Abstract
Nitric oxide-mediated vasodilatation has previously been attributed to the uncharged form of the molecule (NO•), but increasing evidence suggests that nitroxyl (HNO) may play a significant role in endothelium-dependent relaxation. The aim of this study was to investigate the mechanisms underlying HNO-mediated vasodilatation in phenylephrine pre-constricted pressurized (70 mmHg) mesenteric arteries, and on membrane currents in isolated smooth muscle cells using whole cell and perforated patch clamp recordings. Angeli's salt (AS: nitroxyl donor), evoked concentration-dependent vasodilatation that was insensitive to the NO• scavengers carboxy-PTIO and hydroxocobalamin (HXC), but sensitive to either the HNO scavenger L-cysteine, K-channel blockers (4-AP and iberiotoxin), raised [K+]o, or inhibition of soluble guanylyl cyclase (ODQ). AS-evoked smooth muscle hyperpolarization significantly augmented KV current in an ODQ sensitive manner, and also increased the BKCa current. Importantly, 30 μM AS initiated conducted or spreading vasodilatation, and following blockade of endothelial K-channels (TRAM-34 and apamin), ACh was able to evoke similar L-cysteine-sensitive conducted dilatation. These data show that vasodilatation induced by HNO is mediated by both KV and BKCa channels, and suggest a physiological role in vasodilatation through the vasculature. Antioxid. Redox Signal. 14, 1625–1635.
Introduction
The endogenous release of nitric oxide (NO) by the endothelium modulates vascular tone and consequently regulates both blood pressure and flow. NO can exist in several different redox forms, including the uncharged free radical state (NO•), in the oxidized state as nitrosonium cation (NO+), and in the one-electron reduced and protonated form as nitroxyl (HNO) (23).
The vasodilator action of NO has always been thought to be due to uncharged NO•, whether applied exogenously as an NO donor or after direct stimulation of the endothelium (7, 18). However, more recent evidence suggests that HNO may also be active endogenously (22, 33, 35, 38), and therefore likely to be of physiological significance. Indeed a number of potential biosynthetic pathways for HNO generation have been identified, including a) direct oxidation of the nitric oxide synthase (NOS) intermediates hydroxylamine (8) and N-hydroxy-L-arginine (19); b) nitrosothiol decomposition (44); c) direct reduction of NO• by either mitochondrial cytochrome c, ubiquinol, and/or xanthine oxidase (20, 36), and d) by NOS in the absence of the cofactor tetrahydrobiopterin (BH4) (1).
The potential importance of HNO in the vasculature was suggested by the work of Fukuto and colleagues (19), who demonstrated that the HNO donor, Angeli's salt (AS; sodium trioxodinitrate), evoked a marked guanylyl cyclase-dependent vasodilatation in rabbit aorta and bovine intrapulmonary arteries. Further studies in the rat aorta (15, 25, 42), and the resistance vasculature (3, 16, 24), confirmed that like NO•, HNO induces vasodilation via stimulation of soluble guanylyl cyclase and an increase in cGMP. The possibility that HNO is an endothelium-derived relaxing factor (EDRF) then followed the demonstration that this reduced form of NO can be generated endogenously in sufficient amounts to affect vasodilatation, and that endothelium-dependent relaxation is sensitive to scavengers of both NO• and HNO (3, 15, 34, 42). At least in part, this effect of endogenous nitrogen oxides may be due to K channel activation, because in rat mesenteric arteries NO• can activate calcium-activated K channels (KCa) and ATP-sensitive K channels (KATP), causing hyperpolarization (29, 37). But in the same resistance arteries, Irvine and colleagues (24) have shown that HNO can mediate vasodilatation through the activation of KV channels. Together with recent observations, these data suggest that HNO contributes to the endothelial hyperpolarizing effect of acetylcholine, and thus may be an endogenous endothelium-derived smooth muscle hyperpolarizing factor (EDHF) (3). However, it is not clear which K channels are key, and furthermore if HNO really does have a significant input to physiological responses in the vasculature.
The aim of the current study was therefore to address both of these points by investigating the role of HNO in the dilatation of rat resistance arteries under physiological pressure. Furthermore, as agents capable of evoking arterial hyperpolarization can also initiate spread of arterial dilatation over distance, we addressed the possibility that HNO may share this characteristic. Spreading or conducted vasodilatation is thought to be an important physiological mechanism to ensure local dilatation (e.g., of metabolic origin) is amplified sufficiently to ensure an adequate increase in blood flow. We have shown spreading vasodilatation occurs in pressurized mesenteric arteries (39, 43). Finally, we define directly the effect of HNO on specific ion channel currents recorded from individual mesenteric artery smooth muscle cells.
Materials and Methods
Rat mesenteric artery isolation and cannulation
Male Wistar rats (200–250 g) were killed by cervical dislocation in accordance with Schedule 1 of the Animals (Scientific Procedures) Act 1986, UK. The entire mesenteric bed was removed and placed in cold MOPS buffer containing (mM): NaCl, 145.0; KCl, 4.7; CaCl2 · 2H2O, 2.0; MgSO4 · 7H2O, 1.17; MOPS, 2.0; NaH2PO4 · H2O, 1.20; glucose, 5.0; pyruvate, 2.0; EDTA, 0.02; NaOH, 2.75, and adjusted to pH 7.40 ± 0.02. Third-order branches of the superior mesenteric artery were dissected, isolated, and cannulated at each end with small glass pipettes (external diameter ∼180 μm) and positioned near the base of a temperature-regulated chamber (RC-27 chamber, PH-6 platform, Warner Instruments, Hamden, CT) for observation on an IX71 (Olympus) inverted microscope, as described previously (28, 39, 43). The MOPS-buffered solution was heated to 36.5 ± 0.2°C in all experiments. Vessels were pressurized to 70 mmHg by a gravity-fed inflow and outflow system, and the lack of small side-branches was ascertained by absence of diameter change following occlusion of perfusion inflow. Arteries were maintained at 70 mmHg for the duration of the experiment, and submaximally contracted with phenylephrine (0.5–2 μM) to generate a consistent, comparable level of tone in each experiment. Endothelial cell viability was assessed as > 95% relaxation to 1 μM ACh. Relaxation to AS was induced by bath application. Arterial outer diameter was measured online using motion analysis software (Vedi View v.1.2, DMT, Denmark). Dilatation was expressed as a percentage of the maximal diameter of the artery (∼ 300–350 μm), and contraction (or tone) calculated as a percentage of the minimal artery diameter (near 100 μm). Data are summarized as means ± S.E.M. of n replicates, where n is quantity of vessels with each obtained from an individual animal. Statistical analyses were performed using Student's unpaired t-test or one-way ANOVA analysis followed by Bonferroni post test. A value of p < 0.05 was considered to be statistically significant.
