Abstract
Hepatitis C virus (HCV) has a propensity to establish chronic infection that is characterized by attenuated virus-specific T-cell responses. Mechanisms leading to T-cell attenuation are poorly understood and likely involve dysfunctional interactions between antigen-presenting cells (APC) and effector/regulatory T-cells. Reports on dendritic cells (DC) have described only minor dysfunction during HCV infection. However, there is a paucity of reports regarding B-cell function, despite clear associations with B-cell-related secondary sequelae. In this study we evaluated the state of B-cells during chronic HCV infection, and observed a diminished ability to respond to mitogenic stimuli, correlating with increased apoptosis. This was in contrast to their ex vivo phenotype, which indicated ongoing chronic activation in vivo. There was a high association of HCV-positive strand RNA with B-cells in a subset of HCV patients. Interestingly, ex-vivo-derived HCV RNA-positive B-cells induced significantly greater proliferation in allogeneic T-cells than in HCV-negative B-cells, correlating with an increased generation of CD4+CD25+FOXP3+ regulatory T-cells (Tregs). In-vitro exposure of healthy peripheral blood mononuclear cells (PBMC) to HCV resulted in robust activation of resting B-cells. These HCV-exposed B-cells also showed an enhanced ability to generate Tregs. Our results provide strong evidence for a novel and paradoxical link between HCV-induced enhanced APC function and the generation of Tregs.
Introduction
Infection by hepatitis C virus (HCV), a hepatotropic positive-strand RNA virus, is a major worldwide health problem, with over 70–80% of acutely infected individuals unable to clear the virus, leading to chronic infection and associated morbidity. Pegylated interferon and ribavirin remains the standard therapy for HCV, yet it is effective in less than 50% of genotype 1 infections (45). It is well established that the successful clearance of acute HCV infection correlates with the vigor and multi-specificity of virus-specific T-cell responses (10,15,21). It is also well established that chronic infection presents with weakened CD4+ and CD8+ T-cell responses to HCV (4,44). We have previously demonstrated that successful intervention in chronic infection is associated with higher virus-specific CD4+ and CD8+ T-cells, while therapeutic failure correlates with attenuated T-cell responsiveness (29).
The mechanisms of T-cell attenuation remain incompletely understood. CD4+/CD25+/FOXP3+ T-regulatory cells (Tregs) appear to be important contributors to such sustained suppression of HCV-specific T-cell responses (6,9). The source and maintenance of regulatory T-cell generation remains, however, a topic of debate. Antigen-presenting cell (APC) dysfunction has also been implicated as a key component of effector T-cell attenuation during chronic infection, likely involving regulatory T-cell modulation (9,32,36). However, previous reports on the functional capacity of APC from chronic HCV-infected subjects have resulted in conflicting evidence (3,20,38,39), most likely due to the use of bulk APC populations, and/or in vitro-derived dendritic cells (DC). We and others have demonstrated only minor perturbations of APC function in ex-vivo-derived purified DC subsets derived from HCV patients compared to healthy subjects (2,20,38).
B-cells are a major APC population that is understudied in the context of HCV infection. Prior reports suggest only a minor role for B-cells in the outcome of infection, as HCV-specific antibody responses do not necessarily correlate with disease resolution (5,10). Also, individuals with hypogammaglobulinemia spontaneously clear virus at the same rate as the general population (14). However, there are multiple clinical secondary sequelae, including non-Hodgkin's lymphoma and mixed cryoglobulinemia, that are manifestations of aberrant B-cell immunology (25). Thus, while humoral immunity is not predictive of acute infection outcomes, B-cell function does appear to be affected during chronic infection, and this may significantly impact T-cell function.
In addition, there is mounting evidence of HCV utilizing non-hepatic reservoirs as possible propagation sites, including cells of the immune system (7,27,33,42). Previous studies have also shown that interactions between immune cells and HCV gene products may modulate cell function. For example, HCV core protein engagement with the complement receptor, gC1qR, on T-cells reduces their proliferative capacity (17). Also, the HCV envelope glycoprotein E2 inhibits natural killer cell function through interactions with the CD81 cell receptor (12,37).
We therefore hypothesized that the attenuated T-cell phenotype, and the high degree of related B-cell sequelae seen during chronic infection, could both be a result of HCV interactions with immune cells. Herein we report on the functional and phenotypic status of ex-vivo derived B-cells during chronic HCV infection, demonstrating a unique correlation between HCV-harboring B-cells and an increased potential to generate regulatory T-cells. These observations support a novel model of immune interactions during chronic viral infection, and open new possibilities for future immunotherapeutic intervention.
Materials and Methods
Donor recruitment and lymphocyte subset isolation
Donor material was obtained by blood draws per a protocol approved by the University of Texas Southwestern Medical Center institutional review board. Subject characteristics are summarized in Table 1. Peripheral blood mononuclear cells (PBMC) were isolated by a density gradient separation method using Ficoll-Hypaque (GE Healthcare, Piscataway, NJ), and were cryopreserved at 20 × 106/mL in 10% DMSO and 90% FBS (Hyclone, Rochester, NY) until use. Individual cell populations were isolated using magnetic microbeads (Miltenyi Biotec Inc., Auburn, CA). B-cells (CD19 beads and B-cell isolation kit II), monocytes (CD14 beads), CD3+ T-cells (T-cell isolation kit, negative selection), and CD25-depleted T-cells (CD25 beads used for CD25 depletion), were all isolated according to the manufacturer's instructions. Purity of isolated populations ranged from 85–98% across various experiments.
Table 1.
Subject Characteristics
| Characteristic | HCV patients n = 50 | Healthy subjects n = 23 |
|---|---|---|
| Age in years, mean (range) | 46 (24–61) | 45 (20–60) |
| Sex (M/F) | 24/26 | 10/13 |
| Aspartate aminotransferase (IU/L), mean (range) | 62 (14–177) | N/A |
| Alanine aminotransferase (IU/L), mean (range) | 80 (9–259) | N/A |
| Hepatitis C viral load (kIU/mL), mean (range) | 1989 (50–15,100) | N/A |
| Histology: grade/stage, per Batts and Ludwig | I–IV | N/A |
HCV, hepatitis C virus.
