MSRA isolates with high vancomycin MIC have been associated with treatment failure. Predicting CIR090 the likelihood of high vancomycin MIC may be useful for clinical decision making. We present a simple tool to help predict vancomycin MIC in MRSA bacteremia.
Abstract
Background. Increased mortality, treatment failure, and hospital length of stay have been reported in patients treated with vancomycin for methicillin-resistant Staphylococcus aureus (MRSA) bacteremia when their isolates have a vancomycin minimum inhibitory concentration (MIC) > 1 μg/mL. Automated testing often fails to identify these isolates. We developed a simple clinical rule to predict vancomycin MIC of 2 μg/mL in patients with MRSA bacteremia.
Methods. This cohort study was conducted at a tertiary care hospital and an affiliated acute rehabilitation facility. Consecutive patients with MRSA bacteremia from 2001 through 2007 were prospectively identified. Patient characteristics were examined for their association with high vancomycin MIC and a predictive model was created.
Results. A total of 296 MRSA bacteremic episodes among 272 patients were identified; 19% of the episodes had isolates with a vancomycin MIC of 2 μg/mL. Variables associated with a vancomycin MIC of 2 μg/mL included older age (odds ratio [OR], 4.0; 95% confidence interval [CI], 1.5–10.4); prior vancomycin (OR, 3.8; 95% CI, 1.9–7.6) or daptomycin (OR, 7.9; 95% CI, 1.8–34.0) exposure; the presence of a nontunneled central venous catheter (OR, 1.9; 95% CI, 1.1–3.4) or prosthetic heart valve (OR, 3.6; 95% CI, 1.3–10.0); a history of MRSA bacteremia (OR, 3.0; 95% CI, 1.6–5.6); and the presence of sepsis (OR, 2.7; 95% CI, 1.4–5.1) or shock (OR, 2.2; 95% CI, 1.1–4.2) at the time of culture. The final predictive rule included age > 50 years (3 points), prior vancomycin exposure (2 points), history of MRSA bacteremia (2 points), history of chronic liver disease (2 points), and presence of a nontunneled central venous catheter (1 point). A score cutoff of ≥ 4 resulted in a sensitivity of 75% and specificity of 59% (negative predictive value, 91%; positive predictive value, 30%).
Conclusions. Several factors that predict high vancomycin MIC were identified, and a simple predictive tool was created to help clinicians determine which patients are likely to have MRSA isolates with high vancomycin MIC.
Infection with methicillin-resistant Staphylococcus aureus (MRSA) is associated with significant morbidity and mortality, especially when inappropriately treated [1–3]. In US hospitals, the proportion of S. aureus bloodstream infections with methicillin resistance has been increasing over time [4]. There is also evidence suggesting a trend toward higher vancomycin minimum inhibitory concentrations (MICs) in these isolates, also referred to as “MIC creep” [5–7].
Recently, the Clinical and Laboratory Standards Institute (CLSI) changed the cutoff for vancomycin sensitivity to MRSA from an MIC of ≤ 4 to an MIC of ≤ 2 on the basis of studies that suggest a significantly greater treatment failure rate among patients with vancomycin MICs of ≥ 4 [8, 9]. Since this change in laboratory standards, data have emerged demonstrating greater rates of treatment failure and higher mortality among patients treated with vancomycin when MICs are higher, even if those MICs are within the currently accepted range of susceptibility (≤ 2) [10–14].
Most hospitals report estimated vancomycin MICs through automated methods. These MIC values do not accurately reflect those produced with other standardized methods, such as the Etest or microbroth dilution techniques, on which most outcomes data are based [15]. Up to 90% of MRSA isolates with an MIC of 2 μg/mL are missed by the automated systems [16]. Furthermore, even when gold standard methods are used to determine vancomycin MICs, these results may take days to become available, during which time active therapy may be critical to favorable patient outcomes.
