Of 682 antiretroviral-na_ve patients initiating antiretroviral therapy in a prospective, multicenter HIV-1 drug resistance monitoring study involving eight sites in Hong Kong, Malaysia, and Thailand, the prevalence of patients with ≥1 drug resistance mutation(s) was 13.8%. Primary HIV drug resistance is emerging after rapid scaling-up of antiretroviral therapy in Asia.
Abstract
(See editorial commentary by Jordan on pages 1058–1060.)
Of 682 antiretroviral-naïve patients initiating antiretroviral therapy in a prospective, multicenter human immunodeficiency virus type 1 (HIV-1) drug resistance monitoring study involving 8 sites in Hong Kong, Malaysia, and Thailand, the prevalence of patients with ≥1 drug resistance mutation was 13.8%. Primary HIV drug resistance is emerging after rapid scaling-up of antiretroviral therapy use in Asia.
Human immunodeficiency virus type 1 (HIV-1) infection in Asia accounts for a substantial proportion of the global HIV-1 epidemic. Currently, an estimated 4.7 million HIV-1–infected persons are living in Asia [1]. Combination antiretroviral therapy (ART) has significantly reduced mortality and morbidity in the region [2–6]. ART use has been scaling up in Asia for 2–9 years, depending on the country and setting [1, 7]. HIV-1 drug resistance (HIVDR) is a major reason for treatment failure, and primary HIVDR threatens the effectiveness of ART among HIV-1–infected patients who are initiating ART [8–10]. Primary HIVDR is defined as an increase in resistance of HIV-1 to antiretroviral drugs that is seen in individuals who have never received ART and presumably have been infected with drug-resistant virus [11, 12]. The prevalence of primary HIVDR varies from 6.2% to 21% in the United States and Europe, which suggests an increasing trend across the region [8–10]. However, the data on primary HIVDR in Asia is markedly limited. Two small studies in Thailand have reported the prevalence of primary HIVDR as being <5% [13, 14]. There are few widely available antiretroviral regimens in Asia, especially in countries with limited resources, and hence the detection of baseline HIVDR is of great importance.
Therapeutics, Research, Education and AIDS Training in Asia (TREAT Asia) is a network of clinics, hospitals, and research institutions working to ensure the safe and effective delivery of treatments of HIV infection and AIDS in Asia. To assess the extent of HIVDR in Asia, the TREAT Asia Studies to Evaluate Resistance-Monitoring Study (TASER-M) was initiated [15]. The objectives of TASER-M are to assess the prevalence and incidence of emerging HIVDR and to produce evidence to inform future treatment guidelines. This analysis aims to determine the prevalence and risk factors of HIVDR among antiretroviral-naïve HIV-1–infected patients recruited to the TASER-M cohort.
Methods
Patients eligible for TASER-M are those initiating first-line ART or switching to second-line ART [15]. Antiretroviral-naïve patients who initiated ART at participating sites from April 2007 through March 2009 were included in this analysis. Patients with a history of monotherapy or dual therapy or exposure to antiretroviral drugs for prevention of mother-to-child transmission were excluded. Ethics approvals were obtained from local institutional review boards. Informed consent was obtained prior to genotypic resistance testing.
Genotype tests were performed locally with externally quality-controlled in-house and commercial assays on samples collected within 6 months prior to initiating ART. HIV-1 drug-resistance–associated mutations (RAMs) were assessed using International AIDS Society–USA 2009 criteria [16]. Subtype was determined on the basis of genotypes of reverse transcriptase and protease genes. Laboratories providing genotyping results for the TASER-M study were required to participate in the TREAT Asia Quality Assurance Scheme, which is an assessment program to build genotyping laboratory capacity [17].
Data were collected on age, sex, ethnicity, HIV-1 exposure category, Centers for Disease Control and Prevention (CDC) disease stage classification, hepatitis B virus (HBV) or hepatitis C virus (HCV) co-infection status, CD4+ cell count, HIV-1 RNA level, and HIV-1 subtype. The prevalence of primary HIVDR was determined. Predictors of HIVDR were assessed using logistic regression models. A P value of <.05 was considered to be statistically significant.