Rat mesenteric artery electrophysiology
Third-order mesenteric arteries were mounted in a Mulvany-Halpern wire myograph (Model 400A, Danish MyoTech, Aarhus, Denmark) in either Krebs-buffered solution containing (mM): NaCl, 118.0; NaCO3, 25; KCl, 3.6; MgSO4 · 7H2O, 1.2; KH2O4, 1.2; glucose, 11.0; CaCl2, 2.5; and gassed with 95 % O2 and 5 % CO2 or MOPS buffered-solution as above, each at 36.5–36.8°C. Following equilibration, arteries were set to a resting tension equivalent to that generated at 0.9 times the diameter of the vessel at 70 mmHg. Unless otherwise stated, only arteries with functional endothelium were used, assessed as over 95% relaxation to 1 μM ACh. Sharp glass microelectrodes were used to measure the membrane potential of individual smooth muscle cells or endothelial cells (filled with 2 M KCl, tip resistances 70–100 MΩ), as previously described (10). Rapid deflections in membrane potential to near −50 mV were observed upon cell impalement. The membrane potential was recorded via a pre-amplifier (Neurolog system, Digitimer Ltd., Welwyn, U.K.) linked to a MacLab data acquisition system (AD Instruments Model 4e) at 100 Hz. Drugs were added directly to the 10 ml bath, and mixed by gassing. There was no difference in resting membrane potential or in the response to agents with either buffer solution, so data were pooled.
Smooth muscle cell isolation from rat mesenteric artery
Freshly dissected third-order mesenteric arteries were placed in ice-cold calcium-free isolation solution containing (mM): NaCl, 140; KCl, 4.7; MgCl2, 1.2; glucose, 10; HEPES, 10 (pH 7.4). After incubation on ice for 10 min, the arteries were transferred to the calcium-free isolation solution, containing 1 mg/ml papain (Sigma, St. Louis, MO), 1 mg/ml collagenase (Sigma), and 1 mM dithiothreitol, and allowed to digest for 20 min at 37°C. Following digestion, the tissue was twice washed in isolation solution before gentle trituration to disperse cells. After centrifugation for 2 min at 1,000 rpm, the supernatant was removed, and the cells resuspended in fresh isolation solution. The concentration of extracellular calcium was increased over the next 30 min to 750 μM. Freshly isolated cells were maintained on ice for use on the same day.
Electrophysiological recordings
Membrane potential and whole cell recordings were made mainly with voltage clamp electrodes, using the whole-cell and current-clamp modes and an Axopatch 200B amplifier and Digidata 1332A interface (Axon Instruments, Molecular Devices, Sunnyvale, CA), and piezoelectric (PCS-5000 series) precision micromanipulator (Burleigh, New York). Cells were placed in a recording chamber (RC-25F; Warner Instruments, Hamden, CT) and left to adhere to the cover glass for approximately 10 min. Cells were then continually superfused (∼1 ml/min) with heated solution (SH-27B Inline Heater; Warner Instruments; 37°C) via a multi-barrel gravity-fed perfusion system and observed using an inverted microscope (Olympus IX71). The physiological saline solution (PSS) used for superfusion contained (mM): NaCl, 140; KCl, 4; CaCl2, 1.5; MgCl2, 1.2; HEPES, 10; glucose, 5; pH 7.4. Membrane potential recordings were performed in current-clamp mode, using the perforated patch technique, and the pipette solution contained (mM): KCl, 130; NaCl, 10; HEPES, 10; MgCl2, 0.5; CaCl2, 0.5; amphotericin B (200 μg/ml), pH to 7.3.
Voltage-gated potassium channel current (IKv) was recorded in whole cell patch clamp mode, and elicited by 10 mV step depolarization to more positive potentials from a holding potential of −80 mV, using the standard PSS described above. The pipette solution contained the following (mM) KCl, 140; MgCl2, 0.5; HEPES, 10; EGTA, 10; CaCl2, 0.5; Na2ATP, 1; GTP, 0.5; pH 7.3. These recordings were performed in the presence of 1 μM paxilline and 10 μM glibenclamide in the external PSS, in order to block calcium activated and ATP-sensitive K currents, respectively. IKv was inhibited by application of 1 mM 4-AP.
Experiments to record calcium-activated potassium channels utilized the standard PSS with the additional inclusion of 10 μM glibenclamide to inhibit ATP sensitive potassium currents. The patch solution contained (in mM) KCl, 140; MgCl2, 0.5; HEPES, 10; CaCl2, 0.5; Na2ATP, 1; GTP, 0.5; pH 7.3. Paxilline (1 μM) was applied at the end of the protocol, to inhibit calcium activated potassium currents, and currents subtracted from the equivalent records in control and drug to give the paxilline-sensitive current.
The osmolarity of all solutions was measured and corrected to 300 ± 5 mOsm using mannitol. All electrophysiological recordings were performed at 37°C, and using an agar bridge (2% agar filled with 3 M KCl). Cell membrane capacitance was measured using a 10 mV hyperpolarizing step pulse in order to normalize currents to cell size (pA/pF). Angeli's salt was infused with a BeeHive® syringe pump system (Bioanalytical Systems, Lafayette, IN) into an injection port in the superfusion line, directly upstream from the recording chamber.
Data were acquired using Clampex 8.0.2 and Axoclamp 9 (Axon Instruments) and analyzed offline using Clampfit (Axon Instruments). Values are expressed as means ± SEM. Student's paired two-tailed t test was used to compare parameters obtained in control and test conditions in the same cell, followed by the Bonferroni post test. A nonpaired Student's t-test was used to compare the difference between groups of data. A value of p < 0.05 was considered to be statistically significant.
Spreading dilatation to luminally perfused agonists
In this series of experiments, three cannulation pipettes were used as previously described (43). The three ends of an isolated artery with a side branch were cannulated and mounted in a heated chamber and continuously superfused at 2 ml/min with heated MOPS solution. The upstream end of the artery (Feed artery) and one side of the bifurcation (Branch 2) were attached to the gravity-fed pressurizing syringe reservoirs. Phenylephrine was added to the superfusion solution, and each agonist used to study spreading dilatation was infused locally into Branch 1 for at least 2 min at 20 μl/min using a BeeHive® syringe pump system. In all experiments, the movement of perfusion solution was monitored by including 0.1 μM carboxyfluorescein in the agonist solution. Spread of dilatation upstream away from the area of local application could then be assessed throughout the feed artery. Arteries were visualized using a laser scanning confocal microscope (FV500-SU, Olympus, Japan, excitation 488 nm, emission 505 nm) to enable simultaneous fluorescence and brightfield imaging with a 4 × /0.13 NA objective (UplanFl, Olympus, Japan), and images were recorded at 1 Hz using Fluoview software (Olympus, USA). For all measurements of spreading dilatation, artery outer diameter was measured offline with motion analysis software (MetaMorph, Universal Imaging). This enabled simultaneous analysis of multiple, calibrated distances along the artery wall, and direct comparison of local dilatation (Branch 1) to spreading dilatation (0–2.0 mm upstream along the Feed artery) for a single application of agonist. Fluorescence intensity was also measured offline simultaneously at multiple positions in the lumen of arteries, which was temporally matched to diameter measurements. Feed artery diameter was only measured if no upstream flow of fluorescent indicator was detected, as previously shown (43). Values for summary data were taken at the time point where dilatation at the 0 mm position along the feed artery reached 80% of maximal diameter.
Drugs
All drugs were obtained from Sigma (Poole, UK) with the exception of AS (sodium trioxodinitrate) from Axxora (Nottingham, UK) and iberiotoxin (Latoxan, Valence, France). AS was dissolved in 10 mM NaOH to prevent decomposition prior to use (17). Responses to AS and NO• were recorded in the absence and presence of the HNO scavenger L-cysteine (3 mM, at least 5 min), NO• scavengers hydroxocobalamin (HXC, 100 μM, at least 15 min) or carboxy-PTIO (200 μM). In experiments with the sGC inhibitor 1H-(1,2,4)oxadiazole(4,3-a)quinoxalin-1-one (ODQ), cells were incubated in 10 μM ODQ for 15 min, and it was also included in the perfusion solutions. Responses to AS were also performed in the presence of the KV channel inhibitor, 4-aminopyridine (4-AP, 1 mM). All other stock solutions were prepared using MQ water or DMSO.
