Abstract
Chikungunya virus (CHIKV), an emerging mosquito-borne Alphavirus, causes debilitating rheumatic disease in humans that can last for weeks to months. Starting in 2004, a CHIKV outbreak in the Indian Ocean region affected millions of people, and infected travelers introduced CHIKV to new regions. The pathogenesis of CHIKV is poorly understood, and no approved vaccines or specific therapies exist. A major challenge to the study of CHIKV disease is the lack of a small animal model that recapitulates the major outcomes of human infection. In this study, the pathogenesis of CHIKV in C57BL/6J mice was investigated using biological and molecular clones of CHIKV isolated from human serum (CHIKV SL15649). After 14-day-old mice were inoculated with CHIKV SL15649 in the footpad, they displayed reduced weight gain and swelling of the inoculated limb. Histologic analysis of hind limb sections revealed severe necrotizing myositis, mixed inflammatory cell arthritis, chronic active tenosynovitis, and multifocal vasculitis. Interestingly, these disease signs and viral RNA persisted in musculoskeletal tissues for at least 3 weeks after inoculation. This work demonstrates the development of a mouse model of CHIKV infection with clinical manifestations and histopathologic findings that are consistent with the disease signs of CHIKV-infected humans, providing a useful tool for studying viral and host factors that drive CHIKV pathogenesis and for evaluating potential therapeutics against this emerging viral disease.
Chikungunya virus (CHIKV), O'nyong-nyong virus, Mayaro virus, and Ross River virus are among a group of mosquito-transmitted alphaviruses that cause debilitating pain and inflammation of musculoskeletal tissue in humans.1–4 These viruses are an emerging threat because of their ability to initiate explosive epidemics,3,5 including a 1979–1980 epidemic of Ross River fever in the South Pacific, which involved more than 60,000 patients,3 and a 1959–1962 epidemic of O'nyong-nyong fever in Africa, which involved at least 2 million patients.6 In 2004, reemergence of CHIKV resulted in an epidemic that caused millions of cases in multiple countries in the Indian Ocean region, including 270,000 cases on Reunion Island and an estimated 1.4 million to 6.5 million cases in India.7,8 The ability of these viruses to spread in a human to mosquito to human transmission cycle in the absence of animal reservoirs is particularly concerning because this increases the likelihood of establishing epidemics in new areas, including Europe and the Western Hemisphere. This possibility was demonstrated by a CHIKV outbreak in Italy in 2007 that resulted in more than 250 identified cases and included the detection of CHIKV in local mosquito populations.9,10
The name chikungunya, from the Kimakonde language of Tanzania, which translates as “that which bends up the joints,” reflects the severe and debilitating rheumatic symptoms that are experienced by most individuals.11 Although a small subset of people experience atypical outcomes, which include potentially life-threatening multiorgan failure and encephalitis, epidemiologic studies established unusually severe joint pain as the distinguishing and most common feature of CHIKV infection.12,13 In addition to joint pain, recent studies have identified severe tenosynovitis and myositis as prominent clinical features of infection.14–17 The severe pain is chronic, typically lasting from weeks to months. Up to 57% of patients report persistent rheumatic symptoms 15 months after initial diagnosis.18
The study of the pathogenesis of CHIKV disease has been hampered by the lack of a small animal model of disease, although several recent studies in mice have made progress. Neonatal mice and mice deficient in the type I interferon receptor were found to be highly susceptible to CHIKV infection.19 Although CHIKV was detected in muscle and joint tissue in these mice, the pathological outcomes in these tissues were not addressed,19 and these models may be more relevant for studying the pathogenesis of atypical CHIKV disease. Ziegler et al20 described severe skeletal muscle necrosis and inflammation after CHIKV infection of outbred strains of mice. These studies were promising; however, whether infected mice developed other musculoskeletal conditions, such as arthritis, was not reported. In addition to studies in mice, CHIKV pathogenesis was recently investigated in cynomolgus macaques.21 Infected macaques developed features consistent with human disease, such as fever, rash, and swelling in wrist and ankle joints. However, musculoskeletal disease signs were only observed in animals inoculated with very high doses of virus (>107 PFU). Therefore, a critical need remains for a model system that will allow detailed molecular and genetic analyses of the major aspects of CHIKV-induced disease.
In this report, we describe the development of a new small animal model in which infection of young C57BL/6J mice with a clinical isolate of CHIKV (CHIKV SL15649) led to gross swelling of the inoculated foot, induction of mixed inflammatory cell arthritis in the feet and tarsi, and severe tenosynovitis and myositis. Flow cytometric analyses of the musculoskeletal infiltrates identified natural killer cells, neutrophils, monocytes/macrophages, and lymphocytes as major cell infiltrates in musculoskeletal tissues. Similar to human infection, disease signs persisted for at least 3 weeks after inoculation. A full-length molecular clone of CHIKV SL15649 recapitulated the disease induced by the natural isolate, thereby providing us with the tools to study both viral and host determinants that drive CHIKV-induced musculoskeletal inflammatory disease.
Materials and Methods
Biosafety
All studies with viable CHIKV were performed in certified BSL-3 laboratories in biological safety cabinets using biosafety protocols that were approved by the Institutional Biosafety Committees of the University of Colorado, Denver, and the University of North Carolina at Chapel Hill.
Isolation and Sequence Analysis of CHIKV
Serum samples were collected from febrile patients in Sri Lanka during the 2006 CHIKV outbreak.22 Samples were confirmed to be CHIKV positive by a CHIKV-specific PCR assay. A total of 100 μL of serum was inoculated onto Vero cell (ATCC CCL-81) monolayers in 6-well dishes, cells were observed for the development of a cytopathic effect, and culture supernatants were collected at 72 hours after inoculation. These supernatants were diluted onto new Vero cell monolayers and overlayed with 0.5% immunodiffusion-grade agarose (MP Biomedical, Cleveland, OH); then individual, well isolated plaques were collected. Plaque-purified viruses were amplified 1× on Vero cells and viral titers determined by plaque assay on Vero cells. TRIzol reagent (Invitrogen, Carlsbad, CA) was added to an aliquot of infected Vero cell supernatant, and CHIKV RNA was purified using an RNA isolation kit (Invitrogen) according to the manufacturer's instructions. RNA was reverse transcribed, and the CHIKV genome was amplified by PCR using Platinum Pfx Polymerase (Invitrogen). The genome was amplified in a series of 500-bp fragments overlapping by 100 to 150 bp. Amplicons were cloned into pCR-Blunt using the Zero Blunt PCR Cloning Kit (Invitrogen). The sequence of the 5′ untranslated region was determined using the 5′ SMART RACE cDNA Amplification Kit according to the manufacturer's instructions (Clontech, Mountain View, CA). Forward and reverse sequencing of two independent amplicons per region was performed, and the complete genomic sequence was assembled (GenBank: GU189061.1).
