Abstract
AIM: To evaluate whether FDG-positron emission tomography (PET)/computed tomography (CT) may be an accurate technique in the assessment of the T stage in patients with colorectal cancer.
METHODS: Thirty four consecutive patients (20 men and 14 women; mean age: 63 years) with a histologically proven diagnosis of colorectal adenocarcinoma and scheduled for surgery in our hospital were enrolled in this study. All patients underwent FDG-PET/CT preoperatively. The primary tumor site and extent were evaluated on PET/CT images. Colorectal wall invasion was analysed according to a modified T classification that considers only three stages (≤ T2, T3, T4). Assessment of accuracy was carried out using 95% confidence intervals for T.
RESULTS: Thirty five/37 (94.6%) adenocarcinomas were identified and correctly located on PET/CT images. PET/CT correctly staged the T of 33/35 lesions identified showing an accuracy of 94.3% (95% CI: 87%-100%). All T1, T3 and T4 lesions were correctly staged, while two T2 neoplasms were overstated as T3.
CONCLUSION: Our data suggest that FDG-PET/CT may be an accurate modality for identifying primary tumor and defining its local extent in patients with colorectal cancer.
Keywords: Cancer, Colorectal, Positron emission tomography/computed tomography, Staging
INTRODUCTION
In patients with colorectal cancer, accurate preoperative staging is essential for the planning of optimal therapy considering the many therapeutic options available.
Correct evaluation of the local extent (T) of the tumor and of the regional lymph nodes (N) is crucial since it influences the local surgical approach as well as the therapeutic management of the distant metastases (M). Various treatments of the primary tumor are available including radical or limited resection, palliative derivative surgery, local excision, laparoscopic surgical approach, preoperative neoadjuvant chemotherapy and/or radiotherapy: for example the laparoscopic surgical approach is used preferentially for T1 and T2 staged tumors; preoperative neoadjuvant therapy for advanced rectal cancer (T3, T4); limited resection or palliative derivative surgery for diffusely invasive cancers[1-7]. Moreover, only if the whole primary tumor mass can be completely removed, the surgical option for liver, lung or non-regional lymph nodes metastases is considered[8-10].
In daily practice, tumor size and infiltration of adjacent structures in colorectal cancer are assessed initially by contrast-enhanced computed tomography (ceCT)[11-14]. Magnetic resonance imaging (MRI) and endorectal ultrasound (US) represent an accurate diagnostic option for rectal cancer[15-19].
Non-enhanced FDG-positron emission tomography (PET)/CT[20-21] and more recently contrast-enhanced FDG-PET/CT (PET/ceCT)[22-24], are gaining a progressively more important role in the evaluation of the N and the M stages in the staging and follow-up of colorectal cancer, however, the performance of this modality in the evaluation of the T parameter has not been extensively investigated. A single study has recently proposed the evaluation of TNM staging by PET/CT colonography in patients with colorectal cancer and reported good assessment of the T parameter[6-8]; the PET/CT colonography protocol requested previous bowel preparation with a solution containing polyethylene glycol-electrolytes, the iv injection of N-butyl scopolamine, administration of a rectal water enema, a water-based negative oral contrast agent assumption and the iv injection of iodinated contrast medium. This protocol may not be tolerated well by some patients, can be time consuming, is not recommended in patients with impaired renal function and requires additional costs.
The aim of this study was to evaluate whether FDG-PET/CT, without iv contrast medium or colonography technique, may be considered a proper diagnostic tool in the assessment of local primary tumor extent (T) in patients with colorectal cancer.
MATERIALS AND METHODS
Patients
The study protocol was approved by our institutional review board and informed consent was obtained from all patients.
The study population consisted of 34 consecutive patients (20 men and 14 women; age range, 29-81 years; mean age: 63 years) with a histologically proven diagnosis (the histological specimen was obtained during conventional colonoscopy) of colorectal adenocarcinoma and scheduled for surgery in our hospital. Exclusion criteria were refusal to participate in the study.
Before surgery all patients underwent FDG-PET/CT in our Institution. Surgery was scheduled within 10 d of the examination, with the exception of three patients with rectal cancer who underwent neoadjuvant radio-chemotherapy after PET/CT and before surgery.
PET/CT technique
Dual modality imaging was performed with a PET/CT system (Discovery-LS, GE-Medical-Systems, Milwaukee, USA) consisting of a PET scanner and a four-row MDCT system.
All patients had been instructed to fast for a minimum of 6 h prior to the examination. Blood glucose levels were found to be in the normal range prior to 18FDG injection by blood sampling. PET/CT was carried out 60 min after iv administration of 370 MBq of 18FDG.
