Abstract
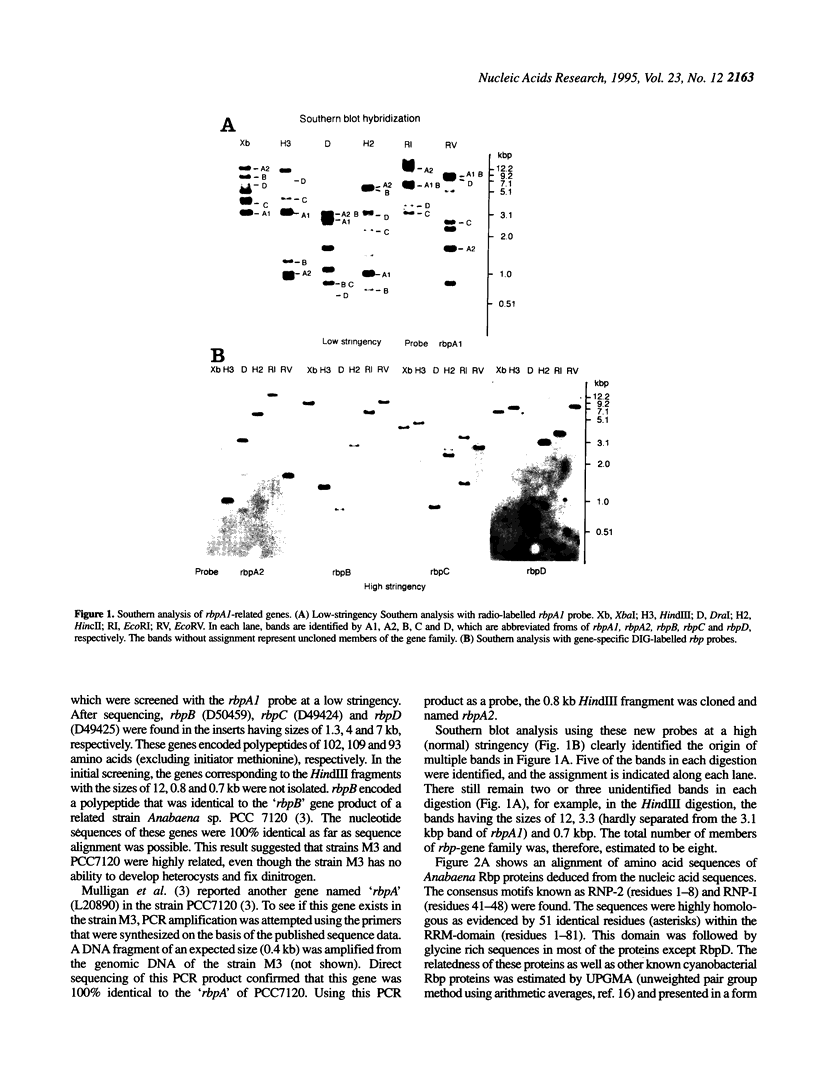
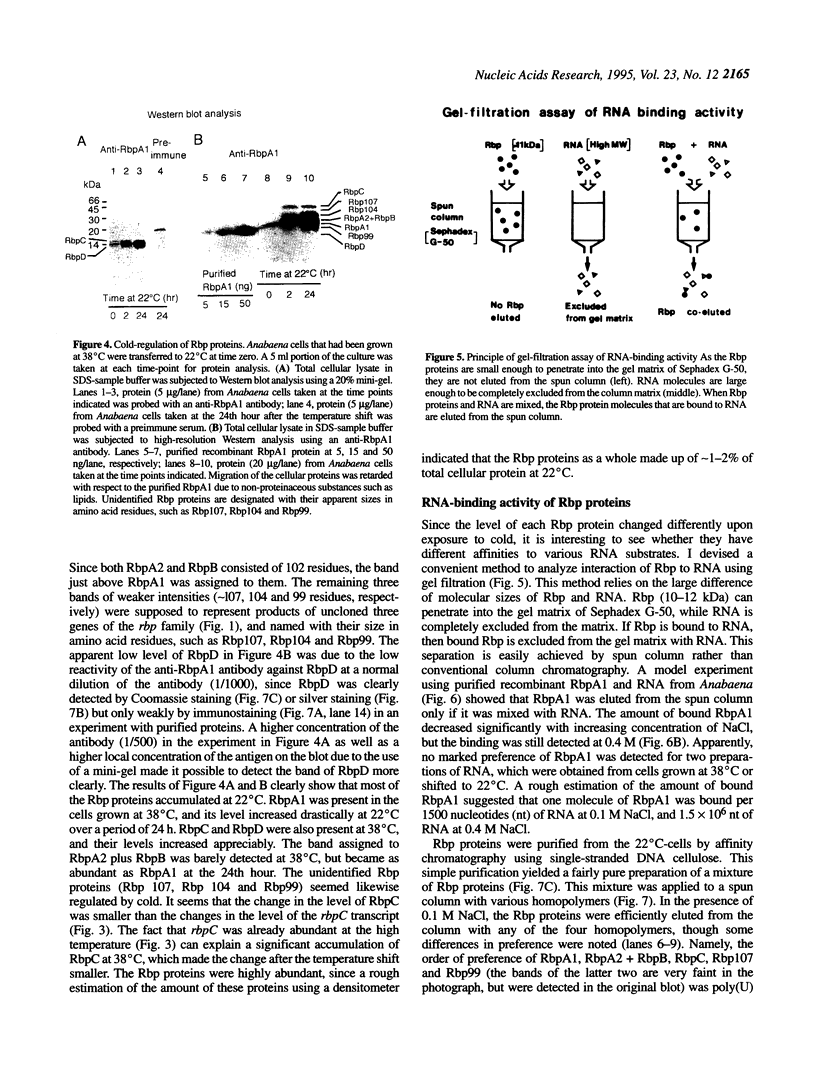
I previously found a cold-regulated RNA-binding protein gene rbpA (now named rbpA1) in Anabaena variabilis M3 [Sato, N. (1994) Plant Mol. Biol. 24, 819-823]. I show here that this gene is a member of a gene family containing at least eight members as evidenced by Southern blot and immunoblot analyses. I have isolated three additional genes (rbpB, rbpC and rbpD) in this family. Of these, rbpB was 100% identical to the rbpB gene of Anabaena 7120 reported previously. Another gene named rbpA in Anabaena 7120 was also found to exist in A.variabilis M3 with identical sequence and named rbpA2. The amino acid sequences of these gene products were highly conserved, except that the RbpD protein lacked glycine-rich C-terminal domain present in all other known members of the gene family. RNA blot and immunoblot analyses showed that the expression of rbpA1, rbpA2, rbpB, rbpC and rbpD, as well as uncloned rbp genes was regulated by cold, though the exact time-course and extent of response to cold were different among these genes. Gel-filtration assay showed that all of the Rbp proteins have higher affinities to poly(G) and poly(U) than to poly(A) and poly(C).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Didierjean L., Frendo P., Burkard G. Stress responses in maize: sequence analysis of cDNAs encoding glycine-rich proteins. Plant Mol Biol. 1992 Feb;18(4):847–849. doi: 10.1007/BF00020034. [DOI] [PubMed] [Google Scholar]
- Gómez J., Sánchez-Martínez D., Stiefel V., Rigau J., Puigdomènech P., Pagès M. A gene induced by the plant hormone abscisic acid in response to water stress encodes a glycine-rich protein. Nature. 1988 Jul 21;334(6179):262–264. doi: 10.1038/334262a0. [DOI] [PubMed] [Google Scholar]
- Jones P. G., Inouye M. The cold-shock response--a hot topic. Mol Microbiol. 1994 Mar;11(5):811–818. doi: 10.1111/j.1365-2958.1994.tb00359.x. [DOI] [PubMed] [Google Scholar]
- Kenan D. J., Query C. C., Keene J. D. RNA recognition: towards identifying determinants of specificity. Trends Biochem Sci. 1991 Jun;16(6):214–220. doi: 10.1016/0968-0004(91)90088-d. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ludevid M. D., Freire M. A., Gómez J., Burd C. G., Albericio F., Giralt E., Dreyfuss G., Pagès M. RNA binding characteristics of a 16 kDa glycine-rich protein from maize. Plant J. 1992 Nov;2(6):999–1003. [PubMed] [Google Scholar]
