Abstract
Partial control of HIV occurs during acute infection, although the mechanisms responsible are poorly understood. We studied the ability of antibody-dependent cellular cytotoxicity (ADCC) antibodies in serum to activate natural killer (NK) cells in longitudinal samples from 8 subjects with well-defined early HIV infection who controlled viremia to low levels. NK cell activation by ADCC antibodies to gp140 Env proteins was detected in half of the subjects at the first time point studied, a mean of 111 d after the estimated time of infection. In contrast, ADCC-mediated NK cell activation in response to linear HIV peptides evolved more slowly, over the first 2 y of infection. Our studies suggest that HIV-specific ADCC responses to conformational epitopes occur early during acute HIV infection, and broaden to include linear epitopes over time. These findings have implications for the immune control of HIV.
Introduction
The partial control of HIV viremia during acute infection has been attributed primarily to HIV-specific CTL responses (1–3). Neutralizing antibodies generally arise too late to control early viremia (4,5). However, several studies now suggest that HIV-specific antibody-dependent cellular cytotoxicity (ADCC) responses could also contribute to this initial control of HIV viremia (6–9). Studies using killing-based ADCC assays have found ADCC antibodies present at around the same time as CTL responses first become detectable (10). Assays that measure the ability of ADCC antibodies to limit virus replication in vitro (ADCVI assays) also show that ADCC antibodies develop early during HIV infection. The strength of ADCC activity has been correlated with decreasing viremia observed during the acute infection (8,11). Many of these studies have analyzed envelope protein as the major antigen targeted by the acute-phase ADCC response (8,11). There is renewed interest in HIV-specific ADCC responses recently, since robust ADCC responses, but minimal neutralizing antibody and CD8 T-cell responses, were induced in the partially successful RV144 HIV vaccine efficacy trial (12).
We recently developed a flow cytometric assay to measure natural killer (NK) cell activation by ADCC antibodies that allows us to study ADCC responses from an alternative perspective to existing assays (13,14). This NK cell activation ADCC assay can evaluate responses to both Env protein and overlapping HIV peptides spanning all nine HIV proteins. The assay uses primary blood cells rather than immortalized cell lines to present HIV antigens, and can measure multiple functions of NK cells triggered by ADCC antibodies (e.g., cytokine expression and degranulation). We employed this ADCC assay to assess the functional role of ADCC antibodies during acute infection.
Materials and Methods
Acute HIV infection cohort
The sample of patients with primary HIV infection (PHI) included in this analysis included patients recruited to two prospective observational PHI cohorts: (1) The Acute Infection and Early Disease Research Program CORE 01 protocol, established by the National Institutes of Health (NIH), Bethesda, Maryland (15); and (2) The Primary HIV and Early Disease Research: Australian Cohort (PHAEDRA), established by the National Centre in HIV Epidemiology and Clinical Research, University of New South Wales, Sydney, N.S.W., Australia (16,17).
Serum samples from 8 primary HIV-1 infection subjects, 7 diagnosed with early infection and 1 with acute infection, from the PHAEDRA and CORE 01 cohorts were studied (Table 1) (16). All subjects were recruited in Sydney. Acute primary infection was defined as a negative or indeterminate serology with positive plasma viremia, and early primary HIV infection was defined as HIV seroconversion within the previous 6–12 mo (defined as either proven negative serology within that period, or a positive result on a detuned ELISA) (15). The estimated time of infection was interpolated as the midpoint between the positive and negative ELISA assays, or 3 mo prior to the detuned ELISA assay. Serum samples were provided for each subject across three time points of early infection. Time point 1 was approximately 22 d after diagnosis, time point 2 was around 69 d after diagnosis, and time point 3 was approximately 2 y after diagnosis (Table 1). None of the subjects was on antiretroviral treatment at any time point. We selected 8 patients with the lowest HIV viral loads during acute/early HIV infection to study (mean viral load 684 copies/mL at diagnosis), as such subjects may provide clues to natural immune control of HIV-1.
Table 1.
