HIV-infected breastfeeding infants acquired multi-class drug resistance (MCR) after their mothers started highly active antiretroviral therapy (HAART). MCR was more frequent in infants whose mothers started HAART by 6 months post-partum or were exclusively breastfeeding when they reported HAART use.
Abstract
Background. The World Health Organization currently recommends initiation of highly active antiretroviral therapy (HAART) for human immunodeficiency virus (HIV)–infected lactating women with CD4+ cell counts <350 cells/μL or stage 3 or 4 disease. We analyzed antiretroviral drug resistance in HIV-infected infants in the Post Exposure Prophylaxis of Infants trial whose mothers initiated HAART postpartum (with a regimen of nevirapine [NVP], stavudine, and lamivudine). Infants in the trial received single-dose NVP and a week of zidovudine (ZDV) at birth; some infants also received extended daily NVP prophylaxis, with or without extended ZDV prophylaxis.
Methods. We analyzed drug resistance in plasma samples collected from all HIV-infected infants whose mothers started HAART in the first postpartum year. Resistance testing was performed using the first plasma sample collected within 6 months after maternal HAART initiation. Categorical variables were compared by exact or trend tests; continuous variables were compared using rank-sum tests.
Results. Multiclass resistance (MCR) was detected in HIV from 11 (29.7%) of 37 infants. Infants were more likely to develop MCR infection if their mothers initiated HAART earlier in the postpartum period (by 14 weeks vs after 14 weeks and up to 6 months vs after 6 months, P = .0009), or if the mother was exclusively breastfeeding at the time of HAART initiation (exclusive breastfeeding vs mixed feeding vs no breastfeeding, P = .003).
Conclusions. postpartum maternal HAART initiation was associated with acquisition of MCR in HIV-infected breastfeeding infants. The risk was higher among infants whose mothers initiated HAART closer to the time of delivery or were still exclusively breastfeeding when they first reported HAART use.
HIV acquisition through breast milk accounts for ∼40% of HIV infections in infants in Sub-Saharan Africa, where >90% of perinatal infections occur [1]. In resource-limited settings, breastfeeding is also critical for infant health and survival [2–4]. Recent studies demonstrate that postnatal HIV acquisition in HIV-exposed breastfeeding infants can be significantly reduced by giving the infant daily nevirapine (NVP), NVP-zidovudine (ZDV), or lamivudine (3TC) prophylaxis [5–8] or by treating the mother with a triple-drug regimen [8–11]. For example, in Malawi, women who started highly active antiretroviral therapy (HAART) postpartum for their own health (CD4+ cell count <250 cells/μL) were 82% less likely to transmit HIV to their breastfeeding infants, compared with treatment-eligible women who did not start therapy [11]. Consequently, the World Health Organization (WHO) recommends that HIV-infected women breastfeed HIV-uninfected infants for 12 months with concomitant administration of either daily infant NVP or a maternal triple-drug regimen (as life-long treatment if required for the mother's health or as prophylaxis during breastfeeding if the mother does not require therapy for her own health) [2]. If the infant is found to be HIV-infected, the WHO recommends continuation of breastfeeding for as long as possible, because studies have demonstrated significant benefit from continued breastfeeding in HIV-infected infants [2, 12].
Both maternal and infant interventions for the prevention of mother-to-child transmission of HIV (pMTCT) appear to be safe in the short-term for women and infants [1]. However, although postnatal infant or maternal antiretroviral prophylaxis use significantly reduces HIV infection in breastfeeding infants, there is some concern that infants who become HIV-infected despite prophylaxis may acquire resistance to drugs used for pMTCT. For example, several studies demonstrate that infants who are HIV-infected despite exposure to extended daily infant NVP or NVP plus ZDV regimens for pMTCT prior to diagnosis of HIV infection are at high risk of developing resistance to nonnucleoside reverse-transcriptase inhibitors (NNRTIs) [13–16], which often persists for at least 6–12 months [13–15]. In contrast, HIV-infected infants rarely acquire resistance to ZDV after exposure to extended ZDV regimens for pMTCT [16, 17].