- Mulligan M. E., Jackman D. M., Murphy S. T. Heterocyst-forming filamentous cyanobacteria encode proteins that resemble eukaryotic RNA-binding proteins of the RNP family. J Mol Biol. 1994 Jan 21;235(3):1162–1170. doi: 10.1006/jmbi.1994.1070. [DOI] [PubMed] [Google Scholar]
- Porter R. D. DNA transformation. Methods Enzymol. 1988;167:703–712. doi: 10.1016/0076-6879(88)67081-9. [DOI] [PubMed] [Google Scholar]
- Sato N. A cold-regulated cyanobacterial gene cluster encodes RNA-binding protein and ribosomal protein S21. Plant Mol Biol. 1994 Mar;24(5):819–823. doi: 10.1007/BF00029864. [DOI] [PubMed] [Google Scholar]
- Sato N. Cloning of a low-temperature-induced gene lti2 from the cyanobacterium Anabaena variabilis M3 that is homologous to alpha-amylases. Plant Mol Biol. 1992 Jan;18(1):165–170. doi: 10.1007/BF00018474. [DOI] [PubMed] [Google Scholar]
- Schnuchel A., Wiltscheck R., Czisch M., Herrler M., Willimsky G., Graumann P., Marahiel M. A., Holak T. A. Structure in solution of the major cold-shock protein from Bacillus subtilis. Nature. 1993 Jul 8;364(6433):169–171. doi: 10.1038/364169a0. [DOI] [PubMed] [Google Scholar]
- Schuster G., Gruissem W. Chloroplast mRNA 3' end processing requires a nuclear-encoded RNA-binding protein. EMBO J. 1991 Jun;10(6):1493–1502. doi: 10.1002/j.1460-2075.1991.tb07669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm A. A Wound-Inducible Glycine-Rich Protein from Daucus carota with Homology to Single-Stranded Nucleic Acid-Binding Proteins. Plant Physiol. 1992 Aug;99(4):1689–1692. doi: 10.1104/pp.99.4.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita M., Sugiura M. The existence of eukaryotic ribonucleoprotein consensus sequence-type RNA-binding proteins in a prokaryote, Synechococcus 6301. Nucleic Acids Res. 1994 Jan 11;22(1):25–31. doi: 10.1093/nar/22.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L. H., Li Y. Q., Fukami-Kobayashi K., Go M., Konishi T., Watanabe A., Sugiura M. Diversity of a ribonucleoprotein family in tobacco chloroplasts: two new chloroplast ribonucleoproteins and a phylogenetic tree of ten chloroplast RNA-binding domains. Nucleic Acids Res. 1991 Dec 11;19(23):6485–6490. doi: 10.1093/nar/19.23.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]