MDCT scans were acquired from the base of the skull to the upper thighs using the following parameters: 4 mm × 5 mm collimation (140 kV, 80 mAs), 0.5 s rotation time, a pitch of 6. 18FDG-PET data were acquired with the patient in the same position on the table at four bed positions (5 min for each bed position) covering the same field of view as CT.
Data obtained from the CT acquisition were used for attenuation correction of 18FDG-PET emission data. 18FDG-PET images were reconstructed with a 4.5 mm thickness.
18FDG-PET, CT and fused 18FDG-PET/CT images were reviewed on the dedicated workstation (Xeleris, GE Medical System).
Imaging interpretation
PET/CT examinations were interpreted before surgery by two pairs of observers, each pair composed of a radiologist and a nuclear medicine physician (each with > 5 years of experience). The images were interpreted jointly within each pair and independently by the two pairs of observers. In cases of disagreement, a consensus panel consisting of the original four observers plus a third blinded party (with 10 years experience) made the final decision.
PET/CT images were evaluated to determine the primary tumor site and extent and the lymph nodes status.
For determination of the primary tumor site and extent, and the lymph nodes status, each pair of observers could use the CT, the PET and the fused PET/CT images individually and simultaneously in no established order. This approach was dictated by the aim of our study which was to evaluate PET/CT exclusively as a single complex technique for local colorectal cancer staging.
For the localization of each lesion, the large intestine was divided into nine anatomic segments: rectum, rectosigmoid colon junction, sigmoid colon, descending colon, splenic flexure, transverse colon, hepatic flexure, ascending colon and caecum.
For the identification of each lesion, the tumor had to be depicted either morphologically or metabolically (a polypoid, annular, semiannular or flat lesion had to be associated with focal abnormal FDG uptake, or focal abnormal FDG uptake had to be associated with a polypoid, annular, semiannular or flat lesion).
Abnormal FDG uptake was defined as focal increased activity higher than the background activity of soft tissues. The evaluation of each focal radiotracer uptake was qualitative and quantitative (the standard maximum uptake value (SUVmax) was calculated; the SUVmax was defined as abnormal when it appeared to be higher than the SUVmax of the background activity of soft tissues). Diffuse radiotracer uptake was assumed to represent normal or non-malignant bowel activity.
Colorectal wall invasion was analysed according to a modified T classification)[1,2] that considers only three stages (≤ T2, T3, T4). A parietal lesion concentrating the 18-FDG in the absence of extra-parietal radio-tracer uptake, was considered as a tumor confined to the bowel wall and defined as a ≤ T2 lesion. A tumor either with a spiculated outer contour or with rounded or nodular advancing edges showing intra- and extra-parietal radio-tracer uptake was defined as a T3 lesion. A tumor infiltrating into adjacent organs as suggested by their increased glucose metabolism was defined as a T4 lesion.
Lymph node metastasis was evaluated in regional lymph nodes. The diagnosis of an abnormal lymph node on PET/CT was based on the presence of focal increased FDG uptake at a location that corresponded to a lymph node regardless of its size on CT scan. N1 was defined as focal FDG uptake in not more than three lymph nodes, while N2 was defined as focal FDG uptake in more than three lymph nodes.
On co-registered PET images, the SUVmax was calculated on the primary tumor as well as on contiguous organs appearing to be involved as well as on the lymph nodes with focal increased glucose metabolism.
Standard of reference
The standard of reference was represented by surgical findings and histopathological analysis of the surgical specimens.
Localization of the tumor was defined during surgical exploration.
Tumour invasion (T) and lymph node status (N) were based on the TNM classification of the surgical specimen.
Three patients with rectal cancer underwent neoadjuvant radio-chemotherapy after PET/CT and before surgery: considering the potential downstaging of the tumor, the T and the N evaluation obtained with both MRI and endorectal US was used as the standard of reference.
Statistical analysis
Data are expressed as mean ± one SD or as proportion as appropriate. A commercial statistical software package was used (MedCalc®). Differences between continuous data were assessed using analysis of variance with post-hoc multiple groups comparison (Student-Newman-Keuls test). Categorical data were evaluated by χ2 analysis, Fisher exact test, and McNemar test, as appropriate. Logistic analysis was used to evaluate significant determinants. A P value < 0.05 was considered significant.
RESULTS
Standard of reference
A total of 37 adenocarcinomas were found in the surgical specimens. Two synchronous lesions were found in 3 out of 34 patients (8.8%). Three adenocarcinomas showed a mucinous component on histopathological examination.
The regional distribution of the 37 tumors was as follows: rectum (n = 6), rectosigmoid colon junction (n = 4), sigmoid colon (n = 15), descending colon (n = 3), transverse colon (n = 1), hepatic flexure (n = 3), ascending colon (n = 2) and caecum (n = 3).