Construction of CHIKV Strain SL15649 cDNA Clone and Generation of Infectious Virus
Genomic cDNA fragments based on the SL15649 genome sequence (GenBank: GU189061.1) were commercially synthesized (BioBasic, Markham, ON, Canada) to create three constructs, each spanning approximately one-third of the CHIKV genome. The three fragments were assembled into the full-length genome using unique AgeI and XhoI sites and inserted into pUC57 to create the infectious clone (icCHIKVSL15649). The icCHIKVSL15649 plasmid was linearized using NotI and transcribed in vitro using an mMessage mMachine SP6 transcription kit (Ambion, Austin, TX) to yield 5′-capped genomic RNA. Viral RNA was electroporated into BHK-21 cells. Culture supernatants were collected at 24 hours after electroporation, centrifuged to remove cellular debris, aliquoted, and stored at −80°C until virus titers were quantified by plaque assay.
Mouse Experiments
C57BL6/J mice were obtained from the Jackson Laboratory and bred in-house. Fourteen-day-old mice were inoculated in the left rear footpad with 100 PFU of virus in diluent (PBS−1% bovine calf serum) in a 10-μL volume. Control animals were inoculated with diluent alone. Mice were housed with the mother until they were 21 days old. Mice were monitored for disease signs and weighed at 24-hour intervals, and no mortality was observed in the CHIKV-infected mice. Calipers were used to perform dorsoventral measurements of the feet and mediolateral measurements of the tarsi. Animal husbandry and experiments were performed in accordance with all University of Colorado, Denver, and University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee guidelines.
Virus Tissue Titers
At the times indicated, mice were sacrificed and perfused by intracardial injection with 1× PBS. Tissues were dissected, weighed, and homogenized, and the amount of infectious CHIKV present was determined by plaque assay on Vero cells.
Histologic Analysis
At the times indicated, mice were sacrificed and perfused by intracardial injection with 4% paraformaldehyde, pH 7.3. Hind limb tissues, not including the femur, were embedded in paraffin and 5-μm sections were prepared. To determine the extent of inflammation and tissue disease, tissues were stained with H&E. Sections were evaluated for bone marrow hyperplasia, necrosis, inflammation, regeneration, mineralization, fibrosis, edema, vasculitis, tenosynovitis, synovitis, and tendonitis. Severity grades were assigned based on the following scale: 1, minimal; 2, mild; 3, moderate; 4, marked; asterisk, present; and minus sign, absent.
Flow Cytometry
Mice were inoculated as described; sacrificed by exsanguination at 3, 5, and 7 days after inoculation; and perfused with 1× PBS. Hind limb skin was removed, and hind limbs below the knee were dissected and incubated for 45 minutes at 37°C in digestion buffer [RPMI, 10% fetal bovine serum, 15 mmol/L HEPES, 2.5 mg/ml of collagenase A (Roche, Basil, Switzerland), and 1.7 mg/ml of DNase I (Sigma, St. Louis, MO)]. Digested tissues were passed through a 40-μm cell strainer, pelleted, washed, and resuspended in RPMI medium. Total viable cells were quantified by counting trypan blue–stained cells. Isolated cells were incubated with antimouse FcγRII/III (2.4G2; BD Pharmingen, San Diego, CA) for 20 minutes on ice and then stained in fluorescence-activated cell sorter staining buffer (1× Hanks balanced salt solution, 1% fetal bovine serum) with the following antibodies: anti-CD45-Biotin, anti-CD11c-phycoerythrin-Texas Red, anti-NK1.1-phycoerythrin, anti-CD3-fluorescein isothiocyanate, anti-CD4-Pacific Blue, anti-CD8-Pacific Orange, anti-F4/80-fluorescein isothiocyanate, CD115-fluorescein isothiocyanate, anti-CD11b-phycoerythrin-Cy7, anti–major histocompatibility complex class II-allophycocyanin, or anti-GR-1-phycoerythrin (all from eBioscience, San Diego, CA). Biotin conjugates were detected with Streptavidin-Peridinin Chlorophyll Protein Complex (eBioscience). Cells were fixed overnight in 2% paraformaldehyde and analyzed on a cyan cytometer (Becton Dickinson, Franklin Lakes, NJ) using Summit software.
RT-PCR
At 2, 14, and 21 days after inoculation, uninfected or CHIKV-infected mice (three mice per group) were sacrificed and perfused by intracardial injection with 1× PBS. Tissues were homogenized in 1 ml of TRIzol (Invitrogen), and total RNA was isolated using the PureLink RNA Kit (Invitrogen). RNA was reverse transcribed with superscript III (Invitrogen) and analyzed by 25 cycles of PCR for the presence of β-actin or 30 cycles of PCR for the presence of CHIKV using previously published primers specific for the E1 region of the genome.23 The icCHIKVSL15649 plasmid was used as a positive control. RNA samples that had not been subjected to reverse transcription were used as a negative control.