There are limited data on the risk of developing drug resistance in HIV-infected infants who are exposed to a maternal triple-drug regimen initiated during pregnancy [18] or postpartum [19, 20], provided either for maternal health or as prophylaxis. Our preliminary studies demonstrated that 75%–86% of infants who were HIV infected by 6 weeks of age developed multiclass resistance (MCR, defined as nucleoside reverse-transcriptase inhibitor [NRTI] resistance plus NNRTI resistance) when their mothers started antiretroviral therapy postpartum [19, 20]. MCR was also documented in 4 (16.7%) of 24 HIV-infected infants whose mothers received a triple-drug regimen during pregnancy [18]. Emergence of MCR in infants is concerning, because infants with MCR may have no viable HIV treatment options. This report assesses the frequency of MCR and risk factors associated with MCR among all HIV-infected infants in the Post Exposure Prophylaxis of Infants (PEPI)-Malawi trial (#NCT00115648) [6] whose mothers initiated antiretroviral treatment during the first postpartum year for their own health.
METHODS
Samples Used for Analysis
Samples were obtained from the PEPI-Malawi trial, a randomized Phase III, controlled trial that enrolled >3000 HIV-infected mothers and infants. The PEPI-Malawi trial compared 3 different regimens for prevention of postnatal HIV transmission. Most women in the PEPI-Malawi trial (∼70%) received single-dose NVP (sdNVP) prior to delivery. All infants received sdNVP at birth and were then randomized to 1 of 3 regimens for pMTCT: (1) sdNVP plus 1 week ZDV (control), (2) control plus daily NVP up to 14 weeks of age (extended NVP), or (3) control plus daily NVP and ZDV up to 14 weeks of age (extended NVP plus ZDV). Prophylaxis was stopped for infected infants following confirmation of an initial positive HIV-DNA polymerase chain reaction test result. Infant plasma was stored at birth and at 3, 6, 9, and 14 weeks and 6, 9, 12, 18, and 24 months of age. Many infants in the extended study arms who were HIV-infected had NNRTI resistance at 14 weeks of age, but none of 85 HIV-infected infants whose mothers did not start treatment by 14 weeks postpartum had ZDV resistance or any other NRTI resistance mutations detected in isolates [16]. Women who met Malawi criteria for treatment initiation initiated a regimen of NVP, 3TC, and stavudine (d4T) during the postpartum period. Maternal antiretroviral therapy was provided outside of the trial. Reported use of maternal antiretroviral drugs was recorded on a structured questionnaire completed at each postpartum visit. The actual date of therapy initiation was not known to the PEPI-Malawi study team. The maximum amount of time between maternal HAART initiation and the mother's report of HAART initiation was 3 weeks for 13 women, 5 weeks for 6 women, and 3 months for 40 women. In this study, we analyzed plasma samples from HIV-infected infants whose mothers initiated HAART postpartum. Only 9 women in the PEPI-Malawi trial initiated HAART before delivery; those women were not included in this study. Infants who received antiretroviral therapy for their own health were excluded from the analysis after infant treatment initiation.
HIV Genotyping
Infant plasma samples (20–100 μL) were analyzed using the ViroSeq HIV-1 Genotyping System, version 2.8 (Celera). Phylogenetic analysis of HIV sequences revealed that all sequences for a given infant grouped together without evidence of sample mix-ups or cross-contamination.
Statistical Analysis
Fisher's exact tests or Cochran–Armitage trend tests were used to compare proportions; Wilcoxon rank-sum tests were used to compare continuous variables. Specifically, Cochran–Armitage trend tests were used to evaluate associations between breastfeeding status and the timing of the mother's first report of HAART use with the probability of MCR. All analyses were performed using SAS, version 9.1 (SAS).
Ethical Considerations
Written informed consent was obtained from all women for participation in the PEPI-Malawi trial. The trial was approved by Institutional Review Boards in Malawi and the United States, including the US Centers for Disease Control and Prevention.
GenBank Accession Numbers
The GenBank accession numbers are HM635477-HM635662.
RESULTS
Overall, 518 women initiated HAART postpartum, 95 of whom had HIV-infected infants. The majority of those infants (90.5%) received a diagnosis of HIV infection before the mother first reported HAART use. Fifty-nine (62.1%) of the 95 women started treatment within 12 months postpartum. Forty-five infants born to those women had at least 1 sample collected within 6 months after the mother first reported HAART use; we analyzed the first sample collected after the mother reported HAART use. Samples from 5 infants could not be analyzed (genotyping failure), and data were censored from 3 infants who were also receiving HAART at the time of the specimen collection; the remaining 37 infants were included in the analysis of MCR. There was no significant difference in clinical or laboratory characteristics of the 37 infants included in the analysis and the 22 infants who were not included (Table 1). To analyze the prevalence of MCR and risk factors associated with MCR, we analyzed drug resistance test results obtained using the first sample collected after the mother first reported HAART use. Thirty (81.1%) of 37 infants had virus with NNRTI resistance mutations, and 11 (29.7%) of the infants had virus with MCR (ie, both NNRTI and NRTI resistance mutations). The proportion of infants who had a sample tested that was collected within 3 months of the mother's first report of HAART use was similar for infants with MCR (9 [81.8%] of 11) and infants without MCR (22 [84.6%] of 26; P = 1.0, by Fisher's exact test). Clinical characteristics of the 11 infants with MCR are shown in Table 2.