Results
A total of 682 patients from 8 sites including Hong Kong (2 sites), Malaysia (2 sites), and Thailand (4 sites) were included in this analysis. The mean age was 38.2 years (SD, 10.1 years); 65.5% of the patients were male. The ethnicities of patients included Thai (500, 73.3%), Chinese (134, 19.6%), Malay (26, 3.8%), Indian (7, 1.0%), Indonesian (3, .4%), Filipino (1, .1%), and others (11, 1.8%). The majority (74.9%) of patients reported heterosexual contact as their primary risk exposure for HIV-1; other risk categories included homosexual contact (18.2%), intravenous drug use (2.3%), receipt of blood products (.3%), and mixed exposure (3.2%). More than one-third of patients were in CDC disease stage C. The median CD4+ cell count was 100 cells/μL (interquartile range, 34–201 cells/μL), and the median HIV-1 RNA level was 100,000 copies/mL (interquartile range, 43,581–6,040,000 copies/mL). Overall, 77.7% of patients were infected with HIV-1 subtype CRF01_AE; other subtypes included B (16.3%), C (.7%), A (.1%), other Circulating Recombinant Forms (CRFs) (2.4%), or were missing (2.9%). Co-infection with HBV was seen in 5.1% of patients, and that with HCV was seen in 7.9% of patients.
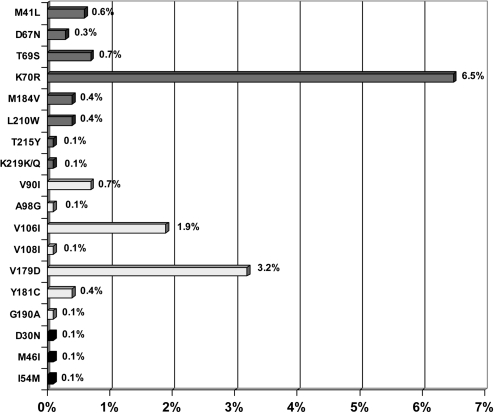
The prevalence of patients with ≥1 RAM to any drug class was 13.8%, including RAMs to nucleoside reverse transcriptase inhibitors (NRTIs; prevalence, 8.4%), nonnucleoside reverse transcriptase inhibitors (NNRTIs; prevalence, 6.5%), and protease inhibitors (PIs; prevalence, .4%). Figure 1 shows the distribution and frequency of each RAM that was detected. K70R was the most common RAM to NRTIs (52 patients [7.6%]); M41L, D67N, T69S, M184V, L210W, T215Y, and K219Q were observed in <1% of patients. RAMs to etravirine were detected in 44 patients (6.5%) and those to efavirenz or nevirapine in 4 patients (.6%). The most common RAMs to etravirine were V179D (22 patients [3.2%]) and V106I (13 patients [1.9%]); other RAMs were found in <1% of patients. The RAMs observed to efavirenz or nevirapine were Y181C (3 patients [.4%]), V108I (1 patient [.1%]), and G190A (1 patient [.1%]). RAMs D30N, M46I, and I54M to PIs were each observed in .1% of patients (1 patient each).
Figure 1.
Distribution of resistance-associated mutations among 682 antiretroviral-naïve human immunodeficiency virus type 1 (HIV-1)–infected patients.
The median CD4+ cell count was significantly lower among patients with RAMs when compared with those without RAMs (66 vs 108 cells/μL, respectively; P = .009). There were no differences between patients with RAMs and those without RAMs in age, sex, site location, ethnicity, risk exposure, HIV-1 subtype, HBV co-infection, HCV co-infection, or HIV-1 RNA level.
Discussion
The emergence of primary HIVDR in antiretroviral-naïve patients has been associated with increased mortality, morbidity, and medical expenditures [8–10, 18, 19]. The longer history of ART availability in the participating sites in the region coupled with early suboptimal treatment regimens [20, 21] may be leading to the higher levels of circulating HIVDR mutations in the community. Because of the limited options available for second- or third-line ART, monitoring of HIVDR is a key to preparing for optimal treatment management in the future. The prevalence of primary HIVDR in the present study was higher than that found in previous surveys of transmitted HIVDR in this region [14, 22]. However, these 2 previous studies were conducted among newly infected patients, whereas the present study was performed among patients with chronic infection prior to ART initiation. The reported prevalence of antiretroviral resistance among ART-naïve HIV-infected persons in Sub-Saharan Africa, where there are also resource-limited settings, ranged from 7.8% to 9.8% [23, 24].