The ability to determine which patients are likely to be infected with MRSA strains that have elevated MICs to vancomycin should be useful clinically. This knowledge could lead to earlier recognition of patients with increased potential for vancomycin treatment failure, allowing for the early use of alternative therapy in appropriate situations. We therefore sought to determine which factors are associated with elevated vancomycin MICs in patients with MRSA bacteremia and to create a predictive tool to help guide clinical decision-making in this context.
METHODS
Study Design and Setting
From January 2001 through December 2007, data were collected as part of a prospective cohort study of MRSA bloodstream infection at Tufts Medical Center and the New England Sinai Hospital at Tufts Medical Center. All MRSA-positive blood cultures during this period were recorded, and the MRSA isolates were saved and underwent further testing. Isolates from admitted patients who were ≥ 18 years of age at the time of culture were included in the study.
Data Collection
Patient comorbidity and demographic data were prospectively collected as part of this cohort study. Laboratory data and vital signs were recorded, as were data on patient location, recent surgeries, and need for mechanical ventilation or hemodialysis. Antibiotic exposure was also recorded with note of antibiotic type(s) and dates of exposure. The presence of foreign bodies (including endovascular devices and lines, orthopedic implants, and foley catheters) was recorded, and markers of severity of illness were calculated and added to the database, including Acute Physiology and Chronic Health Evaluation–II scores and Charlson Cormorbidity scores. Lastly, functional status and the presence of sepsis, systemic inflammatory response syndrome, or shock were recorded for each event.
Microbiologic Methods
S. aureus was identified and methicillin resistance was confirmed according to CLSI methodology. All MRSA isolates were stored in skim milk at −70°C while waiting for further MIC testing. Vancomycin susceptibility was determined through broth microdilution, which was performed in triplicate. At least 2 vancomycin MIC values of 2 μg/mL were required to be defined as having a high MIC to vancomycin.
Definitions
Bacteremia with MRSA is defined as the presence of MRSA in any clinically initiated blood culture. No blood cultures were performed in addition to those that were clinically prompted by the treating clinicians. If multiple positive blood culture results were available for a single patient, isolates were considered as separate events if there were negative intervening blood culture results and at least 30 days had elapsed since the first negative blood culture result. All data were recaptured at the time of their subsequent event. For the purposes of this study, isolates from Tufts Medical Center and the New England Sinai Hospital are not differentiated. Although these hospitals technically represent individual entities, they exist in the same physical structure and share the same services, such as the computerized medical record system and laboratory, including the microbiology laboratory that processes blood cultures. Sepsis and shock are defined by standard clinical definitions [17, 18]. Patients with a history of chronic hepatitis or cirrhosis are defined as having chronic liver disease in this cohort.
Statistical Methods
Patient characteristics were compared between those events that had MRSA isolates with high MICs and those that had MRSA isolates with low MICs with use of χ2 tests and Fisher's exact tests for categorical variables and Student’s t-test for continuous variables. Nonparametric tests were used for continuous variables that were not normally distributed. Factors reaching a significance level of P ≤ .2 were candidates at the first step of a forward selection process used to build a multivariable model. In the course of model building, continuous variables were analyzed for linear relationships with the outcome, and clinically reasonable interactions were evaluated. The final model was chosen on the basis of its ability to predict the outcome of interest and the ease to which the variables could be applied clinically. We re-estimated the coefficients for the terms in the final model in a generalized estimated equations model to account for clustering because some patients contributed > 1 data point (that is, they had > 1 event). The final model underwent the appropriate diagnostic tests to evaluate model fit and to look for outlying data with potential leverage. A scoring system was created on the basis of coefficients generated from this final model, and the model was rerun with the sum score as the only predictor variable. Receiver operating characteristic curves were generated, and sensitivity and specificity were calculated on the basis of this model. All analyses were conducted using SAS, version 9.13 (SAS Institute).
RESULTS
Baseline Data
There were 358 MRSA bacteremia isolates collected from 272 patients during the study period, accounting for 296 separate MRSA bacteremic events. The median age was 65 years (range, 20–93 years), and 62% of the events were in men. The number of events with any microbroth dilution MIC of > 1 was 62 (1 of 3), 19 (2 of 3), and 38 (3 of 3). Thus, 57 (19%) of 296 events had high vancomycin MICs by our definition of majority of triplicate samples having MIC values > 1.