Total RNA isolation of lymphocyte subsets
Microbead-isolated lymphocyte subsets were trypsinized with 0.5% trypsin-EDTA (Invitrogen, Carlsbad, CA), and stored in RNAlater (Applied Biosystems/Ambion, Austin, TX) RNA stabilizing reagent at −80°C until use. Total RNA was extracted from 50 μL of frozen cell pellets (1 × 105 cells/pellet), as described in the RNeasy RNA isolation kit (Qiagen Inc., Valencia, CA), and eluted in 40 μL RNAse free DepC-treated water.
Strand-specific rTth RT-PCR
Nested, real-time reverse transcriptase polymerase chain reaction (RT-PCR) was used to detect positive- and negative-strand HCV RNA, as described previously (18). Up to 1 × 105 microbead-isolated lymphocytes were trypsinized with 0.5% trypsin-EDTA, and stored in RNAlater RNA stabilizing reagent at –80°C until use. Total RNA was extracted using the RNeasy RNA isolation kit. First-round cDNA synthesis was performed on an Eppendorf master cycler (20 pM of forward primer [F], CACTCCCCTGRGAGGAAC, for negative strand, and 20 pM of reverse primer [R], TGCACGGTCTACGAGACCTC for positive strand) using 5 U of the thermostable enzyme Tth DNA polymerase (Applied Biosystems/Ambion), 1 × RT buffer, 1 mM MnCl2, and 200 μm of each dNTP. After 20 min at 70°C, the reaction was chelated with 10 × chelating buffer and 2.2 mM MgCl2, and the reaction volume was adjusted to 100 μL with nuclease-free water, along with the addition of other primer and initial PCR. A second, nested PCR analysis (F: ACTGTCTTCACGCAGAAAGCGTC; R: CAAGCACCCTATCAGGCAGTACC) was performed on 10 μL of the strand-specific products using a real-time thermocycler (Mx3000P; Stratagene, Santa Clara, CA) with SybrGreen incorporated, with the following cycles: 95°C × 10 min × 1; (95°C × 30 min, 57°C × 1 min, 72°C × 30 sec) × 30; 95°C × 1 min, followed by melting curve analysis, as described previously (11).
3H-thymidine-based proliferation assays
Mixed lymphocyte reactions (MLR) were set up as described previously (2). Briefly, stimulator cells were plated in 96-well tissue culture plates at 2 × 104 cells/well combined with 2 × 105 responder cells, in 200 μL of media (RPMI, 5% heat-inactivated human sera, penicillin, and streptomycin). Triplicate cultures were set up for each condition. Cells were pulsed with 0.25 μCi/well of 3H-thymidine for the last 12–18 h of culture. On day 6 of culture, the cells were harvested and analyzed for incorporation of radioactivity using a beta-counter. Results are expressed in counts per minute (CPM) or ΔCPM (background subtracted). For each experimental set-up, at least one pair of HCV and healthy donor stimulator cells were used, and the third-party responder cells were kept constant. For B-cell proliferation, the cells were stimulated for 5 d with staphylococcus protein A (pansorbin; Calbiochem, San Diego, CA), or pokeweed mitogen.
CFSE-based proliferation assays
Magnitude of proliferation of allogeneic CD4+25− T-cells was determined by 5-carboxyfluorescein diacetate succinimidyl ester (CFSE)-based flow cytometric assays, as described previously (11,29). Briefly, CFSE-stained responder cells were re-suspended in cell culture media at 2 × 106 cells/mL, and mixed with allogeneic B-cells or monocytes from healthy donors, or chronic, treatment-naïve HCV patients, at a 10:1 ratio (2 × 105 T-cells:2 × 104 APC) in 96-well plates. The mixed lymphocyte reactions were incubated for 7 d, followed by cell surface staining for CD4 (PE), CD25 (APC), and CD19 (PE-cy7) (all BD Biosciences, San Jose, CA), and intracellular staining for FOXP3 (Alexa Fluor; eBioscience, San Diego, CA). Analysis of flow cytometric data was performed on a BD LSR II flow cytometer (BD Biosciences), and relied on CFSE dilution to assess the magnitude of proliferation. At the same time, CD25 and FOXP3 expression was also evaluated.
Flow cytometric suppression assays
CFSE-stained CD4+CD25− T-cells were used as responders. PKH-stained, irradiated T-depleted PBMC were used as APC. CD25+ T-cells (putative suppressors) from 7-day MLR cultures were stained with CMTPX (Molecular Probes, Eugene, OR), and tested for suppressive activity. A constant number of responder cells and APC were stimulated with anti-CD3 (OKT3). In replicate cultures, increasing numbers of CMTPX-stained putative suppressor cells were added. On day 6 of culture, the cells were stained for CD4 and CD25, and flow cytometric data were collected as described above. CMTPX and PKH staining allowed for exclusion of APC and putative suppressors from the analysis. The magnitude of responder proliferation was quantified based on CFSE dilution.
Flow cytometric evaluation of B-cell activation
CD19-PECy7 staining was used to detect B-cells, either ex vivo or following in-vitro cultures. Expression of CD25, CD81, and CD38 (APC); CD69, CD23, and CD86 (PE); HLA-DR, CD27, and CD71 (FITC) was evaluated based on staining with the relevant isotype controls (BD Biosciences).
JFH-1 virus
Human hepatoma Huh7.5 cells were grown in Dulbecco's minimum essential medium, 10% fetal bovine serum, 1 × penicillin, streptomycin, and 1 × nonessential amino acids (Invitrogen). The S-JFH-1 virus was a kind gift from Drs. Michael Gale Jr. and Julie Pfeiffer. Full-length cDNA was synthesized by the laboratory of Dr. Gale (University of Washington, Seattle, WA), based upon the published sequence of HCV 2a JFH-1 genome RNA (16,41). cDNA assembly was conducted by ligating overlapping length cDNA that was appended with the T7 promoter sequence, and was subsequently cloned into the Xba-1 site of a recipient plasmid. The resulting cDNA clone, pS-JFH1, was sequence-verified as an exact match of the published sequence of HCV strain JFH1 (full details of the pS-JFH1 construction and characterization will be presented elsewhere). The virus was passaged in Huh7.5 cell line. Briefly, 5 × 106 Huh7.5 cells were inoculated with 2 × 104 ffu of JFH1 virus for 48 h, then split into 3 T-150 flasks, and the supernatant was harvested every 5 d thereafter (media contained only 2% FBS). One flask was maintained for the continued propagation of virus. In infection experiments, media from non-infected cells was concentrated in a similar manner to serve as a mock control.