Table 1.
Clinical and Laboratory Characteristics of Human Immunodeficiency Virus (HIV)–infected Infants Whose Mothers Started Highly Active Antiretroviral Therapy (HAART) Postpartum
| Variable | Infants included in the analysisa (n=37) | Infants not included in the analysis (n=22) | Pb |
| Maternal CD4+ cell count at delivery, median cells/mL (IQR) | 186 (103, 221) (n=33) | 215 (105, 266) (n=21) | .59c |
| Maternal HIV load at delivery, median log10 copies/mL (IQR) | 4.93 (4.60–5.29) (n=33) | 4.57 (4.30–5.21) (n=18) | .19c |
| Maternal sdNVP exposure | 25 (67.6) | 15 (68.2) | >.99 |
| Infant HIV-infected at the visit where the mother first reported HAART use | 33 (89.2) | 17 (77.3) | .27 |
| Infant HIV-infected in utero | 17 (45.9) | 8 (36.4) | .59 |
| Infant HIV-infected by 6 weeks | 26 (70.3) | 14 (63.6) | .77 |
| Infant regimen | .48 | ||
| Control | 15 (40.5) | 11 (50.0) | |
| Extended NVP | 8 (21.6) | 6 (27.3) | |
| Extended NVP+ZDV | 14 (37.8) | 5 (22.7) | |
| First visit where the mother reported HAART use | |||
| By 3 months | 13 (35.1) | 6 (27.3) | .57 |
| By 6 months | 26 (70.3) | 11 (50.0) | .17 |
| Status of infant feeding at the visit where the mother first reported HAART used | .08 | ||
| Exclusive breastfeeding | 22 (59.5) | 9 (40.9) | |
| Mixed feeding | 10 (27.0) | 4 (18.2) | |
| No breastfeeding | 5 (13.5) | 9 (40.9) |
NOTE. Data are no. (%) of infants unless otherwise indicated. IQR, interquartile range; NVP, nevirapine; sdNVP, single-dose nevirapine; ZDV, zidovudine.
Infants were included if they had a sample collected within 6 months after the visit where the mother first reported that she was receiving HAART, and if a genotyping result was obtained for that sample.
P values are based on Fisher's exact tests, unless otherwise indicated.
Wilcoxon rank-sum test.
In the Post Exposure Prophylaxis of Infants–Malawi trial, some infants became HIV-infected after the mother reported that she had stopped breastfeeding (unpublished data); therefore, some women are likely to have continued to breastfeed after reporting that they had weaned their infants.
Table 2.
Characteristics of the Infants Who Acquired Multiclass Resistance*
| Infant with MCR | Mother received sdNVP | Infant regimen for pMTCT | Duration of regimen, days | Age at HIV infection diagnosis | Infant age at the visit where mother first reported HAART usea | Last visit when the mother reported EBF | Last visit when the mother reported MBF | Loss to follow-up due to infant death |
| 1 | No | Control | 7 | 14 wk | 6 wk | 6 mo | 18 mo | |
| 2 | Yes | Control | 8 | 6 wk | 6 wk | 14 wk | NA | 18 mo |
| 3 | Yes | Ext NVP | 15 | In utero | 6 wk | 14 wk | 12 mo | |
| 4 | Yes | Ext NVP+ZDV | 37 | In utero | 6 wk | 14 wk | NA | |
| 5 | Yes | Control | 8 | 6 wk | 9 wk | 14 wk | 12 mo | |
| 6 | Yes | Control | 8 | 6 wk | 14 wk | 6 mo | NA | 9 mo |
| 7 | Yes | Ext NVP | 57 | 6 wk | 14 wk | 6 mo | 9 mo | 12 mo |
| 8 | Yes | Ext NVP | 14 | In utero | 14 wk | 14 wk | 18 mo | |
| 9 | Yes | Control | 8 | 6 wk | 6 mo | 6 mo | 18 mo | |
| 10 | Yes | Ext NVP | 35 | In utero | 6 mo | 6 mo | 18 mo | |
| 11 | Yes | Ext NVP+ZDV | 14 | In utero | 6 mo | 6 mo | 9 mo |
NOTE. Characteristics of the 11 infants who had multiclass resistance (MCR) are shown. Control, sdNVP plus 1 week of ZDV; Ext NVP, control plus up to 14 weeks of daily NVP; Ext NVP + ZDV, control plus up to 14 weeks of daily NVP and ZDV. sdNVP, single-dose nevirapine; pMTCT, prevention of mother-to-child transmission of HIV; HAART, highly active antiretroviral therapy; EBF, exclusive breastfeeding; MBF, mixed breastfeeding; wk, weeks; mo, months; NA, not available. a The actual date of maternal HAART initiation was sometime between this visit and the prior study visit.