Resistance mutations to NRTIs and NNRTIs were more commonly observed compared with those to PIs. The most common forms of primary or transmitted HIVDR that are detected globally are resistance mutations to NRTIs [18, 25–29]. In this study, the most common RAM was K70R, a thymidine analogue mutation, which is also consistent with the widespread use of zidovudine and stavudine [30, 31]. The use of zidovudine and stavudine in dual-therapy regimens also has contributed to the increased prevalence of this mutation [14, 32]. Previous studies have demonstrated that K70R was one of the most common RAMs observed among antiretroviral-naïve patients particularly in areas with early scaling-up of ART [33–37].
The low rate of M184V in the present study may be explained by the fact that the present study was conducted among patients with chronic infection prior to ART initiation. It is possible that our patients may have acquired drug-resistant HIV in the earlier period because dual therapy (ie, stavudine or zidovudine plus didanosine) was used in this region.
Interestingly, the prevalence of RAMs to etravirine was higher than that of RAMs to efavirenz or nevirapine. This might be explained by the fact that the most common HIV-1 subtype in the present study was HIV-1 subtype CRF01_AE. A recent study has reported that non-B HIV-1 subtypes have natural polymorphisms that are described as RAMs to etravirine [38]. Further study to evaluate the potential of these polymorphisms to affect etravirine susceptibility is needed.
The pre-ART CD4+ cell counts of patients in this study were much lower than local treatment thresholds, with half of the patients having CD4+ cell counts of <100 cells/μL. In addition, the median CD4+ cell count was significantly lower among patients with RAMs compared with that among patients without RAMs. This finding supports the idea that patients with advanced HIV-1 disease might be at greater risk of having acquired drug-resistant HIV-1 infection earlier in the regional HIV epidemic, when regimens were not as potent.
In Asia, the new local guidelines and World Health Organization (WHO) guidelines recommend the use of nevirapine or efavirenz with lamivudine and zidovudine or tenofovir for first-line ART [39, 40]. According to our findings of a high prevalence of primary HIVDR, particularly RAMs to NRTIs, there is a risk of early treatment failure with the first-line ART in this region. Currently, guidelines in North America and Europe recommend resistance testing prior to initiation of ART [41, 42]. However, treatment guidelines for developing countries in Asia (eg, WHO and Thai guidelines) do not recommend this test in antiretroviral-naïve patients [41, 42], mainly because of the limited data on primary HIVDR in this region and the relatively high cost of the test. This raises concerns regarding the risk of treatment failure among patients with primary HIVDR. Further study to define a cost-effective strategy for detection of primary HIVDR in Asia is needed.
There are some limitations to the present study. First, the patients in the present study were tested for HIV-1 genotypes at pretreatment rather than at the time of diagnosis. Some resistance mutations may have reverted to the wild type. Thus, the prevalence of primary HIVDR could be underestimated. Second, the present study was conducted in a limited region of Asia, including 8 sites in only 3 countries, because of the limited availability of genotype tests in Asia.
In summary, primary HIVDR is emerging in Asia after rapid scale-up of ART use. Patients with a lower pre-ART CD4+ cell count were at higher risk for having primary HIVDR. Although HIV genotype testing prior to ART initiation is not routinely recommended in resource-limited settings, our results raise concerns about the risk of early treatment failure in our cohort if genotype testing is not conducted prior to initiation of ART.
Acknowledgments
The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the funding institutions.