Five out of 37 (13.5%) tumors were classified as stage T1, 5 out of 37 (13.5%) as stage T2, 21 out of 37 (56.8%) as stage T3 and 6 out of 37 (16.2%) as stage T4. All three adenocarcinomas with a mucinous component were classified as T4.
Twenty one out of 37 (57%) lesions were classified as N- and 16 out of 37 (43%) as N+ (13/16 as N1 and 3/16 as N2).
Tumour identification and location
Thirty five out of 37 (94.6%) adenocarcinomas were identified and correctly located on PET/CT images. In two patients no lesions were disclosed on PET/CT images: the two lesions which were missed were located in the transverse colon and the sigmoid colon, respectively, and were flat and confined to the colonic wall resulting in T1 stage on histopathological examination, and measured 15 mm and 16 mm, respectively.
Tumour T staging
PET/CT correctly staged (Table 1) the T of 33/35 lesions identified, showing an accuracy of 94.3% (95% CI: 87%-100%). All T1, T3 and T4 lesions were correctly staged, while two T2 neoplasms, located in the sigmoid colon and rectum, respectively, were overstated as T3 (Figures 1 and 2).
Table 1.
T stage: positron emission tomography/computed tomography vs histology
|
Histology |
||||
| T ≤ 2 | T3 | T4 | Total | |
| PET/CT | ||||
| T ≤ 2 | 6 | 0 | 0 | 6 |
| T3 | 2 | 21 | 0 | 23 |
| T4 | 0 | 0 | 6 | 6 |
| Total | 8 | 21 | 6 | 35 |
PET/CT: Positron emission tomography/computed tomography. χ2: 58.9, (P < 0.0001). Accuracy: 94.3% (95% CI: 87%-100%).
Figure 1.
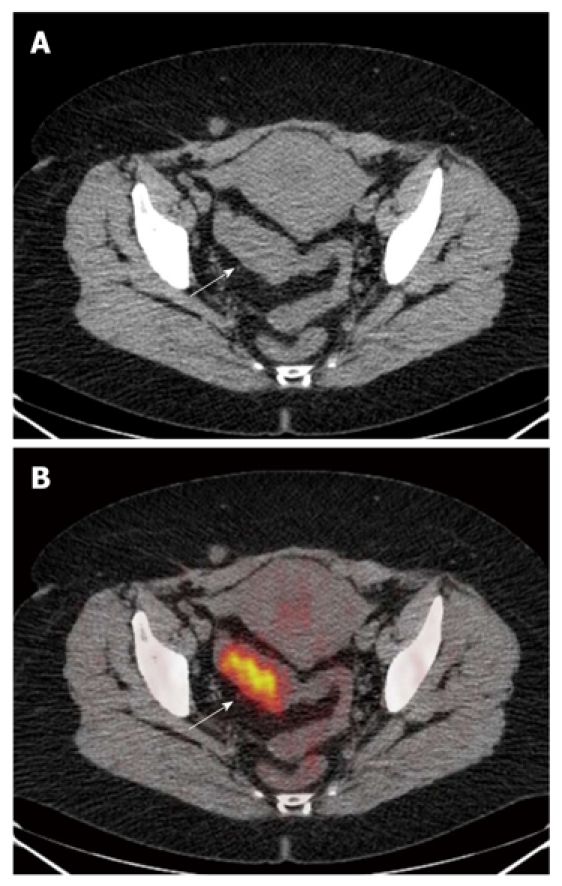
The computed tomography (A) and fused positron emission tomography/computed tomography (B) images. A: Neoplastic thickening of sigmoid colon walls, which show regular profiles with normal appearance of perilesional fat (arrow); B: Uptake of 18-FDG (arrow) (SUVmax 11) in the absence of extra-parietal radio-tracer uptake. The lesion was correctly classified as ≤ T2.
Figure 2.
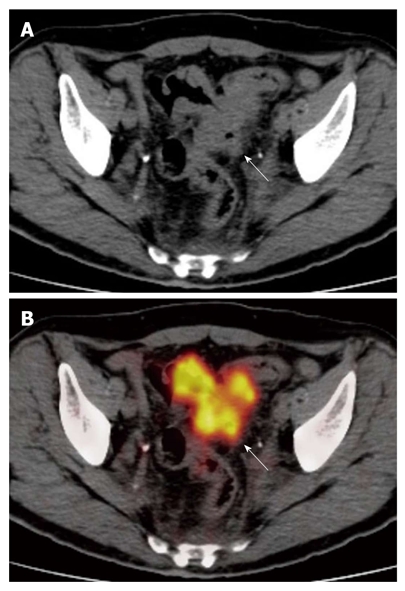
The computed tomography (A) and fused positron emission tomography/computed tomography (B) images show diffuse and irregular neoplastic thickening of sigmoid colon walls, which show rounded advancing margins with intense radiotracer uptake (arrows) (SUVmax 13). The lesion was correctly classified as T3.