Results
To investigate the capacity of a CHIKV clinical isolate to cause disease in a mouse model, 14-day-old C57BL/6J mice were inoculated with 100 PFU of CHIKV SL15649, which was isolated from a febrile CHIKV-positive Sri Lankan patient as described in the “Materials and Methods” section. In addition to the virus that was directly amplified from the human serum, mice were also inoculated with three different plaque-purified CHIKV SL15649 isolates (PL1, PL2, and PL3) or virus inactivated by UV light. Control mice were inoculated with virus diluent alone. Compared with control mice or mice inoculated with UV-treated virus, inoculation with CHIKV SL15649 or any of the three plaque-purified isolates resulted in diminished weight gain (Figure 1, A–C). Strikingly, as evidenced by both caliper measurements and histologic analysis, all mice inoculated with live virus developed intense gross swelling and edema of the left foot (Figure 1, D and E) and tarsus (data not shown). Swelling began 2 days after inoculation (data not shown) and persisted at least 1 week (Figure 1, D and E). Analysis of the noninoculated foot demonstrated that inflammatory infiltrates were present at 7 days after inoculation (Figure 1E). However, these infiltrates appeared less extensive than those observed in the inoculated foot, and no gross or histopathologic evidence of swelling or edema was observed in footpad tissues contralateral to the site of inoculation. To investigate whether tissue swelling and the magnitude of inflammation correlated with differences in the amount of virus present in musculoskeletal tissues ipsilateral or contralateral relative to the site of inoculation, the amounts of infectious virus present in tarsus or foot tissue of mice inoculated with plaque-purified CHIKV SL15649 was quantified by plaque assay at 1 and 4 days after inoculation (Figure 1F). Approximately 100-fold more virus was present in left tarsus or foot tissue compared with the right tarsus or foot tissue at both 1 and 4 days after inoculation, suggesting that CHIKV SL15649 spreads to distal sites, but the peak viral titers at sites distal to the site of inoculation are diminished. These results suggest that the SL15649 virus, which is not mouse adapted, does not spread efficiently from the inoculated foot. It remains to be determined whether the lack of swelling in the noninoculated foot is due to this difference in viral replication or some other mechanism.
Figure 1.
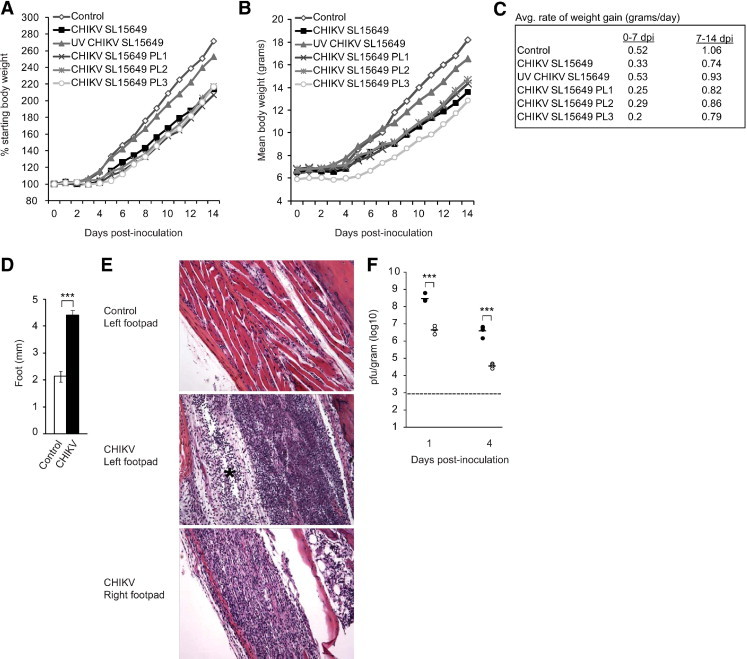
Chikungunya virus (CHIKV)–induced disease signs. Fourteen-day-old C57BL/6J mice were inoculated with virus diluent only (control), 100 PFU of CHIKV SL15649, 100 PFU of UV light–inactivated CHIKV SL15649, or 100 PFU of three independent plaque-purified isolates of CHIKV SL15649 (PL1, PL2, PL3). Mice were weighed at 24-hour intervals, and percentage of starting body weight (A), actual body weights (B), and the average rate of weight gain in grams per day (C) were determined (n = three mice per group). D: Fourteen-day-old C57BL/6J mice were inoculated with virus diluent only (control) or 100 PFU of plaque-purified CHIKV SL15649. At 7 days after inoculation, a dorsoventral measurement of the left foot was performed (n = four mice per group). ***P < 0.001 as determined by 2-tailed t-test. E: Fourteen-day-old C57BL/6J mice were inoculated with virus diluent only (control) or 100 PFU of plaque-purified CHIKV SL15649. At 7 days after inoculation, mice were sacrificed and perfused by intracardial injection with 4% paraformaldehyde. Tissue sections were stained with H&E, and the footpads were assessed for evidence of inflammation and edema (asterisk). Images are representative of at least three mice per group. F: Fourteen-day-old C57BL/6J mice were inoculated with 100 PFU of plaque-purified CHIKV SL15649. At 1 day after inoculation (n = 4) and 4 days after inoculation (n = 4), mice were sacrificed and perfused by intracardial injection with 1× PBS. Left tarsus or foot (filled circles) and right tarsus or foot (open circles) were dissected, weighed, and homogenized, and the amount of infectious virus present was quantified by plaque assays. Dashed line indicates the limit of detection. ***P < 0.0001 as determined by two-tailed t-tests.
