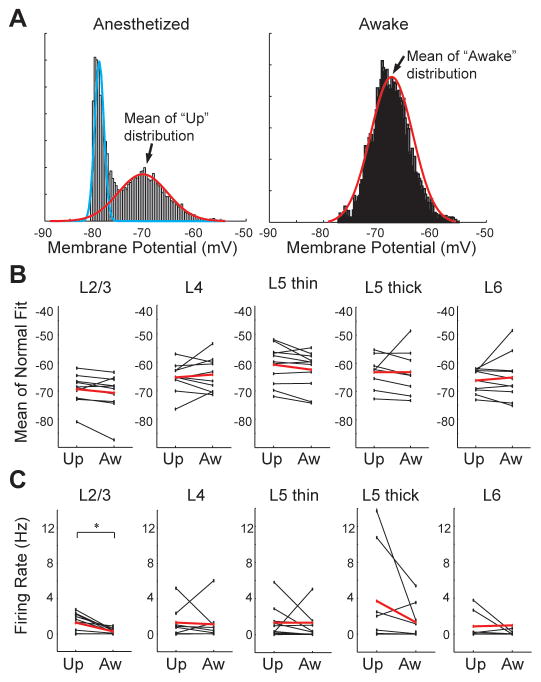
Prolonged periods of synaptic quiescence are commonly referred to as “down states”, and the highest levels of sustained depolarization “up states” (Figure 1A)(Steriade et al., 1993c). Does awake Vm resemble either of these two qualitative states occurring during sleep and anesthesia? Vm during anesthesia was bimodally distributed, reflecting up-down fluctuations, and unimodal during wakefulness (Figure 2A). To characterize the different states of each neuron, anesthetized and awake distributions were fit with a mixture of normals or a single normal (blue and red lines). On average, individual cells’ up and awake states did not differ (Figure 2B; paired t-test, p = 0.79). Similarly, no significant differences were observed within layers or when pooling thalamorecipient layers 4 and 6. Variances were also similar though some differences were detected in L5 (Figure S2A).
Figure 2. The awake state is a persistent up-like state.
(A) Vm histograms of a L5 pyramidal neuron during anesthesia and wakefulness, fit by a mixture of normals and a single normal, respectively.
(B) Summary plots comparing the mean of the up and awake distributions for 52 cells (black, individual cells; red, means).
(C) Summary plots comparing the firing rates during up states (under anesthesia) and awake periods.
To compare neuronal output during up and awake states, up states were algorithmically excerpted from anesthetized data (Seamari et al., 2007). Firing rates were comparable between up and awake states in each layer (Figure 2C) although up states had higher firing rates when data were pooled across all layers (+1.1 +/− 0.37 Hz, paired t-test, p = 0.006). This is likely due to up states’ increased driving force, reduced sodium channel inactivation, and reduced synaptic depression that results from following prolonged periods of synaptic quiescence. Cells exhibited lower overall firing rates during the (unsorted) anesthetized recording phase than during wakefulness (Figure S2B) due to the skewing effect of down states. Lower firing rates may also derive from anesthesia decreasing input resistance relative to wakefulness (Figure S2C), possibly reflecting direct effects of anesthetics on leak channels.
Wakefulness does not necessarily represent a single brain state (Poulet and Petersen, 2008). To investigate more subtle differences among awake brain states, video was used to sort awake data into periods in which the rat was awake but stationary (“quiet wakefulness”), overtly moving its face/jaw/paws (“active”), or rapidly moving its whiskers (“whisking”, a subset of the active category). Vm appeared qualitatively similar during quiet wakefulness and whisking/active (Figure S2D; Movie S1). Average Vm power spectra of these three categories were nearly indistinguishable but exhibited less low-frequency power than under anesthesia (Figure S2E). Quiet wakefulness contained more low-frequency power in EEG than both the active and whisking states (Figure S2F). Nevertheless, we did not observe significant differences in duration of synaptic quiescence or percentage of time spent in quiescent periods between any of the awake groups (p’s > 0.05), suggesting that protracted synaptic quiescence is principally a feature of anesthesia and natural sleep.
Together, the similarity of up and awake states in terms of subthreshold and suprathreshold behavior supports the idea that wakefulness is a persistent up-like state.
Afferent thalamic input is not required for awake Vm
By what mechanism does arousal so dramatically alter the temporal structure of synaptic inputs? Experiments to assess mechanism focused on L4 for two reasons. First, L4 is the principal target of primary sensory nuclei in thalamus, an obvious candidate mechanism. Arousal alters thalamic firing patterns (Steriade et al., 1993b), and pharmacological activation of thalamus in anesthetized animals can generate persistent depolarization of cortex (Hirata and Castro-Alamancos, 2010). Second, a L4 barrel is a relatively self-contained network whereas other layers receive substantial synaptic input from neighboring columns and high-order cortical and thalamic areas. Barrel neurons receive synapses almost entirely from the ventroposterior medial (VPM) nucleus of thalamus, L4 neurons within the same barrel, and L6 neurons (Lubke and Feldmeyer, 2007).