For the six lesions correctly classified as T4, PET/CT showed infiltration of the uterus (n = 1), of the ovary (n = 2), of the small bowel (n = 1) and of the peritoneum (n = 2) (Figures 3 and 4).
Figure 3.
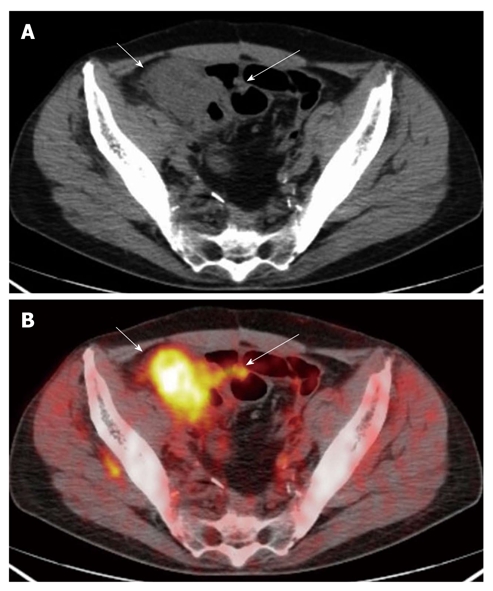
The computed tomography (A) and fused positron emission tomography/computed tomography (B) images show a neoplastic lesion of the caecum concentrating the radio-tracer (short arrows) (SUVmax 9.6). Focal radio-tracer uptake (SUVmax 3.6) of a contiguous ileal loop was disclosed on positron emission tomography/computed tomography (B) (long arrow) consistent with a short concentric wall thickening (A) (long arrow): the finding was suggestive of small bowel infiltration. The lesion was correctly classified as T4.
Figure 4.
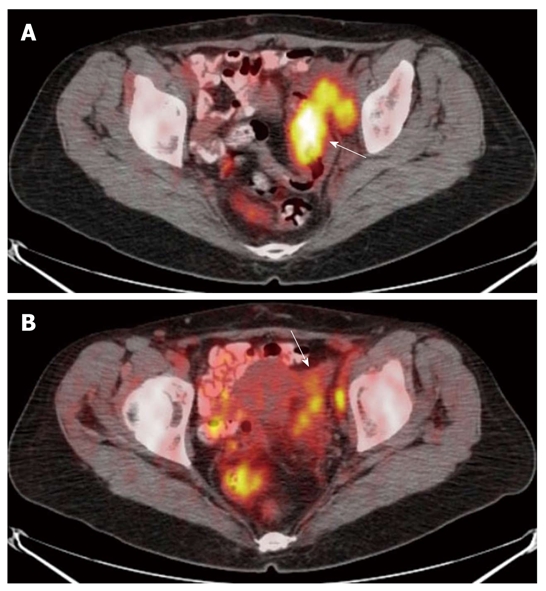
The fused positron emission tomography/computed tomography images show a neoplastic lesion of the sigmoid colon concentrating the radio-tracer (arrow) (SUVmax 14.6) (A) and focal radio-tracer uptake of the left ovary (arrow) (SUVmax 9) (B): the finding was suggestive of ovarian infiltration. The lesion was correctly classified as T4.
The SUVmax of the identified lesions ranged from 1.8 to 27 with a mean of 14.3 ± 5.8. Stratifying for T stages, the SUVmax of the T1 tumors ranged from 1.8 to 14 with a mean of 6.9 ± 6.4, the T2 tumors ranged from 10 to 25 with a mean of 13.9 ± 6.3, the T3 tumors ranged from 5.7 to 27 with a mean of 15.3 ± 5.5, and the T4 tumors ranged from 9.4 to 21 with a mean of 14.7 ± 4.7. No statistically significant difference between the SUVmax of each T group was found.
The SUVmax of the contiguous organs infiltrated ranged between 3.6 and 20 with a media of 11.5 ± 6.2.
Tumour N staging
PET/CT correctly staged (Tables 2 and 3) the N of 27/34 patients, showing an accuracy of 79.4% (95% CI: 66%-93%). There were three false positive and 4 false negative results. 15/18 N0, 9/13 N1 and 3/3 N2 lesions were correctly classified.
Table 2.