In vitro JFH-1 infection assays
Then 2 × 106 PBMC were incubated with JFH-1 virus at an MOI of 0.1 for 3 h, were subsequently washed 2 × with PBS, and then were reseeded in 6-well tissue culture plates. Concentrated media from non-infected Huh7.5 cells was used as a mock infection control. Cells were harvested and stained at the indicated time points, or used as APC in MLR. In some of the experiments, cells were incubated with a cocktail of anti-CD81 antibodies (AbD serotec, clone MCA 1847; BD Pharmingen, and MA clone JS81; RDI Division of Fitzgerald Industries, Concord, MA, at 100 μg/mL) for 30 min prior to the addition of virus.
Statistical analyses
Student's t-tests (paired and unpaired, as appropriate) were used to compare the responses between different groups. p Values <0.05 were considered significant.
Results
B-cells from HCV patients have an attenuated activation potential
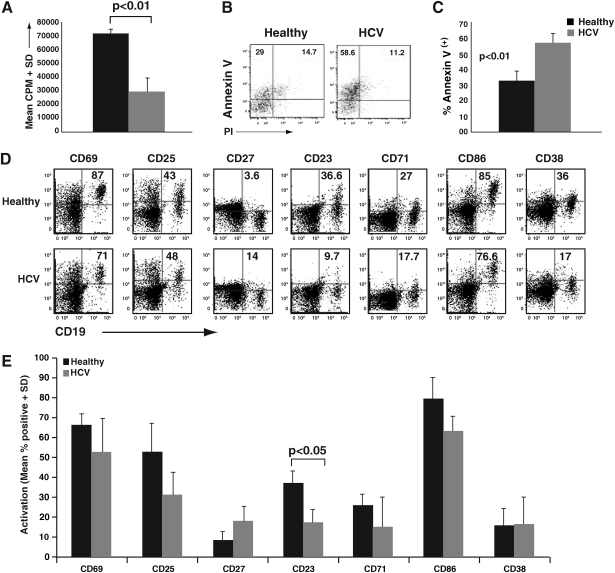
To address the functional status of B-cells in HCV patients, we initially evaluated their ability to respond to activation. Clonal expansion is a hallmark of B-cell activation, and induction of polyclonal proliferation serves as a useful indication of this potential. We isolated CD19-positive B-cells from chronic HCV patients and healthy subjects, and compared their proliferative response following pansorbin stimulation using 3H-thymidine uptake. We found that, compared to healthy controls, B-cells from HCV patients had a significant reduction in proliferation (Fig. 1A; p < 0.01). We subsequently evaluated whether this hypo-responsiveness might be due to increased apoptosis. Indeed, pansorbin-activated B-cells from HCV patients showed a greater degree of early apoptosis (annexin V+/PI−) than B-cells from healthy donors (Fig. 1B and C).
FIG. 1.
B-cells from HCV patients have an attenuated activation potential. (A) B-cells from chronic HCV patients or healthy donors were isolated using CD19 magnetic microbeads. First, 2 × 105 B-cells (triplicate cultures in 96-well plates) were stimulated for 4 d with a 1:1000 dilution of pansorbin (protein A). 3H-thymidine was added in the last 16 h of culture and the scintillation counts were measured. The data are representative of replicate experiments from three separate donor HCV pairs. (B) CD19+ B-cells from chronic HCV patients or healthy donors were incubated for 2 d with a 1:5000 dilution of pansorbin (protein A). The cells were then stained with annexin V-FITC, propidium iodide (PI), and CD19-APC. Flow cytometry dotplots indicate the percent expression of annexin V versus PI, and are representative of five separate experiments. (C) Cumulative data of pansorbin-stimulated CD19+ B-cells, depicting percentages of annexin V+/PI− B-cells, and indicating cells undergoing early apoptosis, showing significant differences in healthy versus HCV B-cells (p < 0.01). (D) Bulk PBMC from chronic HCV patients or healthy donors were incubated for 24 h with 5 μg/mL of pokeweed mitogen. The cells were subsequently stained with the following three panels of activation markers: (1) CD19-PECy7, CD25-APC, and CD69-PE; (2) CD19-PECy7, CD27-FITC, and CD23-PE; and (3) CD19-PECy7, CD71-FITC, CD86-PE, and CD38-APC. Representative dotplots from a single healthy donor and an HCV patient are shown. Numbers indicate percentages of expression of each marker on gated B-cells. (E) Cumulative data from nine HCV patients and four healthy control subjects, depicting percentage expression of the indicated markers. There was an overall trend toward lower induced activation on HCV B-cells. Statistical significance was observed for CD23 differences between healthy subjects and HCV patients (p < 0.05).
To determine if B-cells from HCV patients were concomitantly hypo-responsive to activation, we flow cytometrically evaluated B-cell activation/differentiation marker expression in response to pokeweed mitogen (PWM) stimulation, which did not cause increased annexin V staining (data not shown). PBMC were stimulated with PWM for 24 h, and B-cells were flow cytometrically evaluated for the expression of multiple activation markers (Fig. 1D and E). Overall, there was a trend toward a lower activation potential of B-cells from HCV patients, with CD23 reaching statistical significance (Fig. 1E). Interestingly, CD27 expression (a marker of memory B-cells) trended toward higher expression on HCV B-cells. In all, the innate immune response characteristics of ex-vivo derived B-cells from the HCV cohort suggests an attenuated or exhausted phenotype that potentially results from constant in-vivo stimulation.
B-cells from HCV patients are associated with HCV RNA, which correlates with an enhanced ability to stimulate T-cells
Direct contact of circulating immune cells with the hepatitis C virus is an intriguing possibility that is signified by the expression of a viral co-receptor, CD81, on most immune cell populations. To assess the extent of immune cell tropism, we adopted an HCV strand-specific RT-PCR protocol from Lanford et al. (as summarized in the materials and methods section) that we confirmed to amplify a minimum of 10 viral RNA copies, and to have the capacity to detect the correct RNA strand in the presence of 10,000-fold excess of the opposite strand (19). Using this approach, we evaluated for HCV RNA in PBMC from chronic HCV patients. Using magnetic microbeads, PBMC were first separated into CD19-positive B-cells, CD14-positive monocytes, and CD19/CD14-depleted PBMC, followed by trypsin treatment, and a nested real-time RT-PCR. As shown in Table 2, we detected HCV positive-strand RNA in the B-cell fraction of 15/50 patients (30%). Only 3/42 (∼7%) of patients showed detectable virus in their monocyte and/or CD19/CD14-depleted PBMC cell fractions. In all cases in whom RNA was detected in the non-B-cell fractions, it was also found in B-cells.