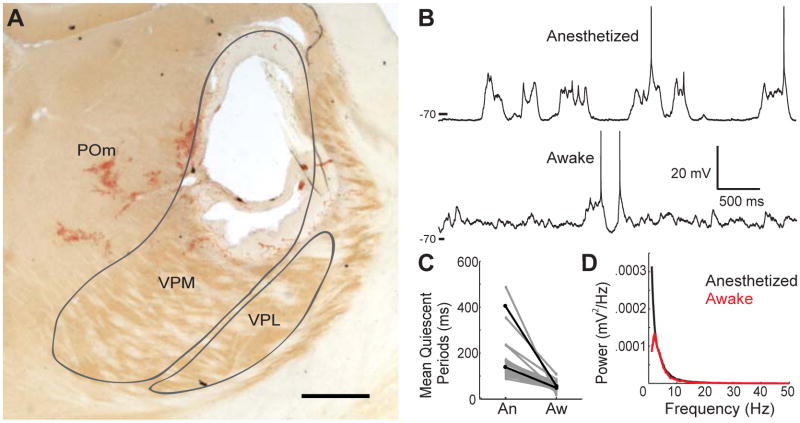
To test whether afferent thalamic input is required to achieve awake patterns of synaptic inputs, L4 barrel neurons were recorded following electrolytic lesions, centered on the somatotopically aligned thalamic “barreloid” and large enough (~1 mm) to destroy the entire VPM representation of the large whiskers (Figure 3A, S3). Lesions additionally severed connections from 1) the secondary somatosensory thalamic area, the posterior medial (POm) nucleus, whose axons traverse VPM to reach barrel cortex (Wimmer et al., 2010), and 2) the central lateral nucleus, an intralaminar nucleus whose fibers course immediately dorsal of VPM and innervate diverse cortical areas (Van der Werf et al., 2002). Consistent with previous studies (Steriade et al., 1993c; Timofeev et al., 2000), slow-wave patterns of synaptic inputs under anesthesia were independent of thalamus (Figure 3B, upper). We discovered, however, that the disappearance of protracted periods of quiescence during wakefulness is also independent of thalamus (lower). Neurons were patched after thalamic lesions in 2 experiments (Figure 3C, black) or lesions combined with pharmacology in cortex (see below) in 20 additional experiments (grey). In all 22 cases, quiescent periods disappeared during wakefulness (Figures 3C,D). Wakefulness still abolished quiescent states in L4 neurons following L6 lesions directly below the barrel, which disrupt both L6 and VPM input (n = 2; data not shown). Thus, afferent thalamic input is not the mechanism that produces the awake state of cortical neurons.
Figure 3. Thalamus is not required for producing an awake Vm.
(A) Coronal section showing an electrolytic VPM lesion, centered on the C2 barreloid. Scale bar, 500 μm.
(B) A L4 star pyramid in the C2 barrel following the lesion shown in A.
(C) Mean length of quiescent periods in L4 neurons following lesions.
(D) Average Vm power spectra of lesion-only data in C.
Acetylcholine is not required for awake Vm but modifies sensory responses
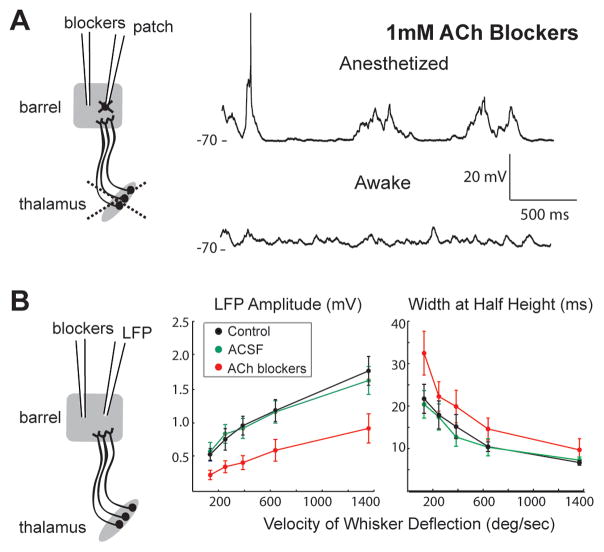
We initially suspected that release of the neuromodulator acetylcholine (ACh) in the cortex was responsible for the switch in cortical dynamics. Electrical stimulation of cholinergic nuclei in anesthetized animals is well known to simulate awake-like EEG, local field potential, and cortical Vm, effects which are blocked by antagonists of muscarinic ACh receptors (Goard and Dan, 2009; Metherate et al., 1992; Steriade et al., 1993a). If the awake state indeed depended on ACh, the inverse experiment should be possible: blocking muscarinic receptors should induce synaptic quiescence during wakefulness. Systemic injections of the muscarinic antagonist scopolamine, applied in even higher doses than in previous studies (5 mg/kg IV or IP), failed to induce quiescent states in L4 neurons of awake rats (Figure S4A). To ensure that antagonists reached their targets, we recorded from L4 neurons while locally perfusing 1 mM muscarinic (atropine) and nicotinic (mecamylamine) antagonists from a pipette whose tip was positioned 50–75 μm from the patch pipette tip. In each case, ACh blockers did not affect awake Vm (Figure S4B,C).