Univariate Analyses
A number of variables were found to be significantly associated with high vancomycin MIC in univariate analysis, including age > 50 years, the presence of sepsis or shock at the time of culture, a known history of MRSA bacteremia, recent exposure to vancomycin or daptomycin, and the presence of a prosthetic heart valve or nontunneled central line (Table 1). Recent exposure to piperacillin-tazobactam was found to be associated with low vancomycin MIC.
Table 1.
Predictors of High Vancomycin Minimum Inhibitory Concentration (MIC)
| Variable | No (%) of events with high MICs (n = 57) | No.(%) of events with low MICs (n = 239) | OR (95% CI) | P value |
| Male sex | 39 (68) | 145 (61) | 1.4 (0.8–2.6) | .27 |
| Age > 50 years | 52 (91) | 173 (72) | 4.0 (1.5–10.4) | < .01 |
| Any malignancy | 11 (19) | 70 (29) | 0.6 (0.3–1.2) | .12 |
| Hematologic malignancy | 3 (5) | 34 (14) | 0.3 (0.1–1.1) | .05 |
| Diabetes mellitus | 23 (40) | 93 (39) | 1.1 (0.6–1.9) | .84 |
| Immunosuppressive therapy | 16 (28) | 81 (34) | 0.8 (0.4–1.4) | .40 |
| Chronic cardiovascular disease | 38 (67) | 132 (55) | 1.6 (0.9–3.0) | .11 |
| Chronic liver disease | 18 (32) | 51 (21) | 1.7 (0.9–3.2) | .11 |
| Chronic lung disease | 20 (35) | 80 (33) | 1.1 (0.6–2.0) | .82 |
| Chronic kidney disease | 25 (44) | 85 (36) | 1.4 (0.8–2.5) | .25 |
| Hemodialysis | 13 (23) | 45 (19) | 1.3 (0.6–2.6) | .50 |
| Dementia | 3 (5) | 27 (11) | 0.4 (0.1–1.5) | .15 |
| Transplant recipient | 3 (5) | 19 (8) | 0.6 (0.2–2.3) | .47 |
| SIRS on bacteremia presentation | 42 (88) | 163 (76) | 2.2 (0.9–5.6) | .06 |
| Sepsis on bacteremia presentation | 31 (65) | 87 (41) | 2.7 (1.4–5.1) | < .01 |
| Septic shock on bacteremia presentation | 18 (38) | 47 (22) | 2.2 (1.1–4.2) | .03 |
| Apache II ≥ 5 | 28 (57) | 92 (43) | 1.8 (0.9–3.3) | .08 |
| Charlson score > 5 | 18 (36) | 103 (47) | 0.6 (0.3–1.2) | .17 |
| ICU at time of diagnosis of bacteremia | 15 (26) | 83 (35) | 0.7 (0.4–1.3) | .22 |
| Health care associated bacteremia | 50 (91) | 210 (90) | 1.1 (0.4–3.0) | .86 |
| History of MRSA | 31 (54) | 105 (44) | 1.5 (0.9–2.7) | .16 |
| History of MRSA bacteremia | 20 (35) | 37 (16) | 3.0 (1.6–5.6) | < .01 |
| Any antibiotics in prior 30 days | 43 (77) | 179 (75) | 1.1 (0.6–2.2) | .80 |
| Piperacillin/tazobactam in prior month | 1 (2) | 25 (11) | 0.2 (0.0–1.2) | .02 |
| Daptomycin in prior 30 days | 5 (9) | 3 (1) | 7.9 (1.8–34.0) | < .01 |
| Vancomycin > 48 h in last week | 18 (32) | 26 (11) | 3.8 (1.9–7.6) | < .01 |
| Foreign bodya | 51 (90) | 189 (80) | 2.1 (0.9–5.2) | .08 |
| Joint implant | 10 (18) | 26 (11) | 1.8 (0.8–4.0) | .17 |
| Any endovascular | 48 (84) | 174 (73) | 1.9 (0.9–4.2) | .08 |
| Heart valve | 7 (12) | 9 (4) | 3.6 (1.3–10.0) | .02 |
| Tunneled central line | 9 (16) | 42 (18) | 0.9 (0.4–1.9) | .72 |
| Nontunneled central line | 29 (51) | 85 (36) | 1.9 (1.1–3.4) | .03 |
| Subcutaneous device | 21 (37) | 75 (32) | 1.3 (0.7–2.3) | .44 |
NOTE. Univariate analyses of 296 episodes of MRSA bacteremia to identify factors that are associated with a vancomycin MIC of 2 mg/L. P values of < 0.5 are presented in bold.