Members of the TASER study. P.C.K. Li (steering committee member and protocol co-chair) and M.P. Lee, Queen Elizabeth Hospital, Hong Kong, China; K.H. Wong, Integrated Treatment Centre, Hong Kong, China; N. Kumarasamy (steering committee member and protocol chair) and S. Saghayam, YRG Centre for AIDS Research and Education, Chennai, India; S. Pujari (steering committee member) and K. Joshi, Institute of Infectious Diseases, Pune, India; T.P. Merati (steering committee member) and F. Yuliana, Faculty of Medicine, Udayana University and Sanglah Hospital, Bali, Indonesia; C.K.C. Lee (steering committee member) and L.L. Low, Hospital Sungai Buloh, Kuala Lumpur, Malaysia; A. Kamarulzaman (steering committee member Protocol co-chair) and L.Y. Ong, University of Malaya, Kuala Lumpur, Malaysia; M. Mustafa (steering committee member) and N. Nordin, Hospital Raja Perempuan Zainab II, Kota Bharu, Malaysia; R. Ditangco (steering committee chair) and R.O. Bantique, Research Institute for Tropical Medicine, Manila, Philippines; Y.M.A. Chen (steering committee member Protocol chair), Y.J. Chen, and Y.T. Lin, AIDS Prevention and Research Centre, National Yang-Ming University, Taipei, Taiwan; P. Phanuphak (steering committee member) and S. Sirivichayakul, HIV-NAT/Thai Red Cross AIDS Research Centre, Bangkok, Thailand; S. Sungkanuparph (steering committee member) and S. Kiertiburanakul, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; T. Sirisanthana (steering committee member) and J Praparattanapan, Research Institute for Health Sciences, Chiang Mai University, Chiang Mai, Thailand; P. Kantipong (steering committee co-chair) and P. Kambua, Chiang Rai Regional Hospital, Chiang Rai, Thailand; J.Y. Choi (steering committee member) and S.H. Han, Division of Infectious Diseases, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, South Korea; W. Ratanasuwan (steering committee member) and R. Sriondee, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand; R. Kantor (steering committee member), Brown University, Rhode Island; A.H. Sohn, L. Messerschmidt (steering committee member), and T. Singtoroj, TREAT Asia, The Foundation for AIDS Rsearch (amfAR), Bangkok, Thailand; and D.A. Cooper, M.G. Law (steering committee member), A. Jiamsakul, and J. Zhou, National Centre in HIV Epidemiology and Clinical Research, University of New South Wales, Sydney, Australia.
Financial Support. This work was supported by the Dutch Ministry of Foreign Affairs through a partnership with Stichting AIDS Fonds; the National Institute of Allergy and Infectious Diseases and the National Cancer Institute at the US National Institutes of Health as part of the International Epidemiologic Databases to Evaluate AIDS (grant number U01-AI069907); TREAT Asia, amfAR; and the National Centre in HIV Epidemiology and Clinical Research, University of New South Wales. Queen Elizabeth Hospital and the Integrated Treatment Centre are supported by the Hong Kong Council for AIDS Trust Fund. The National Centre in HIV Epidemiology and Clinical Research is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, University of New South Wales.
Potential conflicts of interest: M.G.L. is a consultant for Johnson & Johnson Research, Janssen-Cilag, New South Wales Health, Roche, Gilead, and Merck and has provided expert testimony for DLA Phillips Fox. M.G.L. has also received grant support from the US National Institutes of Health, The Foundation for AIDS Research (amfAR), Australian National Health and Medical Research Council, Abbott, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Janssen-Cilag, Johnson & Johnson, Merck Sharp & Dohme, Pfizer, Roche, and CSL and meeting expenses/travel reimbursement from amfAR. P.C.K.L. has received institutional grant support from the Hong Kong Council for AIDS Trust fund, amfAR, and University of New South Wales and meeting expenses/travel reimbursement from amfAR, Bristol-Myers Squibb, Bayer, GlaxoSmithKline, Boehringer Ingelheim, Janssen-Cilag, and Merck Serono. P.C.K.L. is also on the advisory boards of Abbott, Merck Sharp & Dohme, Janssen, and Pfizer. All other authors: no conflicts.
