Abstract
Objective
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) exhibits biological activity on vascular cells in vitro. Rapid variation of circulating TRAIL levels occurs during acute coronary ischemia, suggesting that biological pathways involving TRAIL may be activated during ischemic heart disease. However, whether differential levels of soluble TRAIL in normal individuals are associated with adverse health outcomes has not been investigated. We tested the hypothesis that TRAIL levels predict mortality in a population based sample of community dwelling men and women.
Methods
Plasma TRAIL level was measured by ELISA at baseline in 1282 adults (mean age 68 years) enrolled in the InCHIANTI study. Vital status was ascertained over a the six-year follow-up.
Results
In multivariable Cox regression analysis adjusted for potential confounders including prevalent cardiovascular diseases (CVD), ankle-brachial index, electrocardiogram abnormalities, and inflammatory markers, baseline TRAIL levels were inversely related to all-cause mortality (p=0.008). In stratified analyses, the prognostic effect of TRAIL level was strong and highly significant in participants with prevalent CVD (N=321), (lowest versus highest quartile: HR 3.1; 95% CI 1.5–6.5) while it was negligible in those free of CVD (P value for the interaction term between CVD status and TRAIL levels= 0.038). Similar findings were obtained when CVD mortality was considered as the outcome of interest.
Conclusions
. In older patients with CVD, low levels of TRAIL were associated increased risk of death over a period of 6 years. Lower concentration of circulating TRAIL may be related to the clinical evolution of older adults with CVD.
Keywords: cardiovascular disease, TRAIL, mortality, epidemiology, aging
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is a member of the TNF ligand superfamily. TRAIL is expressed as a type II TNF family transmembrane protein, but its extracellular domain can be proteolytically cleaved from the cell surface and act as a soluble cytokine.1 Although its primarily recognized biological activity is the induction of apoptosis in cancer cells through its interaction with two trans-membrane death domain containing receptors,2 accumulating experimental evidence suggests that soluble TRAIL is active on vascular cells at picomolar to nanomolar concentrations.1, 3–9 However, the exact role of TRAIL in the vascular system is incompletely understood. In fact, while some in vitro studies showed that TRAIL might induce endothelial cell demage3,4 and is a target of thrombin activity5 on endothelial cells, other studies of our and other groups have suggested that TRAIL exerts anti-inflammatory and anti-atherosclerotic activity in vitro6–8 and in animal models.9 Recent clinical studies found that in patients with coronary artery disease10–12 TRAIL levels tend to be lower and are inversely correlated with biomarkers of myocardial damage or dysfunction and with C-reactive protein (CRP),13 a powerful predictor of future cardiovascular events.14 Finally, in samples of selected patients with acute myocardial infarction11 or advanced heart failure,12 lower levels of soluble TRAIL predicted the risk of death or congestive heart failure over the follow-up.
On these bases, the aim of the present study was to investigate the long-term prognostic value of TRAIL in terms of all-cause and cardiovascular mortality, by exploring its potential clinical value in the InCHIANTI study, a large Italian prospective population-based epidemiological study with a six-year follow-up.
METHODS
Study population
The InCHIANTI study is a prospective, population-based study of older people living in 2 cities in the Chianti area, Tuscany, Italy. The study was designed by the Laboratory of Clinical Epidemiology of the Italian Research Council of Aging (Florence, Italy) to identify risk factors for late-life disability, as previously described.15 Briefly, in 1998, 1270 persons age 65 years or older were randomly selected from the population of Greve in Chianti and Bagno a Ripoli using a multistage sampling method. Additionally, 30 men and 30 women randomly sampled from the age strata 20 to 29, 30 to 39, 40 to 49, 50 to 59, and 60 to 69 years were enrolled. Of the 1714 eligible persons, 1453 (84%) agreed to participate in the project, 1343 donated a blood sample, and 1282 had complete data for the analysis presented here. Participants excluded from this analysis because of incomplete data were older and less educated, but they were not different in other demographic and clinical characteristics. The Italian National Research Council on Aging Ethical Committee ratified the study protocol and participants provided written consent to participate.