N stage: positron emission tomography/computed tomography vs histology
| Istologia |
Histology |
||
| N+ | N- | Total | |
| PET/CT | |||
| N+ | 12 | 3 | 15 |
| N- | 4 | 15 | 19 |
| Total | 16 | 18 | 34 |
PET/CT: Positron emission tomography/computed tomography. χ2: 9.5, (P < 0.005). Accuracy: 79.4% (95% CI: 66%-93%).
Table 3.
N stage: positron emission tomography/computed tomography vs histology with N1 and N2 stratification
|
Histology |
||||
| N0 | N1 | N2 | Total | |
| PET/CT | ||||
| N0 | 15 | 4 | 0 | 19 |
| N1 | 3 | 9 | 0 | 12 |
| N2 | 0 | 0 | 3 | 3 |
| Total | 18 | 13 | 3 | 34 |
PET/CT: Positron emission tomography/computed tomography. χ2: 43.6, (P < 0.001). Accuracy: 79.4% (95% CI: 66%-93%).
The SUVmax of the lymph nodes with focal increased radio-tracer uptake ranged from 1.6 to 10.3 with a mean of 5.5 ± 3.1. No statistically significant differences between the SUVmax of each N group were observed.
DISCUSSION
Our data shows that FDG-PET/CT is a useful diagnostic tool in identifying primary tumor extent in patients with colorectal cancer: the T stage of 33 out of 35 (94.3%) neoplastic colorectal lesions was correctly identified as well as the N stage of 27/34 (79.4%).
One of the major strengths of PET/CT as a cancer staging modality is its ability to identify systemic metastases. At any phase of cancer evaluation, the demonstration of systemic metastases has profound therapeutic and prognostic implications. Only in the absence of systemic metastases does nodal status become important, and only when unresectable nodal metastasis has been excluded does T stage become important. There are now accumulating data to suggest that PET/CT could be used as the first, rather than the last test to assess M and N stage in the evaluation of cancers[25]. In this scenario, it would also be desirable for PET/CT to be accurate in the evaluation of the T stage. As a consequence, there is a great opportunity to use PET/CT as an all-in-one staging imaging modality in oncologic patients, and subsequently selecting and tailoring the performance of anatomically-based imaging modalities without or with iv contrast medium (US, MR, CT) to better define the abnormalities identified by PET/CT, when this information would be of relevance to management planning.
For these reasons, neither comparing the performance of PET/CT with other non-invasive imaging modalities nor investigating the added value of FDG information to CT data nor defining what was the contribution of each component (PET, CT and fused PET/CT images) were our goals. On the contrary, the aim of our study was exclusively to evaluate PET/CT as a single complex technique in colorectal cancer T staging, comparing its performance directly with the gold standard of histological results.
In colorectal cancer, accurate assessment of the T stage and tumor size may aid in determining the correct way to access the lesion (local endoscopic excision, laparotomy, laparoscopy, or transanally), or the modality of surgery (radical or limited resection, palliative derivative surgery). Moreover, for rectal cancer, the T stage will be of major clinical relevance since its accurate preoperative assessment may help to select patients who will benefit from neoadjuvant therapy compared with resection alone.
In colorectal cancer, although various imaging modalities have been proposed for TNM staging, ceCT widely represents the first diagnostic step due to relatively low cost and widespread availability. For T staging, endorectal US and MRI are becoming mandatory in the management of rectal cancer[15-19,26]. For N staging, malignant lymph node identification remains a problem and the use of the size criteria may lead to misdiagnosis: evaluation of the outline of the node and the features of signal intensity with MRI[15-19,26] as well as the assessment of glucose metabolism with PET/CT[22,23,27] have been shown to be more reliable. For M staging, particularly for liver metastases, the optimal imaging staging strategy has not yet been defined and the role of CT, MRI, PET/CT and US is still debated[22,28-32].
As a result, if PET/CT is used as the all-in-one imaging modality in the staging of colo-rectal cancer, it is mandatory that it is demonstrated to be accurate in the evaluation of the T parameter, it adopts a minimal complex procedure, is well tolerated by patients, is less time consuming and is as inexpensive as possible.
Thus, the choice to perform PET/CT without the aid of iv contrast medium and colonography has been dictated by our interest in evaluating how accurate this modality, in its basal condition, is in T staging. The use of iv contrast medium allows better definition of the boundaries of structures and colonography permits easy identification of the primary tumor and a more accurate assessment of its local extent[13,14]. However, the radio-tracer may play the role of “metabolic contrast agent” and is able to increase the contrast resolution of the structures, to characterize the perilesional tissues and to compensate for the absence of luminal distension on the unenhanced CT images, as demonstrated in our series in which 95% of adenocarcinomas were correctly identified and staged.