Table 2.
HCV RNA Detection in Cells from Treatment-Naïve, Chronic HCV Patients
| Positive-strand frequency | Negative-strand frequency | |
|---|---|---|
| B-cells (CD19) | 15/50 (30%) | 2/50 (4%) |
| Monocytes (CD14) | 3/42 (7%) | 0/42 (0%) |
| CD19/CD14-depleted PBMC | 2/42 (5%) | 1/42 (2%) |
| Healthy subject B-cells | 0/23 (0%) | 0/23 (0%) |
| Healthy subject B-cells pre-exposed in vitro to HCV-positive serum | 0/4 (0%) | 0/4 (0%) |
PBMC, peripheral blood mononuclear cell; HCV, hepatitis C virus.
We did not detect HCV negative-strand RNA to any appreciable degree. In the 2 of 50 cases it was detected in the B-cell fraction, yet the level was very close to the sensitivity limit of the assay. Thus overall, we did not find strong evidence for viral replication among ex-vivo-derived PBMC subsets. Of note, the possibility of passive carriage of virus by the B-cells was tackled in two ways. First, all preparations were initially trypsinized to eliminate such carryover. Furthermore, control experiments were performed in which PBMC from healthy donors were incubated with serum from HCV-infected patients, followed by nested RT-PCR evaluation, confirming no detection of virus (Table 2). The PCR results suggest the possibility of immune-cell tropism with a high viral propensity for B-cells.
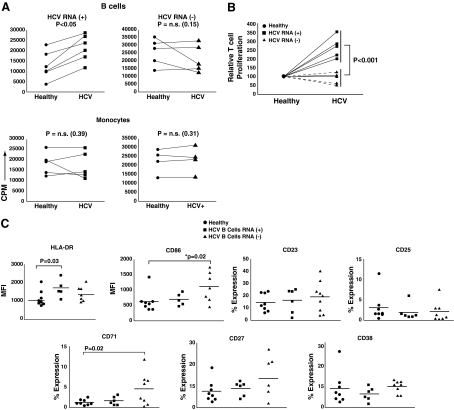
Regardless of active viral replication, it is entirely possible that HCV association with B-cells may result in functional consequences (e.g., via virus entry mechanisms and/or viral protein production). We therefore evaluated whether the presence of HCV RNA in B-cells affected their antigen-presenting function, using an MLR. We cultured ex-vivo-isolated B-cells from either healthy donors or chronic HCV patients with third-party healthy T-cells, and determined the proliferation response at 6 days of culture using 3H-thymidine incorporation. Each experiment contained at least one healthy-HCV pair, while the same responder T-cells from a third-party healthy donor were used across all experiments (controlling for HLA variability) (2). As shown in Fig. 2A, B-cells that harbored detectable HCV RNA showed significantly higher allostimulation (compared to their paired healthy subjects), while the HCV RNA−/healthy B-cell pairs did not show significant differences. Importantly, monocytes from either cohort were not significantly different from healthy subjects, further ruling out HLA differences as a possible explanation for these findings. Fig. 2B shows the data from all B-cell-induced MLR, normalized to responses induced by healthy B-cell controls (designated as 100). This depiction of response magnitude clearly illustrates the significantly different activating potential of HCV RNA+ B-cells, compared to B-cells in which no RNA was detected.
FIG. 2.
HCV RNA+ B-cells induce significantly greater MLR responses and exhibit an activated phenotype. (A) Microbead-purified B-cells (top panels), and monocytes (bottom panels), from up to 13 healthy donors and 13 HCV-infected patients were used to stimulate purified allogeneic CD3+ T-cells from one of two healthy third-party subjects. Every experimental set-up consisted of at least one healthy-HCV pair against the same third-party donor. Allogeneic cultures were pulsed with 3H-thymidine on day 5 and harvested on day 6. Responses of T-cells at a stimulator:responder ratio of 1:10 are represented as CPM on the y-axis. Data are separated based on HCV RNA status of the patients' B-cells: patients with HCV RNA+ B-cells are represented in the left two panels, whereas those with HCV RNA− B-cells are represented in the right two panels. (B) B-cell data from panel A were normalized for responses generated from healthy control subjects (assigned as 100). Responses induced by HCV RNA+ B-cells were significantly greater than those from HCV RNA− B-cells (p < 0.001). (C) PBMC from 14 HCV patients (6 B-cell HCV RNA+ and 8 HCV RNA−), and 8 healthy donors were stained ex-vivo for HLA-DR, CD23, CD25, CD27, CD38, CD71, and CD86. The expression level of each marker was evaluated on a gated CD19+ B-cell population. Isotypic controls were used to determine cutoffs. Mean fluorescence intensity (MFI) levels were used for HLA-DR and CD86, whereas percentage expression was determined for the remaining markers.
The MLR data measured T-cell responses to bulk CD19+ B-cells. Experimental limitations did not allow determination of the difference in MLR stimulation between those B-cells that actually harbor virus versus those without in the same patient. However, we evaluated phenotypic marker expression, ex-vivo, between the patient cohorts to determine any potential phenotypic correlations. Of the markers analyzed on B-cells, we found a significantly higher expression percentage of HLA-DR (Fig. 2C) in the HCV RNA+ B-cell cohort, correlating with the higher allostimulatory capacity of these cells. Interestingly, there were significant differences in CD86 and CD71 expression prominent in the HCV RNA− B-cell cohort. This difference suggests the possibility of cumulative B-cell phenotypic changes occurring without stable HCV integration, and perhaps early in the course of infection.
Exposure of healthy PBMC to HCV results in robust B-cell activation
Results from our studies of ex-vivo-derived B-cells suggest that interactions of B-cells with HCV may lead to a hyperstimulatory ability. To further evaluate this potential, we obtained PBMC from healthy subjects and exposed them, in vitro, to the JFH-1 strain of HCV, representing the best characterized whole virus with the capacity to set up productive infection in hepatocyte cultures. After exposing PBMC to 0.1 MOI of S-JFH-1 for 3 h, the cells were washed and allowed to expand in culture for up to 14 d. The B-cells from these cultures were then phenotyped for activation status. We observed robust B-cell activation within 7 d of culture, with significant upregulation of multiple activation markers, including CD25, CD27, CD69, CD71, and CD86 (Fig. 3A). Conditioned media (mock) from Huh7.5 cell cultures did not have this effect (p < 0.05).