Includes all foreign bodies, except Foley catheter.
Predictive Model
A multivariate model that incorporates variables with high predictive value that are likely to be readily available at the time of clinical decision making was created. This model includes age > 50 years, history of chronic liver disease, recent vancomycin exposure (> 48 h during the previous 7 days), presence of a nontunneled central venous catheter at the time of culture, and a history of MRSA bacteremia. The predictive area for this model (c statistic) was 0.74, and the adjusted ORs are provided in table 2. After adjustment for clustering, there was little change in the ORs for the variables in the model, and the overall model performance remained relatively stable (Table 2). A scoring system for the predictor variables was created (Table 2), and total scores were calculated for each of the bacteremic events. These total scores were evaluated for their ability to predict the outcome of high vancomycin MIC in the prediction data set, and their performance was similar to the individually weighted variables from the final multivariable logistic regression model (c statistic, 0.73). The sensitivity, specificity, and negative and positive predictive values for the various score cutoffs and for the final model are described in table 3. Model calibration demonstrates that the use of the predictive rule results in a slight over-prediction in quintiles 1, 2, and 5; slight under-prediction in quintile 4; and predicts equally in quintile 3.
Table 2.
Final Predictive Model, with Results of a Multivariate Analysis for Independent Factors that Predict a Vancomycin Minimum Inhibitory Concentration (MIC) of 2 μg/L in 296 Episodes of Methicillin-Resistant Staphylococcus aureus (MRSA) Bloodstream Infection
| Variable | AdjustedaOR (95% CI) | P value | GEE ModelOR (95% CI) | P value | Score |
| Age > 50 years | 5.8 (2.1–16.3) | < .01 | 5.7 (2.2–15.0) | < .01 | 3 |
| Vancomycin > 48 h in previous week | 2.5 (1.1–5.5) | .03 | 2.5 (1.1–5.4) | .02 | 2 |
| Chronic liver disease | 2.6 (1.2–5.3) | .01 | 2.5 (1.2–5.2) | .01 | 2 |
| History of MRSA bacteremia | 2.5 (1.2–5.3) | .02 | 2.5 (1.2–5.0) | .01 | 2 |
| Nontunneled central line | 1.6 (0.9–3.1) | .14 | 1.7 (0.9–3.1) | .12 | 1 |
Receiver operating characteristic area (c-statistic) = 0.74.
Table 3.
Performance of the Predictive Rule by Score Cutoff
| Score | Sensitivity | Specificity | Positive predictive value | Negative predictive value | Percent of the entire cohort |
| 10 | 4 | 100 | 100 | 81 | 0.7 |
| ≥ 9 | 5 | 100 | 75 | 82 | 1.4 |
| ≥ 8 | 16 | 97 | 60 | 83 | 5.1 |
| ≥ 7 | 26 | 96 | 60 | 85 | 8.4 |
| ≥ 6 | 40 | 87 | 42 | 86 | 18.6 |
| ≥ 5 | 58 | 74 | 35 | 88 | 31.8 |
| ≥ 4 | 75 | 59 | 30 | 91 | 48.0 |
| ≥ 3 | 97 | 19 | 22 | 96 | 83.8 |
| ≥ 2 | 100 | 12 | 21 | 100 | 90.2 |
| ≥ 1 | 100 | 8 | 21 | 100 | 93.6 |
DISCUSSION
The use of early appropriate antibiotic therapy is crucial to the overall care of infected patients. However, the initial choice of an antibiotic regimen is often made empirically, awaiting microbiologic test results. Although the results of microbiology tests are delayed, and in the case of automated testing of vancomycin MIC, inaccurate, the early use of readily available patient characteristics can help stratify patients according to their risk of treatment failure. Thus, the application of a clinical prediction rule may lead to better outcomes. We have identified a number of patient characteristics that are associated with having an MRSA bloodstream isolate with a high vancomycin MIC. Using this information, we were able to construct a simple predictive tool to help clinicians determine the likelihood that their patients with MRSA bacteremia have isolates with high MIC to vancomycin. The tool that we created uses binary (yes/no) variables that are readily available at the time of bacteremia and does not require knowledge of laboratory data or determination of severity of illness in its application. This tool can be used to either rule in or rule out patients with bacteremia, according to the circumstances and clinical need.