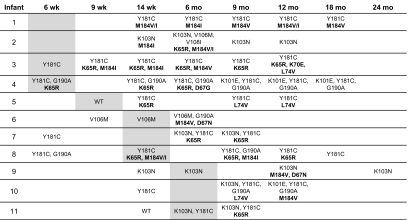
Additional studies were performed for the 11 infants with MCR to examine the emergence and fading of resistance mutations (all available plasma samples were tested; Figure 1, infants 1–11). For each infant, the study visit at which the mother first reported HAART use is indicated in the figure by shading. For 8 infants, NRTI mutations were only detected at or after the study visit where the mother first reported HAART use (infants 3 and 5–11); the other 3 infants did not have samples from earlier study visits to assess timing of emergence of NRTI resistance mutations. The most common NNRTI resistance mutations detected were K103N and Y181C; the most common NRTI resistance mutations detected were M184V/I and K65R. Other NRTI resistance mutations detected included D67N/G, K70E, and L74V. Two infants had MCR detected at the study visit where the mother first reported HAART use (note that the actual date of maternal HAART initiation was sometime between the prior visit and that visit). In the other infants, MCR was first detected 3 months (n = 8) or 6 months (n = 1) after the mother first reported HAART use. All 11 infants still had NNRTI resistance, and 7 still had NRTI resistance detected in the last sample tested (median time, 10.5 months after the mother first reported HAART use; range, 3–18 months). The M184V/I mutations appeared to fade more quickly than did the K65R mutation; in cases where the NRTI mutations faded from detection, NNRTI resistance mutations (K103N, Y181C, and G190A) were still detected 6–12 months after the last NRTI resistance mutation was detected. All 7 women whose infants had MCR detected at the last visit analyzed were still breastfeeding at that visit (Figure 1 and Table 2). In contrast, only 1 of 4 women whose infants no longer had NRTI resistance detected were still breastfeeding at the last visit analyzed (Figure 1 and Table 2).
Figure 1.
Human immunodeficiency virus (HIV) genotyping results obtained for infants who acquired multiclass resistance (MCR). HIV genotyping results are shown for 11 infants who acquired MCR after their mothers started highly active antiretroviral therapy (HAART); test results from all available samples are shown. The top row indicates the PEPI-Malawi study visit. Shaded boxes indicate the study visit at which the mothers first reported that they were receiving HAART. Non-nucleoside reverse transcriptase inhibitor (NNRTI)-resistance mutations are shown in plain text and nucleoside reverse transcriptase inhibitor (NRTI)-resistance mutations are shown in bold. Infant designations 1-11 correspond to the designations in Table 2. Wk: weeks; Mo: months; WT: wild type (no resistance mutations detected).
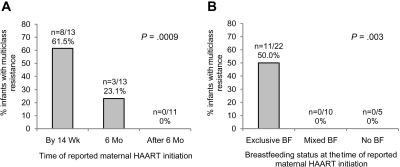
We also analyzed the association between timing of the first report of maternal HAART use and acquisition of MCR (Figure 2A). Earlier postpartum maternal HAART use was strongly associated with acquisition of MCR in the infant (first reported HAART use by 14 weeks vs at 6 months vs after 6 months, P = .0009 by Cochran–Armitage trend test). Based on maternal breastfeeding history, all 11 infants who acquired MCR were exclusively breastfeeding at the visit where the mother first reported HAART use (Figure 2B). The association of MCR and exclusive breastfeeding was highly significant (exclusive breastfeeding vs mixed feeding vs no breastfeeding, P = .003 by Cochran–Armitage trend test). Among the 22 infants who were exclusively breastfeeding when the mother first reported HAART use, we tested whether other clinical or laboratory variables were associated with acquisition of MCR (Table 3). None of the following variables were associated with MCR: maternal CD4+ cell count and log10 viral load at delivery, maternal sdNVP exposure, time of infant HIV infection, detection of NVP resistance in the infant prior to maternal HAART exposure, and infant prophylactic regimen (control vs extended NVP vs extended NVP plus ZDV). Similar results were obtained when the analysis was expanded from these 22 infants to all 37 infants in the sub-study (not shown).