Biochemical Measures
At baseline, blood samples were obtained from participants after a 12-hour fast. Aliquots of serum and plasma were stored at −80°C and were not thawed until analyzed. Plasma TRAIL was measured in duplicate by specific, commercially available ELISA kit (R&D Systems, Minneapolis, MN), in accordance with the manufacturer’s instructions and analyzed with an ELISA reader at 450 nm. Sensitivity of the assay was 2.86 pg/ml and the intra- and inter-assay coefficients of variation (CV) were 3.9% and 6%, respectively and the upper limits of detection was 1000 pg/ml. Total cholesterol and high-density lipoprotein cholesterol were determined using commercial enzymatic tests (Roche Diagnostics, Mannheim, Germany). Serum creatinine and urinary creatinine from the 24-hour urine collection were measured using a modified Jaffe method and used to calculate creatinine clearance as a measure of glomerular filtration rate. Serum interleukin (IL)-6 and Tumor Necrosis Factor-α (TNF-α), were measured in duplicate by high-sensitivity enzyme-linked immunoabsorbent assays (kits from BIOSOURCE, Camarillo, CA). Sensitivity was 0.1 pg/ml for IL-6 and 0.09 pg/ml for TNF-α and the CV was <7% for both tests. C-reactive protein (CRP) was measured in duplicate using an ELISA and colorimetric competitive immunoassay (Sensitivity 0.03 mg/l and interassay CV < 5%).
Mortality Follow-up
Participants were evaluated for a 3-year follow-up visit from 2001 to 2003 and 6-year follow-up visit from 2004 to 2006. At the end of the field data collection, mortality data of the original InCHIANTI cohort were collected using data from the Mortality General Registry maintained by the Tuscany Region and the death certificates that are deposited immediately after death at the Registry office of the municipality of residence. Death certificates were obtained for all but 18 of the 259 participants who died over the six-year follow-up. Cardiovascular mortality, based on underlying cause of death, was defined as any cardiovascular mortality (codes 390–459), coded using the International Classification of Diseases, Ninth Revision.
Other Measures
Socio-demographic characteristics included age, gender, and self-reported education level. History of smoking and medication use was ascertained from baseline interview. Daily alcohol intake (g/day) were estimated by the European Prospective Investigation into Cancer and Nutrition Food Frequency Questionnaire.16 The prevalence of specific medical conditions was established using standardized criteria that combined information from self-reported history, medical records, and a clinical medical examination. Diagnostic algorithms were modified versions of those created for the Women’s Health and Aging Study.17 The following diseases were assessed: coronary heart disease (angina and acute myocardial infarction, CHD), peripheral arterial disease (PAD), stroke (and/or transient ischemic attack), hypertension, diabetes, congestive heart failure, chronic obstructive pulmonary disease (COPD), and cancer. Prevalent cardiovascular disease (CVD) was defined as the as presence of one of the following: coronary heart disease, congestive heart failure, peripheral arterial disease, and stroke. Indicators of subclinical or otherwise undiagnosed cardiovascular diseases were assessed at baseline and included major electrocardiogram abnormalities (major Q or QS abnormality, major ST or T wave abnormality, ventricular conduction defects, or left ventricular hypertrophy) and the ankle-brachial index, used as indicator of severity of atherosclerosis.18 The ankle-brachial index was measured using a Doppler stethoscope (Parks model 41-A; Parks Medical Electronics, Inc, Aloha, Ore). Individuals with an ankle-brachial index ≥1.50 were excluded because this indicates poorly compressible leg arteries and inability to gauge arterial perfusion accurately.19 Weight and height were measured using objective standard techniques and used to calculate body mass index (BMI, Kg/m2).