The relationship between prior vancomycin exposure and elevated MIC has been described elsewhere [19, 20]. Prior studies have also found associations between intensive care unit exposure, female sex, elevated body mass index, recent surgery, and cardiovascular disease and elevated vancomycin MIC [20, 21]. The results of our study complement these data and are further strengthened by the large size of our cohort, the use of only bacteremic isolates, and our use of microbroth dilution in triplicate, which is unique and leads to a more accurate measure of high vancomycin MIC. To our knowledge, this is the first known predictive model to examine the question of which patients are likely to have MRSA bacteremic isolates with elevated MICs to vancomycin.
A potential weakness to our study is related to differences in vancomycin MIC among MRSA isolates that are community acquired versus those that are classically thought of as health care associated or hospital acquired, with lower MIC values typically seen in community-acquired strains [22, 23]. Therefore, as the proportion of community-acquired MRSA isolates increases as a cause of bacteremia, differences may arise in the predictors of high MIC. In our study, we examined health care exposure and antibiotic susceptibility pattern as predictor variables and found no association with high vancomycin MIC. However, we do not have strain typing data for our isolates, so do not know the extent to which strain type may have contributed to our findings.
Recently published guidelines on the use of vancomycin suggest considering alternative antibiotics in complicated MRSA infections, including bacteremia, when the isolates are found to have MICs ≥ 2 [24]. Knowing that poor outcomes are associated with inappropriate empirical antibiotics and that there are now data and guidelines suggesting that vancomycin is not the appropriate choice for patients with MRSA bacteremia when the MIC is ≥ 2, the ability to accurately discriminate between patients who have high versus low MIC values to vancomycin will be a critical step in the overall clinical decision-making process.
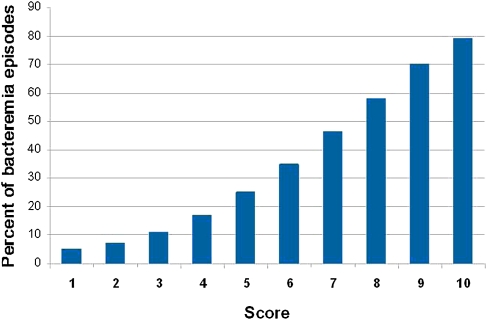
Depending on the clinical circumstances, this model can be used to rule in or rule out high vancomycin MIC in patients with MRSA bacteremia. Typical rule out situations are those in which the consequences of mistreating a high MIC isolate are severe. These include patients who are very sick because of their bacteremia or infections that affect hard-to-treat sites (eg, osteomyelitis). Rule in situations include less severe illness involving tissues where adequate vancomycin concentrations are easier to achieve. To ensure high performance as a rule out tool, the score cutoff should be chosen to maximize the negative predictive value of the rule. A cutoff score of ≥ 4 performs well as a rule out cutoff. Although it maintains a high sensitivity and negative predictive value, it eliminates approximately half of the cohort of patients with MRSA bacteremia (Table 3). A score cutoff of ≥ 8 performs well as a rule in cutoff. It defines a group of patients with a likelihood of a high vancomycin MIC of ≥ 60% (Figure 1). Although the performance of this model, in terms of sensitivity or specificity, is modest, the model produces a high negative predictive value, which represents a substantial improvement to our current ability to rule out a high vancomycin MIC.