Results
Effect of the HNO donor Angeli's salt in pressurized mesenteric arteries
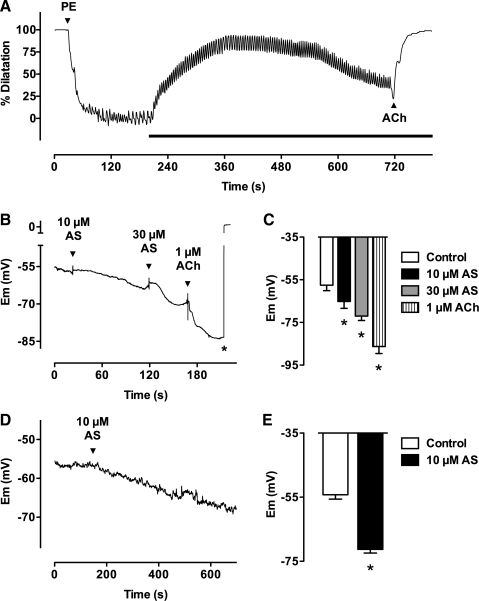
AS relaxes rat mesenteric arteries under isometric tension with a log EC50 of ∼ −7.7 (24). Herein, AS was administered to phenylephrine pre-constricted small mesenteric arteries under physiological pressure (70 mmHg), and a representative trace of vasodilatation to 30 μM AS is shown in Figure 1A. 30 μM AS evoked close to maximal dilatation within 5 min (relative to 1 μM ACh). With longer periods of application, the magnitude of vasodilatation decreased. Mean data (not shown), demonstrated that the concentration-dependent vasodilatation to AS occurred over the concentration range of 100 nM–100 μM and logEC50 −4.7 ± 0.03 (n = 8), a less potent effect than observed with wire myography. Furthermore, phenylephrine induced pre-constriction resulted in vasomotion that was consistently augmented upon application of AS.
FIG. 1.
Angeli's salt induces vasodilatation and smooth muscle cell hyperpolarization. (A) Typical trace illustrating the dilatation in response to Angeli's salt. A mesenteric artery mounted in a pressure myograph was preconstricted with phenylephrine (PE, 0.8 μM, 73% tone), and Angeli's salt (AS, 30 μM) added to the bath during the period indicated by the bar. ACh (1 μM) was added at the end. Following 5 min exposure to AS, almost complete vasodilatation was achieved, and over a longer period of time the degree of vasodilatation was reduced, perhaps resulting from decomposition of AS. Note that vasomotion was observed in these experiments, and was consistently augmented by Angeli's salt. The NO• scavenger carboxy-PTIO (200 μM) was present throughout. Maximum and minimum outer diameters were 320 and 110 μm. (B) Typical trace showing hyperpolarization to AS in an artery. Smooth muscle cell membrane potential (Em) was recorded using sharp microelectrodes in a mesenteric artery mounted in a wire myograph. Both AS (10 and 30 μM) and ACh (1 μM) evoked hyperpolarization from resting membrane potential. The microelectrode was manually pulled out of the cell at the asterisk. Data are summarized in (C), n = 6–7 from three arteries; *p < 0.05 vs control. The NO• scavenger carboxy-PTIO (200 μM) was present throughout all experiments. (D) Typical trace showing hyperpolarization to AS in a single smooth muscle cell. Perforated patch-clamp recording in current-clamp mode of membrane potential in a mesenteric artery smooth muscle cell in the presence of the NO• scavenger HXC (100 μM). The arrow indicates where 10 μM AS's was applied, resulting in a significant hyperpolarization. Data are summarized in (E), n = 5 cells from four animals; *p < 0.05 vs control.
Hyperpolarization of the resting membrane potential to HNO
Measurement of change in smooth muscle membrane potential, recorded either with sharp microelectrodes (Figs. 1B and 1C) or patch electrodes in current-clamp mode (Fig. 1D and 1E) in the presence of the NO• scavengers hydroxcobalamin (HXC) or carboxy-PTIO, revealed hyperpolarization of circa 15–20 mV. The patch clamp recordings were made from cells perforated with amphotericin B, to minimize changes to the intracellular milieu. In the sharp electrode studies, the mean resting membrane potential was −57.6 ± 2.5 mV (n = 7), increasing by 7.3 ± 2.0 mV and 14.5 ± 1.6 mV to 10 and 30 μM AS, respectively (n = 6, 7). In the perforated patch experiments, the mean resting membrane potential was −54.2 ± 1.2 mV (n = 5). Rapid bath infusion of 10 μM AS induced marked hyperpolarization by 17.0 ± 1.0 mV (n = 5), measured after 5 minutes application (p < 0.05) (Fig. 1C).
AS was diluted in 10 mM NaOH to prevent decomposition (17), and 10 mM NaOH vehicle had no significant effect on vascular tone in phenylephrine pre-constricted vessels (p > 0.05, n = 2).
Augmented K channel activity with Angeli's salt
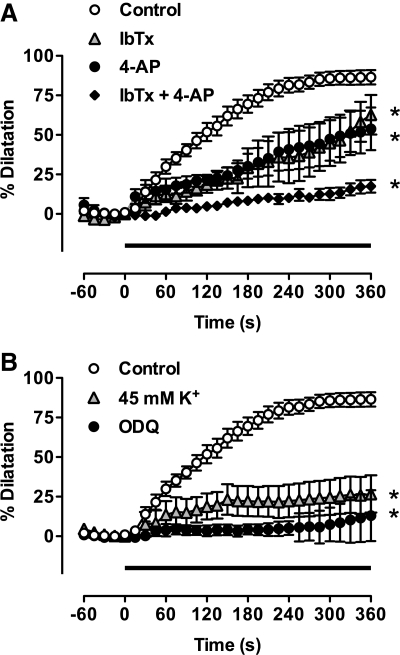
4-AP potently inhibits KV1 and KV3 type voltage-gated potassium channels (GRAC) (2). When applied in low concentrations (150 μM), 4-AP inhibited relaxation to the submaximal concentration of AS (30 μM), and slowed the time-course of the response (Fig. 2A, n = 4). Previous work in mesenteric artery by Irvine et al. (24) indicated AS-induced vasodilatation was insensitive to iberiotoxin (IbTx), suggesting that BKCa channels may not provide a significant contribution to HNO-evoked hyperpolarization/dilatation. However, in pressurized mesenteric arteries these channels seemed clearly to contribute to AS vasodilatation. Pre-incubation with the specific BKCa inhibitor IbTx (100 nM) not only caused further constriction of the vessel (to 35.3 ± 8.4% of the maximal response), but also induced vasomotion in four of seven experiments (n = 7). After 5 min application of AS, in the presence of HXC (100 μM) and IbTx, vasodilatation was significantly attenuated (Fig. 2A, n = 4; p < 0.05), and the time-course of the persistent vasodilatation considerably slower than with AS alone. In combination, 4-AP and IbTx almost completely inhibited the vasodilatation to AS, demonstrating significant input through both BKCa and KV channels (Fig. 2A). Increasing external K+ (45 mM), which causes vasoconstriction following smooth muscle membrane depolarization and abolishes ACh-evoked hyperpolarization (40), almost totally abolished vasodilatation to AS in pressurized arteries (Fig. 2B, n = 3).