Statistical analysis
Soluble TRAIL distribution only approximated a normal distribution but a statistical test for normality formally demonstrated that TRAIL was not normally distributed(Shapiro-Wilk V= 447; p <0.0001). Log-transformation of TRAIL values only marginally improved the normality of distribution (Shapiro-Wilk V= 47; p <0.0001). Consequently TRAIL data were analyzed as quartiles of the original TRAIL distribution. Analysis of variance for continuous variables and χ2 test for categorical variables were used to test differences in baseline characteristics across quartiles of TRAIL (<59.8 pg/ml, n=321; 59.8–<71.3 pg/ml, n=321; 71.3– <84.5 pg/ml, n=320, and > 84.5, n=320). Kaplan-Meier curves were fitted to explore the relation between baseline TRAIL quartiles and time to death. Death rates per 1,000-person years were calculated. Hazard Ratios (HR) for each quartile of TRAIL were estimated by Cox proportional hazard regression adjusting for potential confounders, with the highest quartile as the reference category. The assumption of proportionality of all variables introduced in the models was assessed through the analysis of Schoenfeld residuals. The proportional hazards assumption held for all models. These models were adjusted for variables found to be associated with TRAIL in univariate analysis, as well as other known risk factor for total and cardiovascular mortality in older persons. First, we estimated hazard ratios (HR) and 95% confidence intervals (CI) adjusting for age and gender, then we adjusted for prevalent diseases, history of smoking, BMI, electrocardiogram abnormalities, total cholesterol and ankle-brachial index, and, finally, markers of inflammation levels were included into the multivariable model. Tests for trend were ascertained from models with quartiles of TRAIL concentration entered as an ordinal variable. Interactions of TRAIL quartiles with age, sex, prevalent cardiovascular diseases and the risk of mortality were tested. Finally, a restricted cubic spline model with five knots was used to explore departure from linearity in the relationship between TRAIL level and mortality risk, using TRAIL values as continuous data. These models were fitted also after log-transformation of the TRAIL values and the results were identical. In order to increase the clinical interpretation of the figure we decided to present the data with the original distribution. All analyses were performed using Stata 11.0 statistical package. All reported P values are two sided.
RESULTS
Participants’ characteristics by quartile of baseline TRAIL concentration show that those with lower level of TRAIL were older, more often were men, tended to have greater prevalence of CHD, PAD, any CVD, and diabetes. In addition, they were more likely to have prevalent COPD and major electrocardiographic abnormalities, had lower BMI, lower total cholesterol, were less likely to use statins, and had higher level of IL-6 (Table 1). At baseline, there was no significant association between TRAIL and TNF-α levels.
Table 1.
Selected Characteristics of Study Participants according to baseline TRAIL distribution
| Characteristics | Quartiles of plasma TRAIL levels (pg/ml) | P* | ||||
|---|---|---|---|---|---|---|
| 19.6–<59.8 | 59.8–<71.3 | 71.3–<84.5 | 84.5–652.3 | |||
| (n = 321) | (n = 321) | (n = 320) | (n = 320) | |||
| Age, mean (SD), y | 68.8 (18.8) | 68.4 (14.4) | 68.4 (15.2) | 67.4 (13.9) | 0.670 | |
| Age >75, No. (%) | 143 (44.6) | 119 (37.1) | 111 (34.7) | 86 (26.9) | <0.001 | |
| Women, No. (%) | 157 (48.9) | 169 (52.7) | 184 (57.5) | 200 (62.5) | 0.004 | |
| Education > Elementary school No.(%) | 233 (72.6) | 253 (78.8) | 235 (73.4) | 254 (79.4) | 0.086 | |
| Prevalent health conditions, No. (%) | ||||||
| Coronary heart disease | 46 (14.3) | 38 (11.8) | 41 (12.38 | 26 (8.1) | 0.092 | |
