Abstract
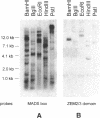
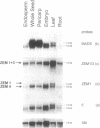
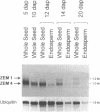
The identification of a number of cis-elements which direct gene expression in maize endosperm, and the characterization of corresponding DNA binding proteins, point to the interaction of different classes of transcription factors in this tissue. To assess whether MADS box genes are also involved in maize endosperm development, cDNA and genomic MADS box clones have been isolated. The three cDNA clones ZEM1, ZEM2 and ZEM3 were cloned from a maize endosperm cDNA library using a probe based on sequences conserved in plant MADS box genes. Further transcripts were cloned by RT-PCR experiments and designated ZEM4 and ZEM5. Analysis of the corresponding genomic clones led to the identification of the ZEM2 MADS box gene family, three members of which were characterized sharing 97% sequence identity in corresponding domains. 100% sequence identities between cDNA and one of the genomic clones, conserved exon-intron boundaries and the demonstration of in vivo splicing in a maize endosperm transient expression system, show that the transcripts ZEM1-5 are derived by alternative splicing of ZEMa, one ZEM2 member. The ZEMa transcripts are present in almost all maize tissues, but specific differentially spliced forms accumulate preferentially in maturing endosperm and leaf. The function of the ZEMa gene is discussed in the light of similarities in the expression pattern with members of the human MEF2/RSRF gene family.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi M., Hornig H., Padgett R. A., Reiser J., Weissmann C. Sequence requirements for splicing of higher eukaryotic nuclear pre-mRNA. Cell. 1986 Nov 21;47(4):555–565. doi: 10.1016/0092-8674(86)90620-3. [DOI] [PubMed] [Google Scholar]
- Alvarez E., Northwood I. C., Gonzalez F. A., Latour D. A., Seth A., Abate C., Curran T., Davis R. J. Pro-Leu-Ser/Thr-Pro is a consensus primary sequence for substrate protein phosphorylation. Characterization of the phosphorylation of c-myc and c-jun proteins by an epidermal growth factor receptor threonine 669 protein kinase. J Biol Chem. 1991 Aug 15;266(23):15277–15285. [PubMed] [Google Scholar]
- Bartels D., Thompson R. D. The characterization of cDNA clones coding for wheat storage proteins. Nucleic Acids Res. 1983 May 25;11(10):2961–2977. doi: 10.1093/nar/11.10.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbart R. E., Liang C. S., Smoot L. B., Laheru D. A., Mahdavi V., Nadal-Ginard B. A fourth human MEF2 transcription factor, hMEF2D, is an early marker of the myogenic lineage. Development. 1993 Aug;118(4):1095–1106. doi: 10.1242/dev.118.4.1095. [DOI] [PubMed] [Google Scholar]
- Cohen P. Protein phosphorylation and hormone action. Proc R Soc Lond B Biol Sci. 1988 Jul 22;234(1275):115–144. doi: 10.1098/rspb.1988.0040. [DOI] [PubMed] [Google Scholar]
- Cully D. E., Gengenbach B. G., Smith J. A., Rubenstein I., Connelly J. A., Park W. D. Endosperm Protein Synthesis and l-[S]Methionine Incorporation in Maize Kernels Cultured In Vitro. Plant Physiol. 1984 Feb;74(2):389–394. doi: 10.1104/pp.74.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois E., Bercy J., Messenguy F. Characterization of two genes, ARGRI and ARGRIII required for specific regulation of arginine metabolism in yeast. Mol Gen Genet. 1987 Apr;207(1):142–148. doi: 10.1007/BF00331501. [DOI] [PubMed] [Google Scholar]
- Ellis D. D., McCabe D., Russell D., Martinell B., McCown B. H. Expression of inducible angiosperm promoters in a gymnosperm, Picea glauca (white spruce). Plant Mol Biol. 1991 Jul;17(1):19–27. doi: 10.1007/BF00036802. [DOI] [PubMed] [Google Scholar]