FIG. 2.
The vasodilatation to Angeli's salt is mediated by BKCa and KV channels and soluble guanylyl cyclase. Summary data showing the effect of K-channel blockers (A) and high K+ and ODQ (B) on dilatation to Angeli's salt (AS). AS (30 μM) was added to the bath during the period indicated by the bar (0–360 s). The dilatation under control conditions (n = 13) was significantly reduced in the presence of either iberiotoxin (IbTx, 100 nM, n = 5) or 4-AP (150 μM, n = 5) at the 5 min point. The combination of IbTx and 4-AP almost fully inhibited dilatation to AS (n = 4). This block was matched in the presence of either 45 mM K+ (n = 5) or the sGC inhibitor ODQ (10 μM, n = 5). *p < 0.05 vs control, 5 min.
HNO and whole cell potassium channel activity
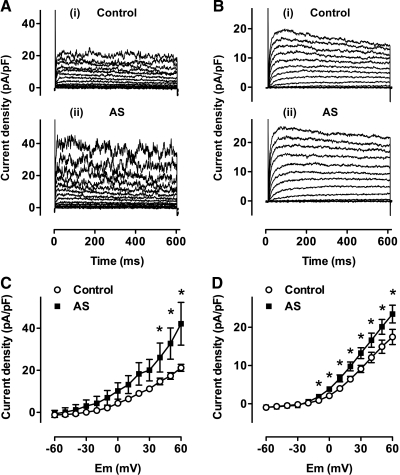
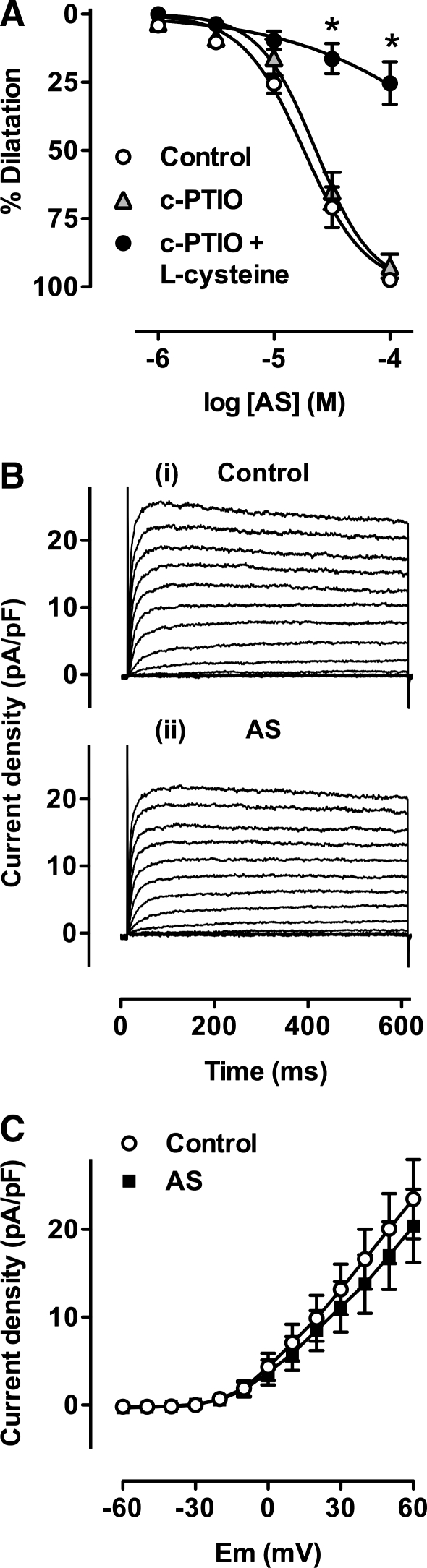
To probe further the ion channels involved in vasodilatation to HNO, experiments were performed in the whole-cell mode of the patch clamp technique using intracellular and extracellular solutions designed to minimise contamination from interfering conductances (see Materials and Methods). The NO• scavenger HXC (100 μM) was also included in all externally applied solutions. Families of BKCa channel currents were elicited by sequential step depolarization to positive test potentials, from a holding potential of −80 mV. Currents under control conditions are shown in Figure 3A (i). Application of 10 μM AS significantly enhanced these currents, as shown in the typical traces in Figure 3A (ii). Mean data in Figure 3C show enhanced BKCa channel activity at positive test potentials in 6 cells (p < 0.05), confirming directly that HNO enhances BKCa activity in small mesenteric artery smooth muscle cells.
FIG. 3.
Angeli's salt activates both BKCa and KV channels in single smooth muscle cells. (A-i) Family of BKCa currents in an isolated mesenteric artery smooth muscle cell. Control currents shown are those sensitive to 1 μM paxilline, and elicited upon 10 mV step depolarizations to more positive potentials from holding potential of −80 mV in the presence of 10 μM glibenclamide and 100 μM HXC. (A-ii) Currents elicited under the same conditions as above, but in the presence of 10 μM AS. (B-i) A family of KV currents recorded in an isolated mesenteric artery smooth muscle cell. Control currents shown are those sensitive to 1 mM 4-AP, and elicited upon 10 mV step depolarizations to more positive potentials from holding potential of − 80 mV in the presence of 1 μM paxilline, 10 μM glibenclamide, and 100 μM HXC. (B-ii) Currents elicited under the same conditions as above, but in the presence of 10 μM AS. (C, D) Mean current-voltage relationship showing an augmentation in the BKCa current (C, n = 7) and KV current (D, n = 15) to 10 μM AS at test potentials indicated by an asterisk (p < 0.05 vs control).
Figure 3B (i) shows a family of KV currents elicited by step depolarization from a holding potential of −80 mV to more positive potentials, under control conditions. Rapid bath infusion of 10 μM AS significantly augmented current amplitude (Fig. 3B (ii)). The mean current voltage relationship for 15 cells is shown in Figure 3D. Potentials marked by an asterisk in the presence of 10 μM AS are significantly greater than control (p < 0.05, n = 15). These data support the pressurized artery studies, indicating that HNO induced vasodilatation is mediated by enhancement of both KV and BKCa channel activity.
HNO augments Kv activity via soluble guanylyl cyclase
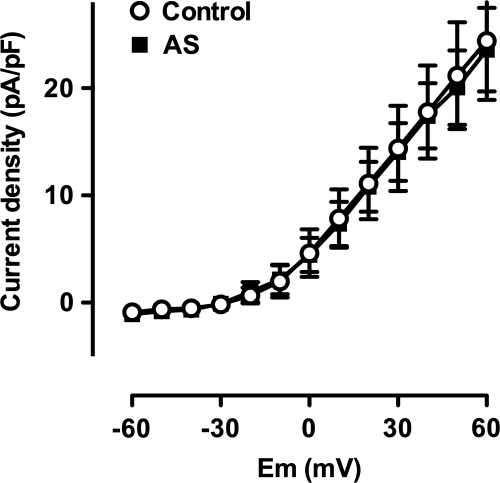
Pre-incubation of pressurized mesenteric vessels with ODQ (10 μM, n = 3), completely abolished vasodilatation. This indicated a central role for soluble guanylyl cyclase (Fig. 2B). Experiments with isolated smooth muscle cells confirmed these observations. The presence of ODQ (10 μM) completely inhibited the augmentation of KV current normally induced by AS in mesenteric artery smooth muscle cells (Fig. 4, n = 9).