| Peripheral arterial disease | 38 (11.8) | 34 (10.6) | 22 (6.7) | 27 (8.4) | 0.139 | |
| Stroke | 23 (7.2) | 22 (6.9) | 22 (6.9) | 23 (7.2) | 0.997 | |
| Congestive heart failure | 15 (4.7) | 14 (4.4) | 16 (5.0) | 11 (3.4) | 0.791 | |
| Any cardiovascular disease | 94 (20.3) | 84 (26.2) | 79 (24.7) | 64 (20.0) | 0.054 | |
| Hypertension | 161 (50.2) | 172 (53.6) | 180 (56.3) | 189 (59.1) | 0.133 | |
| Diabetes | 35 (9.9) | 43 (15.0) | 36 (10.3) | 22 (7.0) | 0.058 | |
| COPD | 49 (15.3) | 31 (9.7) | 39 (12.2) | 15 (4.7) | <0.001 | |
| Cancer | 17 (5.3) | 18 (5.9) | 19 (5.5) | 11 (3.4) | 0.477 | |
| Medications, No. (%) | ||||||
| Beta-blocker | 12 (3.7) | 7 (2.2) | 14 (4.4) | 12 (3.8) | 0.478 | |
| Antiplatelet | 35 (10.9) | 23 (7.2) | 26 (8.1) | 30 (9.4) | 0.375 | |
| ACE/RAAS-inhibitor | 58 (18.1) | 61 (19.0) | 73 (22.8) | 76 (23.8) | 0.209 | |
| Statins | 2 (0.6) | 8 (2.5) | 11 (3.4) | 14 (4.4) | 0.026 | |
| Risk factors, No., (%) | ||||||
| Alcohol consumption (g/day) | ||||||
| >0–20 | 147 (45.9) | 165 (51.9) | 144 (45.3) | 156 (49.1) | ||
| >20 | 91 (28.4) | 81 (25.5) | 82 (25.8) | 79 (24.8) | 0.516 | |
| Smoking | Former | 82 (25.6) | 90 (28.0) | 76 (23.8) | 68 (21.3) | |
| Current | 59 (18.4) | 64 (19.9) | 52 (16.3) | 62 (19.4) | 0.336 | |
| Major ECG abnormalities | 77 (24.1) | 56 (17.5) | 58 (18.2) | 50 (15.6) | 0.039 | |
| Ankle-brachial index, mean (SD) | 1.03 (0.18) | 1.03 (0.16) | 1.05 (0.14) | 1.05 (0.15) | 0.255 | |
| Body mass index, mean (SD) Kg/m2 | 26.2 (4.0) | 26.9 (4.1) | 27.2 (4.1) | 28.2 (4.1) | <0.001 | |
| Creatinine clearance, mean (SD) mL/min | 81.7 (32.9) | 83.1 (27.3) | 84.3 (32.1) | 83.0 (29.5) | 0.783 | |
| Total Cholesterol, mean (SD) mg/dl | 203.9 (42.3) | 215.7 (39.0) | 218.2 (39.8) | 220.7 (38.0) | <0.001 | |
| HDL-Cholesterol, mean (SD) mg/dl | 55.4 (15.3) | 55.0 (14.5) | 56.6 (14.9) | 56.0 (15.0) | 0.567 | |
| Interleukin-6, median (IQR) pg/ml | 1.36 (0.7–2.6) | 1.38 (0.8–2.1) | 1.17 (0.7–1.8) | 1.17 (0.8–1.8) | 0.002† | |
| C-reactive protein, median (IQR) μg/ml | 2.83 (1.1–6.2) | 2.28 (1.1–5.4) | 2.22 (1.1–4.5) | 2.35 (1.2–4.7) | 0.119† | |
| Tumor necrosis factor-α (IQR) pg/ml | 4.38 (2.9–5.9) | 4.19 (2.9–5.8) | 4.22 (3.0–5.7) | 4.47 (3.3–5.8) | 0.771† | |
Abbreviations: ECG, electrocardiogram, COPD, chronic obstructive pulmonary disease, IQR, inter-quartile range
P values are from χ2 test of proportion for categorical variables and analysis of variance for comparison of mean values of continuous variables
analysis of variance after log-transformation of the variables to approximate a normal distribution
After 6 years, 259 participants had died, of these deaths, 112 were from CVD. There was a strong relationship between TRAIL concentration and survival, with participants in the lowest quartile having the lowest survival, participants in the second and third quartiles having a similar, intermediate, survival probability, and participants in the highest quartile having the best survival (Table 2). In the analysis adjusted for age and gender those in the lowest quartile of TRAIL had a 2-fold risk of all-cause mortality (HR 1.98; 95% CI 1.3–2.9) compared to participants in the highest quartile. The point estimate was only modestly attenuated with further adjustment for smoking history, BMI, prevalent conditions, indicators of sub-clinical cardiovascular disease, and cardiovascular risk factors. After adding IL-6 and CRP values to the model, the HR was further attenuated but remained significantly greater than 1.00 (HR 1.86; 95% CI 1.2–2.9). In all the multivariable models participants in the second and third quartile of TRAIL had a significantly increased risk of death compared to participants in the highest quartile. There was no significant difference in mortality risk according to TRAIL levels in men and women. The analysis limited to individuals aged 65 and older produced identical results.