- Fischer A., Baum N., Saedler H., Theissen G. Chromosomal mapping of the MADS-box multigene family in Zea mays reveals dispersed distribution of allelic genes as well as transposed copies. Nucleic Acids Res. 1995 Jun 11;23(11):1901–1911. doi: 10.1093/nar/23.11.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming A. J., Mandel T., Roth I., Kuhlemeier C. The patterns of gene expression in the tomato shoot apical meristem. Plant Cell. 1993 Mar;5(3):297–309. doi: 10.1105/tpc.5.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S. A., Cone K. C., Fromm M. E. Identification of functional domains in the maize transcriptional activator C1: comparison of wild-type and dominant inhibitor proteins. Genes Dev. 1991 Feb;5(2):298–309. doi: 10.1101/gad.5.2.298. [DOI] [PubMed] [Google Scholar]
- Green M. R. Biochemical mechanisms of constitutive and regulated pre-mRNA splicing. Annu Rev Cell Biol. 1991;7:559–599. doi: 10.1146/annurev.cb.07.110191.003015. [DOI] [PubMed] [Google Scholar]
- Grotewold E., Athma P., Peterson T. Alternatively spliced products of the maize P gene encode proteins with homology to the DNA-binding domain of myb-like transcription factors. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4587–4591. doi: 10.1073/pnas.88.11.4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes T. E., Sengupta P., Cochran B. H. The human c-fos serum response factor and the yeast factors GRM/PRTF have related DNA-binding specificities. Genes Dev. 1988 Dec;2(12B):1713–1722. doi: 10.1101/gad.2.12b.1713. [DOI] [PubMed] [Google Scholar]
- Jack T., Brockman L. L., Meyerowitz E. M. The homeotic gene APETALA3 of Arabidopsis thaliana encodes a MADS box and is expressed in petals and stamens. Cell. 1992 Feb 21;68(4):683–697. doi: 10.1016/0092-8674(92)90144-2. [DOI] [PubMed] [Google Scholar]
- Janknecht R., Hipskind R. A., Houthaeve T., Nordheim A., Stunnenberg H. G. Identification of multiple SRF N-terminal phosphorylation sites affecting DNA binding properties. EMBO J. 1992 Mar;11(3):1045–1054. doi: 10.1002/j.1460-2075.1992.tb05143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal S., Schneider J. W., Nadal-Ginard B., Mahdavi V. Activation of the myogenic lineage by MEF2A, a factor that induces and cooperates with MyoD. Science. 1994 Nov 18;266(5188):1236–1240. doi: 10.1126/science.7973707. [DOI] [PubMed] [Google Scholar]
- Kawalleck P., Somssich I. E., Feldbrügge M., Hahlbrock K., Weisshaar B. Polyubiquitin gene expression and structural properties of the ubi4-2 gene in Petroselinum crispum. Plant Mol Biol. 1993 Feb;21(4):673–684. doi: 10.1007/BF00014550. [DOI] [PubMed] [Google Scholar]
- Kennelly P. J., Krebs E. G. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem. 1991 Aug 25;266(24):15555–15558. [PubMed] [Google Scholar]
- Leifer D., Krainc D., Yu Y. T., McDermott J., Breitbart R. E., Heng J., Neve R. L., Kosofsky B., Nadal-Ginard B., Lipton S. A. MEF2C, a MADS/MEF2-family transcription factor expressed in a laminar distribution in cerebral cortex. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1546–1550. doi: 10.1073/pnas.90.4.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmer S., Maddaloni M., Motto M., Di Fonzo N., Hartings H., Salamini F., Thompson R. D. The maize regulatory locus Opaque-2 encodes a DNA-binding protein which activates the transcription of the b-32 gene. EMBO J. 1991 Mar;10(3):617–624. doi: 10.1002/j.1460-2075.1991.tb07989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan S., Bogorad L. A rice cab gene promoter contains separate cis-acting elements that regulate expression in dicot and monocot plants. Plant Cell. 1992 Aug;4(8):971–981. doi: 10.1105/tpc.4.8.