Debilitating rheumatic disease signs and symptoms, such as severe joint pain and tenosynovitis, are prominent features of CHIKV infection of humans.14–16 Therefore, we next investigated whether inoculation of mice with CHIKV SL15649 resulted in musculoskeletal tissue disease consistent with the disease signs of CHIKV-infected humans. For these experiments, 14-day-old C57BL/6J mice were inoculated with diluent alone (control) or 100 PFU of plaque-purified CHIKV SL15649 in the footpad, which resulted in 100% morbidity and 0% mortality. At 1, 3, 7, 10, 14, and 21 days after inoculation, mice were sacrificed, perfused by intracardial injection with 4% paraformaldehyde, and H&E-stained tissue sections generated from the left and right hind limbs were evaluated for disease. The major pathological findings from these studies are given in Figure 2 and Table 1. In contrast to control-inoculated mice (Figure 2A), by 7 days after inoculation mice inoculated with CHIKV SL15649 had developed a mixed inflammatory cell arthritis containing histiocytes, neutrophils, lymphocytes, and rare plasma cells in various joints of the feet (Figure 2B) and the tarsi (data not shown). Virus-infected mice displayed severe chronic active tenosynovitis (Figures 2, C and D) with coagulative necrosis that did not involve the blood vessels, and this was not observed in control-inoculated mice. Furthermore, in contrast to skeletal muscle tissue of control mice (Figure 2, E and G), necrotizing myositis was observed in skeletal muscle tissues of CHIKV SL15649–inoculated mice, such as the gastrocnemius muscle (Figures 2, F and H) and quadriceps femoris muscle (data not shown). In addition to the major pathological findings of arthritis, tenosynovitis, and myositis, CHIKV SL15649–inoculated mice displayed multifocal vasculitis (Figure 2, I and J) and an expansion of myeloid cells in the bone marrow compared with control-inoculated mice (Figures 2, K and L). Although viral loads at 1 and 4 days after inoculation were significantly higher in tissues in the left hind limb (site of inoculation), similar types of inflammatory lesions were present in right hind limb tissues (Table 1). The lesions in the right hind limb tended to be milder in severity at 3 and 7 days after inoculation and similar in severity at 10 and 14 days after inoculation (Table 1). Although many healthy adults recover from CHIKV infection within days or weeks after the onset of disease signs, a number of studies indicate that a large fraction (up to 57%) of CHIKV-infected humans have persistent arthralgia for months to years.18,24–28 Importantly, ongoing signs of inflammation were observed in joint tissues of CHIKV SL15649–inoculated mice at 21 days after inoculation, whereas both a fibrous response and regenerative response were evident in skeletal muscle tissue (Table 1). Taken together, these findings indicate that inoculation of 14-day-old C57BL/6J mice with CHIKV strain SL15649 results in acute histopathologic inflammatory changes of musculoskeletal tissues, including joints, tendons, and skeletal muscle, which is consistent with the disease signs of CHIKV-infected humans.29 To further characterize the inflammatory response in musculoskeletal tissues, infiltrating leukocytes were isolated from hind limbs at 3, 5, and 7 days after inoculation and quantified by flow cytometry as described in the “Material and Methods” section. At early times after inoculation (3 and 5 days after inoculation), natural killer (CD3−/NK1.1+) cells (Figure 2M, first panel) and neutrophils (data not shown) were the predominant cell types detected in musculoskeletal tissues of the left hind limb. In contrast, by this method there was no detectable increase above background numbers of leukocytes in musculoskeletal tissues of the right hind limb (Figure 2M, first panel). This finding is consistent with the histologic results, where tissues in the noninoculated limb exhibited inflammatory cell infiltrates, but these were reduced compared with the inoculated limb. By 7 days after inoculation, the inflammatory lesions in the inoculated foot were characterized by the presence of natural killer cells, monocytes/macrophages (F4/80+/CD11b+), and both CD4 and CD8 T lymphocytes (CD3+/CD4+/CD8− and CD3+/CD4−/CD8+, respectively) (Figure 2M). In addition, analyses with additional cell surface markers suggested the presence of a heterogenous mix of myeloid cell phenotypes and other cell types (data not shown); however, future studies are required to fully characterize the complexities of the inflammatory response to CHIKV infection.
Figure 2.
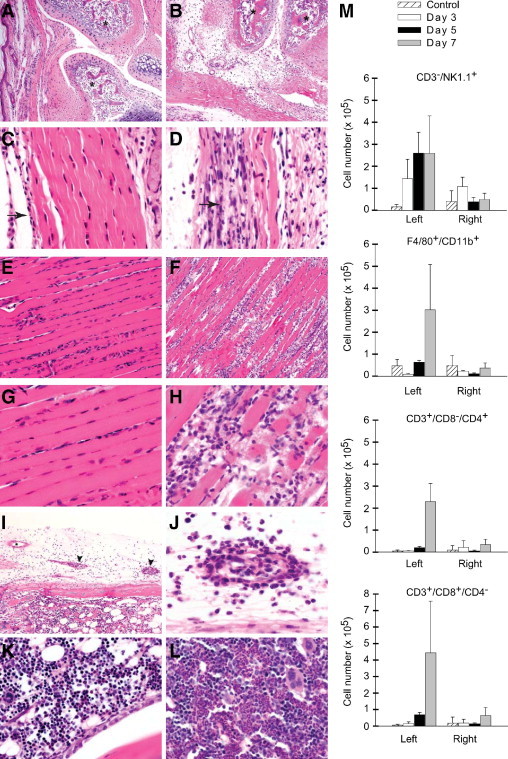
Chikungunya virus (CHIKV) SL15649-induced musculoskeletal disease. Fourteen-day-old C57BL/6J mice were inoculated with virus diluent only (control) or 100 PFU of plaque-purified CHIKV SL15649. At 7 days after inoculation, mice were sacrificed and perfused by intracardial injection with 4% paraformaldehyde. Tissue sections were stained with H&E and evaluated for tissue disease. Metatarsophalangeal joint of control-inoculated (A) or CHIKV-inoculated (B) inoculated mouse. Asterisks indicate bone. Pelvic limb tendon of control-inoculated (C) or CHIKV-inoculated (D) mouse. Arrows indicate tendon sheath. Gastrocnemius muscle of control-inoculated (E and G) or CHIKV-inoculated (F and H) mouse. Pelvic limb vasculitis of CHIKV-inoculated mouse (I and J). Asterisk indicates normal vessel (I). Arrowheads indicate vessels with vasculitis (I). Pelvic limb bone marrow of control-inoculated (K) or CHIKV-inoculated (L) mouse. Images are representative of at least three mice per group (M). At 3 (n = 3), 5 (n = 3), and 7 (n = 3) days after inoculation, leukocytes were isolated from hind limb tissues of control-inoculated and CHIKV SL15649–inoculated mice. Isolated cells were counted and analyzed by flow cytometry as described in the “Materials and Methods” section. Total numbers of natural killer cells (NK1.1+/CD3−), monocytes/macrophages (F4/80+/CD11b+), CD4 T lymphocytes (CD3+/CD8−/CD4+), and CD8 T lymphocytes (CD3+/CD8−/CD4+) isolated from the left and right hind limbs are shown. Each bar represents the arithmetic mean ± SEM of three mice per group.
Table 1.