References
- 1.Joint United Nations Programme on HIV/AIDS and World Health Organization. AIDS epidemic update December 2009. WHO Library Cataloguing-in-Publication Data November 2009; [Google Scholar]
- 2.Manosuthi W, Chottanapand S, Thongyen S, Chaovavanich A, Sungkanuparph S. Survival rate and risk factors of mortality among HIV/tuberculosis-coinfected patients with and without antiretroviral therapy. J Acquir Immune Defic Syndr. 2006;43:42–6. doi: 10.1097/01.qai.0000230521.86964.86. [DOI] [PubMed] [Google Scholar]
- 3.Kumarasamy N, Solomon S, Chaguturu SK, et al. The changing natural history of HIV disease: before and after the introduction of generic antiretroviral therapy in southern India. Clin Infect Dis. 2005;41:1525–8. doi: 10.1086/497267. [DOI] [PubMed] [Google Scholar]
- 4.Jongwutiwes U, Kiertiburanakul S, Sungkanuparph S. Impact of antiretroviral therapy on the relapse of cryptococcosis and survival of HIV-infected patients with cryptococcal infection. Curr HIV Res. 2007;5:355–60. doi: 10.2174/157016207780636551. [DOI] [PubMed] [Google Scholar]
- 5.Sungkanuparph S, Chakriyanuyok T, Butthum B. Antiretroviral therapy in AIDS patients with CMV disease: impact on the survival and long-term treatment outcome. J Infect. 2008;56:40–3. doi: 10.1016/j.jinf.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Zhou J, Paton NI, Ditangco R, et al. Experience with the use of a first-line regimen of stavudine, lamivudine and nevirapine in patients in the TREAT Asia HIV Observational Database. HIV Med. 2007;8:8–16. doi: 10.1111/j.1468-1293.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Towards universal access scaling up priority HIV/AIDS interventions in the health sector, progress report 2010. WHO Library Cataloguing-in-Publication Data September 2010. [Google Scholar]
- 8.Little SJ, Holte S, Routy JP, et al. Antiretroviral-drug resistance among patients recently infected with HIV. N Engl J Med. 2002;347:385–94. doi: 10.1056/NEJMoa013552. [DOI] [PubMed] [Google Scholar]
- 9.Balotta C, Berlusconi A, Pan A, et al. Prevalence of transmitted nucleoside analogue-resistant HIV-1 strains and pre-existing mutations in pol reverse transcriptase and protease region: outcome after treatment in recently infected individuals. Antivir Ther. 2000;5:7–14. [PubMed] [Google Scholar]
- 10.Grant RM, Hecht FM, Warmerdam M, et al. Time trends in primary HIV-1 drug resistance among recently infected persons. JAMA. 2002;288:181–8. doi: 10.1001/jama.288.2.181. [DOI] [PubMed] [Google Scholar]
- 11.Division of HIV/AIDS Epidemiology and Surveillance, National HIV and Retrovirology Laboratories, Centre for Infectious Disease Prevention and Control, Public Health Agency of Canada, Health Canada. HIV-1 strain and primary drug resistance in Canada. Surveillance report to June 30, 2001. http://www.phacaspc.gc.ca/publicat/hiv1-vih1/pdf/hiv-1-strain-01-e.pdf. Accessed 10 December 2010. [Google Scholar]
- 12.The International Consultation on Monitoring the Emergence of Antiretroviral Resistance sponsored by WHO, UNAIDS and ISS (October, 2000) http://www.who.int/csr/resources/publications/drugresist/WHO_CDS_CSR_DRS_2001_11/en/. Accessed 10 December 2010. [Google Scholar]
- 13.Apisarnthanarak A, Jirayasethpong T, Sa-nguansilp C, et al. Antiretroviral drug resistance among antiretroviral-naive persons with recent HIV infection in Thailand. HIV Med. 2008;9:322–5. doi: 10.1111/j.1468-1293.2008.00562.x. [DOI] [PubMed] [Google Scholar]
- 14.Sirivichayakul S, Phanuphak P, Pankam T, O-Charoen R, Sutherland D, Ruxrungtham K. HIV drug resistance transmission threshold survey in Bangkok, Thailand. Antivir Ther. 2008;13(suppl 2):109–13. [PubMed] [Google Scholar]