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig S. R., Habera L. F., Dellaporta S. L., Wessler S. R. Lc, a member of the maize R gene family responsible for tissue-specific anthocyanin production, encodes a protein similar to transcriptional activators and contains the myc-homology region. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7092–7096. doi: 10.1073/pnas.86.18.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luehrsen K. R., Taha S., Walbot V. Nuclear pre-mRNA processing in higher plants. Prog Nucleic Acid Res Mol Biol. 1994;47:149–193. doi: 10.1016/s0079-6603(08)60252-4. [DOI] [PubMed] [Google Scholar]
- Ma H. The unfolding drama of flower development: recent results from genetic and molecular analyses. Genes Dev. 1994 Apr 1;8(7):745–756. doi: 10.1101/gad.8.7.745. [DOI] [PubMed] [Google Scholar]
- Ma H., Yanofsky M. F., Meyerowitz E. M. AGL1-AGL6, an Arabidopsis gene family with similarity to floral homeotic and transcription factor genes. Genes Dev. 1991 Mar;5(3):484–495. doi: 10.1101/gad.5.3.484. [DOI] [PubMed] [Google Scholar]
- Manak J. R., Prywes R. Mutation of serum response factor phosphorylation sites and the mechanism by which its DNA-binding activity is increased by casein kinase II. Mol Cell Biol. 1991 Jul;11(7):3652–3659. doi: 10.1128/mcb.11.7.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manak J. R., de Bisschop N., Kris R. M., Prywes R. Casein kinase II enhances the DNA binding activity of serum response factor. Genes Dev. 1990 Jun;4(6):955–967. doi: 10.1101/gad.4.6.955. [DOI] [PubMed] [Google Scholar]
- Mandel T., Lutziger I., Kuhlemeier C. A ubiquitously expressed MADS-box gene from Nicotiana tabacum. Plant Mol Biol. 1994 May;25(2):319–321. doi: 10.1007/BF00023247. [DOI] [PubMed] [Google Scholar]
- Marais R. M., Hsuan J. J., McGuigan C., Wynne J., Treisman R. Casein kinase II phosphorylation increases the rate of serum response factor-binding site exchange. EMBO J. 1992 Jan;11(1):97–105. doi: 10.1002/j.1460-2075.1992.tb05032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson P., Rutherford G., Banks J. A., Fedoroff N. Essential large transcripts of the maize Spm transposable element are generated by alternative splicing. Cell. 1989 Aug 25;58(4):755–765. doi: 10.1016/0092-8674(89)90109-8. [DOI] [PubMed] [Google Scholar]
- McCarty D. R., Hattori T., Carson C. B., Vasil V., Lazar M., Vasil I. K. The Viviparous-1 developmental gene of maize encodes a novel transcriptional activator. Cell. 1991 Sep 6;66(5):895–905. doi: 10.1016/0092-8674(91)90436-3. [DOI] [PubMed] [Google Scholar]
- Minty A., Kedes L. Upstream regions of the human cardiac actin gene that modulate its transcription in muscle cells: presence of an evolutionarily conserved repeated motif. Mol Cell Biol. 1986 Jun;6(6):2125–2136. doi: 10.1128/mcb.6.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller C. G., Nordheim A. A protein domain conserved between yeast MCM1 and human SRF directs ternary complex formation. EMBO J. 1991 Dec;10(13):4219–4229. doi: 10.1002/j.1460-2075.1991.tb05000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman C., Runswick M., Pollock R., Treisman R. Isolation and properties of cDNA clones encoding SRF, a transcription factor that binds to the c-fos serum response element. Cell. 1988 Dec 23;55(6):989–1003. doi: 10.1016/0092-8674(88)90244-9. [DOI] [PubMed] [Google Scholar]
- Passmore S., Elble R., Tye B. K. A protein involved in minichromosome maintenance in yeast binds a transcriptional enhancer conserved in eukaryotes. Genes Dev. 1989 Jul;3(7):921–935. doi: 10.1101/gad.3.7.921. [DOI] [PubMed] [Google Scholar]