Table 2.
Event Rates and Hazard Ratios for All-Cause Mortality according to baseline TRAIL distribution
| No. of Participants | No. of Events | Events per 1000 person-Years | Hazard Ratio (95 % Confidence Interval) | |||
|---|---|---|---|---|---|---|
| Age- and Sex-Adjusted | Multivariable* Adjustment | Further Adjustment for IL-6 and CRP | ||||
| TRAIL plasma levels (pg/ml) | ||||||
| 19.6–<59.8 | 321 | 102 | 59.7 | 1.98 (1.33–2.95) | 1.92 (1.26–2.93) | 1.86 (1.22–2.85) |
| 59.8–<71.3 | 321 | 64 | 34.7 | 1.69 (1.11–2.55) | 1.69 (1.10–2.58) | 1.66 (1.08–2.55) |
| 71.3– <84.5 | 320 | 58 | 31.8 | 1.44 (0.94–2.19) | 1.63 (1.05–2.52) | 1.58 (1.02–2.46) |
| 84.5–652.3 | 320 | 35 | 18.3 | 1 | 1 | 1 |
| P for trend | <0.001 | 0.005 | 0.008 | |||
Adjusted for: age, gender, education, coronary heart disease, stroke, peripheral arterial disease, congestive heart failure, hypertension, diabetes, COPD, smoking history, BMI, total cholesterol, major electrocardiogram abnormalities, statin use
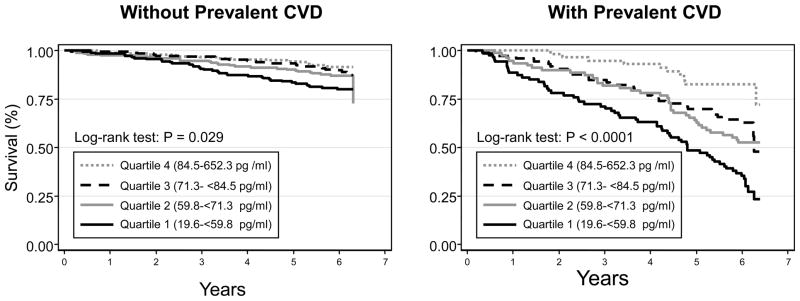
We found an interaction between prevalent CVD status and TRAIL concentration (P value for the interaction term=0.038 in fully adjusted model), suggesting that the excess risk of mortality associated with low TRAIL levels was larger in participants with CVD compared to those without; therefore, the analyses were stratified according to baseline prevalence of CVD. Figure 1 shows survival curves according to TRAIL quartile categories in participants with and without prevalent CVD. Table 3 shows participants’ characteristics according to baseline CVD status. As expected patients with prevalent CVD were older, more often were men, had greater prevalence of and diabetes and hypertension and other risk factors for CVD. Patients with prevalent CVD also had a modest, albeit statistically significant, reduction in TRAIL levels. Table 4 and Table 5 show the relationship between quartile of TRAIL and incidence of all-cause and CVD mortality, respectively, according to prevalent CVD. Among the 321 participants (25.0%) who had prevalent CVD there was a strong inverse relationship between baseline TRAIL concentration and all-cause and cardiovascular mortality. Sixty-eight percent of patients with prevalent-CVD in the lowest quartile of the TRAIL distribution had died at the end of the six-year follow-up. Conversely, among participant free of CVD at baseline neither all-cause mortality nor CVD mortality were associated with the TRAIL distribution. These associations were not modified after further adjustment for potential confounders including smoking history, BMI, prevalent chronic conditions, indicators of sub-clinical cardiovascular disease, cardiovascular risk factors, statin use, and biomarkers of inflammation. In the fully adjusted model (Table 4), among those with prevalent CVD, participants in the first, second, and third TRAIL quartile had a significantly increased risk of all-cause mortality compared to participants with the highest TRAIL concentrations (fourth quartile). In this analysis, of note, participants with the lowest levels of TRAIL (first and second quartiles) have a three-fold increased risk of death, compared to those with highest TRAIL levels (HR, 95% CI 3.1, 1.5–6.3 and 3.3, 1.6–6.7, respectively). This same pattern was seen when evaluating CVD mortality as the outcome (Table 5). Consistent with results in Table 4 and 5, the non-linear association between TRAIL and mortality adjusted for potential confounders is shown in Figure 2. The overall shape of the curves suggests that the risk of all-cause and CVD mortality linearly increases as TRAIL levels decreases up to 70 pg/ml, and after this level there is a curvilinear relationship between TRAIL and the risk of death, with very high mortality risk associated with the lowest TRAIL levels.