Figure 2.
Factors associated with multiclass resistance (MCR) in infants whose mothers started highly active antiretroviral therapy (HAART) postpartum. A, Panel A shows the proportion of infants with MCR whose mothers reported that they had started HAART by 14 weeks postpartum (by 14 wk), at 6 months postpartum (6 mo), or after 6 months postpartum (after 6 mo). The P value for the comparison of all three groups is shown (Cochran–Armitage trend test). B, Panel B shows the proportion of infants with MCR whose mothers reported that they were exclusively breastfeeding, providing mixed feeding, or not breastfeeding at the visit where they first reported that they were receiving HAART. The P value for the comparison of all three groups is shown (Cochran–Armitage trend test). BF, breastfeeding.
Table 3.
Association of Multiclass Resistance to Clinical and Laboratory Variables
| Variable | Infants without MCR (n=11) | Infants with MCR (n=11) | Pa |
| Maternal CD4+ cell count at delivery, median cells/mL (IQR) | 194 (103–294) (n=10) | 178 (90–193) | .37b |
| Maternal HIV load at delivery, median log10 copies/mL (IQR) | 4.95 (4.55–5.15) (n=10) | 4.62 (4.29–5.21) (n=9) | .90b |
| Maternal sdNVP exposure | 8/11 (72.7) | 10/11 (90.9) | .59 |
| Infant HIV-infected at the time of visit where the mother first reported HAART use | 9/11 (81.8) | 10/11 (90.9) | >.99 |
| Infant HIV-infected in utero | 4/11 (36.4) | 5/11 (45.5) | >.99 |
| Infant HIV-infected by 6 weeks | 5/11 (45.5) | 10/11 (90.9) | .06 |
| NVP resistance in the infant at baseline | 3/4 (75.0) | 5/6 (83.3) | >.99 |
| Infant regimen | |||
| Control | 6/11 (54.5) | 5/11 (45.5) | .40 |
| Ext NVP (vs control) | 1/11 (9.1) | 4/11 (36.4) | |
| Ext NVP+ZDV (vs control) | 4/11 (36.4) | 2/11 (22.2) | |
| Duration of prophylaxis, median days (IQR) | 8 (8–36) (n=10) | 14 (8–35) (n=11) | .91b |
NOTE. Data are no. or proportion (%) of infants, unless otherwise indicated. Results are shown for the subset of 22 infants whose mothers reported that they were exclusively breastfeeding at the visit where they first reported that they were receiving highly active antiretroviral therapy (HAART). Control, sdNVP 1 + week of ZDV; Ext NVP, control plus up to 14 weeks of daily NVP; Ext NVP + ZDV, control plus up to 14 weeks of daily NVP and ZDV. MCR, multiclass resistance; IQR, interquartile range; sdNVP, single-dose nevirapine. NVP, nevirapine; ZDV, zidovudine.
P values are based on Fisher's exact tests, unless otherwise indicated.
Wilcoxon rank-sum test.
DISCUSSION
In the PEPI-Malawi trial, a substantial proportion of the HIV-infected breastfeeding infants whose mothers started HAART postpartum developed MCR. Although the NNRTI resistance mutations in these HIV-infected infants may have arisen due to infant receipt of sdNVP (in the control study arm) or extended NVP (in the extended NVP and extended NVP plus ZDV study arms), four lines of evidence suggest that acquisition of NRTI resistance in the infants resulted from maternal HAART use and not from exposure to ZDV in the infant regimens used for pMTCT. First, in a previous study, we analyzed resistance in infants in the PEPI-Malawi trial whose mothers did not initiate HAART by 14 weeks; none of the 85 infants in that study had virus with ZDV resistance mutations or any other NRTI mutations at 14 weeks of age (the maximum length of prophylaxis) [16]. Second, in this report, we did not detect NRTI resistant virus in any infant prior to the mother's first report of HAART use; among the 11 infants who subsequently developed MCR, none of the 6 infants with pre-HAART samples had NRTI resistance before the mother's first report of HAART use, and 2 of the remaining infants did not have NRTI resistance, at the time the mother first reported HAART use. Third, in this report, acquisition of MCR was not associated with the type or duration of the regimen used for pMTCT (eg, MCR virus was not more frequent among infants in the extended NVP plus ZDV arm than among infants in the control or extended NVP study arms). Finally, the NRTI mutations detected in infants with MCR virus were typical of those selected by drugs in the maternal HAART regimen (3TC and d4T). We did not detect any mutations that are typically associated with ZDV resistance.