FIG. 3.
Exposure of healthy PBMC to HCV results in robust B-cell activation. (A) PBMC from healthy donors were exposed to JFH-1 virus (or mock media) for 3 h, washed, and cultured for 7 d. B-cell activation was monitored at various time points (days 1 and 7 are shown). CD19 is shown on the x-axis, and staining for the indicated activation markers is represented on the corresponding y-axes. The percentages of B-cells that were positive for each marker (i.e., within gated CD19+ cells) is indicated. The data are representative of replicate experiments from five separate donors. (B) PBMC were incubated with viable virus, UV-inactivated virus (UV virus), or were pre-incubated with anti-CD81 (αCD81), or anti-E2 antibody (αE2+), for 1 h prior to the addition of viable virus for 3 h at 37°C, followed by washing and culture for 7 d. PBMC were then harvested and analyzed for B-cell activation (CD71 is shown as a representative activation marker). Data are representative of three replicate experiments, using PBMC from different donors. (C) Bulk PBMC, monocyte-depleted PBMC (PBMC-M), T-cell-depleted PBMC (PBMC-T), purified B-cells + monocytes (B + M), B-cells + T-cells (B + T), and purified B-cells + monocytes + T-cells (B + M + T), were incubated with viable JFH-1 virus at a 0.01 virus:cell ratio. All cell combinations were incubated for 7 d, and gated B-cells were subsequently analyzed for activation (represented by CD71-FITC and CD86-PE expression). Data are representative of three replicate experiments, using PBMC from different donors.
Purified viral glycoprotein E2 contact with its cognate co-receptor, CD81, has been previously shown to induce polyclonal B-cell activation events (30). To evaluate a similar mechanism in our system, we evaluated JFH exposure in the presence of anti-CD81 or anti-HCV-E2 antibodies, as well as using UV-inactivated virus. None of these manipulations were able to inhibit the ability of the virus to activate B-cells (Fig. 3B). These results suggest that viral effects on B-cells may not be exclusive to HCV-E2/CD81 interactions, and do not require viable virions. Moreover, activation of B-cells may involve complex pathways that involve other PBMC subtypes, through cellular interactions and/or secreted molecules. In fact, when monocytes were removed prior to PBMC cultures, we found a decrease (or absence) of B-cell activation (Fig. 3C). In fact, activation was not possible unless B-cells were cultured at least in the presence of monocytes and T-cells, suggesting a multiple cell- or cytokine-mediated process.
HCV-exposed B-cells are potent inducers of regulatory T-cells
Our data demonstrate that while being attenuated in their own response to mitogenic stimulation, HCV RNA+ B-cells are able to induce increased responses in T-cells, which is in contrast to the known attenuation of T-cell responses that characterizes chronic HCV infection. We thus hypothesized that this enhanced T-cell activation may result in greater generation of T-cells with suppressor activity. The biology of CD4+CD25+FOXP3+ regulatory T-cells (Tregs) in humans appears to be quite distinct from that in mice (29,40). We have shown that every human T-cell has the opportunity to attain a CD25+FOXP3+ phenotype upon activation, with associated regulatory function (29). We thus asked whether HCV-associated B-cells resulted in greater generation of suppressive T-cells. Using a CFSE-based proliferation assay, we first confirmed the increased proliferation of responder T-cells when stimulated by HCV RNA+ B-cells (Fig. 4A, top panels). Of note, in the absence of APC, background T-cell proliferation in these assays was consistently <5%. Interestingly, HCV+ B-cell-stimulated cultures also had a greater proportion of CD4+CD25+FOXP3+ T-cells, compared to cultures stimulated by healthy B-cells or HCV RNA− B-cells from HCV-infected patients (Fig. 4A, bottom panels). Fig. 4B shows cumulative data from multiple experiments, showing a clear trend toward increased induction of CFSE-low(proliferating)/CD25+/FOXP3+ T-cells by HCV RNA+ B-cells (p = 0.06).
FIG. 4.
HCV-modified B-cells are potent inducers of regulatory T-cells. (A) Bead-sorted B-cells from healthy subjects and treatment-naïve HCV patients were used as APC in CFSE-based MLR assays, using sorted third-party CD4+CD25− T-cells as responders. The top panels show representative CFSE histograms obtained from assays with healthy B-cells, HCV RNA+ B-cells, and HCV RNA− B-cells as APC. The percentages of proliferation are indicated, demonstrating a significantly greater response from HCV RNA+ B-cells. The bottom panels demonstrate CD25 versus FOXP3 staining on gated CD4+ T-cells from the same cultures. The percentages of CD25+ and FOXP3+ are indicated, showing higher Treg generation in HCV RNA+ MLR. Data are representative of 11 replicate experiments, using different healthy and HCV donors [a total of 11 healthy and 11 HCV (6 HCV+ and 5 HCV−) subjects]. (B) Cumulative graph of the CFSE-based MLR assays described in panel A. Results are represented as percentages of CFSElow(proliferating)/CD25+/FOXP3+ cells induced by HCV B-cells relative to corresponding healthy donor B-cells (normalized to 100%). These data confirm a trend toward higher proliferation and increased CD25/FOXP3 induction by HCV+ B-cells (*p = 0.06). (C) B-cell-induced MLR were set up as described for panel A. Activated CD25+ T-cells from these MLR were then tested for suppressor activity, using flow cytometry–based, anti-CD3-stimulated suppression assays. Autologous T-cells were incubated with increasing numbers of putative suppressor T-cells, derived from MLR. The percentage of proliferation was normalized to the proliferation seen in the absence of any suppressors (designated as 100). Both healthy (black bars) and HCV (gray bars) B-cell-induced CD4+CD25+FOXP3+ T-cells demonstrated comparable Treg activity on a per-cell basis. These data are representative of five separate MLR-induced suppressor pair replicate experiments. (D) JFH- or mock-exposed B-cells were used as APC in allogeneic MLR with CD4+25− T-cells. The percentages of CD4+CD25+FOXP3+ Tregs from the indicated cultures are demonstrated. Data are representative of three replicate experiments.