Chikungunya Virus SL15649 Musculoskeletal Tissue Disease
| Days afterinoculation | Leg | Bone marrowhyperplasia | Tibia muscle |
Metatarsal muscle |
V | TS | S | T | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | I | R | N | I | R | M | F | E | |||||||
| Control | R | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Control | L | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Control | L | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 1 | R | — | — | — | — | — | 1 | — | — | — | — | — | — | — | — |
| 1 | L | — | — | — | — | 3 | 2 | — | — | — | 3 | — | 2 | 1 | 2 |
| 3 | R | 2 | — | 1 | — | 1 | 1 | — | — | — | — | — | |||
| 3 | L | 2 | — | 2 | — | 3 | 3 | — | — | — | 3 | * | 2 | 2 | 3 |
| 7 | R | 4 | 2 | 2 | — | 4 | 4 | 1 | — | — | 1 | * | 1 | 2 | 3 |
| 7 | L | 4 | 4 | 4 | 4 | 4 | 4 | 1 | 4 | — | 4 | * | 4 | 4 | 4 |
| 10 | R | 3 | 2 | 3 | 4 | 4 | 4 | 2 | 4 | — | 3 | * | 4 | 3 | 4 |
| 10 | L | 3 | 2 | 2 | 2 | 2 | 3 | 2 | — | — | 1 | * | 3 | 2 | 2 |
| 14 | R | 2 | 2 | 3 | 2 | 3 | 3 | 3 | — | 1 | 1 | — | 2 | 2 | 2 |
| 14 | L | 2 | 2 | 3 | 4 | 3 | 3 | — | 3 | 3 | 1 | * | 4 | 3 | 3 |
| 21 | R | 2 | 1 | 2 | 2 | 2 | 2 | 4 | — | 2 | — | * | 2 | 2 | 1 |
| 21 | L | 2 | 1 | 2 | 4 | 4 | 3 | — | 3 | 4 | 2 | * | 4 | 2 | 3 |
E, edema; F, fibrosis; I, inflammation; M, mineralization; N, necrosis; R, regeneration; S, synovitis; T, tendonitis; TS, tenosynovitis; V, vasculitis; —, absent; *, present; 1, minimal; 2, mild; 3, moderate; 4, marked.
The data described thus far indicate that inoculation of C57BL/6J mice with CHIKV SL15649 provides a robust animal model of CHIKV-induced musculoskeletal tissue disease to investigate mechanisms of pathogenesis. To establish a viral reverse genetics system for this model and confirm that the inflammatory condition associated with CHIKV SL15649 was due to CHIKV and not an unidentified contaminant within the virus preparation, CHIKV SL15649 genomic cDNA fragments were commercially synthesized and assembled into a full-length cDNA (icCHIKV SL15649) as described in the “Materials and Methods” section. Comparative analysis of multistep replication kinetics of icCHIKV SL15649 and CHIKV SL15649 in both Vero cells and C6/36 mosquito cells indicated that in vitro replication characteristics of the molecularly and biologically cloned viruses were indistinguishable (data not shown). To assess the in vivo virulence of the molecular clone–derived virus, C57BL/6J mice were inoculated with either the natural isolate SL15649 virus or the clone-derived icCHIKV SL15649 virus, and the mice were evaluated for virus-induced disease signs and inflammatory musculoskeletal tissue disease. Similar to CHIKV SL15649 (Figure 1B), inoculation of icCHIKV SL15649 induced intense swelling of the left foot and tarsus (Figure 3D). In addition, the distribution and degree of inflammation within musculoskeletal tissues, including the tarsi (Figure 3, A–C), various joints of the feet (data not shown), and hind limb skeletal muscle tissue (data not shown), were similar in mice inoculated with either CHIKV SL15649 or icCHIKV SL15649. To assess whether icCHIKV SL15649 replicated in tarsus or foot tissues similar to CHIKV SL15649, the amounts of infectious virus was quantified by plaque assay at 1, 4, and 7 days after inoculation (Figure 3E). Similar to CHIKV SL15649 (Figure 1D), significantly more virus was present in left tarsus or foot tissue compared with the right tarsus or foot tissue at all times evaluated. Notably, despite the presence of inflammatory histopathologic changes, the amount of infectious virus present in right tarsus or foot tissue was below the limit of detection by 7 days after inoculation. To determine whether the histopathologic changes observed at late times after inoculation (14 and 21 days after inoculation; Table 1) were associated with the persistence of CHIKV, we isolated total RNA at 2, 14, and 21 days after inoculation from the left ankle, right ankle, left quadriceps, right quadriceps, and the spleen and performed RT-PCR analyses. As expected, CHIKV RNA was readily detected in all tissues at 2 days after inoculation (Figure 3F). Interestingly, CHIKV RNA was detected in the left and right ankles at 14 and 21 days after inoculation but not the other tissues (Figure 3F). No CHIKV-specific RT-PCR signal was detected in RNA samples isolated from tissues of mock-inoculated control mice or in the absence of reverse transcription (Figure 3F). These findings indicate that though CHIKV is readily cleared from most tissues after the acute stage of infection, CHIKV RNA persists in joint tissues for at least 3 weeks after inoculation, and the persistence of viral RNA is associated with ongoing inflammation within joint-associated tissues.
Figure 3.