- Passmore S., Maine G. T., Elble R., Christ C., Tye B. K. Saccharomyces cerevisiae protein involved in plasmid maintenance is necessary for mating of MAT alpha cells. J Mol Biol. 1988 Dec 5;204(3):593–606. doi: 10.1016/0022-2836(88)90358-0. [DOI] [PubMed] [Google Scholar]
- Pearson R. B., Kemp B. E. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- Pereira A., Schwarz-Sommer Z., Gierl A., Bertram I., Peterson P. A., Saedler H. Genetic and molecular analysis of the Enhancer (En) transposable element system of Zea mays. EMBO J. 1985 Jan;4(1):17–23. doi: 10.1002/j.1460-2075.1985.tb02311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pnueli L., Abu-Abeid M., Zamir D., Nacken W., Schwarz-Sommer Z., Lifschitz E. The MADS box gene family in tomato: temporal expression during floral development, conserved secondary structures and homology with homeotic genes from Antirrhinum and Arabidopsis. Plant J. 1991 Sep;1(2):255–266. [PubMed] [Google Scholar]
- Pollock R., Treisman R. Human SRF-related proteins: DNA-binding properties and potential regulatory targets. Genes Dev. 1991 Dec;5(12A):2327–2341. doi: 10.1101/gad.5.12a.2327. [DOI] [PubMed] [Google Scholar]
- Schmidt R. J., Burr F. A., Aukerman M. J., Burr B. Maize regulatory gene opaque-2 encodes a protein with a "leucine-zipper" motif that binds to zein DNA. Proc Natl Acad Sci U S A. 1990 Jan;87(1):46–50. doi: 10.1073/pnas.87.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R. J., Veit B., Mandel M. A., Mena M., Hake S., Yanofsky M. F. Identification and molecular characterization of ZAG1, the maize homolog of the Arabidopsis floral homeotic gene AGAMOUS. Plant Cell. 1993 Jul;5(7):729–737. doi: 10.1105/tpc.5.7.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz-Sommer Z., Hue I., Huijser P., Flor P. J., Hansen R., Tetens F., Lönnig W. E., Saedler H., Sommer H. Characterization of the Antirrhinum floral homeotic MADS-box gene deficiens: evidence for DNA binding and autoregulation of its persistent expression throughout flower development. EMBO J. 1992 Jan;11(1):251–263. doi: 10.1002/j.1460-2075.1992.tb05048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz-Sommer Z., Huijser P., Nacken W., Saedler H., Sommer H. Genetic Control of Flower Development by Homeotic Genes in Antirrhinum majus. Science. 1990 Nov 16;250(4983):931–936. doi: 10.1126/science.250.4983.931. [DOI] [PubMed] [Google Scholar]
- Shiraishi H., Okada K., Shimura Y. Nucleotide sequences recognized by the AGAMOUS MADS domain of Arabidopsis thaliana in vitro. Plant J. 1993 Aug;4(2):385–398. doi: 10.1046/j.1365-313x.1993.04020385.x. [DOI] [PubMed] [Google Scholar]
- Tyner A. L., Eichman M. J., Fuchs E. The sequence of a type II keratin gene expressed in human skin: conservation of structure among all intermediate filament genes. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4683–4687. doi: 10.1073/pnas.82.14.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky M. F., Ma H., Bowman J. L., Drews G. N., Feldmann K. A., Meyerowitz E. M. The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature. 1990 Jul 5;346(6279):35–39. doi: 10.1038/346035a0. [DOI] [PubMed] [Google Scholar]
- Yu Y. T., Breitbart R. E., Smoot L. B., Lee Y., Mahdavi V., Nadal-Ginard B. Human myocyte-specific enhancer factor 2 comprises a group of tissue-restricted MADS box transcription factors. Genes Dev. 1992 Sep;6(9):1783–1798. doi: 10.1101/gad.6.9.1783. [DOI] [PubMed] [Google Scholar]
- van der Krol A. R., Brunelle A., Tsuchimoto S., Chua N. H. Functional analysis of petunia floral homeotic MADS box gene pMADS1. Genes Dev. 1993 Jul;7(7A):1214–1228. doi: 10.1101/gad.7.7a.1214. [DOI] [PubMed] [Google Scholar]