Although we found that arousal-induced changes were independent of thalamic afferents, thalamocortical input and ACh could conceivably interact to alter cortical dynamics. We therefore combined local perfusion of blockers with somatotopically aligned thalamic lesions (Figure 4A). In this preparation, a L4 barrel becomes a relatively isolated cortical network (see Discussion). In every recording following thalamic lesion (n = 8), perfusing 100 μM-1 mM atropine and mecamylamine failed to prevent awake patterns of synaptic inputs (Figure 4A; 5E, black). Inclusion of an α7 nicotinic antagonist (methyllycaconitine) similarly had no effect on awake Vm (n = 3; data not shown). Thus, 18 out of 18 cholinergic blocker experiments yielded negative results, in which wakefulness continued to abolish synaptic quiescence.
Figure 4. ACh is not required for awake Vm but modifies sensory responses.
(A) Left, schematic of experiment combining thalamic lesion with local perfusion of 1 mM blockers of muscarinic and nicotinic ACh receptors. Right, ACh blockers do not prevent transition to awake Vm patterns in example L4 star pyramid.
(B) Local perfusion of blockers during LFP recording with thalamus intact (left). ACh blockers but not aCSF vehicle suppress amplitudes (middle) and extend durations (width at half amplitude) of whisker-evoked LFPs across velocities (right).
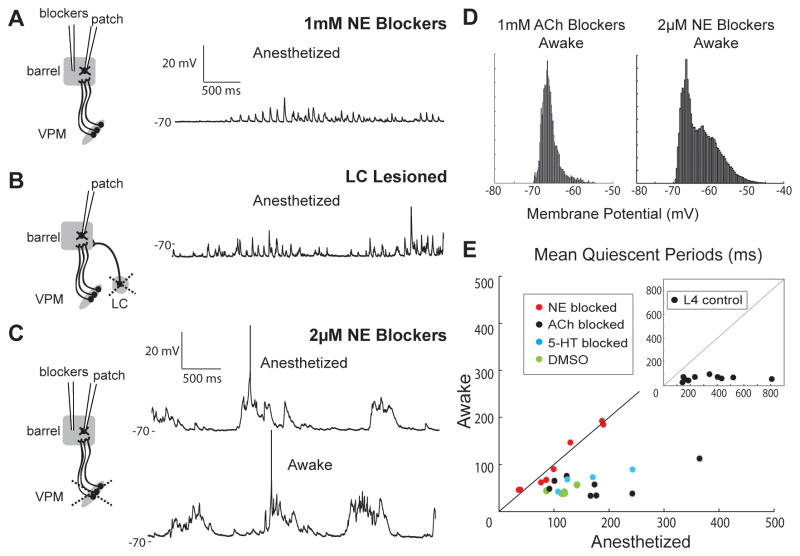
Figure 5. Blocking NE prevents awake Vm and induces synaptic quiescence.
(A) L4 star pyramid in the presence of 1 mM NE blockers (thalamus intact).
(B) L4 star pyramid after lesion of ipsilateral locus coeruleus (thalamus intact, no blockers).
(C) L4 spiny stellate during local perfusion of 2 μM NE blockers after thalamic lesion.
(D) Vm histograms of the cells shown in Figures 4A (left) and 5C (right) during wakefulness.
(E) Scatter plot of the mean quiescent periods of L4 neurons during wakefulness and anesthesia in the presence of NE, ACh or 5-HT1A/B blockers or DMSO vehicle following thalamic lesion. Inset, L4 control data (from Figure 1C).
Effective delivery of blockers was verified by a positive control. ACh enhances contrast sensitivity of L4 neurons in macaque visual cortex via nicotinic receptors on thalamocortical terminals (Disney et al., 2007), which similarly exist in rodent somatosensory cortex (Gil et al., 1997). Cholinergic blockade should therefore shift the sensitivity of L4 neurons to the velocity of whisker movements, the tactile analog of visual contrast. The time course of L4 integration should also differ due to muscarinic receptors on corticocortical terminals (Eggermann and Feldmeyer, 2009; Kruglikov and Rudy, 2008). Therefore, instead of recording whole-cell, we acquired local field potential (LFP) in the barrel and kept thalamus intact so that sensory responses could be evoked (Figure 4B, left). LFP samples neurons over a 300–400 μm wide region (Katzner et al., 2009), so our positive control assesses the synaptic input to the vast majority of neurons in a barrel (~200–300 μm wide).
Amplitudes of sensory-evoked LFPs were proportional to velocity. In individual experiments (Figure S4D,E) as in the average (Figure 4B, middle, black; n = 5), cholinergic blockers consistently decreased LFP responses across velocities (red) with no effect of artificial cerebrospinal fluid (aCSF; green). LFP time course was also impacted by blockers but not vehicle (Figure 4B, right). Blockers ejected 250 μm from the LFP pipette similarly reduced responses (Figure S4F), indicating that drugs impacted an area of at least an entire barrel. We conclude that cholinergic receptors in rat barrel cortex modulate sensory responses and are antagonized by our local perfusion method.
Together, these results show that ACh is not necessary to produce awake patterns of Vm in cortical neurons.