Figure 1.
Kaplan-Meier estimates of survival according to quartile of TRAIL and prevalent CVD.
Table 3.
Selected Characteristics of Study Participants according to prevalent Cardiovascular disease
| Without Prevalent CVD (N.961) | With Prevalent CVD (N.321) | P* | |
|---|---|---|---|
| Age >75, No. (%) | 257 (26.7) | 202 (62.9) | <0.001 |
| Women, No. (%) | 561 (58.4) | 149 (46.4) | <0.001 |
| Prevalent health conditions, No. (%) | |||
| Hypertension | 480 (49.9) | 222 (69.2) | <0.001 |
| Diabetes | 86 (8.9) | 50 (15.6) | <0.001 |
| Medications, No. (%) | |||
| Beta-blocker | 27 (2.8) | 18 (5.6) | 0.018 |
| Antiplatelet | 49 (5.1) | 65 (20.3) | <0.001 |
| ACE/RAAS-inhibitor | 164 (17.1) | 104 (32.4) | <0.001 |
| Statins | 22 (2.3) | 13 (4.1) | 0.094 |
| Risk factors, No., (%) | |||
| Major ECG abnormalities | 118 (12.3) | 123 (38.6) | <0.001 |
| Body mass index, mean (SD) Kg/m2 | 27.1 (4.1) | 27.2 (4.1) | 0.767 |
| Total Cholesterol, mean (SD) mg/dl | 214.5 (40.8) | 215.1 (38.9) | 0.819 |
| HDL-Cholesterol, mean (SD) mg/dl | 56.9 (14.7) | 52.5 (15.4) | <0.001 |
| Interleukin-6, median (IQR) pg/ml | 1.09 (0.7–1.8) | 1.89 (1.1–3.1) | <0.001† |
| C-reactive protein, median (IQR) μg/ml | 2.10 (1.1–4.6) | 3.39 (1.6–7.8) | <0.001† |
| Tumor necrosis factor-α, median (IQR) pg/ml | 5.01 (2.9–5.6) | 4.19 (3.7–6.6) | <0.001† |
| TRAIL, median (IQR) pg/ml | 72.5 (60.4–85.6) | 68.7 (57.3–79.7) | <0.001† |
Abbreviations: ECG, electrocardiogram, COPD, chronic obstructive pulmonary disease, IQR, inter-quartile range
P values are from χ2 test of proportion for categorical variables and analysis of variance for comparison of mean values of continuous variables
analysis of variance after log-transformation of the variables to approximate a normal distribution
Table 4.
Adjusted Hazard Ratios for All-Cause Mortality According to Baseline quartile of TRAIL and Prevalent Cardiovascular Disease
| Without Prevalent CVD |
With Prevalent CVD |
|||||||
|---|---|---|---|---|---|---|---|---|
| N | % Deaths | Hazard Ratio (95 % Confidence Interval) |
N | % Deaths | Hazard Ratio (95 % Confidence Interval) |
|||
| Age- and Sex-Adjusted | Multivariable* Adjustment | Age- and Sex-Adjusted | Multivariable† Adjustment | |||||
| TRAIL plasma levels (pg/ml) | ||||||||
| 19.6–<59.8 | 227 | 16.7 | 1.52 (0.88–2.61) | 1.27 (0.66–2.14) | 94 | 68.1 | 2.67 (1.40–5.07) | 3.11 (1.53–6.30) |
| 59.8–<71.3 | 237 | 9.7 | 1.15 (0.66–2.02) | 0.90 (0.49–1.64) | 84 | 48.8 | 2.57 (1.34–4.92) | 3.28 (1.61–6.69) |
| 71.3–<84.5 | 241 | 11.2 | 1.07 (0.60–1.91) | 1.19 (0.66–2.14) | 79 | 39.2 | 1.95 (0.93–3.67) | 2.45 (1.19–5.03) |
| 84.5–652.3 | 256 | 8.9 | 1 | 1 | 64 | 18.8 | 1 | 1 |
Adjusted for: age, gender, education, hypertension, diabetes, COPD, smoking history, BMI, total cholesterol, major electrocardiogram abnormalities, ankle-brachial index, IL-6 quartiles.