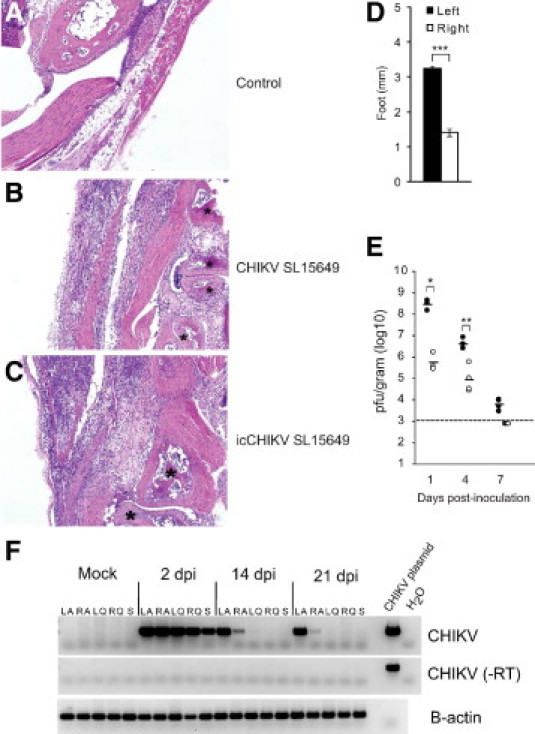
A cDNA clone of chikungunya virus (CHIKV) SL15649 induces inflammation of the musculoskeletal tissues. Fourteen-day-old C57BL/6J mice were inoculated with virus diluent only (control), 100 PFU of plaque-purified CHIKV SL15649, or 100 PFU of cDNA clone–derived CHIKV SL15649 (icCHIKV SL15649). At 10 days after inoculation, mice were sacrificed and perfused by intracardial injection with 4% paraformaldehyde. Tissue sections from control-inoculated (A), CHIKV SL15649–inoculated (B), and icCHIKV SL15649–inoculated mice (C) were stained with H&E and evaluated for tissue disease. Shown are images of the left tarsus. Asterisks indicate bone. Images are representative of at least three mice per group. D: Fourteen-day-old C57BL/6J mice were inoculated with virus diluent only (control) or 100 PFU of icCHIKV SL15649. At 7 days after inoculation, dorsoventral measurements of the left and the right foot were performed (n = four mice per group). Data are representative of at least two independent experiments. ***P < 0.0001 as determined by two-tailed t-test. E: Fourteen-day-old C57BL/6J mice were inoculated with 100 PFU of icCHIKV SL15649. At 1 (n = 4), 4 (n = 4), and 7 (n = 4) days after inoculation, mice were sacrificed and perfused by intracardial injection with 1× PBS. Left tarsus or foot (filled circles) and right tarsus or foot (open circles) were dissected, weighed, and homogenized, and the amount of infectious virus present was quantified by plaque assays. Dashed line indicates the limit of detection. *P = 0.0006 as determined by two-tailed t-test. **P = 0.002 as determined by two-tailed t-test. F: Fourteen-day-old C57BL/6J mice were mock inoculated or inoculated with 100 PFU of icCHIKV SL15649. At 2 (n = 3), 14 (n = 3), and 21 (n = 3) days after inoculation, mice were sacrificed and perfused by intracardial injection with 1× PBS. Left tarsus/foot (LA), right tarsus/foot (RA), left quadriceps femoris (LQ), right quadriceps femoris (RQ), and spleens (S) were homogenized in TRIzol. Isolated RNA was analyzed for the presence of CHIKV RNA by RT-PCR. Gel images are representative of three mice per group.
Discussion
These results demonstrate that infection of young mice with a clinical CHIKV isolate results in a spectrum of histopathologic changes, including mixed inflammatory cell arthritis, tenosynovitis, and myositis, that is similar to the distinguishing features of classic CHIKV-induced disease in humans. A similar mouse model for the related arthritic alphavirus, Ross River virus, has led to the identification of several host pathways, including the host complement cascade, as key drivers of virus-induced arthritis and myositis,30–32 and this newly developed mouse model will allow analysis of host pathways that contribute to the pathogenesis of CHIKV-induced disease. Furthermore, reproduction of the disease caused by the clinical isolate by the full-length infectious clone-derived virus not only demonstrates a direct role for CHIKV in driving the inflammatory pathological changes but also allows for the use of this molecular clone to identify viral determinants that regulate viral virulence and virus-induced disease.
Recently, evidence of arthritis, tenosynovitis, and myositis after inoculation of adult C57BL/6 mice with a Reunion Island CHIKV isolate (LR2006-OPY1) or a presumably mouse-adapted Asian CHIKV isolate was reported.33 Similar to our findings, Gardner et al33 observed that CHIKV-induced swelling was restricted to the inoculated foot, suggesting that neither the SL15649 virus used in our studies nor the LR2006-OPY1 virus used by Gardner et al disseminated efficiently in the mouse. Interestingly, some of the findings from Gardner et al differed from our findings. Whereas we observed clear evidence of severe tendonitis (Figure 2D and Table 1), Gardner et al reported that hind limb tendons appeared unaffected. Furthermore, Gardner et al did not report any evidence for CHIKV persistence in their model, although this has been reported both in CHIKV-infected humans and nonhuman primates.21,34 In contrast, we observed ongoing inflammation in joint tissues at least 3 weeks after inoculation, as well as joint-specific persistence of CHIKV RNA. These differences may reflect the differences in mouse age or the virus isolate used in the two studies and require further investigation.
A current limitation of our model is the unequal replication of the virus in tissues near and distal to the site of inoculation, which is likely due to inefficient viral dissemination. However, our generation of a molecular clone of CHIKV SL15649, which exhibits the same phenotype, combined with the availability of genetically engineered mice provides the opportunity to investigate both the host factors and virus genetic determinants that regulate CHIKV dissemination and spread. Our analysis has focused on the early acute stages of CHIKV-induced inflammation; however, a subset of CHIKV-infected humans exhibit long-term joint pain,18 and it will be important to assess the long-term consequences of CHIKV infection in this model to determine whether it will reproduce the chronic aspects of the human disease and thereby allow analysis of mechanisms underlying chronic infection. As previously noted, our study demonstrated that severe histopathologic changes persisted in musculoskeletal tissues of CHIKV-infected mice for at least 3 weeks after inoculation (Table 1). Furthermore, although CHIKV RNA was cleared from other peripheral tissues, viral RNA was readily detected in ankle tissue at 3 weeks after tinoculation, suggesting that the persistence of viral RNA may drive chronic inflammatory responses. It will be important to evaluate the mechanisms leading to CHIKV persistence, including determination of whether the persistence of viral RNA reflects ongoing viral replication or the maintenance of some type of defective viral genome within the joints. It is possible that viral persistence is due to active evasion of host innate or adaptive immune responses by the virus. Interestingly, we and others have recently demonstrated that several alphaviruses, including CHIKV, actively antagonize STAT activation by both type I and type II interferon, and it will be important to assess whether evasion of the interferon response plays any role in promoting persistence of CHIKV RNA within the joints.35–37
Finally, there is a critical need to develop new therapies for CHIKV-induced disease because there are currently no specific therapies to treat Alphavirus-induced rheumatic disease. Treatment options are limited primarily to nonsteroidal anti-inflammatory drugs, which provide relief for a subset of patients.38 Therefore, in addition to allowing for an in-depth analysis of the viral and host factors that drive CHIKV-induced inflammatory disease, this model should prove useful for testing potential therapeutics for preventing or ameliorating the effects of CHIKV-induced rheumatic disease.