FIG. 4.
Activation of IKv depends on soluble guanylyl cyclase. Mean current-voltage relationship showing full block of the augmentation in the KV current (same conditions as in Figs. 3B and 3D) to 10 μM AS in the presence of ODQ (10 μM, paired data, n = 9).
L-Cysteine ameliorates the response to Angeli's salt
To demonstrate directly if the current augmentation observed in the presence of AS was due to HNO, and not partially due to NO•, experiments were performed in the presence of the nitroxyl scavenger L-cysteine. Figure 5A shows a cumulative concentration-response curve to AS in pressurized mesenteric arteries. Whilst the NO• scavenger carboxy-PTIO did not alter vasodilatation to AS, 3 mM L-cysteine, applied together with carboxy-PTIO (24), markedly inhibited this response (n = 6). Similar responses were also observed using an alternate NO• scavenger, hydroxcobalamin (HXC) (4, 34), data not shown.
FIG. 5.
Angeli's salt induced KV augmentation is ameliorated by L-cysteine. (A) The effects of the NO• scavenger carboxy-PTIO (c-PTIO, 200 μM) alone (n = 2) or together with the HNO scavenger L-cysteine (3 mM, n = 6), on the cumulative concentration response curves to Angeli's salt (AS). *p < 0.05 vs control. (B-i) A family of KV currents recorded in an isolated mesenteric artery smooth muscle cell. Control currents shown are those sensitive to 1 mM 4-AP, and elicited upon 10 mV step depolarizations to more positive potentials from holding potential of −80 mV in the presence of 1 μM paxilline, 10 μM glibenclamide, and 100 μM HXC. (B-ii) Currents elicited under the same conditions as above, but in the presence of both L-cysteine (3 mM) and 10 μM AS. (C) Mean current-voltage relationship showing full block of the augmentation in the KV current to 10 μM AS in the presence of L-cysteine (3 mM, paired data, n = 5).
In single smooth muscle cells, AS significantly augmented KV currents as described previously (Fig. 5B upper panel). In the presence of L-cysteine and HXC, the same concentration of AS now failed to augment current amplitude (Fig. 5B lower panel). There was no significant difference in current amplitude in the presence or absence of AS in five cells incubated with L-cysteine and HXC, indicating that current augmentation is explained solely by the action of HNO.
Angeli's salt evokes spreading vasodilatation in pressurized mesenteric arteries
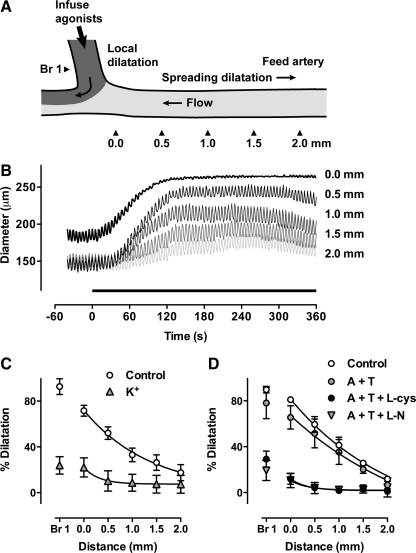
As previous studies have demonstrated that conducted vasodilatation is usually associated with agonists that either directly hyperpolarize the smooth muscle layer (39), or act indirectly by stimulating endothelium-dependent hyperpolarization (12, 39, 43), we investigated whether AS could evoke spreading responses in this preparation. Figure 6 shows that AS (30 μM), infused locally into branch 1 of a triple-cannulated artery, evoked local dilatation (of phenylephrine-induced preconstriction) that then spread upstream in the feed artery. Each individual trace shows the dilatation developed during 6 min luminal application of AS and measured at 500 μm intervals along the feed artery away from the point of infusion. Spreading dilatation was decremental, but was still significant beyond 1.5 mm (Fig. 6C). Note also that the presence of AS in Branch 1 was associated with synchronized vasomotion observed along the length of the artery. In the presence of elevated external K+ (45 mM) or the K-channel blockers TEA (1 mM) and 4-AP (150 μM), the spreading response to AS was completely abolished (Fig. 6C).
FIG. 6.
HNO induces spreading dilatation responses in pressurized rat mesenteric arteries. (A) Using a third pipette, one branch at an arterial bifurcation (Br 1) was cannulated, through which perfusate containing an agonist and fluorescent marker was infused. (B) Simultaneous traces of outer diameter in response to luminal infusion of 30 μM Angeli's salt (AS) and 0.1 μM carboxyfluorescein into Branch 1 (Br 1). The arrowheads in (A) indicate the positions of diameter and fluorescence measurement in Br 1 and upstream from the bifurcation into the Feed artery (0–2.0 mm). Phenylephrine (0.5 μM) was present in the superfusion solution, resulting in 79% of maximum tone and synchronized vasomotion along the entire arterial segment. AS was infused during the period indicated by the bar (0–360 s) and clearly evoked spreading dilatation that decayed with distance from the bifurcation, but was associated with continued synchronized vasomotion (compare to Fig. 1A). (C) Summary data showing local (Br 1) and spreading dilatation (0–2.0 mm) to luminal perfusion of 30 μM AS into Br 1 (n = 3). The data are normalized to ∼ 80% dilatation at 0 mm. The local and spreading dilatation were markedly reduced in the presence of either 45 mM K+ (n = 1) or TEA (1 mM) and 4-AP (150 μM) (n = 2), and these data were combined (K+, n = 3). (D) Summary data showing local (Br 1) and spreading dilatation (0–2.0 mm) to luminal perfusion of ACh (10 μM) into Br 1. Responses were obtained in the absence (Control) and presence of apamin (50 nM) and TRAM-34 (1 μM, A + T) (n = 4), and were normalized to ∼ 80% dilatation at 0 mm. The local and spreading dilatation were markedly reduced by the additional presence of either L-cysteine (3 mM, A + T + L-cys, n = 4), or the NOS inhibitor L-NAME (100 μM, A + T + L-N, n = 4).
Spreading dilatation with HNO is sensitive to L-cysteine
Previous work has shown that ACh, by increasing endothelial cell Ca2+, stimulates small conductance (SKCa, apamin sensitive) and intermediate conductance (IKCa, TRAM-34 sensitive) calcium-activated potassium channels (9, 10, 13, 14, 41), leading to smooth muscle cell hyperpolarization and consequent dilatation/relaxation. This hyperpolarization is observed in the presence of NOS inhibitors, which are routinely utilized in studies investigating EDHF responses, and would therefore preclude effects mediated by NO. As a recent study (3) has demonstrated that ACh, following blockade of SKCa and IKCa channels, but in the absence of NOS inhibitors, can evoke hyperpolarization and relaxation of rat mesenteric arteries via the release of HNO, we sought to establish whether this response was associated conducted vasodilatation. Figure 6D shows summary data for dilatation at 500 μm distances along the feed artery, following luminal perfusion of ACh (10 μM) and in the presence of apamin (50 nM) and TRAM-34 (1 μM) (n = 4). As with responses to AS, robust spreading vasodilatation was stimulated, and decreased with distance along the feed artery. It too was accompanied by significant vasomotion. The local dilatation in Branch 1 was reduced by 3 mM L-cysteine, and spreading dilatation was not evoked. The residual dilatation to ACh was further reduced by the NOS inhibitor L-NAME (n = 4). These data indicate that the spreading responses induced by both Angeli's salt and ACh are likely to be initiated by HNO-dependent hyperpolarization of the smooth muscle.