Adjusted for: age, gender, education, coronary heart disease, peripheral arterial disease, stroke, congestive heart failure, hypertension, diabetes, smoking history, COPD, BMI, total cholesterol, major electrocardiogram abnormalities, statins use, IL-6 quartiles
Table 5.
Adjusted Hazard Ratios for Cardiovascular Mortality According to Baseline quartile of TRAIL and Prevalent Cardiovascular Disease
| Without Prevalent CVD |
With Prevalent CVD |
|||||||
|---|---|---|---|---|---|---|---|---|
| N | % Deaths | Hazard Ratio (95 % Confidence Interval) |
N | % Deaths | Hazard Ratio (95 % Confidence Interval) |
|||
| Age- and Sex-Adjusted | Multivariable* Adjustment | Age- and Sex-Adjusted | Multivariable† Adjustment | |||||
| TRAIL plasma levels (pg/ml) | ||||||||
| 19.6–<59.8 | 225 | 7.1 | 0.99 (0.42–2.32) | 0.89 (0.36–2.20) | 88 | 33.0 | 2.75 (1.09–6.91) | 2.90 (1.12–7.53) |
| 59.8–<71.3 | 235 | 2.7 | 0.51 (0.19–1.39) | 0.34 (0.11–1.03) | 82 | 20.1 | 2.32 (0.89–6.05) | 2.23 (0.84–5.98) |
| 71.3–<84.5 | 240 | 3.3 | 0.59 (0.23–1.49) | 0.57 (0.22–1.49) | 77 | 23.4 | 2.13 (0.82–5.51) | 2.49 (0.96–6.46) |
| 84.5–652.3 | 253 | 4.3 | 1 | 1 | 64 | 10.9 | 1 | 1 |
Adjusted for: age, gender, education, hypertension, diabetes, smoking history, COPD, BMI, total cholesterol, major electrocardiogram abnormalities, ankle-brachial index, IL-6 quartiles.
Adjusted for: age, gender, education, coronary heart disease, peripheral arterial disease, stroke congestive heart failure, hypertension, diabetes, smoking history, COPD, BMI, total cholesterol, major electrocardiogram abnormalities, statins use, IL-6 quartiles
Figure 2.
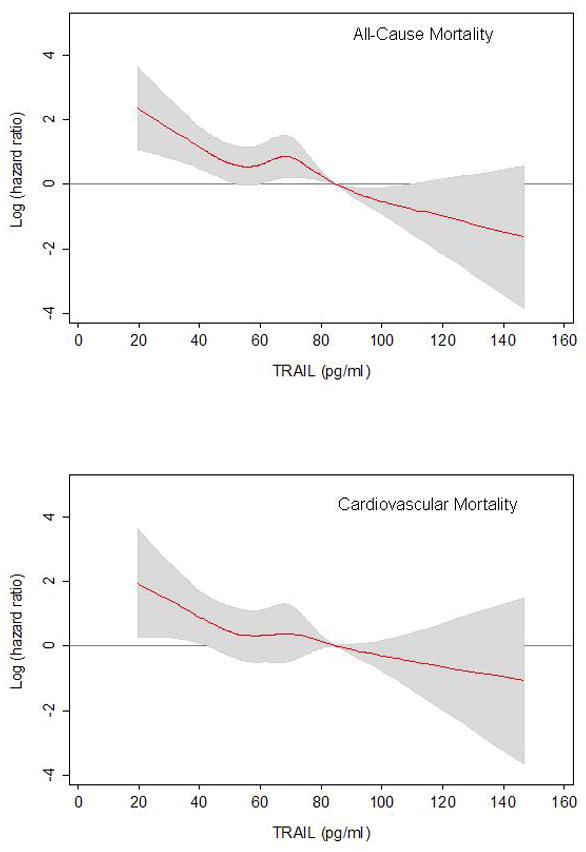
Adjusted association (log Hazard Ratio) of baseline TRAIL concentration with all-cause mortality and cardiovascular mortality in individuals with prevalent CVD.