As neither CD25 nor FOXP3 may be reliable markers of Tregs, we decided to confirm the suppressive ability of these induced T-cells by isolating the CD25+ cells from these MLR cultures, and assessing their suppressor ability, using flow cytometry–based in-vitro suppression assays. CD4+CD25+ T-cells derived from all cultures showed similar abilities to suppress the proliferation of CD4+CD25− responder T-cells, confirming similar regulatory functionality on a per-cell basis (Fig. 4C). Therefore we conclude that HCV RNA+ B cells induced a greater suppressive ability, as they were capable of generating greater numbers of CD25+FOXP3+ T-cells.
Finally, we also tested the ability of JFH-1-activated B-cells to induce Tregs, using them as APC in MLR cultures. Along with robust MLR responses, JFH-1-activated B-cells generated increased numbers of CD4+CD25+FOXP3+ Tregs (Fig. 4D), similarly to ex-vivo-derived HCV+ B-cells. These results demonstrate that HCV-modified B-cells are capable of inducing greater numbers of CD4+CD25+FOXP3+ Tregs, providing a possible mechanistic link between B-cell hyperstimulation and T-cell attenuation/suppression, which is characteristic of chronic HCV infection.
Discussion
Multiple immune mechanisms have been proposed to explain the suppression of virus-specific T-cell responses during HCV infection (8), including dysregulated APC function (2,32), and increased numbers of CD4+CD25+FOXP3+ regulatory/suppressor T-cells (Tregs) (9). Whereas a direct association of HCV with immune cells has been proposed (7,34,42), it is still unclear whether viral replication occurs in extra-hepatic sites (18). While earlier assays could not differentiate between active infection and passive absorption (27,43,46), the possibility of direct infection has garnered some support from the finding of HCV RNA species in PBMC populations in the absence of serum HCV RNA (34). Moreover, experiments evaluating HCV quasi-species show that serum-associated sequences are a product of extra-hepatic origin, suggesting a lympho-specific contribution (28). In the current study, we evaluated the association of HCV with various immune cell subsets. We found that, similarly to prior reports, there was an almost exclusive association of HCV with B-cells. There were a few instances in which HCV RNA was detected in non-B-cell PBMC fractions (Table 2); however, we believe that this may be a reflection of a small proportion of contaminating B-cells in these preparations, especially since in all instances where HCV was found in non-B-cell fractions, there was detection in the B-cell fraction as well. Also, we found little evidence of active viral replication in PBMC (Table 2, 3 out of 50 samples). However, the mere presence of the positive-strand RNA and/or resultant viral proteins may be sufficient to affect the function of HCV-harboring B-cells. Whereas HCV-specific antibodies do not appear to make a significant contribution to the resolution or chronicity of infection (8), a multitude of extra-hepatic diseases, such as lymphoproliferative disorders and cryoglobulinemia, are related to abnormal B-cell function and polyclonal activation (25). It is unclear whether these associations may be causally linked to the presence of HCV. It has been proposed that this may involve either chronic antigenic stimulation of HCV-specific B-cells (35), or the cross-linking of CD81, a ubiquitously expressed receptor that is known to bind HCV glycoprotein E2, and is associated with CD19 and CD21 on B-cells (22). Engagement or cross-linking of this complex results in several B-cell activation events, including enhanced TNF-α production, cellular proliferation, and Ig gene hyper-mutation (1,23). These processes can eventually lead to an attenuated B-cell phenotype (26), characterized by a reduced capacity to proliferate in response to exogenous stimulation. A significantly inhibited proliferation was found in B-cells from HCV-infected patients (Fig. 1A). This correlated with increased apoptosis (Fig. 1B and C), that could not be reversed by the addition of IL-4 and/or IL-2 (data not shown). Moreover, there was trend toward lower induction of activation markers following B-cell stimulation. These findings suggest a large population of “exhausted” or “hyporesponsive” B-cells during HCV infection, rather than effects restricted to HCV-specific B-cells. This attenuated function is in paradoxical contrast to the ex-vivo phenotype of B-cells (Fig. 2C), which showed enhanced expression of certain activation markers. Therefore, it appears that there is a sustained level of chronic B-cell activation during HCV infection, which correlates with paradoxical hyporesponsiveness to mitogenic stimuli.
As B-cells are a critical APC population during ongoing immune responses, we asked whether B-cell:T-cell interactions were affected as a result of HCV. We discovered several novel and interesting features, including another apparent paradox. B-cells associated with HCV RNA had an increased ability to induce T-cell responses, compared to both healthy subjects and those with B-cells that were HCV RNA−. However, this enhanced APC function resulted in the generation of increased numbers of CD4+CD25+FOXP3+ T-cells with immune regulatory function. Exposure of PBMC to HCV in vitro also replicated the ex-vivo phenotype, in that HCV-modulated B-cells showed an activated phenotype, and could generate greater numbers of Tregs from primary CD4+ T-cells. HCV-induced activation of B-cells appears to be a multifactorial process, depending on the complex immune interactions between monocytes, T-cells, and B-cells. Future studies will need to address the mechanisms in greater detail.
Several reports have shown increased numbers of CD4+CD25+FOXP3+ Tregs in HCV-infected patients (6,9,24,31,36), with some reports suggesting HCV specificity in this population (13). In separate studies, we have also confirmed the presence of increased Tregs in chronic HCV patients, in whom the depletion of these cells results in significant enhancement of HCV-specific, but not CMV-specific, T-cell responses in vitro, suggesting HCV specificity (data not shown). Our results provide a mechanistic link between HCV-induced B-cell activation and the generation and maintenance of Tregs. This link may also explain the relative specificity of the T-cell attenuation seen during HCV infection. Thus, either an HCV-specific B-cell or an HCV-stimulated B-cell is likely to serve as an APC for an HCV-specific T-cell, causing inadvertent chronic hyperstimulation, resulting in the generation of HCV-specific Tregs, and the suppression of HCV-specific effector CD4+ and CD8+ responses. Of note, increased Treg activity is observed in almost all patients, regardless of the HCV RNA status of the B-cells. While distinct mechanisms may be involved in different patients, it is tempting to speculate that the association of B-cells with HCV is an early immunological event, leading to the generation of HCV-specific Tregs early during the infection. This model also explains the altered immunophenotype of HCV RNA− B-cells, which may represent sequelae of prior HCV association and activation without subsequent viral uptake.
In summary, our findings provide evidence for a paradoxical link between B-cell hyperactivation and the generation of Tregs, resulting in overall suppression of HCV-specific responses. This suggests a more complex model of interaction between various immune cell types, wherein not every cell type needs to have suppressed function to cause an overall attenuation. As a corollary, immunologic therapeutic intervention may then be targeted toward inhibiting these immune interactions once the molecular mechanisms are further sorted out in future studies.