Discussion
This study provides direct functional evidence from mesenteric resistance arteries, under physiological pressure, that HNO can evoke significant vasodilatation. Electrophysiological data from both whole tissue and single smooth muscle cells support the suggestion that HNO affects dilatation by augmenting current flow through both voltage gated and large conductance calcium-activated potassium channels. Finally, we provide the first direct observations to suggest a physiological role for endogenous HNO, in initiating spreading vasodilatation.
A key finding in the present study is that AS can induce vasodilatation in resistance arteries under physiological pressure, and that this effect correlates with smooth muscle hyperpolarization. The fact that both hyperpolarization and dilatation were abolished in the presence of raised extracellular K+ suggests a cause and effect relationship. These data are consistent with previous observations in a variety of arterial preparations assessed mainly by wire myography (15, 16, 19, 24), demonstrating that nitroxyl, derived from AS, mediates vasodilatation in both the intact circulation and isolated arteries.
The hyperpolarization evoked by HNO appeared to be explained by the activity of both Kv and BKCa channels in the smooth muscle layers. A central role for Kv channels was suggested originally from sharp electrode and isometric tension measurements in the mesenteric artery (17, 24). Kv1.2, 1.3, and 1.5 isoforms are expressed in these arteries and may therefore underlie both the resting membrane potential and the hyperpolarization (45). As 4-AP significantly attenuated responses to AS, our data confirm an action through Kv channels. Furthermore, selective recording of whole cell Kv currents showed AS induced a significant increase in whole current density, without altering the voltage dependent activation. Taken together, these observations demonstrate that Kv channels mediate a significant part of the hyperpolarization and dilatation to AS and thus HNO.
In the coronary vasculature, KATP channels contribute to AS-mediated vasodilatation (16), and in the mesenteric artery they underlie hyperpolarization from resting potential to NO gas or acidified sodium nitrite (21). However, we did not observe any significant effect of AS on direct recordings of KATP currents in isolated smooth muscle cells (data not shown), indicating that neither vasodilatation nor the underlying hyperpolarisation receive input through KATP activation. The ability of NO to activate KATP is lost once the muscle cells are depolarized, so this characteristic may explain our observations in the pressurized arteries (21).
BKCa channels in vascular smooth muscle contribute to both the repolarization and relaxation to NO gas, and the NO donors 3-morpholino-sydnonimine (SIN-1), sodium nitroprusside (SNP) and S-nitroso-N-acetylpenicillamine (SNAP) (5, 31, 32). However, vasorelaxation induced by AS appears insensitive to the BKCa inhibitor IbTx (24). However, in this previous study IbTx was tested alone, so AS-evoked vasorelaxation might still be mediated through KV activation. We demonstrated a partial attenuation of the AS-evoked vasodilatation with IbTx. This observation was consistent with our single cell experiments, where AS clearly activated BKCa channels at positive test potentials. Furthermore, in combination 4-AP and IbTx abolished the effect of AS. So it seems reasonable to conclude that both channel types contribute to vasodilatation and the underlying smooth muscle hyperpolarization to AS.
Previous studies have suggested that NO in the form of NO• is responsible for stimulating soluble guanylyl cyclase (7). However, as discussed in a recent review by Irvine et al. (26), these studies were carried out under high-thiol-containing conditions that would be predicted to scavenge HNO. Consistent with this suggestion, is the fact that vasodilatation to AS in aorta (15, 42), anococcygeus (27), urethral (6), and mesenteric artery smooth muscle (24), among others, is sensitive to the soluble guanylyl cyclase inhibitor ODQ, indicating that HNO can affect vasodilatation by increasing intracellular cGMP. Recently, ODQ has also been shown by one of us to block both the AS-evoked repolarization and relaxation of mesenteric arteries, and to have far less effect against NO gas. These observations are therefore consistent with previous data and strongly indicate that soluble guanylyl cyclase is the signaling mechanism linking HNO to activation of KV (17). Albeit that the possibility of significant intracellular oxidation of HNO to NO• still remains to be discounted.
Our data appear to be quite clear. Vasodilatation to AS was effectively blocked with ODQ, suggesting that soluble guanylyl cyclase is the central signaling transducer in the activation of both KV and BKCa. In patch-clamp experiments, ODQ blocked the augmented KV current with AS, consistent with our data in Favaloro and Kemp–Harper (17). We are currently investigating whether BKCa channels are subject to a similar influence. However, the activation of BKCa with NO, presumably as NO•, appeared not to be sensitive to ODQ (29).
To ensure, as far as reasonably possible, that we were recording only the effect of HNO on pressurized arteries and isolated smooth muscle cells, we used pharmacological agents to distinguish between the different redox states of NO. The free radical scavengers carboxy-PTIO and HXC, both inactivate NO•, with no effect against HNO (15, 27, 42), whereas mM concentrations of L-cysteine scavenge only HNO (15, 30, 42). We found that carboxy-PTIO did not modify vasodilatation to AS, consistent with this agent acting purely via HNO, not NO•. This conclusion is supported by the fact that L-cysteine (4) applied together with carboxy-PTIO did eliminate vasodilatation. Further support comes from the single cell electrophysiological experiments, where the direct effect of AS on Kv current was markedly reduced in the presence of L-cysteine.
Probably the most important observation in the present study is the demonstration that AS stimulates spreading or conducted vasodilatation. To achieve the increases in tissue blood flow necessary to meet changing metabolic demand, the restricted local action of vasodilator agents must be conducted upstream in order to provide a significant drop in vascular resistance. Whether initiated in the endothelium or the smooth muscle, hyperpolarization can spread axially through the walls of small arteries and arterioles to affect this response (see Dora, (11) for a recent review). In small mesenteric arteries, increasing endothelial cell calcium with agonists such as ACh as well as generating NO also activates the SKCa and IKCa channels within these cells causing hyperpolarization. The hyperpolarization spreads radially into the smooth muscle to affect ‘EDHF’-mediated dilatation, but in addition somehow initiates a calcium-independent hyperpolarization at upstream sites (39). This axial hyperpolarization forms the basis of spreading dilatation. A similar response occurs when hyperpolarization is initiated in the smooth muscle by directly activating KATP channels with levcromakalim (39). Interestingly, a fundamental feature of spreading dilation is that it has not been observed following local application of NO (likely NO• with NONOate) (43), presumably because hyperpolarization was not induced. By blocking the ‘EDHF’ response to ACh specifically with blockers with apamin and TRAM-34 together, we now show that an endogenous nitrogen oxide can actually cause spreading dilatation, and that this ability appears to be explained by the generation and action of HNO. Also of note is the fact that the dilatation to HNO was superimposed with vasomotion. The possibility that HNO has an important role in vasomotion, a physiological response that remains to be fully defined, is an exciting development that merits further investigation.