Acknowledgments
We thank Aravinda deSilva and Nalaka Kanakaratne for essential assistance with these studies.
Footnotes
Supported by National Institutes of Health (NIH) research grant K22 AI079163 (T.E.M.), NIH research grants U54 AI 057157-07 (SERCEB Project 2.1) and 2 R01 AR 047190 (M.T.H.), and in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
References
- 1.Griffin D.E. Alphaviruses. In: Knipe D.M., Howley P.M., editors. Fields Virology. 5th edition. Williams, & Wilkins; Philadelphia, Lippincott: 2007. pp. 1023–1067. [Google Scholar]
- 2.Staples J.E., Breiman R.F., Powers A.M. Chikungunya fever: an epidemiological review of a re-emerging infectious disease. Clin Infect Dis. 2009;49:942–948. doi: 10.1086/605496. [DOI] [PubMed] [Google Scholar]
- 3.Harley D., Sleigh A., Ritchie S. Ross River virus transmission, infection, and disease: a cross-disciplinary review. Clin Microbiol Rev. 2001;14:909–932. doi: 10.1128/CMR.14.4.909-932.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tesh R.B., Watts D.M., Russell K.L., Damodaran C., Calampa C., Cabezas C., Ramirez G., Vasquez B., Hayes C.G., Rossi C.A., Powers A.M., Hice C.L., Chandler L.J., Cropp B.C., Karabatsos N., Roehrig J.T., Gubler D.J. Mayaro virus disease: an emerging mosquito-borne zoonosis in tropical South America. Clin Infect Dis. 1999;28:67–73. doi: 10.1086/515070. [DOI] [PubMed] [Google Scholar]
- 5.Johnston R.E., Peters C.J. Alphaviruses. In: Fields B.N., Knipe D.M., Howley P.M., editors. Fields Virology. 3rd edition. Lippincott-Raven; Philadelphia: 1996. pp. 843–898. [Google Scholar]
- 6.Williams M.C., Woodall J.P., Gillett J.D. O'nyong-Nyong fever: an epidemic virus disease in East Africa: Vii virus isolations from man and serological studies up to July 1961. Trans R Soc Trop Med Hyg. 1965;59:186–197. doi: 10.1016/0035-9203(65)90080-5. [DOI] [PubMed] [Google Scholar]
- 7.Mavalankar D., Shastri P., Raman P. Chikungunya epidemic in India: a major public-health disaster. Lancet Infect Dis. 2007;7:306–307. doi: 10.1016/S1473-3099(07)70091-9. [DOI] [PubMed] [Google Scholar]
- 8.Charrel R.N., de Lamballerie X., Raoult D. Chikungunya outbreaks: the globalization of vectorborne diseases. N Engl J Med. 2007;356:769–771. doi: 10.1056/NEJMp078013. [DOI] [PubMed] [Google Scholar]
- 9.ProMED-mail: Chikungunya-Italy (Emilia Romagna). ProMED mail 2007; 7 Sept: 20070907.2957. Available online at http://www.promedmail.org. Accessed October 2009.
- 10.Watson R. Chikungunya fever is transmitted locally in Europe for first time. BMJ. 2007;335:532–533. doi: 10.1136/bmj.39332.708738.DB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross R.W. The Newala epidemic, III: the virus: isolation, pathogenic properties and relationship to the epidemic. J Hyg (Lond) 1956;54:177–191. doi: 10.1017/s0022172400044442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powers A.M., Logue C.H. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol. 2007;88:2363–2377. doi: 10.1099/vir.0.82858-0. [DOI] [PubMed] [Google Scholar]
- 13.Brighton S.W., Prozesky O.W., de la Harpe A.L. Chikungunya virus infection: a retrospective study of 107 cases. S Afr Med J. 1983;63:313–315. [PubMed] [Google Scholar]
- 14.Simon F., Parola P., Grandadam M., Fourcade S., Oliver M., Brouqui P., Hance P., Kraemer P., Ali Mohamed A., de Lamballerie X., Charrel R., Tolou H. Chikungunya infection: an emerging rheumatism among travelers returned from Indian Ocean islands: report of 47 cases. Medicine (Baltimore) 2007;86:123–137. doi: 10.1097/MD/0b013e31806010a5. [DOI] [PubMed] [Google Scholar]
- 15.Parola P., de Lamballerie X., Jourdan J., Rovery C., Vaillant V., Minodier P., Brouqui P., Flahault A., Raoult D., Charrel R.N. Novel chikungunya virus variant in travelers returning from Indian Ocean islands. Emerg Infect Dis. 2006;12:1493–1499. doi: 10.3201/eid1210.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parola P., Simon F., Oliver M. Tenosynovitis and vascular disorders associated with Chikungunya virus-related rheumatism. Clin Infect Dis. 2007;45:801–802. doi: 10.1086/521171. [DOI] [PubMed] [Google Scholar]
- 17.Ozden S., Huerre M., Riviere J.P., Coffey L.L., Afonso P.V., Mouly V., de Monredon J., Roger J.C., El Amrani M., Yvin J.L., Jaffar M.C., Frenkiel M.P., Sourisseau M., Schwartz O., Butler-Browne G., Despres P., Gessain A., Ceccaldi P.E. Human muscle satellite cells as targets of Chikungunya virus infection. PLoS ONE. 2007;2:e527. doi: 10.1371/journal.pone.0000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sissoko D., Malvy D., Ezzedine K., Renault P., Moscetti F., Ledrans M., Pierre V. Post-epidemic chikungunya disease on Reunion Island: course of rheumatic manifestations and associated factors over a 15-month period. PLoS Negl Trop Dis. 2009;3:e389. doi: 10.1371/journal.pntd.0000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Couderc T., Chretien F., Schilte C., Disson O., Brigitte M., Guivel-Benhassine F., Touret Y., Barau G., Cayet N., Schuffenecker I., Despres P., Arenzana-Seisdedos F., Michault A., Albert M.L., Lecuit M. A mouse model for Chikungunya: young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog. 2008;4:e29. doi: 10.1371/journal.ppat.0040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ziegler S.A., Lu L., da Rosa A.P., Xiao S.Y., Tesh R.B. An animal model for studying the pathogenesis of chikungunya virus infection. Am J Trop Med Hyg. 2008;79:133–139. [PubMed] [Google Scholar]