Early HIV Infection Cohort
| Subject | Estimated days from infectionato diagnosis | First sample time point (days from diagnosis) | Second sample time point (days from diagnosis) | Third sample time point (years from diagnosis) | Total follow-up (y) | CD4 count at diagnosis (cells/μL) | Viral load at diagnosis (copies/mL) | Last CD4 count (cells/μL) | Last viral load (copies/mL) | CD4 T-cell slopeb |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 82 | 22 | 69 | 985 | 3.9 | 798 | 440 | 780 | 556 | −0.01 |
| 2 | 100 | 13 | 86 | 710 | 4.7 | 595 | 400 | 544 | 5800 | −0.19 |
| 3 | 67 | 16 | 49 | 733 | 5.7 | 980 | 2200 | 916 | 400 | −0.15 |
| 4 | 153 | 49 | 81 | 550 | 3.5 | 845 | 50 | 1063 | 40 | 0.22 |
| 5 | 75 | 24 | 70 | 665 | 4.4 | 414 | 400 | 616 | 120 | 0.35 |
| 6 | 92 | 20 | 154 | 535 | 4.1 | 448 | 190 | 430 | 543 | −0.04 |
| 7 | 28 | 22 | 35 | 840 | 4.5 | 655 | 1090 | 643 | 400 | −0.29 |
| 8 | 47 | 76 | 52 | 698 | 4.3 | 1135 | 700 | 655 | 1090 | −0.26 |
| Median | 78.5 | 22 | 69.5 | 703 | 4.4 | 726 | 420 | 649 | 471.5 | −0.095 |
Time from infection to diagnosis was interpolated as either midpoint between a negative and positive enzyme-linked immunosorbent assay (ELISA), or 3 mo prior to a positive detuned ELISA assay.
CD4 T-cell slope calculated from all CD4 measurements available between diagnosis and last follow-up.
NK cell activation ADCC Assay
ADCC activity in the serum samples was determined by the NK cell activation ADCC assay, using serum added to healthy donor whole blood as previously described (14). In brief, 50 μL of serum was incubated with 150 μL of HIV-negative donor blood, together with either the overlapping 15-mer peptide pools spanning the HIV-1 consensus subtype B proteins (1 μg/mL, kindly provided by the NIH AIDS reagent repository), or HIV-1AD8 gp140 protein (1 μg/mL, produced from stably-transfected HeLa cells and purified by lentil lectin affinity chromatography), in the presence of brefeldin A and monensin (10 μg/mL; Sigma-Aldrich, St. Louis, MO). We studied responses to Env and Gag peptide pools, two separate Pol peptide pools (the first 125 and the last 124 peptides of the 249-peptide set), and combined pools encompassing peptides spanning either Rev, Tat, and Vpu (RTV), or Vif, Vpr, and Nef (VVN). At the end of incubation, CD3-CD56+ NK lymphocytes were studied by flow cytometry for the expression of intracellular IFN-γ, and the surface degranulation marker CD107a. Cut-off values for positive responses to each HIV antigen were calculated at 2 SD above the average of results of 10 HIV-uninfected subjects.
Results
The NK cell activation ADCC assay was used on serial serum samples from 8 subjects with early HIV infection. The assay measures the ability of ADCC antibodies to trigger IFN-γ and CD107a expression from NK cells (Fig. 1). At the first time point available (a median of 22 d after diagnosis, and an estimated 100 d after infection; Table 1), 4 of 8 subjects tested had detectable ADCC activity to the recombinant glycosylated HIV-1AD8 gp140 protein (Fig. 2). ADCC activity to the pool of linear Env peptides was not detected in any of the 8 subjects at this early time point. Responses to peptide pools were only detected within a single individual by the NK cell activation ADCC assay, a response to Pol peptides. At the second time point (a median of after diagnosis), detectable ADCC activity was observed in at least one of the 8 samples across all peptide pools, with the exception of Gag protein peptide pool, to which none of the subjects responded at any of the three time points. By the third time point, around 2 y after diagnosis, all 8 subjects had an ADCC response to either gp140 protein or to one of the protein peptide pools.
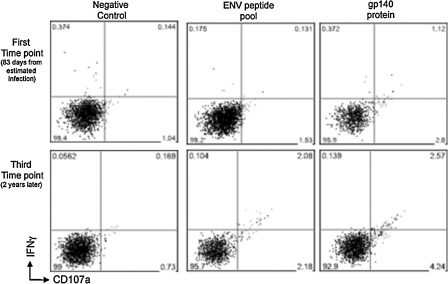
FIG. 1.