In conclusion, we show that AS can induce robust and sustained vasodilatation in mesenteric resistance-size arteries under physiological pressure. This response is caused by muscle hyperpolarization, and appears to reflect the activation of both KV and BKCa channels possibly via soluble guanylyl cyclase. Based on the sensitivity of hyperpolarization and dilatation to the thiol L-cysteine, and resistance to block with carboxy-PTIO and HXC, the effects appear to be due solely to HNO. Finally, it appears that HNO can be generated endogenously by the endothelium in quantities sufficient to initiate spreading vasodilatation, suggesting an important physiological role for this reduced form of NO.
Abbreviations Used
- 4-AP
4-aminopyridine
- Ach
acetylcholine
- AS
Angeli's salt
- BKCa
big conductance calcium activated potassium channel
- cGMP
cyclic-guanosine-monophosphate
- EDHF
endothelium derived hyperpolarizing facor
- EDRF
endothelium derived relaxation factor
- HNO
nitroxyl
- HXC
hydroxocobalamin, NO• scavenger
- IbTx
Iberiotoxin, BKCa inhibitor.
- IKv
voltage-gated potassium channel current
- IKCa
intermediate conductance calcium-activated potassium channel
- KV
voltage-gated potassium channel
- NO
nitric oxide
- NO•
uncharged free radical state of NO molecule
- NO+
the nitrosonium cation in oxidized state
- NO-
the nitroxyl anion in reduced state
- NOS
nitric oxide synthase
- ODQ
soluble guanylyl cyclase inhibitor (1H-(1,2,4)oxadiazole(4,3-a)quinoxalin-1-one)
- sGC
soluble guanylyl cyclase
- SKCa
small conductance calcium-activated potassium channel
Acknowledgments
This work was supported by grants from the British Heart Foundation and Wellcome Trust, UK. KAD is a British Heart Foundation Senior Basic Science Research Fellow. CJG is a Royal Society Wolfson Merit Award holder.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Adak S. Wang Q. Stuehr DJ. Arginine conversion to nitroxide by tetrahydrobiopterin-free neuronal nitric-oxide synthase. Implications for mechanism. J Biol Chem. 2000;275:33554–33561. doi: 10.1074/jbc.M004337200. [DOI] [PubMed] [Google Scholar]
- 2.Alexander SP. Mathie A. Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(Suppl 2):S1–209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews KL. Irvine JC. Tare M. Apostolopoulos J. Favaloro JL. Triggle CR. Kemp-Harper BK. A role for nitroxyl (HNO) as an endothelium-derived relaxing and hyperpolarizing factor in resistance arteries. Br J Pharmacol. 2009;157:540–550. doi: 10.1111/j.1476-5381.2009.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonaventura D. Oliveira FS. Lunardi CN. Vercesi JA. da Silva RS. Bendhack LM. Characterization of the mechanisms of action and nitric oxide species involved in the relaxation induced by the ruthenium complex. Nitric Oxide. 2006;15:387–394. doi: 10.1016/j.niox.2006.04.260. [DOI] [PubMed] [Google Scholar]
- 5.Carrier GO. Fuchs LC. Winecoff AP. Giulumian AD. White RE. Nitrovasodilators relax mesenteric microvessels by cGMP-induced stimulation of Ca-activated K channels. Am J Physiol Heart Circ Physiol. 1997;273:H76–84. doi: 10.1152/ajpheart.1997.273.1.H76. [DOI] [PubMed] [Google Scholar]
- 6.Costa G. Labadia A. Triguero D. Jimenez E. Garcia–Pascual A. Nitrergic relaxation in urethral smooth muscle: Involvement of potassium channels and alternative redox forms of NO. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:516–523. doi: 10.1007/s002100100480. [DOI] [PubMed] [Google Scholar]
- 7.Dierks EA. Burstyn JN. Nitric oxide (NO•), the only nitrogen monoxide redox form capable of activating soluble guanylyl cyclase. Biochem Pharmacol. 1996;51:1593–1600. doi: 10.1016/0006-2952(96)00078-0. [DOI] [PubMed] [Google Scholar]
- 8.Donzelli S. Espey MG. Flores–Santana W. Switzer CH. Yeh GC. Huang J. Stuehr DJ. King SB. Miranda KM. Wink DA. Generation of nitroxyl by heme protein-mediated peroxidation of hydroxylamine but not N-hydroxy-L-arginine. Free Radic Biol Med. 2008;45:578–584. doi: 10.1016/j.freeradbiomed.2008.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dora KA. Hinton JM. Walker SD. Garland CJ. An indirect influence of phenylephrine on the release of endothelium-derived vasodilators in rat small mesenteric artery. Br J Pharmacol. 2000;129:381–387. doi: 10.1038/sj.bjp.0703052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dora KA. Gallagher NT. McNeish A. Garland CJ. Modulation of endothelial cell KCa3.1 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries. Circ Res. 2008;102:1247–1255. doi: 10.1161/CIRCRESAHA.108.172379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dora KA. Coordination of vasomotor responses by the endothelium. Circ J. 2010;74:226–232. doi: 10.1253/circj.cj-09-0879. [DOI] [PubMed] [Google Scholar]
- 12.Doyle MP. Duling BR. Acetylcholine induces conducted vasodilation by nitric oxide-dependent and -independent mechanisms. Am J Physiol Heart Circ Physiol. 1997;272:H1364–H1371. doi: 10.1152/ajpheart.1997.272.3.H1364. [DOI] [PubMed] [Google Scholar]
- 13.Edwards G. Dora KA. Gardener MJ. Garland CJ. Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- 14.Edwards G. Feletou M. Gardener MJ. Thollon C. Vanhoutte PM. Weston AH. Role of gap junctions in the responses to EDHF in rat and guinea-pig small arteries. Br J Pharmacol. 1999;128:1788–1794. doi: 10.1038/sj.bjp.0703009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellis A. Li CG. Rand MJ. Differential actions of L-cysteine on responses to nitric oxide, nitroxyl anions and EDRF in the rat aorta. Br J Pharmacol. 2000;129:315–322. doi: 10.1038/sj.bjp.0703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Favaloro JL. Kemp–Harper BK. The nitroxyl anion (HNO) is a potent dilator of rat coronary vasculature. Cardiovasc Res. 2007;73:587–596. doi: 10.1016/j.cardiores.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 17.Favaloro JL. Kemp–Harper BK. Redox variants of NO (NO• and HNO) elicit vasorelaxation of resistance arteries via distinct mechanisms. Am J Physiol Heart Circ Physiol. 2009;296:H1274–1280. doi: 10.1152/ajpheart.00008.2009. [DOI] [PubMed] [Google Scholar]
- 18.Feelisch M. Biotransformation to nitric oxide of organic nitrates in comparison to other nitrovasodilators. Eur Heart J 14 Suppl. 1993;I:123–132. [PubMed] [Google Scholar]
- 19.Fukuto JM. Chiang K. Hszieh R. Wong P. Chaudhuri G. The pharmacological activity of nitroxyl: A potent vasodilator with activity similar to nitric oxide and/or endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1992;263:546–551. [PubMed] [Google Scholar]