- 15.Hamers RL, Oyomopito R, Kityo C, et al. Cohort profile: The PharmAccess African (PASER-M) and the TREAT Asia (TASER-M) Monitoring Studies to Evaluate Resistance—HIV drug resistance in sub-Saharan Africa and the Asia-Pacific. Int J Epidemiol. 2010 doi: 10.1093/ije/dyq192. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson VA, Brun-Vezinet F, Clotet B, et al. Update of the drug resistance mutations in HIV-1: December 2009. Top HIV Med. 2009;17:138–45. [PubMed] [Google Scholar]
- 17.Land S, Cunningham P, Zhou J, et al. TREAT Asia Quality Assessment Scheme (TAQAS) to standardize the outcome of HIV genotypic resistance testing in a group of Asian laboratories. J Virol Methods. 2009;159:185–93. doi: 10.1016/j.jviromet.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geretti AM. Epidemiology of antiretroviral drug resistance in drug naïve persons. Curr Opin Infect Dis. 2007;20:22–32. doi: 10.1097/QCO.0b013e328013caff. [DOI] [PubMed] [Google Scholar]
- 19.Sax PE, Islam R, Walensky RP, et al. Should resistance testing be performed for treatment-naive HIV-infected patients? A cost effectiveness analysis. Clin Infect Dis. 2005;41:1316–23. doi: 10.1086/496984. [DOI] [PubMed] [Google Scholar]
- 20.Nuesch R, Ananworanich J, Sirivichayakul S, et al. Development of HIV with drug resistance after CD4 cell count-guided structured treatment interruptions in patients treated with highly active antiretroviral therapy after dual-nucleoside analogue treatment. Clin Infect Dis. 2005;40:728–34. doi: 10.1086/427878. [DOI] [PubMed] [Google Scholar]
- 21.Ungsedhapand C, Srasuebkul P, Cardiello P, et al. Three-year durability of dual-nucleoside versus triple-nucleoside therapy in a Thai population with HIV infection. J Acquir Immune Defic Syndr. 2004;36:693–701. doi: 10.1097/00126334-200406010-00006. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen HT, Duc NB, Shrivastava R, et al. HIV drug resistance threshold survey using specimens from voluntary counselling and testing sites in Hanoi, Vietnam. Antivir Ther. 2008;13(suppl 2):115–21. [PubMed] [Google Scholar]
- 23.Vergne L, Diagbouga S, Kouanfack C, et al. HIV-1 drug-resistance mutations among newly diagnosed patients before scaling-up programmes in Burkina Faso and Cameroon. Antivir Ther. 2006;11:575–9. [PubMed] [Google Scholar]
- 24.Koizumi Y, Ndembi N, Miyashita M, et al. Emergence of antiretroviral therapy resistance-associated primary mutations among drug-naive HIV-1-infected individuals in rural western Cameroon. J Acquir Immune Defic Syndr. 2006;43:15–22. doi: 10.1097/01.qai.0000226793.16216.55. [DOI] [PubMed] [Google Scholar]
- 25.Booth CL, Garcia-Diaz AM, Youle MS, et al. Prevalence and predictors of antiretroviral drug resistance in newly diagnosed HIV-1 infection. J Antimicrob Chemother. 2007;59:517–24. doi: 10.1093/jac/dkl501. [DOI] [PubMed] [Google Scholar]
- 26.Corvasce S, Violin M, Romano L, et al. Evidence of differential selection of HIV-1 variants carrying drug-resistant mutations in seroconverters. Antivir Ther. 2006;11:329–34. [PubMed] [Google Scholar]
- 27.Oette M, Kaiser R, Daumer M, et al. Primary HIV drug resistance and efficacy of first-line antiretroviral therapy guided by resistance testing. J Acquir Immune Defic Syndr. 2006;41:573–81. doi: 10.1097/01.qai.0000214805.52723.c1. [DOI] [PubMed] [Google Scholar]
- 28.Shet A, Berry L, Mohri H, et al. Tracking the prevalence of transmitted antiretroviral drug-resistant HIV-1: a decade of experience. J Acquir Immune Defic Syndr. 2006;41:439–46. doi: 10.1097/01.qai.0000219290.49152.6a. [DOI] [PubMed] [Google Scholar]