ADCC responses as measured by the NK cell activation assay in a subject with acute HIV infection. Gated CD3-CD56+ NK cells were assessed for IFN-γ and CD107a expression after in-vitro stimulation with either overlapping HIV-1 peptide pools (envelope peptides are shown), or gp140 protein. Responses are shown of serum samples from a single subject early during infection (first time point, 83 d from estimated infection), and 2 y later, during chronic infection (third time point).
FIG. 2.
NK cell-mediated ADCC responses over time in subjects with acute HIV infection. Serum samples from 8 subjects with acute HIV infection were assayed for ADCC responses via ADCC ICS assay. Progressive serum samples were provided at three time points of infection after recruitment. Time point 1: median 100 d after estimated infection; time point 2: median 148 d from infection; time point 3: median 2 y after infection (see Table 1). All subjects were tested for responses to HIV protein overlapping 15-mer peptide pools spanning all nine HIV-1 proteins, along with glycosylated recombinant gp140 protein. (A) The number of subjects who had detectable ADCC responses to each of the test antigens at different time points of infection. (B) Mean (±SE) NK cell-mediated ADCC responses as measured by percentage of NK cells expressing both CD107a and IFN-γ of all subjects are shown at each time point in ADCC responses between time points (*p < 0.05).
A significant increase in the magnitude of the mean ADCC response to peptide pools, but not to the whole gp140 protein, was also observed across the cohort of subjects over the three time points assessed. Mean NK cell-mediated ADCC responses as measured by NK cells expressing CD107a+IFN-γ+, were significantly larger between the first and third time points for Env peptides (p = 0.043), and the second pool of Pol peptides (p = 0.022), as well as the combined regulatory/accessory protein peptide pools RTV (p = 0.047) and VVN (p = 0.043). The combined summation of responses to all of the HIV peptide pools was also larger (p = 0.03). Enhanced ADCC responses were also observed between the first and second time points to the regulatory/accessory protein pools (RTV [p = 0.013] and VVN [p = 0.039]), and when the sum of responses across all HIV peptide pools was analyzed (p = 0.013). In contrast, ADCC activity to the whole gp140 protein did not significantly change across the cohort over the 2 y of follow-up.
Discussion
We observed ADCC responses to HIV during early HIV infection as measured by activation of NK cells in the presence of serum. The early appearance of ADCC responses to Env protein is consistent with a role for ADCC in the suppression of viremia during acute infection (6–8,11). Interestingly, we found that ADCC responses to linear HIV peptide pools were nearly completely absent at the earliest time point studied, but these responses gradually strengthened over the 2 y of follow-up. This suggests that HIV-specific ADCC responses are primarily being directed toward conformational epitopes early in infection, which broaden to linear responses later. This is consistent with other aspects of antiviral antibodies (18). Remarkably, we frequently detected ADCC responses to non-Env peptide pools, including Pol and the regulatory/accessory peptide pools, beyond the acute infection period. We studied a cohort of HIV-infected subjects that naturally controlled HIV viremia and had not yet required ART. Whether the ADCC responses detected play a role in maintaining control of viremia later during chronic HIV infection requires further study in larger comparative cohorts, controlled for other factors that also influence the outcome of infection. The epitopes targeted during early HIV infection are of considerable interest as vaccine antigens. Although mapping linear ADCC epitopes is possible with this assay of NK cell activation in responses to peptides, mapping important conformational epitopes will require studying a larger panel of deleted Env proteins, cyclic peptides, and the use of blocking antibodies. Furthermore, we only measured responses to one set of peptides and glycoslated gp140. It is likely that ADCC responses to other reference strains, or to the subject's own viral sequences, play an important role in viral control and warrant further study. In summary, we detected ADCC responses to Env protein very early during HIV infection using a novel flow cytometric assay of NK cell activation. Further studies of the ability of ADCC responses to prevent HIV infection and control acute infection are warranted.