Norepinephrine is critical for awake Vm
The locus coeruleus (LC)-norepinephrine (NE) system is also a plausible mechanism of the switch in cortical dynamics. Pharmacologically stimulating LC desynchronizes EEG (Berridge et al., 1993), and the firing rates of noradrenergic LC neurons change with arousal (Aston-Jones and Bloom, 1981). To examine a possible role of NE, we initially locally perfused 1 mM antagonists of α1 (prazosin), α2 (yohimbine), and β (propranolol) noradrenergic receptors while recording from L4 neurons with thalamus intact. This high concentration prevented cells from achieving/maintaining prolonged depolarization under both anesthesia and wakefulness (Figure 5A, S5A). Ipsilateral LC lesion also prevented sustained depolarization (Figure 5B), indicating that our pharmacology results were due to NE receptor blockade rather than non-specific drug effects. Thus, some minimal amount of NE appears required for prolonged depolarizations normally observed during sleep/anesthesia, consistent with tonic LC firing (Aston-Jones and Bloom, 1981) that likely maintains non-zero levels of NE in cortex under these conditions.
We predicted that clear slow-wave fluctuations should emerge in awake animals for low levels of NE. To test this, we locally perfused lower concentrations of antagonists in L4 barrels, again after thalamic lesion to ensure that measurements reflected synaptic input from the local network and not thalamic afferents (Figure 5C, left). A wide range of concentrations of NE blockers (1–100μM; Figure S5B) were sufficient to induce periodic synaptic quiescence in awake animals (Figure 5C). In stark contrast to ACh antagonists, NE blockers induced clear bimodality of cortical Vm during wakefulness (Figure 5D). Under NE blockade, wakefulness and anesthesia had comparably long quiescent states (Figure 5E, red; n = 7, p = 0.69; Figure S5B, right) whereas perfusion of DMSO vehicle resembled control (green; n = 5).
We ruled out possible cross-reactivity of propranolol with serotonin (5-HT) 1A/1B receptors by patching L4 neurons in the presence of 1 mM blockers for 5-HT1A (WAY-100635) and 5-HT1B (GR 127935) receptors (n = 4). Serotonergic blockers did not prevent the disappearance of slow waves upon waking (Figures 5E, blue; S5C), validating a role for norepinephrine in switching cortical dynamics.
DISCUSSION
We conclude that arousal dramatically transforms the temporal pattern of spontaneous synaptic inputs in cortical networks. Local recurrent networks appear able to generate a relatively constant level of background synaptic input. Our study demonstrates that wakeful patterns of synaptic input can occur independent of primary and secondary sensory thalamic nuclei, contrary to the idea that global brain states influence local cortical networks via thalamic afferents (Hirata and Castro-Alamancos, 2010; Steriade et al., 1993b). Cholinergic modulation was also unnecessary to achieve awake cortical dynamics. ACh levels vary with arousal (Jones, 2004), but we found that ACh more noticeably impacts sensory-evoked responses, a capacity which may subserve attentional focusing on selected stimuli. In contrast, the powerful influence of arousal on cortical dynamics required norepinephrine.
Electrical stimulation of non-specific intralaminar thalamic nuclei, which diffusely project across cortex, initially implicated them in arousal (Hunter and Jasper, 1949). Lesions of intralaminar nuclei do not, however, alter EEG patterns (Buzsaki et al., 1988; Vanderwolf and Stewart, 1988). Indeed, we found that wakefulness still profoundly affected cortical dynamics after our thalamic lesions, which severed connections between cortex and the central lateral intralaminar nucleus, the most investigated for a role in arousal. Our results do not rule out possible contributions of the central medial nucleus, parafascicular complex, or rhomboid nucleus. These, however, seem unlikely given that sparse axons from these intralaminar nuclei avoid L4 (Van der Werf et al., 2002), where we investigated mechanism. Moreover, these projections would have to act through L2/3-L4 and L5/6-L4 synapses, which are also anatomically sparse and, in those rare instances when observed, substantially (~2–6 fold) weaker than L4-L4 synapses (Gottlieb and Keller, 1997; Lefort et al., 2009; Schubert et al., 2003; Sehara et al., 2010). A more likely explanation for the switch in cortical dynamics, therefore, is that NE directly modulates synapses among L4 neurons.
Natural versus stimulated cholinergic release
Electrical stimulation of cholinergic nuclei is sufficient to produce awake-like cortical activity in anesthetized animals (Goard and Dan, 2009; Metherate et al., 1992; Steriade et al., 1993a). We found however that cholinergic modulation is unnecessary to achieve wakeful cortical dynamics. Our experiments do not rule out possible behavioral contexts in which natural ACh release could alter dynamics. For example, attention is widely suspected to involve cholinergic modulation, and highly attentive states might also produce a constant bombardment of synaptic input.
Nonetheless, stimulation techniques have two important limitations. First, the various neuromodulatory nuclei are densely and reciprocally interconnected (Briand et al., 2007). Electrical or optogenetic stimulation of one center likely activates multiple neuromodulatory nuclei, including LC. Thus, stimulation of cholinergic nuclei likely has effects on levels of norepinephrine, serotonin, and other neuromodulators.