Moreover, a basal PET/CT protocol also offers the chance of colorectal cancer staging to those patients in whom the administration of iv contrast medium is contraindicated or not recommended, such as patients with impaired renal function or with an allergic history.
CT and PET/CT have limitations in distinguishing the wall layers of the colon, as a consequence, the differentiation of T1 and T2 tumors is not accurate[13,14,22]. For this reason we decided to classify T1 and T2 tumors as a single group (≤ T2). Further technique developments concerning CT and PET resolution may improve their ability to differentiate the colonic wall layers and as a result T1 from T2 tumors. The distinction between T1 and T2 stage is not crucial for the therapeutic management of colorectal cancer because the mandatory information relates to whether the tumor is confined to the colonic wall or infiltrates the surrounding tissues.
In our series, a significant difference in SUVmax between each T group was not observed. As a result, it was not possible to identify a potential cut-off value of SUVmax for each T stage in our population.
It is well known that normal gastro-intestinal tract can accumulate FDG extensively, hindering pathological focal tracer uptake or simulating the presence of a tumor. The physiological FDG gastro-intestinal uptake was not the cause of misinterpretation in our series, as tumors were identified either morphologically or metabolically by the readers.
Neither bowel peristaltism nor respiratory motion resulted in mis-registered PET and CT datasets, hindering the interpretation of images in our population.
In our series, two lesions located in the transverse colon and in the sigmoid colon, respectively, were missed. In both cases, retrospective re-evaluation of the colonic segment involved allowed us to conclude that the flat morphology of the two lesions rather than their dimensions or the physiological bowel uptake hindered the identification of these lesions.
Although mucinous adenocarcinoma is a histopathological type of colorectal cancer known to have limited FDG PET sensitivity, the three mucinous adenocarcinomas were correctly identified and staged in our series.
Nodal status was correctly evaluated in 27/34 patients with an accuracy of 79.4%. The use of an un-enhanced PET/CT protocol did not invalidate the N stage as shown by our findings which are similar to those reported in other papers[22,23]. Moreover, it has been demonstrated that contrast-enhanced PET/CT shows a trend towards more accurate N-staging of rectal cancer compared with non-contrast-enhanced PET/CT[23]. Subcentimeter positive nodes are the major source of false negative results in nodal staging, being missed by both PET/CT with and PET/CT without contrast enhancement[23]. As a result, the spatial resolution of PET/CT is not sufficient to detect small lymph node metastases and this limitation can not be obviated by the administration of iv contrast material.
In conclusion, our data suggest that FDG-PET/CT, without administration of iv contrast medium or colonography may be an accurate modality for identifying primary tumor and defining its local extent in patients with colorectal cancer. Further investigations using larger populations and PET/CT devices with improved spatial resolution need to be performed to confirm our observations.
COMMENTS
Background
In oncologic patients, the accurate evaluation of the T (tumor depth), the N (lymph node status) and M (distant metastases) of the primary neoplasm is a crucial point relative to therapeutic management. Although multiple imaging modalities, such as ultrasound (US), computed tomography (CT), magnetic resonance imaging and positron emission tomography (PET)/CT, are available for local tumor and distant staging, an all-in-one staging imaging modality would be a great opportunity to reduce the costs and the time of hospitalization.
Research frontiers
Un-enhanced FDG-PET/CT and more recently contrast-enhanced FDG-PET/CT (PET/ceCT) are gaining a progressively more important role in the evaluation of the N and the M stages in the staging and follow-up of colorectal cancer, however, the performance of this modality in the evaluation of the T parameter has not been extensively investigated.
Innovations and breakthroughs
Our findings suggest that FDG-PET/CT may be an accurate modality for defining local extent (T) of colorectal cancer.
Applications
If PET/CT accurately evaluated the T parameter, this would be a great opportunity to use PET/CT as an all-in-one staging imaging modality in colorectal cancer patients, and subsequently selecting and tailoring the performance of anatomically-based imaging modalities without or with iv contrast medium (US, magnetic resonance, CT) to better define the abnormalities identified by PET/CT, when this information would be of relevance to management planning.
Peer review
The article gives a new idea for the diagnosis and staging of colorectal cancer.