Figure 1.
Percentage of bacteremic events predicted to have a vancomycin MIC of 2 mg/L by total score.
Rates of high vancomycin MIC may vary by hospital. The rate of high vancomycin MIC (∼ 20%) at our center is similar to rates described in other tertiary care centers. In settings in which the rate of high vancomycin MIC is less than the rate in our cohort, the use of our prediction model would result in a lower positive predictive value and a higher negative predictive value. In this case, the use of a higher prediction score threshold will improve the model performance. In settings in which the rate of high vancomycin MIC is greater than the rate in our cohort, the use of our prediction model will result in a higher positive predictive value and a lower negative predictive value. In these settings, the use of a lower prediction score threshold (ie, 3) is preferred, because it will result in missing fewer patients with a high vancomycin MIC.
As new alternative antimicrobial agents become available for the treatment of serious MRSA infections and as currently available agents become more cost-effective, the ability to accurately predict vancomycin MIC in MRSA isolates will only become more relevant. The more effective these agents are found to be, the lower the threshold for risk of having an isolate with high vancomycin MIC will need to be to consider using alternative therapy. Until that time, we would still argue that, because of the very high morbidity and mortality associated with MRSA bloodstream infection and with worse outcomes associated with inappropriate treatment, the presence of even a modest amount of risk for having a high MIC should lead to consideration of alternative and/or more aggressive therapy.
Acknowledgments
We thank Drs Robert Arbeit and Debra Poutsiaka for reviewing the project in its various stages and providing useful advice.
Potential conflicts of interest. Y.G. has received a research grant from, served on the speakers’ bureau for, and been a consultant for Cubist Pharmaceuticals and has received a research grant from and been a consultant for Pfizer. D.R.S. has been a consultant for CSL Behring, Novartis, Boeringer INgelheim, Mass Biologic Public Health Laboratories, Genzyme, Millenium, and Genentech; has received grant support from Optimer, Merck, Cubist, Genentech, Pfizer, and Forrest; and has served on the speakers’ bureau and given lectures for Merck CSL Behring. All other authors: no conflicts.
Financial support. National Institutes of Health Training Grant (5 T32 AI055412 to A.S.L.), Tufts Medical Center (to A.S.L.), Tufts CTSI grant (UL1 RR025752 to RR), Cubist Pharmaceuticals (to Y.G.), and National Center for Research Resources (UL1 RR025752 to R.R.).
References
- 1.Soriano A, Martinez JA, Mensa J, et al. Pathogenic significance of methicillin resistance for patients with Staphylococcus aureus bacteremia. Clin Infect Dis. 2000;30:368–73. doi: 10.1086/313650. [DOI] [PubMed] [Google Scholar]
- 2.Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus: a meta-analysis. Clin Infect Dis. 2003;36:53–9. doi: 10.1086/345476. [DOI] [PubMed] [Google Scholar]
- 3.Kaye KS, Anderson DJ, Choi J, Link K, Thacker P, Sexton DJ. The deadly toll of invasive methicillin-resistant Staphylococcus aureus infection in community hospitals. Clin Infect Dis. 2008;46:1568–77. doi: 10.1086/587673. [DOI] [PubMed] [Google Scholar]
- 4.Wisplighoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–17. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 5.Wang G, Hindler JF, Ward KW, Bruckner DA. Increased vancomycin MICs for Staphylococcus aureus clinical isolates from a university hospital during a 5-year period. J Clin Microbiol. 2006;44:3883–6. doi: 10.1128/JCM.01388-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinkraus G, White R, Friedrich L. Vancomycin MIC creep in non-vancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-susceptible clinical methicillin-resistant S. aureus (MRSA) blood isolates from 2001–05. J Antimicrob Chemother. 2007;60:788–94. doi: 10.1093/jac/dkm258. [DOI] [PubMed] [Google Scholar]