In contrast to our findings related to NRTI resistance, infants in the PEPI-Malawi trial frequently acquired NNRTI resistance as a result of exposure to the infant regimens used for pMTCT. Therefore, exposure to both infant NVP used for pMTCT and NVP in the mother's HAART regimen may have contributed to acquisition of NNRTI resistance in the infants. This study did not find an association between acquisition of MCR and maternal sdNVP administration but was not powered to detect small associations. We also did not find an association between acquisition of MCR and the type of regimen that the infant received for pMTCT, the duration of prophylaxis, or detection of NNRTI resistance in the infant at the study visit prior to the mother's first report of HAART use.
Two factors were statistically associated with acquisition of MCR in these infants: (1) earlier initiation of maternal HAART, and (2) exclusive breastfeeding. Mothers of all 11 infants who developed MCR first reported HAART use by 6 months post-partum (8 of 11 mothers reported HAART use by 14 weeks), and all 11 were providing exclusive breastfeeding when they first reported HAART use. We recognize that the small number of infants in this sub-study may have limited the power to detect associations between MCR and other variables examined. Also, because we did not have information on maternal HAART adherence, we were not able to assess the association between maternal HAART adherence and acquisition of MCR in the infants. Acquisition of MCR occurred soon after maternal HAART initiation. Two infants had MCR detected at the visit where the mother first reported HAART use (note that the mother initiated HAART at some point between that visit and the prior study visit), 8 infants developed MCR within 3 months after the mother first reported HAART use, and 1 developed MCR within 6 months after that visit.
We are currently investigating the mechanism(s) responsible for acquisition of MCR in this setting, which include direct transmission of resistant strains to the infant through breast milk and/or transfer of antiretroviral drugs through breast milk that result in sub-therapeutic infant plasma concentrations and selection of MCR virus after infant HIV infection. Women in the PEPI-Malawi trial who received HAART postpartum received the first-line treatment regimen of NVP-3TC-d4T. NVP and 3TC are transferred into breast milk and breastfeeding infants when women receive HAART [21, 22], but less is known about the transfer of d4T into breast milk and breastfeeding infants.
The results of this study should also be considered in the context of the availability of treatment programs for HIV-infected infants. A recent study demonstrated dramatic improvement in the survival of HIV-infected infants who started HAART by 3 months postpartum [23]. As antiretroviral treatment for HIV-infected infants becomes more widely available, more infants may initiate HAART near the time of birth. Current WHO recommendations for infants who are HIV infected despite exposure to NVP prophylaxis are to initiate therapy with protease inhibitor–based regimens [24]. Our data suggest that the choice of antiretroviral drugs for treatment may be limited in HIV-infected infants whose mothers initiate HAART postpartum while they are still breastfeeding. It is not known whether concurrent use of HAART in women and their breastfeeding HIV-infected infants reduces the infants’ risk of MCR or whether concurrent antiretroviral treatment of women and their breastfeeding infants exposes the infants to levels of antiretroviral drugs that may be associated with toxicity. Exposure to maternal HAART in utero and during breastfeeding has been found to increase the risk of neutropenia in infants who are not receiving any antiretroviral drugs [25]. These issues are likely to become increasingly important as treatment programs for HIV-infected women and infants are scaled up around the world.
As antiretroviral drug availability continues to improve in resource-limited settings, the issues surrounding use of antiretroviral drugs for pMTCT and for treatment of HIV-infected women and their infants are becoming increasingly complex. Although significant advances have been made in pMTCT, there is still an urgent need to address issues associated with effective antiretroviral interventions, such as acquisition of MCR, that could compromise the safety and scale-up of these programs.
To optimally preserve maternal health and reduce mother-to-child transmission of HIV, women who need antiretroviral treatment for their own health need to be identified and to initiate HAART early in pregnancy; this will significantly reduce the risk of in utero as well as intrapartum HIV transmission, compared with initiation of antiretroviral drugs late in pregnancy or postpartum. By reducing the number of infants who become infected, the problem of MCR will also be reduced. The WHO recommendations for pMTCT include use of extended maternal triple-drug regimens when treatment is not required for maternal health; this approach is being used with increasing frequency [26]. Our data, along with other reports [18–20], indicate that maternal triple-drug use, either for treatment or solely for prophylaxis, particularly when first initiated postpartum, may be associated with development of MCR in the infants who are infected with HIV despite prophylaxis for pMTCT. These data emphasize the importance of prompt identification of HIV infection in pregnant women, initiation of antiretroviral treatment early in pregnancy when needed for maternal health, and initiation of effective prophylaxis regimens beginning in pregnancy when treatment is not needed, to reduce the risk of mother-to-child transmission of HIV. Finally, when initiating antiretroviral therapy in infants who have HIV infection despite maternal HAART or triple-drug prophylaxis, the risk of MCR infection in infants should be taken into account when choosing an appropriate treatment regimen.