- 20.Fukuto JM. Bianco CL. Chavez TA. Nitroxyl (HNO) signaling. Free Radic Biol Med. 2009;47:1318–1324. doi: 10.1016/j.freeradbiomed.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Garland CJ. McPherson GA. Evidence that nitric oxide does not mediate the hyperpolarization and relaxation to acetylcholine in the rat small mesenteric artery. Br J Pharmacol. 1992;105:429–435. doi: 10.1111/j.1476-5381.1992.tb14270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hobbs AJ. Soluble guanylate cyclase: The forgotten sibling. Trends Physiol Sci. 1997;18:484–486. doi: 10.1016/s0165-6147(97)01137-1. [DOI] [PubMed] [Google Scholar]
- 23.Hughes MN. Relationships between nitric oxide, nitroxyl ion, nitrosonium cation and peroxynitrite. Biochim Biophys Acta. 1999;1411:263–272. doi: 10.1016/s0005-2728(99)00019-5. [DOI] [PubMed] [Google Scholar]
- 24.Irvine JC. Favaloro JL. Kemp–Harper BK. NO− activates soluble guanylate cyclase and KV channels to vasodilate resistance arteries. Hypertension. 2003;41:1301–1307. doi: 10.1161/01.HYP.0000072010.54901.DE. [DOI] [PubMed] [Google Scholar]
- 25.Irvine JC. Favaloro JL. Widdop RE. Kemp–Harper BK. Nitroxyl anion donor, Angeli's salt, does not develop tolerance in rat isolated aortae. Hypertension. 2007;49:885–892. doi: 10.1161/01.HYP.0000259328.04159.90. [DOI] [PubMed] [Google Scholar]
- 26.Irvine JC. Ritchie RH. Favaloro JL. Andrews KL. Widdop RE. Kemp–Harper BK. Nitroxyl (HNO): The Cinderella of the nitric oxide story. Trends Pharmacol Sci. 2008;29:601–608. doi: 10.1016/j.tips.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Li CG. Rand MJ. Effects of hydroxocobalamin and carboxy-PTIO on nitrergic transmission in porcine anococcygeus and retractor penis muscles. Br J Pharmacol. 1999;127:172–176. doi: 10.1038/sj.bjp.0702496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mather S. Dora KA. Sandow SL. Winter P. Garland CJ. Rapid endothelial cell-selective loading of connexin 40 antibody blocks endothelium-derived hyperpolarizing factor dilation in rat small mesenteric arteries. Circ Res. 2005;97:399–407. doi: 10.1161/01.RES.0000178008.46759.d0. [DOI] [PubMed] [Google Scholar]
- 29.Mistry DK. Garland CJ. Nitric oxide (NO)-induced activation of large conductance Ca2+-dependent K+ channels (BKCa) in smooth muscle cells isolated from the rat mesenteric artery. Br J Pharmacol. 1998;124:1131–1148. doi: 10.1038/sj.bjp.0701940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pino RZ. Feelisch M. Bioassay discrimination between nitric oxide (NO•) and nitroxyl (NO−) using L-cysteine. Biochem Biophys Res Commun. 1994;201:54–62. doi: 10.1006/bbrc.1994.1668. [DOI] [PubMed] [Google Scholar]
- 31.Plane F. Wiley KE. Jeremy JY. Cohen RA. Garland CJ. Evidence that different mechanisms underlie smooth muscle relaxation to nitric oxide and nitric oxide doners in the rabbit isolated carotid artery. Br J Pharmacol. 1998;123:1351–1358. doi: 10.1038/sj.bjp.0701746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plane F. Sampson LJ. Smith JJ. Garland CJ. Relaxation to authentic nitric oxide and SIN-1 in rat isolated mesenteric arteries: Variable role for smooth muscle hyperpolarization. Br J Pharmacol. 2001;133:665–672. doi: 10.1038/sj.bjp.0704127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pufahl RA. Wishnok JS. Marletta MA. Hydrogen peroxide-supported oxidation of NG-hydroxy-L-arginine by nitric oxide synthase. Biochemistry. 1995;34:1930–1941. doi: 10.1021/bi00006a014. [DOI] [PubMed] [Google Scholar]
- 34.Rajanayagam MA. Li CG. Rand MJ. Differential effects of hydroxocobalamin on NO-mediated relaxations in rat aorta and anococcygeus muscle. Br J Pharmacol. 1993;108:3–5. doi: 10.1111/j.1476-5381.1993.tb13429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rusche KM. Spiering MM. Marletta MA. Reactions catalyzed by tetrahydrobiopterin-free nitric oxide synthase. Biochemistry. 1998;37:15503–15512. doi: 10.1021/bi9813936. [DOI] [PubMed] [Google Scholar]
- 36.Saleem M. Ohshima H. Xanthine oxidase converts nitric oxide to nitroxyl that inactivates the enzyme. Biochem Biophys Res Commun. 2004;315:455–462. doi: 10.1016/j.bbrc.2004.01.081. [DOI] [PubMed] [Google Scholar]
- 37.Sampson LJ. Plane F. Garland CJ. Involvement of cyclic GMP and potassium channels in relaxation evoked by the nitric oxide donor, diethylamine NONOate, in the rat small isolated mesenteric artery. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:220–225. doi: 10.1007/s002100100453. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt HH. Hofmann H. Schindler U. Shutenko ZS. Cunningham DD. Feelisch M. No · NO from NO synthase. Proc Natl Acad Sci USA. 1996;93:14492–14497. doi: 10.1073/pnas.93.25.14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takano H. Dora KA. Spitaler MM. Garland CJ. Spreading dilatation in rat mesenteric arteries associated with calcium-independent endothelial cell hyperpolarization. J Physiol. 2004;556:887–903. doi: 10.1113/jphysiol.2003.060343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waldron GJ. Garland CJ. Contribution of both nitric oxide and a change in membrane potential to acetylcholine-induced relaxation in the rat small mesenteric artery. Br J Pharmacol. 1994;112:831–836. doi: 10.1111/j.1476-5381.1994.tb13154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walker SD. Dora KA. Ings NT. Crane GJ. Garland CJ. Activation of endothelial cell IKCa and 1-ethyl-2-benzimidazolinone evokes smooth muscle hyperpolarization in rat isolated mesenteric artery. Br J Pharmacol. 2001;134:1548–1554. doi: 10.1038/sj.bjp.0704415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wanstall JC. Jeffery TK. Gambino A. Lovren F. Triggle CR. Vascular smooth muscle relaxation mediated by nitric oxide donors: A comparison with acetylcholine, nitric oxide and nitroxyl ion. Br J Pharmacol. 2001;134:463–472. doi: 10.1038/sj.bjp.0704269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winter P. Dora KA. Spreading dilatation to luminal perfusion of ATP and UTP in rat isolated small mesenteric arteries. J Physiol. 2007;582:335–347. doi: 10.1113/jphysiol.2007.135202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong PS. Hyun J. Fukuto JM. Shirota FN. DeMaster EG. Shoeman DW. Nagasawa HT. Reaction between S-nitrosothiols and thiols: Generation of nitroxyl (HNO) and subsequent chemistry. Biochemistry. 1998;37:5362–5371. doi: 10.1021/bi973153g. [DOI] [PubMed] [Google Scholar]
- 45.Xu C. Lu Y. Tang G. Wang R. Expression of voltage-dependent K+ channel genes in mesenteric artery smooth muscle cells. Am J Physiol Gas Liv Phy. 1999;277:G1055–1063. doi: 10.1152/ajpgi.1999.277.5.G1055. [DOI] [PubMed] [Google Scholar]