- 7.Ho PL, Lo PY, Chow KH, et al. Vancomycin MIC creep in MRSA isolates from 1997 to 2008 in a healthcare region in Hong Kong. J Infect. 2010;60:140–5. doi: 10.1016/j.jinf.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 8.CLSI. CLSI approved standard M100-S16. Wayne, PA: CLSI; 2006. Performance standards for antimicrobial susceptibility testing. [Google Scholar]
- 9.Tenover FC, Moellering RC., Jr The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin Infect Dis. 2007;44:1208–15. doi: 10.1086/513203. [DOI] [PubMed] [Google Scholar]
- 10.Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC, Jr., Eliopoulos GM. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Micro. 2004;42:2398–402. doi: 10.1128/JCM.42.6.2398-2402.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soriano A, Marco F, Martinez JA, et al. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46:193–200. doi: 10.1086/524667. [DOI] [PubMed] [Google Scholar]
- 12.Lodise TP, Graves J, Evans A, et al. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob agents chemother. 2008;52:3315–20. doi: 10.1128/AAC.00113-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med. 2006;166:2138–44. doi: 10.1001/archinte.166.19.2138. [DOI] [PubMed] [Google Scholar]
- 14.Yoon YK, Kim JY, Park DW, Sohn JW, Kim MJ. Predictors of persistent methicillin-resistant Staphylococcus aureus bacteraemia in patients treated with vancomycin. J Antimicrob Chemother. 2010;65:1015–18. doi: 10.1093/jac/dkq050. [DOI] [PubMed] [Google Scholar]
- 15.Hsu DI, Hidayat LK, Quist R, et al. Comparison of method-specific vancomycin minimum inhibitory concentration values and their predictability for treatment outcome of meticillin-resistant Staphylococcus aureus (MRSA) infections. Int J Antimicrob Agents. 2008;32:378–85. doi: 10.1016/j.ijantimicag.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Golan Y, Baez-Giangreco C, O'Sullivan C, Snydman DR. The 44th Annual Meeting of the Infectious Disease Society of America (IDSA) Toronto, Canada: The American Society of Microbiology: 2006. Trends in vancomycin susceptibility among consecutive MRSA bacteremia isolates[abstract LB-11] [Google Scholar]
- 17.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 18.Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;365:63–78. doi: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- 19.Moise PA, Smyth DS, El-Fawal N, et al. Microbiological effects of prior vancomycin use in patients with methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother. 2008;61:85–90. doi: 10.1093/jac/dkm445. [DOI] [PubMed] [Google Scholar]
- 20.Lodise TP, Miller CD, Graves J, et al. Predictors of high MIC values among patients with methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother. 2008;62:1138–41. doi: 10.1093/jac/dkn329. [DOI] [PubMed] [Google Scholar]
- 21.Maclayton DO, Suda KJ, Coval KA, York CB, Garey KW. Case-control study of the relationship between MRSA bacteremia with vancomycin MIC of 2 microg/mL and risk factors, cost, and outcomes in inpatients undergoing hemodialysis. Clin Ther. 2006;28:1208–16. doi: 10.1016/j.clinthera.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 22.McDougal LK, Steward CD, Killgore GE, Chaitram JM, McAllister SK, Tenover FC. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: stablishing a national database. J Clin Microbiol. 2003;41:5113–20. doi: 10.1128/JCM.41.11.5113-5120.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moise PA, Smyth DS, Robinson DA, El-Fawal N, McCalla C, Sakoulas G. Genotypic and phenotypic relationships among methicillin-resistant Staphylococcus aureus from three multicentre bacteraemia studies. J Anitimicrob Chemother. 2009;63:873–6. doi: 10.1093/jac/dkp047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rybak MJ, Lomaestro BM, Rotschafer JC, et al. Vancomycin therapeutic guidelines: a summary of concensus recommendations from the Infectious diseases Society of America, the American Society of health-system Pharmacists, and the Society of Infectious diseases Pharmacists. Clin Infect Dis. 2009;49:325–7. doi: 10.1086/600877. [DOI] [PubMed] [Google Scholar]