Second, stimulation might induce aphysiological release, both in terms of timing and quantity. Neuromodulatory centers switch between tonic and phasic firing modes according to behavioral state, eliciting different temporal dynamics of release. The time course of release is probably especially important for neuromodulators like ACh that target both ionotropic and metabotropic receptors. Ionotropic nicotinic receptors exhibit a brief window of activation often followed by desensitization (Dani et al., 2000) whereas metabotropic muscarinic effects begin tens of milliseconds after ligand exposure and persist for hundreds of milliseconds (McCormick and Prince, 1987). Moreover, the activation rate of a receptor depends on its affinity and ligand concentration. Therefore, stimulated bulk release may abnormally engage receptor subtypes in terms of both temporal recruitment and degree of activation.
While difficult, local perfusion of blockers in awake animals has two key advantages. First, neuromodulatory systems other than the one targeted are not affected. Second, neuromodulator release is naturally set by global brain state.
Norepinephrine as a “sliding scale” for cortical dynamics
We have shown that norepinephrine exerts powerful effects on cortical dynamics. Consistent with this, optogenetic LC stimulation wakes sleeping animals and is sufficient to extend the duration of wakefulness (Carter et al., 2010). Bilateral ablation of ~70% of the LC can induce coma and anomalous EEG slow waves during the first 24 hours (Jones et al., 1977). Interestingly, normal behavior and EEG return during subsequent days (Blanco-Centurion et al., 2004; Jones et al., 1977). Thus, long-term compensatory changes are possible given LC dysfunction—either an increase in norepinephrine release from surviving LC neurons and/or changes in the levels of other neuromodulators.
We hypothesize that in the absence of NE local cortical networks are functionally uncoupled and fail to achieve/sustain depolarizations. Basal levels of NE during SWS and anesthesia may partially couple cortical neurons to produce up states. During wakefulness, when the firing rates of LC neurons increase (Aston-Jones and Bloom, 1981), even higher concentrations of NE may alter release at recurrent corticocortical synapses and/or intrinsic membrane properties of cortical neurons to produce awake Vm. Awake Vm likely requires more than simply a recurrent network and NE. Other endogenous neuromodulators, specific patterns of connections among and between excitatory and inhibitory neurons, certain ranges of release probabilities, and so on are probably additionally required. Our results nevertheless raise the possibility that the LC-NE system may act as a “sliding scale” by which arousal controls the dynamics of cortical networks.
The various noradrenergic receptors exhibit different affinities for NE. Low levels of NE during SWS and anesthesia may preferentially recruit the high-affinity α2 receptors, which are coupled to Gi proteins that suppress synaptic release and Vm (Ramos and Arnsten, 2007). High levels of NE during wakefulness may recruit low-affinity α1 and β receptors, which are generally coupled to Gq and Gs proteins whose effects oppose Gi. β receptors are also expressed by astrocytes, which modulate slow waves during sleep and anesthesia (Halassa et al., 2009). Determining the sites of receptor expression will be critical for understanding how NE modulates circuit dynamics.
The nature of awake synaptic input patterns
Our recordings from barrel cortex clearly demonstrate that any given cortical neuron experiences dramatically different patterns of synaptic input during wakefulness and anesthesia. Wakefulness, however, includes many distinct brain states. For example, other studies in barrel cortex have reported that Vm of L2/3 neurons can, but does not always, exhibit slow (1–5 Hz) fluctuations during “quiet wakefulness” (Petersen et al., 2003; Poulet and Petersen, 2008). Similar fluctuations have also been inferred from extracellular recording in the auditory cortex of awake rats (Sakata and Harris, 2009). While such fluctuations are faster and not identical to those under anesthesia (Haider and McCormick, 2009), they are substantially diminished by active whisking (Poulet and Petersen, 2008). In our recordings, we did not see major differences in Vm between quiet wakefulness and active/whisking periods. Previous recordings from a very different type of cortical region (multimodal) in a different species (cat) mirror our results in rat barrel cortex, in which neurons are continually bombarded with synaptic input during wakefulness (Steriade et al., 2001).
Our study and those conducted in cats employed animals unhabituated to the experimental setup. In contrast, reports of slow fluctuations during wakefulness utilized habituated animals trained to sit still. Unhabituated animals are likely in a heightened state of arousal and/or attention during wakefulness. Indeed, the LC-NE system was recently demonstrated to sustain wakefulness and aroused EEG patterns in rats exposed to novel stimuli or environments (Gompf et al., 2010). Therefore, habituation likely leads to lower levels of cortical NE during wakefulness.
State and neuronal response properties
Cortical activity has been and continues to be widely studied in anesthetized animals, in which prominent subthreshold slow waves dramatically impact synaptic inputs. Ideally all studies could be conducted in awake animals, but the need for careful stimulus control or sensitive physiological recording often precludes this. Our data suggests that sedation and local anesthesia could simultaneously satisfy such requirements and avoid confounds of general anesthesia. We have shown that anesthetized and awake studies clearly sample cortical networks in different regimes in which not only long-range synaptic inputs differ. Neuromodulation of the local circuit alone produces different Vm profiles which via driving force, sodium channel inactivation, and short-term synaptic plasticity will impact reliability, synchrony, and tuning of sensory-evoked suprathreshold responses. The persistent depolarization of neurons induced by wakefulness therefore is a likely cellular explanation of why single-unit and imaging studies observe less sensory-evoked activity during wakefulness than anesthesia (Ferezou et al., 2007; Simons et al., 1992).