Acknowledgments
We thank the women and infants who participated in the PEPI-Malawi trial; the PEPI-Malawi study team in Malawi; the laboratory staff at the College of Medicine, University of Malawi-Johns Hopkins University Research Project in Blantyre, Malawi, for their assistance with sample processing and shipping; and Dr Mark Mirochnick, for critical review of the manuscript.
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the U.S. Centers for Disease Control and Prevention. Use of trade names is for identification purposes only and does not constitute endorsement by the U.S. Centers for Disease Control and Prevention or the Department of Health and Human Services.
Financial support. The National Institute of Allergy and Infectious Diseases (NIAID/NIH) (R01 HD050180), the Eunice Kennedy Shriver National Institutes of Child Health and Human Development of the National Institutes of Health (NICHD/NIH) (R03 HD061299), the Centers for Disease Control and Prevention (Cooperative Agreement U50/CCU022061), the International Maternal Pediatric and Adolescent AIDS Clinical Trials (IMPAACT) Network (U01 AI068633), the HIV Prevention Trials Network (HPTN) sponsored by NIAID, the National Institute on Drug Abuse, the National Institute of Mental Health, and the Office of AIDS Research of the NIH, DHHS (U01 AI068613), and the Intramural Research Program of the National Human Genome Research Institute, NIH.
Potential conflicts of interest.S.H.E. has served as a consultant for Abbott Diagnostics and has received payment for presentations from Abbott Diagnostics and Celera. All other authors: no conflicts.
References
- 1.Mofenson LM. Protecting the next generation–eliminating perinatal HIV-1 infection. N Engl J Med. 2010;362:2316–2318. doi: 10.1056/NEJMe1004406. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO) HIV and infant feeding. Rapid advice. 2009. Available at: http://whqlibdoc.who.int/publications/2009/9789241598873_eng.pdf. Accessed 5 March 2010. [Google Scholar]
- 3.Joint United Nations Programme on HIV/AIDS (UNAIDS) and the World Health Organization. AIDS Epidemic Update. Geneva, Switzerland: UNAIDS; 2009. Available at: http://data.unaids.org/pub/Report/2009/JC1700_Epi_Update_2009_en.pdf. Accessed 6 June 2010. [Google Scholar]
- 4.Kuhn L, Reitz C, Abrams EJ. Breastfeeding and AIDS in the developing world. Curr Opin Pediatr. 2009;21:83–93. doi: 10.1097/MOP.0b013e328320d894. [DOI] [PubMed] [Google Scholar]
- 5.Kilewo C, Karlsson K, Massawe A, et al. Prevention of mother-to-child transmission of HIV-1 through breast-feeding by treating infants prophylactically with lamivudine in Dar es Salaam, Tanzania: the Mitra Study. J Acquir Immune Defic Syndr. 2008;48:315–323. doi: 10.1097/QAI.0b013e31816e395c. [DOI] [PubMed] [Google Scholar]
- 6.Kumwenda NI, Hoover DR, Mofenson LM, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N Engl J Med. 2008;359:119–129. doi: 10.1056/NEJMoa0801941. [DOI] [PubMed] [Google Scholar]
- 7.Six Week Extended-Dose Nevirapine (SWEN) Study Team. Extended-dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: an analysis of three randomised controlled trials. Lancet. 2008;372:300–313. doi: 10.1016/S0140-6736(08)61114-9. [DOI] [PubMed] [Google Scholar]
- 8.Chasela CS, Hudgens MG, Jamieson DJ, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010;362:2271–2281. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shapiro RL, Hughes MD, Ogwu A, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. 2010;362:2282–2294. doi: 10.1056/NEJMoa0907736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kilewo C, Karlsson K, Ngarina M, et al. Prevention of mother-to-child transmission of HIV-1 through breastfeeding by treating mothers with triple antiretroviral therapy in Dar es Salaam, Tanzania: the Mitra Plus study. J Acquir Immune Defic Syndr. 2009;52:406–416. doi: 10.1097/QAI.0b013e3181b323ff. [DOI] [PubMed] [Google Scholar]
- 11.Taha TE, Kumwenda J, Cole SR, et al. Postnatal HIV-1 transmission after cessation of infant extended antiretroviral prophylaxis and effect of maternal highly active antiretroviral therapy. J Infect Dis. 2009;200:1490–1497. doi: 10.1086/644598. [DOI] [PubMed] [Google Scholar]