Acknowledgments
Supported by National Health & Medical Research Council (NHMRC) award 510448, and NIH awards U01AI069907 and R21AI081541. The authors thank the study participants, Pat Grey (study coordinator, National Centre for HIV Epidemiology and Clinical Research), and recruiting physicians Tim Read (Melbourne Sexual Health Clinic), Jennifer Hoy (Alfred Hospital), David Cooper (St. Vincent's Hospital, Sydney), and David Baker (East Sydney Doctors).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Koup RA. Safrit JT. Cao Y, et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin X. Bauer DE. Tuttleton SE, et al. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borrow P. Lewicki H. Hahn BH, et al. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sawyer LA. Katzenstein DA. Hendry RM, et al. Possible beneficial effects of neutralizing antibodies and antibody-dependent, cell-mediated cytotoxicity in human immunodeficiency virus infection. AIDS Res Hum Retroviruses. 1990;6:341–356. doi: 10.1089/aid.1990.6.341. [DOI] [PubMed] [Google Scholar]
- 5.McMichael AJ. Borrow P. Tomaras GD, et al. The immune response during acute HIV-1 infection: clues for vaccine development. Nat Rev Immunol. 2010;10:11–23. doi: 10.1038/nri2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomez-Roman VR. Patterson LJ. Venzon D, et al. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J Immunol. 2005;174:2185–2189. doi: 10.4049/jimmunol.174.4.2185. [DOI] [PubMed] [Google Scholar]
- 7.Xiao P. Zhao J. Patterson LJ, et al. Multiple vaccine-elicited non-neutralizing anti-envelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following SHIV89.6P challenge in rhesus macaques. J Virology. 2010;84:7161–7173. doi: 10.1128/JVI.00410-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Florese RH. Van Rompay KK. Aldrich K, et al. Evaluation of passively transferred, nonneutralizing antibody-dependent cellular cytotoxicity-mediating IgG in protection of neonatal rhesus macaques against oral SIVmac251 challenge. J Immunol. 2006;177:4028–4036. doi: 10.4049/jimmunol.177.6.4028. [DOI] [PubMed] [Google Scholar]
- 9.Florese RH. Demberg T. Xiao P, et al. Contribution of nonneutralizing vaccine-elicited antibody activities to improved protective efficacy in rhesus macaques immunized with Tat/Env compared with multigenic vaccines. J Immunol. 2009;182:3718–3727. doi: 10.4049/jimmunol.0803115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connick E. Marr DG. Zhang XQ, et al. HIV-specific cellular and humoral immune responses in primary HIV infection. AIDS Res Hum Retroviruses. 1996;12:1129–1140. doi: 10.1089/aid.1996.12.1129. [DOI] [PubMed] [Google Scholar]
- 11.Forthal DN. Landucci G. Daar ES. Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural-killer effector cells. J Virol. 2001;75:6953–6961. doi: 10.1128/JVI.75.15.6953-6961.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rerks-Ngarm S. Pitisuttithum P. Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 13.Chung AW. Rollman E. Center RJ, et al. Rapid degranulation of NK cells following activation by HIV-specific antibodies. J Immunol. 2009;182:1202–1210. doi: 10.4049/jimmunol.182.2.1202. [DOI] [PubMed] [Google Scholar]
- 14.Stratov I. Chung A. Kent SJ. Robust NK cell-mediated human immunodeficiency virus (HIV)-specific antibody-dependent responses in HIV-infected subjects. J Virol. 2008;82:5450–5459. doi: 10.1128/JVI.01952-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hecht FM. Wang L. Collier A, et al. A multicenter observational study of the potential benefits of initiating combination antiretroviral therapy during acute HIV infection. J Infect Dis. 2006;194:725–733. doi: 10.1086/506616. [DOI] [PubMed] [Google Scholar]
- 16.Falster K. Gelgor L. Shaik A, et al. Trends in antiretroviral treatment use and treatment response in three Australian states in the first decade of combination antiretroviral treatment. Sex Health. 2008;5:141–154. doi: 10.1071/sh07082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnott A. Jardine D. Wilson K, et al. High viral fitness during acute HIV-1 infection. PLoS One. 2010:5. doi: 10.1371/journal.pone.0012631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baumeister MA. Medina-Selby A. Coit D, et al. Hepatitis B virus e antigen specific epitopes and limitations of commercial anti-HBe immunoassays. J Med Virol. 2000;60:256–263. [PubMed] [Google Scholar]