Synaptic quiescence during sleep
Is periodic synaptic quiescence during sleep an epiphenomenon of cortical circuitry? Transcranial stimulation to induce slow waves during non-REM sleep enhances declarative memory of previously learned word lists in humans, suggesting slow-wave activity facilitates memory consolidation (Marshall et al., 2006). Slow-wave activity has also been shown to promote ocular dominance plasticity in cats (Frank et al., 2001). These studies suggest that slow waves during sleep instead serve a biological purpose.
Periodic synaptic quiescence brought about by natural sleep may promote plasticity. One hypothesis is that sleep homeostatically downscales synapses potentiated during wakefulness, perhaps via long-term depression triggered by alternating periods of synaptic quiescence and spiking (Tononi and Cirelli, 2006). We further propose another mechanism, not mutually exclusive, in which quiescence promotes potentiation. Quiescent periods will enhance the efficacy of synaptic inputs driven by replay during sleep and consequently the number and timing of action potentials evoked by those inputs. This feature could facilitate spike-timing dependent plasticity and thereby memory consolidation.
CONCLUSION
We have demonstrated that a single neuromodulator can alter the dynamics of local cortical networks according to global brain state. Selective dynamics may be a ubiquitous means by which behavioral state optimizes circuits for specific tasks.
EXPERIMENTAL PROCEDURES
Anesthetized-Awake Preparation
77 female Wistar rats (94–245 g, mean 178 g) were anesthetized with isoflurane (1–3% in O2). Body temperature was kept at 37°C by a heating blanket. Eyes were coated with lubricating ointment to prevent drying. One or two metal posts for stabilizing the head were attached to skull by dental acrylic. Screws were inserted in the right frontal and parietal bones for electrocorticogram (“EEG”) recording. Small (<0.5 mm2) craniotomies were made over left barrel cortex, and the dura removed. Animals were wrapped in a blanket and secured in a plastic tube to reduce movement. The local anesthetic bupivacaine was regularly applied to the area of the head surrounding the acrylic. To avoid startling the rat, a black curtain was placed around the air table, and noise in the lab minimized. Movements were recorded by an infrared camera.
Sedation
Sedated rats were further prepared as described previously (Bruno and Sakmann, 2006). Briefly, cannulae were inserted into the trachea (for mechanical ventilation), femoral artery (for blood pressure monitoring) and jugular vein (for drug infusion). All wounds were infiltrated with bupivacaine. Fentanyl (~10 μg/kg/hr) and pancuronium bromide (1.6 mg/kg/hr) were continuously infused after discontinuation of general anesthesia, and rats were ventilated (~90 breaths/min). Mean arterial blood pressure was typically ~120 mm Hg. No restraints were used.
Electrophysiology
Patch pipettes (4–7 MΩ) were pulled from borosilicate glass and tip-filled with (in mM) 135 K-gluconate, 10 HEPES, 10 phosphocreatin-Na2, 4 KCl, 4 ATP-Mg, 0.3 GTP, and 0.2–0.4% biocytin (pH 7.2, osmolarity 291). Pipette capacitance was neutralized prior to break-in, and access resistance was 1–60 MΩ. Recordings were digitized at 32 kHz. LFP pipettes (2–3 MΩ) were filled with aCSF (in mM: 135 NaCl, 5.4 KCl, 1.8 CaCl2, 1.0 MgCl2, and 50 HEPES; pH 7.2). LFPs were bandpassed 1–325 Hz.
Pharmacology
Drugs were purchased from Sigma except for atropine (Henry Schein), dissolved in ddH2O, diluted in aCSF, and filtered. NE blockers were initially dissolved in 0.01% DMSO in aCSF and sonicated. Drugs were ejected from a pipette (3–5 μm I.D.) by applying 100 mbar between recordings and 30 mbar during.
Lesions
VPM was accessed by a ~2 × 2 mm craniotomy and mapping using a metal electrode. Lesions were made by passing 100–400 μA for 1–5 min through the electrode. For L6 lesions, a ~1 × 1 mm craniotomy was made ~1.2 mm medial of a cortical craniotomy, and 40 μA passed for 20 sec.
Sensory Stimulation
Individual whiskers were deflected by multi-directional piezoelectric stimulators (Bruno and Sakmann, 2006). Directional tuning was determined by ramp-and-hold movements (1-mm amplitude at ~10 mm from follicle, ~5.7°; peak velocity 1360°/sec) in each of eight directions. The angle evoking the largest LFP was deemed the preferred direction. A hundred blocks of deflections with randomized onset velocities were applied in this direction (500 total stimuli) with 4-sec interstimulus intervals to avoid short-term plasticity.
Histology
Rats were deeply anesthetized with sodium pentobarbital and perfused transcardially with phosphate buffer followed by 4% paraformaldehyde. Cortex was cut tangentially in 100-μm sections and stained for cytochrome oxidase (CO) and biocytin. Cells morphology and layer were classified as previously described (de Kock et al., 2007). Thalamus was cut in 100-μm coronal sections and stained for CO.
Analysis
Data were analyzed using custom Matlab routines. Power analysis was performed with Chronux. DC was subtracted from Vm prior to power analysis.