Footnotes
Peer reviewers: Guideng Li, MD, Department of Biological Chemistry, University of California, 140 Sprague Hall, 839 Health Sciences Road, 92617 Irvine, United States; Ali Harlak, MD, Department of General Surgery, Gulhane Military Medical Academy, 06018 Ankara, Turkey
S- Editor Sun H L- Editor Webster JR E- Editor Zheng XM
References
- 1.Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, Starling N. Colorectal cancer. Lancet. 2010;375:1030–1047. doi: 10.1016/S0140-6736(10)60353-4. [DOI] [PubMed] [Google Scholar]
- 2.A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–2059. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 3.Leung KL, Kwok SP, Lam SC, Lee JF, Yiu RY, Ng SS, Lai PB, Lau WY. Laparoscopic resection of rectosigmoid carcinoma: prospective randomised trial. Lancet. 2004;363:1187–1192. doi: 10.1016/S0140-6736(04)15947-3. [DOI] [PubMed] [Google Scholar]
- 4.Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 5.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 6.Habr-Gama A, de Souza PM, Ribeiro U Jr, Nadalin W, Gansl R, Sousa AH Jr, Campos FG, Gama-Rodrigues J. Low rectal cancer: impact of radiation and chemotherapy on surgical treatment. Dis Colon Rectum. 1998;41:1087–1096. doi: 10.1007/BF02239429. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham D, Starling N. Adjuvant chemotherapy of colorectal cancer. Lancet. 2007;370:1980–1981. doi: 10.1016/S0140-6736(07)61841-8. [DOI] [PubMed] [Google Scholar]
- 8.Penna C, Nordlinger B. Colorectal metastasis (liver and lung) Surg Clin North Am. 2002;82:1075–1090. doi: 10.1016/s0039-6109(02)00051-8. [DOI] [PubMed] [Google Scholar]
- 9.Guenette JP, Dupuy DE. Radiofrequency ablation of colorectal hepatic metastases. J Surg Oncol. 2010;102:978–987. doi: 10.1002/jso.21658. [DOI] [PubMed] [Google Scholar]
- 10.Inoue M, Ohta M, Iuchi K, Matsumura A, Ideguchi K, Yasumitsu T, Nakagawa K, Fukuhara K, Maeda H, Takeda S, et al. Benefits of surgery for patients with pulmonary metastases from colorectal carcinoma. Ann Thorac Surg. 2004;78:238–244. doi: 10.1016/j.athoracsur.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 11.Hundt W, Braunschweig R, Reiser M. Evaluation of spiral CT in staging of colon and rectum carcinoma. Eur Radiol. 1999;9:78–84. doi: 10.1007/s003300050632. [DOI] [PubMed] [Google Scholar]
- 12.Kulinna C, Eibel R, Matzek W, Bonel H, Aust D, Strauss T, Reiser M, Scheidler J. Staging of rectal cancer: diagnostic potential of multiplanar reconstructions with MDCT. AJR Am J Roentgenol. 2004;183:421–427. doi: 10.2214/ajr.183.2.1830421. [DOI] [PubMed] [Google Scholar]
- 13.Filippone A, Ambrosini R, Fuschi M, Marinelli T, Genovesi D, Bonomo L. Preoperative T and N staging of colorectal cancer: accuracy of contrast-enhanced multi-detector row CT colonography--initial experience. Radiology. 2004;231:83–90. doi: 10.1148/radiol.2311021152. [DOI] [PubMed] [Google Scholar]
- 14.Mainenti PP, Cirillo LC, Camera L, Persico F, Cantalupo T, Pace L, De Palma GD, Persico G, Salvatore M. Accuracy of single phase contrast enhanced multidetector CT colonography in the preoperative staging of colo-rectal cancer. Eur J Radiol. 2006;60:453–459. doi: 10.1016/j.ejrad.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Brown G, Richards CJ, Newcombe RG, Dallimore NS, Radcliffe AG, Carey DP, Bourne MW, Williams GT. Rectal carcinoma: thin-section MR imaging for staging in 28 patients. Radiology. 1999;211:215–222. doi: 10.1148/radiology.211.1.r99ap35215. [DOI] [PubMed] [Google Scholar]
- 16.Bartram C, Brown G. Endorectal ultrasound and magnetic resonance imaging in rectal cancer staging. Gastroenterol Clin North Am. 2002;31:827–839. doi: 10.1016/s0889-8553(02)00027-4. [DOI] [PubMed] [Google Scholar]
- 17.Brown G, Richards CJ, Bourne MW, Newcombe RG, Radcliffe AG, Dallimore NS, Williams GT. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology. 2003;227:371–377. doi: 10.1148/radiol.2272011747. [DOI] [PubMed] [Google Scholar]