Acknowledgments
These studies were supported by research grants (to N.J.K.) from the National Institutes of Health. The authors are indebted to all the subjects who participated in this study. We also thank Drs. Jennifer Cuthbert and Dwain Thiele, and Ms. Ann Varghese and Ms. Griselda Soto for their help with patient recruitment, and Sterling Ortega, Andrew Benagh, and Maycie Garibay for experimental support. We thank Drs. Jason Mendoza and Venkatesh Kashi for review of manuscript and helpful discussions. We also thank Drs. Michael Gale, Andrea Erickson, and Julie Pfeiffer, and Ms. Kristie Ibarra for their help in obtaining the S-JFH-1 HCV clone.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Altomonte M. Montagner R. Pucillo C. Maio M. Triggering of target of an antiproliferative antibody-1 (TAPA-1/CD81) up-regulates the release of tumour necrosis factor-alpha by the EBV-B lymphoblastoid cell line JY. Scand J Immunol. 1996;43:367–373. doi: 10.1046/j.1365-3083.1996.d01-64.x. [DOI] [PubMed] [Google Scholar]
- 2.Averill L. Lee WM. Karandikar NJ. Differential dysfunction in dendritic cell subsets during chronic HCV infection. Clin Immunol. 2007;123:40–49. doi: 10.1016/j.clim.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bain C. Fatmi A. Zoulim F, et al. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology. 2001;120:512–524. doi: 10.1053/gast.2001.21212. [DOI] [PubMed] [Google Scholar]
- 4.Barnes E. Lauer G. Walker B. Klenerman P. T cell failure in hepatitis C virus infection. Viral Immunol. 2002;15:285–293. doi: 10.1089/08828240260066233. [DOI] [PubMed] [Google Scholar]
- 5.Bassett SE. Brasky KM. Lanford RE. Analysis of hepatitis C virus-inoculated chimpanzees reveals unexpected clinical profiles. J Virol. 1998;72:2589–2599. doi: 10.1128/jvi.72.4.2589-2599.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boettler T. Spangenberg HC. Neumann-Haefelin C, et al. T cells with a CD4+ CD25+ regulatory phenotype suppress in vitro proliferation of virus-specific CD8+ T cells during chronic hepatitis C virus infection. J Virol. 2005;79:7860–7867. doi: 10.1128/JVI.79.12.7860-7867.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouffard P. Hayashi PH. Acevedo R, et al. Hepatitis C virus is detected in a monocyte/macrophage subpopulation of peripheral blood mononuclear cells of infected patients. J Infect Dis. 1992;166:1276–1280. doi: 10.1093/infdis/166.6.1276. [DOI] [PubMed] [Google Scholar]
- 8.Bowen DG. Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005;436:946–952. doi: 10.1038/nature04079. [DOI] [PubMed] [Google Scholar]
- 9.Cabrera R. Tu Z. Xu Y, et al. An immunomodulatory role for CD4(+)CD25(+) regulatory T lymphocytes in hepatitis C virus infection. Hepatology. 2004;40:1062–1071. doi: 10.1002/hep.20454. [DOI] [PubMed] [Google Scholar]
- 10.Cooper S. Erickson AL. Adams EJ, et al. Analysis of a successful immune response against hepatitis C virus. Immunity. 1999;10:439–449. doi: 10.1016/s1074-7613(00)80044-8. [DOI] [PubMed] [Google Scholar]
- 11.Crawford MP. Yan SX. Ortega SB, et al. High prevalence of autoreactive, neuroantigen-specific CD8+ T cells in multiple sclerosis revealed by novel flow cytometric assay. Blood. 2004;103:4222–4231. doi: 10.1182/blood-2003-11-4025. [DOI] [PubMed] [Google Scholar]
- 12.Crotta S. Stilla A. Wack A, et al. Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein. J Exp Med. 2002;195:35–41. doi: 10.1084/jem.20011124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ebinuma H. Nakamoto N. Li Y, et al. Identification and in vitro expansion of functional antigen-specific CD25+ FoxP3+ regulatory T cells in hepatitis C virus infection. J. Virol. 2008;82:5043–5053. doi: 10.1128/JVI.01548-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gates JA. Post JJ. Kaldor JM, et al. Risk factors for hepatitis C infection and perception of antibody status among male prison inmates in the Hepatitis C Incidence and Transmission in Prisons Study cohort, Australia. J Urban Health. 2004;81:448–452. doi: 10.1093/jurban/jth129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerlach JT. Diepolder HM. Jung MC, et al. Recurrence of hepatitis C virus after loss of virus-specific CD4(+) T-cell response in acute hepatitis C. Gastroenterology. 1999;117:933–941. doi: 10.1016/s0016-5085(99)70353-7. [DOI] [PubMed] [Google Scholar]
- 16.Kato T. Date T. Miyamoto M, et al. Efficient replication of the genotype 2a hepatitis C virus subgenomic replicon. Gastroenterology. 2003;125:1808–1817. doi: 10.1053/j.gastro.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 17.Kittlesen DJ. Chianese-Bullock KA. Yao ZQ, et al. Interaction between complement receptor gC1qR and hepatitis C virus core protein inhibits T-lymphocyte proliferation. J Clin Invest. 2000;106:1239–1249. doi: 10.1172/JCI10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanford RE. Chavez D. Chisari FV. Sureau C. Lack of detection of negative-strand hepatitis C virus RNA in peripheral blood mononuclear cells and other extrahepatic tissues by the highly strand-specific rTth reverse transcriptase PCR. J Virol. 1995;69:8079–8083. doi: 10.1128/jvi.69.12.8079-8083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanford RE. Sureau C. Jacob JR, et al. Demonstration of in vitro infection of chimpanzee hepatocytes with hepatitis C virus using strand-specific RT/PCR. Virology. 1994;202:606–614. doi: 10.1006/viro.1994.1381. [DOI] [PubMed] [Google Scholar]
- 20.Larsson M. Babcock E. Grakoui A, et al. Lack of phenotypic and functional impairment in dendritic cells from chimpanzees chronically infected with hepatitis C virus. J Virol. 2004;78:6151–6161. doi: 10.1128/JVI.78.12.6151-6161.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lechner F. Wong DK. Dunbar PR, et al. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–1512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy S. Todd SC. Maecker HT. CD81 (TAPA-1): a molecule involved in signal transduction and cell adhesion in the immune system. Annu Rev Immunol. 1998;16:89–109. doi: 10.1146/annurev.immunol.16.1.89. [DOI] [PubMed] [Google Scholar]