Supplementary Material
Acknowledgments
We thank Anita Disney, Attila Losonczy, Charles Zuker, Nate Sawtell, Elaine Zhang, and Alejandro Ramirez for comments on the manuscript and Drew Baughman for histology. This work was supported by NIH R01 NS069679-01 and Rita Allen Foundation grants (RMB) and an NSF Student Fellowship (CMC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Page ME, Valentino RJ, Foote SL. Effects of locus coeruleus inactivation on electroencephalographic activity in neocortex and hippocampus. Neuroscience. 1993;55:381–393. doi: 10.1016/0306-4522(93)90507-c. [DOI] [PubMed] [Google Scholar]
- Blake H, Gerard RW. Brain potential during sleep. American Journal of Physiology. 1937;119:692–702. [Google Scholar]
- Blanco-Centurion C, Gerashchenko D, Salin-Pascual RJ, Shiromani PJ. Effects of hypocretin2-saporin and antidopamine-beta-hydroxylase-saporin neurotoxic lesions of the dorsolateral pons on sleep and muscle tone. Eur J Neurosci. 2004;19:2741–2752. doi: 10.1111/j.1460-9568.2004.03366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand LA, Gritton H, Howe WM, Young DA, Sarter M. Modulators in concert for cognition: modulator interactions in the prefrontal cortex. Prog Neurobiol. 2007;83:69–91. doi: 10.1016/j.pneurobio.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno RM, Sakmann B. Cortex is driven by weak but synchronously active thalamocortical synapses. Science. 2006;312:1622–1627. doi: 10.1126/science.1124593. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Bickford RG, Ponomareff G, Thal LJ, Mandel R, Gage FH. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci. 1988;8:4007–4026. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, Deisseroth K, de Lecea L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–1533. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, Radcliffe KA, Pidoplichko VI. Variations in desensitization of nicotinic acetylcholine receptors from hippocampus and midbrain dopamine areas. Eur J Pharmacol. 2000;393:31–38. doi: 10.1016/s0014-2999(00)00003-0. [DOI] [PubMed] [Google Scholar]
- de Kock CP, Bruno RM, Spors H, Sakmann B. Layer- and cell-type-specific suprathreshold stimulus representation in rat primary somatosensory cortex. J Physiol. 2007;581:139–154. doi: 10.1113/jphysiol.2006.124321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disney AA, Aoki C, Hawken MJ. Gain modulation by nicotine in macaque v1. Neuron. 2007;56:701–713. doi: 10.1016/j.neuron.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermann E, Feldmeyer D. Cholinergic filtering in the recurrent excitatory microcircuit of cortical layer 4. Proc Natl Acad Sci U S A. 2009;106:11753–11758. doi: 10.1073/pnas.0810062106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferezou I, Haiss F, Gentet LJ, Aronoff R, Weber B, Petersen CC. Spatiotemporal dynamics of cortical sensorimotor integration in behaving mice. Neuron. 2007;56:907–923. doi: 10.1016/j.neuron.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Frank MG, Issa NP, Stryker MP. Sleep enhances plasticity in the developing visual cortex. Neuron. 2001;30:275–287. doi: 10.1016/s0896-6273(01)00279-3. [DOI] [PubMed] [Google Scholar]
- Gil Z, Connors BW, Amitai Y. Differential regulation of neocortical synapses by neuromodulators and activity. Neuron. 1997;19:679–686. doi: 10.1016/s0896-6273(00)80380-3. [DOI] [PubMed] [Google Scholar]
- Goard M, Dan Y. Basal forebrain activation enhances cortical coding of natural scenes. Nat Neurosci. 2009;12:1444–1449. doi: 10.1038/nn.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompf HS, Mathai C, Fuller PM, Wood DA, Pedersen NP, Saper CB, Lu J. Locus ceruleus and anterior cingulate cortex sustain wakefulness in a novel environment. J Neurosci. 2010;30:14543–14551. doi: 10.1523/JNEUROSCI.3037-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb JP, Keller A. Intrinsic circuitry and physiological properties of pyramidal neurons in rat barrel cortex. Exp Brain Res. 1997;115:47–60. doi: 10.1007/pl00005684. [DOI] [PubMed] [Google Scholar]
- Haider B, McCormick DA. Rapid neocortical dynamics: cellular and network mechanisms. Neuron. 2009;62:171–189. doi: 10.1016/j.neuron.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata A, Castro-Alamancos MA. Neocortex network activation and deactivation states controlled by the thalamus. J Neurophysiol. 2010;103:1147–1157. doi: 10.1152/jn.00955.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter J, Jasper HH. Effects of thalamic stimulation in unanaesthetised animals. Electroencephalogr Clin Neurophysiol. 1949;1:305–324. doi: 10.1016/0013-4694(49)90043-7. [DOI] [PubMed] [Google Scholar]
- Jones BE. Activity, modulation and role of basal forebrain cholinergic neurons innervating the cerebral cortex. Prog Brain Res. 2004;145:157–169. doi: 10.1016/S0079-6123(03)45011-5. [DOI] [PubMed] [Google Scholar]
- Jones BE, Harper ST, Halaris AE. Effects of locus coeruleus lesions upon cerebral monoamine content, sleep-wakefulness states and the response to amphetamine in the cat. Brain Res. 1977;124:473–496. doi: 10.1016/0006-8993(77)90948-9. [DOI] [PubMed] [Google Scholar]