- 18.Smith N, Brown G. Preoperative staging of rectal cancer. Acta Oncol. 2008;47:20–31. doi: 10.1080/02841860701697720. [DOI] [PubMed] [Google Scholar]
- 19.Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging--a meta-analysis. Radiology. 2004;232:773–783. doi: 10.1148/radiol.2323031368. [DOI] [PubMed] [Google Scholar]
- 20.Cohade C, Osman M, Leal J, Wahl RL. Direct comparison of (18)F-FDG PET and PET/CT in patients with colorectal carcinoma. J Nucl Med. 2003;44:1797–1803. [PubMed] [Google Scholar]
- 21.Gearhart SL, Frassica D, Rosen R, Choti M, Schulick R, Wahl R. Improved staging with pretreatment positron emission tomography/computed tomography in low rectal cancer. Ann Surg Oncol. 2006;13:397–404. doi: 10.1245/ASO.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 22.Veit-Haibach P, Kuehle CA, Beyer T, Stergar H, Kuehl H, Schmidt J, Börsch G, Dahmen G, Barkhausen J, Bockisch A, et al. Diagnostic accuracy of colorectal cancer staging with whole-body PET/CT colonography. JAMA. 2006;296:2590–2600. doi: 10.1001/jama.296.21.2590. [DOI] [PubMed] [Google Scholar]
- 23.Tateishi U, Maeda T, Morimoto T, Miyake M, Arai Y, Kim EE. Non-enhanced CT versus contrast-enhanced CT in integrated PET/CT studies for nodal staging of rectal cancer. Eur J Nucl Med Mol Imaging. 2007;34:1627–1634. doi: 10.1007/s00259-007-0455-9. [DOI] [PubMed] [Google Scholar]
- 24.Soyka JD, Veit-Haibach P, Strobel K, Breitenstein S, Tschopp A, Mende KA, Lago MP, Hany TF. Staging pathways in recurrent colorectal carcinoma: is contrast-enhanced 18F-FDG PET/CT the diagnostic tool of choice? J Nucl Med. 2008;49:354–361. doi: 10.2967/jnumed.107.048249. [DOI] [PubMed] [Google Scholar]
- 25.Hicks RJ, Ware RE, Lau EW. PET/CT: will it change the way that we use CT in cancer imaging? Cancer Imaging. 2006;6:S52–S62. doi: 10.1102/1470-7330.2006.9012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor FG, Swift RI, Blomqvist L, Brown G. A systematic approach to the interpretation of preoperative staging MRI for rectal cancer. AJR Am J Roentgenol. 2008;191:1827–1835. doi: 10.2214/AJR.08.1004. [DOI] [PubMed] [Google Scholar]
- 27.Davey K, Heriot AG, Mackay J, Drummond E, Hogg A, Ngan S, Milner AD, Hicks RJ. The impact of 18-fluorodeoxyglucose positron emission tomography-computed tomography on the staging and management of primary rectal cancer. Dis Colon Rectum. 2008;51:997–1003. doi: 10.1007/s10350-008-9244-1. [DOI] [PubMed] [Google Scholar]
- 28.Kinkel K, Lu Y, Both M, Warren RS, Thoeni RF. Detection of hepatic metastases from cancers of the gastrointestinal tract by using noninvasive imaging methods (US, CT, MR imaging, PET): a meta-analysis. Radiology. 2002;224:748–756. doi: 10.1148/radiol.2243011362. [DOI] [PubMed] [Google Scholar]
- 29.Bipat S, van Leeuwen MS, Comans EF, Pijl ME, Bossuyt PM, Zwinderman AH, Stoker J. Colorectal liver metastases: CT, MR imaging, and PET for diagnosis--meta-analysis. Radiology. 2005;237:123–131. doi: 10.1148/radiol.2371042060. [DOI] [PubMed] [Google Scholar]
- 30.Rappeport ED, Loft A, Berthelsen AK, von der Recke P, Larsen PN, Mogensen AM, Wettergren A, Rasmussen A, Hillingsoe J, Kirkegaard P, et al. Contrast-enhanced FDG-PET/CT vs. SPIO-enhanced MRI vs. FDG-PET vs. CT in patients with liver metastases from colorectal cancer: a prospective study with intraoperative confirmation. Acta Radiol. 2007;48:369–378. doi: 10.1080/02841850701294560. [DOI] [PubMed] [Google Scholar]
- 31.Mainenti PP, Mancini M, Mainolfi C, Camera L, Maurea S, Manchia A, Tanga M, Persico F, Addeo P, D'Antonio D, et al. Detection of colo-rectal liver metastases: prospective comparison of contrast enhanced US, multidetector CT, PET/CT, and 1.5 Tesla MR with extracellular and reticulo-endothelial cell specific contrast agents. Abdom Imaging. 2010;35:511–521. doi: 10.1007/s00261-009-9555-2. [DOI] [PubMed] [Google Scholar]
- 32.Niekel MC, Bipat S, Stoker J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology. 2010;257:674–684. doi: 10.1148/radiol.10100729. [DOI] [PubMed] [Google Scholar]