- 21.Labadie K., Larcher T., Joubert C., Mannioui A., Delache B., Brochard P., Guigand L., Dubreil L., Lebon P., Verrier B., de Lamballerie X., Suhrbier A., Cherel Y., Le Grand R., Roques P. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J Clin Invest. 2010;120:894–906. doi: 10.1172/JCI40104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seneviratne S.L., Perera J. Fever epidemic moves into Sri Lanka. BMJ. 2006;333:1220–1221. doi: 10.1136/bmj.39051.725729.3A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laurent P., Le Roux K., Grivard P., Bertil G., Naze F., Picard M., Staikowsky F., Barau G., Schuffenecker I., Michault A. Development of a sensitive real-time reverse transcriptase PCR assay with an internal control to detect and quantify chikungunya virus. Clin Chem. 2007;53:1408–1414. doi: 10.1373/clinchem.2007.086595. [DOI] [PubMed] [Google Scholar]
- 24.Borgherini G., Poubeau P., Jossaume A., Gouix A., Cotte L., Michault A., Arvin-Berod C., Paganin F. Persistent arthralgia associated with chikungunya virus: a study of 88 adult patients on reunion island. Clin Infect Dis. 2008;47:469–475. doi: 10.1086/590003. [DOI] [PubMed] [Google Scholar]
- 25.Staikowsky F., Le Roux K., Schuffenecker I., Laurent P., Grivard P., Develay A., Michault A. Retrospective survey of Chikungunya disease in Reunion Island hospital staff. Epidemiol Infect. 2008;136:196–206. doi: 10.1017/S0950268807008424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larrieu S, Pouderoux N, Pistone T, Filleul L, Receveur MC, Sissoko D, Ezzedine K, Malvy D: Factors associated with persistence of arthralgia among Chikungunya virus-infected travellers: report of 42 French cases. J Clin Virol 47:85–88 [DOI] [PubMed]
- 27.Bouquillard E., Combe B. A report of 21 cases of rheumatoid arthritis following Chikungunya fever: a mean follow-up of two years. Joint Bone Spine. 2009;76:654–657. doi: 10.1016/j.jbspin.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Malvy D., Ezzedine K., Mamani-Matsuda M., Autran B., Tolou H., Receveur M.C., Pistone T., Rambert J., Moynet D., Mossalayi D. Destructive arthritis in a patient with chikungunya virus infection with persistent specific IgM antibodies. BMC Infect Dis. 2009;9:200. doi: 10.1186/1471-2334-9-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaffar-Bandjee M.C., Das T., Hoarau J.J., Krejbich Trotot P., Denizot M., Ribera A., Roques P., Gasque P. Chikungunya virus takes centre stage in virally induced arthritis: possible cellular and molecular mechanisms to pathogenesis. Microbes Infect. 2009;11:1206–1218. doi: 10.1016/j.micinf.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Morrison T.E., Fraser R.J., Smith P.N., Mahalingam S., Heise M.T. Complement contributes to inflammatory tissue destruction in a mouse model of Ross River virus-induced disease. J Virol. 2007;81:5132–5143. doi: 10.1128/JVI.02799-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrison T.E., Simmons J.D., Heise M.T. Complement receptor 3 promotes severe Ross River virus-induced disease. J Virol. 2008;82:11263–11272. doi: 10.1128/JVI.01352-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrison T.E., Whitmore A.C., Shabman R.S., Lidbury B.A., Mahalingam S., Heise M.T. Characterization of Ross River virus tropism and virus-induced inflammation in a mouse model of viral arthritis and myositis. J Virol. 2006;80:737–749. doi: 10.1128/JVI.80.2.737-749.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gardner J., Anraku I., Le T.T., Larcher T., Major L., Roques P., Schroder W.A., Higgs S., Suhrbier A. Chikungunya virus arthritis in adult wild-type mice. J Virol. 2010;84:8021–8032. doi: 10.1128/JVI.02603-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoarau J.J., Jaffar Bandjee M.C., Trotot P.K., Das T., Li-Pat-Yuen G., Dassa B., Denizot M., Guichard E., Ribera A., Henni T., Tallet F., Moiton M.P., Gauzere B.A., Bruniquet S., Jaffar Bandjee Z., Morbidelli P., Martigny G., Jolivet M., Gay F., Grandadam M., Tolou H., Vieillard V., Debre P., Autran B., Gasque P. Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. J Immunol. 2010;184:5914–5927. doi: 10.4049/jimmunol.0900255. [DOI] [PubMed] [Google Scholar]
- 35.Simmons J.D., White L.J., Morrison T.E., Montgomery S.A., Whitmore A.C., Johnston R.E., Heise M.T. Venezuelan equine encephalitis virus disrupts STAT1 signaling by distinct mechanisms independent of host shutoff. J Virol. 2009;83:10571–10581. doi: 10.1128/JVI.01041-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fros J.J., Liu W.J., Prow N.A., Geertsema C., Ligtenberg M., Vanlandingham D.L., Schnettler E., Vlak J.M., Suhrbier A., Khromykh A.A., Pijlman G.P. Chikungunya virus nonstructural protein 2 inhibits type I/II interferon-stimulated JAK-STAT signaling. J Virol. 2010;84:11429–11439. doi: 10.1128/JVI.00949-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simmons J.D., Wollish A.C., Heise M.T. A determinant of Sindbis virus neurovirulence enables efficient disruption of Jak/STAT signaling. J Virol. 2010;84 doi: 10.1128/JVI.00577-10. 11429–11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mylonas A.D., Brown A.M., Carthew T.L., McGrath B., Purdie D.M., Pandeya N., Vecchio P.C., Collins L.G., Gardner I.D., de Looze F.J., Reymond E.J., Suhrbier A. Natural history of Ross River virus-induced epidemic polyarthritis. Med J Aust. 2002;177:356–360. doi: 10.5694/j.1326-5377.2002.tb04837.x. [DOI] [PubMed] [Google Scholar]