- Katzner S, Nauhaus I, Benucci A, Bonin V, Ringach DL, Carandini M. Local origin of field potentials in visual cortex. Neuron. 2009;61:35–41. doi: 10.1016/j.neuron.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruglikov I, Rudy B. Perisomatic GABA release and thalamocortical integration onto neocortical excitatory cells are regulated by neuromodulators. Neuron. 2008;58:911–924. doi: 10.1016/j.neuron.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefort S, Tomm C, Floyd Sarria JC, Petersen CC. The excitatory neuronal network of the C2 barrel column in mouse primary somatosensory cortex. Neuron. 2009;61:301–316. doi: 10.1016/j.neuron.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Lubke J, Feldmeyer D. Excitatory signal flow and connectivity in a cortical column: focus on barrel cortex. Brain Struct Funct. 2007;212:3–17. doi: 10.1007/s00429-007-0144-2. [DOI] [PubMed] [Google Scholar]
- Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Prince DA. Actions of acetylcholine in the guinea-pig and cat medial and lateral geniculate nuclei, in vitro. J Physiol. 1987;392:147–165. doi: 10.1113/jphysiol.1987.sp016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metherate R, Cox CL, Ashe JH. Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J Neurosci. 1992;12:4701–4711. doi: 10.1523/JNEUROSCI.12-12-04701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CC, Hahn TT, Mehta M, Grinvald A, Sakmann B. Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc Natl Acad Sci U S A. 2003;100:13638–13643. doi: 10.1073/pnas.2235811100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulet JF, Petersen CC. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature. 2008;454:881–885. doi: 10.1038/nature07150. [DOI] [PubMed] [Google Scholar]
- Ramos BP, Arnsten AF. Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol Ther. 2007;113:523–536. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigas P, Castro-Alamancos MA. Impact of persistent cortical activity (up States) on intracortical and thalamocortical synaptic inputs. J Neurophysiol. 2009;102:119–131. doi: 10.1152/jn.00126.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev RN, Ebner FF, Wilson CJ. Effect of subthreshold up and down states on the whisker-evoked response in somatosensory cortex. J Neurophysiol. 2004;92:3511–3521. doi: 10.1152/jn.00347.2004. [DOI] [PubMed] [Google Scholar]
- Sakata S, Harris KD. Laminar structure of spontaneous and sensory-evoked population activity in auditory cortex. Neuron. 2009;64:404–418. doi: 10.1016/j.neuron.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D, Kotter R, Zilles K, Luhmann HJ, Staiger JF. Cell type-specific circuits of cortical layer IV spiny neurons. J Neurosci. 2003;23:2961–2970. doi: 10.1523/JNEUROSCI.23-07-02961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamari Y, Narvaez JA, Vico FJ, Lobo D, Sanchez-Vives MV. Robust off- and online separation of intracellularly recorded up and down cortical states. PLoS One. 2007;2:e888. doi: 10.1371/journal.pone.0000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehara K, Toda T, Iwai L, Wakimoto M, Tanno K, Matsubayashi Y, Kawasaki H. Whisker-related axonal patterns and plasticity of layer 2/3 neurons in the mouse barrel cortex. J Neurosci. 2010;30:3082–3092. doi: 10.1523/JNEUROSCI.6096-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons DJ, Carvell GE, Hershey AE, Bryant DP. Responses of barrel cortex neurons in awake rats and effects of urethane anesthesia. Exp Brain Res. 1992;91:259–272. doi: 10.1007/BF00231659. [DOI] [PubMed] [Google Scholar]
- Steriade M, Amzica F, Nunez A. Cholinergic and noradrenergic modulation of the slow (approximately 0.3 Hz) oscillation in neocortical cells. J Neurophysiol. 1993a;70:1385–1400. doi: 10.1152/jn.1993.70.4.1385. [DOI] [PubMed] [Google Scholar]
- Steriade M, Dossi RC, Nunez A. Network modulation of a slow intrinsic oscillation of cat thalamocortical neurons implicated in sleep delta waves: cortically induced synchronization and brainstem cholinergic suppression. J Neurosci. 1991;11:3200–3217. doi: 10.1523/JNEUROSCI.11-10-03200.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993b;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993c;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Grenier F, Bazhenov M, Sejnowski TJ, Steriade M. Origin of slow cortical oscillations in deafferented cortical slabs. Cereb Cortex. 2000;10:1185–1199. doi: 10.1093/cercor/10.12.1185. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH, Stewart DJ. Thalamic control of neocortical activation: a critical re-evaluation. Brain Res Bull. 1988;20:529–538. doi: 10.1016/0361-9230(88)90143-8. [DOI] [PubMed] [Google Scholar]
- Watson BO, MacLean JN, Yuste R. UP states protect ongoing cortical activity from thalamic inputs. PLoS One. 2008;3:e3971. doi: 10.1371/journal.pone.0003971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer VC, Bruno RM, de Kock CP, Kuner T, Sakmann B. Dimensions of a projection column and architecture of VPM and POm axons in rat vibrissal cortex. Cereb Cortex. 2010;20:2265–2276. doi: 10.1093/cercor/bhq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.