- 23.Machida K. Cheng KT. Pavio N, et al. Hepatitis C virus E2-CD81 interaction induces hypermutation of the immunoglobulin gene in B cells. J Virol. 2005;79:8079–8089. doi: 10.1128/JVI.79.13.8079-8089.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manigold T. Shin EC. Mizukoshi E, et al. Foxp3+ CD4+ CD25+ T cells control virus-specific memory T cells in chimpanzees that recovered from hepatitis C. Blood. 2006;107:4424–4432. doi: 10.1182/blood-2005-09-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayo MJ. Extrahepatic manifestations of hepatitis C infection. Am J Med Sci. 2003;325:135–148. doi: 10.1097/00000441-200303000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Montes CL. Acosta-Rodriguez EV. Merino MC, et al. Polyclonal B cell activation in infections: infectious agents' devilry or defense mechanism of the host? J Leukoc Biol. 2007;82:1027–1032. doi: 10.1189/jlb.0407214. [DOI] [PubMed] [Google Scholar]
- 27.Muller HM. Pfaff E. Goeser T, et al. Peripheral blood leukocytes serve as a possible extrahepatic site for hepatitis C virus replication. J Gen Virol. 1993;74:669–676. doi: 10.1099/0022-1317-74-4-669. [DOI] [PubMed] [Google Scholar]
- 28.Pal S. Sullivan DG. Kim S, et al. Productive replication of hepatitis C virus in perihepatic lymph nodes in vivo: implications of HCV lymphotropism. Gastroenterology. 2006;130:1107–1116. doi: 10.1053/j.gastro.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 29.Pillai V. Karandikar NJ. Human regulatory T cells: a unique, stable thymic subset or a reversible peripheral state of differentiation? Immunol Lett. 2007;114:9–15. doi: 10.1016/j.imlet.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosa D. Saletti G. De Gregorio E, et al. Activation of naive B lymphocytes via CD81, a pathogenetic mechanism for hepatitis C virus-associated B lymphocyte disorders. Proc Natl Acad Sci USA. 2005;102:18544–18549. doi: 10.1073/pnas.0509402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rushbrook SM. Ward SM. Unitt E, et al. Regulatory T cells suppress in vitro proliferation of virus-specific CD8+ T cells during persistent hepatitis C virus infection. J Virol. 2005;79:7852–7859. doi: 10.1128/JVI.79.12.7852-7859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarobe P. Lasarte JJ. Casares N, et al. Abnormal priming of CD4(+) T cells by dendritic cells expressing hepatitis C virus core and E1 proteins. J Virol. 2002;76:5062–5070. doi: 10.1128/JVI.76.10.5062-5070.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt WN. Klinzman D. LaBrecque DR, et al. Direct detection of hepatitis C virus (HCV) RNA from whole blood, and comparison with HCV RNA in plasma and peripheral blood mononuclear cells. J Med Virol. 1995;47:153–160. doi: 10.1002/jmv.1890470208. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt WN. Wu P. Han JQ, et al. Distribution of hepatitis C virus (HCV) RNA in whole blood and blood cell fractions: plasma HCV RNA analysis underestimates circulating virus load. J Infect Dis. 1997;176:20–26. doi: 10.1086/514024. [DOI] [PubMed] [Google Scholar]
- 35.Suarez F. Lortholary O. Hermine O. Lecuit M. Infection-associated lymphomas derived from marginal zone B cells: a model of antigen-driven lymphoproliferation. Blood. 2006;107:3034–3044. doi: 10.1182/blood-2005-09-3679. [DOI] [PubMed] [Google Scholar]
- 36.Sugimoto K. Ikeda F. Stadanlick J, et al. Suppression of HCV-specific T cells without differential hierarchy demonstrated ex vivo in persistent HCV infection. Hepatology. 2003;38:1437–1448. doi: 10.1016/j.hep.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 37.Tseng CT. Klimpel GR. Binding of the hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions. J Exp Med. 2002;195:43–49. doi: 10.1084/jem.20011145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsubouchi E. Akbar SM. Murakami H, et al. Isolation and functional analysis of circulating dendritic cells from hepatitis C virus (HCV) RNA-positive and HCV RNA-negative patients with chronic hepatitis C: role of antiviral therapy. Clin Exp Immunol. 2004;137:417–423. doi: 10.1111/j.1365-2249.2004.02544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ulsenheimer A. Gerlach JT. Gruener NH, et al. Detection of functionally altered hepatitis C virus-specific CD4 T cells in acute and chronic hepatitis C. Hepatology. 2003;37:1189–1198. doi: 10.1053/jhep.2003.50194. [DOI] [PubMed] [Google Scholar]
- 40.Vukmanovic-Stejic M. Zhang Y. Cook JE, et al. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J Clin Invest. 2006;116:2423–2433. doi: 10.1172/JCI28941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wakita T. Pietschmann T. Kato T, et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11:791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang JT. Sheu JC. Lin JT, et al. Detection of replicative form of hepatitis C virus RNA in peripheral blood mononuclear cells. J Infect Dis. 1992;166:1167–1169. doi: 10.1093/infdis/166.5.1167. [DOI] [PubMed] [Google Scholar]
- 43.Willems M. Moshage H. Yap SH. PCR and detection of negative HCV RNA strands. Hepatology. 1993;17:526. [PubMed] [Google Scholar]
- 44.Wong DK. Dudley DD. Afdhal NH, et al. Liver-derived CTL in hepatitis C virus infection: breadth and specificity of responses in a cohort of persons with chronic infection. J Immunol. 1998;160:1479–1488. [PubMed] [Google Scholar]
- 45.Zeuzem S. Feinman SV. Rasenack J, et al. Peginterferon alfa-2a in patients with chronic hepatitis C. N Engl J Med. 2000;343:1666–1672. doi: 10.1056/NEJM200012073432301. [DOI] [PubMed] [Google Scholar]
- 46.Zignego AL. Macchia D. Monti M, et al. Infection of peripheral mononuclear blood cells by hepatitis C virus. J Hepatol. 1992;15:382–386. doi: 10.1016/0168-8278(92)90073-x. [DOI] [PubMed] [Google Scholar]