- 12.Kuhn L, Aldrovandi GM, Sinkala M, et al. Effects of early, abrupt weaning on HIV-free survival of children in Zambia. N Engl J Med. 2008;359:130–141. doi: 10.1056/NEJMoa073788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Church JD, Omer SB, Guay LA, et al. Analysis of nevirapine (NVP) resistance in Ugandan infants who were HIV infected despite receiving single-dose (SD) NVP versus SD NVP plus daily NVP up to 6 weeks of age to prevent HIV vertical transmission. J Infect Dis. 2008;198:1075–1082. doi: 10.1086/591503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moorthy A, Gupta A, Bhosale R, et al. Nevirapine resistance and breast-milk HIV transmission: effects of single and extended-dose nevirapine prophylaxis in subtype C HIV-infected infants. PLoS ONE. 2009;4:e4096. doi: 10.1371/journal.pone.0004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Persaud D, Bedri A, Ziemniak C, et al. AIDS Res Hum Retroviruses. 2011. Slower clearance of nevirapine resistant virus in infants failing extended nevirapine prophylaxis for prevention of mother-to-child HIV-transmission. E-Pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lidstrom J, Li Q, Hoover DR, et al. Addition of extended zidovudine to extended nevirapine prophylaxis reduces nevirapine resistance in infants who were HIV-infected in utero. AIDS. 2010;24:381–386. doi: 10.1097/QAD.0b013e3283352ef1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McSherry GD, Shapiro DE, Coombs RW, et al. The effects of zidovudine in the subset of infants infected with human immunodeficiency virus type-1 (Pediatric AIDS Clinical Trials Group Protocol 076) J Pediatr. 1999;134:717–724. doi: 10.1016/s0022-3476(99)70287-8. [DOI] [PubMed] [Google Scholar]
- 18.Zeh C, Weidle P, Nafisa L, et al. 15th Conference on Retroviruses and Opportunistic Infections. Boston, MA: Emergence of HIV-1 drug resistance among breastfeeding infants born to HIV-infected mothers taking antiretrovirals for prevention of mother-to-child transmission of HIV: the Kisumu Breastfeeding Study, Kenya (abstract #84 LB) 3–6 February 2008. [Google Scholar]
- 19.Lidstrom J, Guay LA, Musoke P, et al. 17th Conference on Retroviruses and Opportunistic Infections. San Francisco, CA: Multi-class drug resistance arises frequently in HIV-infected breastfeeding infants whose mothers initiate highly active antiretroviral therapy (HAART) post-partum (abstract #920) 16–19 February 2010. [Google Scholar]
- 20.Lidstrom J, Kumwenda N, Kafulafula G, et al. Antiretroviral treatment of HIV-infected women can induce multi-class drug resistance in their breastfeeding infants. Antivir Ther. 2009;14(Suppl 1):135. [Google Scholar]
- 21.Mirochnick M, Thomas T, Capparelli E, et al. Antiretroviral concentrations in breast-feeding infants of mothers receiving highly active antiretroviral therapy. Antimicrob Agents Chemother. 2009;53:1170–1176. doi: 10.1128/AAC.01117-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shapiro RL, Holland DT, Capparelli E, et al. Antiretroviral concentrations in breast-feeding infants of women in Botswana receiving antiretroviral treatment. J Infect Dis. 2005;192:720–727. doi: 10.1086/432483. [DOI] [PubMed] [Google Scholar]
- 23.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization (WHO) Report of the WHO Technical Reference group, Paediatric HIV/ART Care Guideline Group Meeting. 2009. Available at: http://www.who.int/hiv/pub/paediatric/WHO_Paediatric_ART_guideline_rev_mreport_2008.pdf. Accessed 6 June 2010. [Google Scholar]
- 25.Bae WH, Wester C, Smeaton LM, et al. Hematologic and hepatic toxicities associated with antenatal and postnatal exposure to maternal highly active antiretroviral therapy among infants. AIDS. 2008;22:1633–1640. doi: 10.1097/QAD.0b013e328307a029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization (WHO) Use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Rapid advice. 2009. Available at: http://www.who.int/hiv/pub/mtct/rapid_advice_mtct.pdf. Accessed 28 May 2010. [PubMed] [Google Scholar]