- 29.Jayaraman GC, Archibald CP, Kim J, et al. A population-based approach to determine the prevalence of transmitted drug-resistant HIV among recent versus established HIV infections: results from the Canadian HIV strain and drug resistance surveillance program. J Acquir Immune Defic Syndr. 2006;42:86–90. doi: 10.1097/01.qai.0000196666.16616.fe. [DOI] [PubMed] [Google Scholar]
- 30.Larder BA, Kemp SD. Multiple mutations in HIV-1 reverse transcriptase confer high-level resistance to zidovudine (AZT) Science. 1989;246:1155–8. doi: 10.1126/science.2479983. [DOI] [PubMed] [Google Scholar]
- 31.Picard V, Angelini E, Maillard A, et al. Comparison of genotypic and phenotypic resistance patterns of human immunodeficiency virus type 1 isolates from patients treated with stavudine and didanosine or zidovudine and lamivudine. J Infect Dis. 2001;184:781–4. doi: 10.1086/323088. [DOI] [PubMed] [Google Scholar]
- 32.Sirivichayakul S, Ruxrungtham K, Ungsedhapand C, et al. Nucleoside analogue mutations and Q151M in HIV-1 subtype A/E infection treated with nucleoside reverse transcriptase inhibitors. AIDS. 2003;17:1889–96. doi: 10.1097/00002030-200309050-00007. [DOI] [PubMed] [Google Scholar]
- 33.Erice A, Mayers DL, Strike DG, et al. Primary infection with zidovudine-resistant human immunodeficiency virus type 1. N Engl J Med. 1993;328:1163–5. doi: 10.1056/NEJM199304223281605. [DOI] [PubMed] [Google Scholar]
- 34.Césaire R, Dos Santos G, Abel S, et al. Drug resistance mutations among HIV-1 strains from antiretroviral-naive patients in Martinique, French West Indies. J Acquir Immune Defic Syndr. 1999;22:401–5. doi: 10.1097/00126334-199912010-00012. [DOI] [PubMed] [Google Scholar]
- 35.Eiros JM, Labayru C, Hernández B, et al. Prevalence of genotypic resistance in untreated HIV patients in Spain. Eur J Clin Microbiol Infect Dis. 2002;21:310–3. doi: 10.1007/s10096-002-0711-7. [DOI] [PubMed] [Google Scholar]
- 36.Ammaranond P, Cunningham P, Oelrichs R, et al. Rates of transmission of antiretroviral drug resistant strains of HIV-1. J Clin Virol. 2003;26:153–61. doi: 10.1016/s1386-6532(02)00114-2. [DOI] [PubMed] [Google Scholar]
- 37.Juhász E, Ghidán A, Kemény B, Nagy K. Emergence of antiretroviral drug resistance in therapy-naive HIV infected patients in Hungary. Acta Microbiol Immunol Hung. 2008;55:383–94. doi: 10.1556/AMicr.55.2008.4.3. [DOI] [PubMed] [Google Scholar]
- 38.Maïga AI, Descamps D, Morand-Joubert L, et al. Resistance-associated mutations to etravirine (TMC-125) in antiretroviral-naïve patients infected with non-B HIV-1 subtypes. Antimicrob Agents Chemother. 2010;54:728–33. doi: 10.1128/AAC.01335-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sungkanuparph S, Techasathit W, Utaipiboon C, et al. Thai national guidelines for antiretroviral therapy in HIV-1 infected adults and adolescents 2010. Asian Biomed. 2020;4:515–28. [Google Scholar]
- 40.World Health Organization . Rapid advice: antiretroviral therapy for HIV infection in adults and adolescents. November 2009. pp. 1–24. http://www.who.int/HIV/pub/arv/rapid_advice_art.pdf. Accessed 25 April 2010. [Google Scholar]
- 41.Hirsch MS, Gunthard HF, Schapiro JM, et al. Antiretroviral drug resistance testing in adult HIV-1 infection: 2008 recommendations of an International AIDS Society-USA panel. Clin Infect Dis. 2008;47:266–85. doi: 10.1086/589297. [DOI] [PubMed] [Google Scholar]
- 42.Gazzard BG, Anderson J, Babiker A, et al. BHIVA Treatment Guidelines Writing Group. British HIV Association guidelines for the treatment of HIV-1-infected adults with antiretroviral therapy 2008. HIV Med. 2008;9:563–608. doi: 10.1111/j.1468-1293.2008.00636.x. [DOI] [PubMed] [Google Scholar]