Hazard ratios are calculated by restricted-cubic-spline models and are adjusted for all variables included in the most adjusted model in Table 3 and 4. Solid curve represents point estimates, and gray area represent 95% confidence intervals. Both models are centered on the cut point for the highest TRAIL quartile (84.5 pg/ml)
Finally, to assess the hypothesis of the effect of pre-existing diseases presence and severity on baseline TRAIL level, we also examined the relation between TRAIL and mortality after exclusion of participants who had died during the first year of follow-up. Again, among those with prevalent CVD, participants with lowest TRAIL concentrations (first and second quartile) have a three-fold increased risk of death, compared to those with highest TRAIL levels, independent of potential confounders (HR, 95% CI 3.0, 1.4–6.5 and 3.2, 1.5–6.9, respectively).
DISCUSSION
In this study, we have demonstrated for the first time a strong, independent, association between soluble TRAIL levels and subsequent long-term risk of all-cause and cardiovascular mortality among older adults with pre-existing CVD. Using data from a sample of randomly selected subjects representative of people living in the community, we found that the risk of death associated with low TRAIL levels was limited to participants with prevalent CVD. Indeed, among individuals with prevalent CVD, those with baseline TRAIL levels < 84.5 pg/ml (quartile 1–3) had a 2 to 3 times higher rate of total and cardiovascular mortality, whereas among those without CVD we found a weak and non significant association. Several potential risk factors for mortality in older adults20–23 were associated with baseline TRAIL level; nevertheless, in multivariable analyses adjusted for prevalent chronic conditions, indicators of subclinical disease, statin use, and biomarkers, the association between TRAIL and the risk of death in persons with CVD was not substantially reduced, suggesting that the effect on mortality of this multifunctional cytokine might be explained by a different, independent, and still unknown mechanism.
Our results confirm and expand, at a population level and with a longer follow-up period, the findings of recent clinical studies on the potential prognostic role of circulating soluble TRAIL in patients with acute coronary syndrome or congestive heart failure. In particular, in a pilot study performed in a small sample of adults with acute myocardial infarction,11 soluble TRAIL not only was inversely associated with biochemical indicators of myocardial injury (creatine kinase-MB fraction) and cardiac dysfunction (B-type natriuretic peptide), but also predicted short (in-hospital) and mid-term (12 months) mortality or incident congestive heart failure. Moreover, in patients with advanced heart failure,12 soluble TRAIL is an independent predictor of the risk of re-hospitalization or death over a 16-month follow-up. Our current study, however, extends the prognostic value of soluble TRAIL to individuals with stable cardiac disease and to a wider range of cardiovascular conditions. Indeed, among the 321 individuals with prevalent CVD, only 32% had either prevalent heart failure or myocardial infarction, and even after exclusion of these patients from the analysis, the association between TRAIL and total mortality remained strong and statistically significant (data not shown), suggesting that TRAIL might predict the risk of death also in patients affected by stable angina, stroke, peripheral arterial disease, or cardiovascular subclinical conditions. We found that the association between TRAIL and mortality risk was not limited to CVD mortality, but it was consistent and strong also for all-cause mortality. This finding is not unexpected as in older patients the presence of CVD might interact with other associated comorbidities including, but not limited to, cancer and pneumonia, in the process leading to death.21 Furthermore, our findings are also in agreement with a recent paper that demonstrated an inverse and independent association of soluble TRAIL and all-cause mortality in a sample patients with chronic kidney disease, a condition characterized by accelerated atherosclerosis and underlying chronic inflammation.24
While previous studies showed that serum levels of TRAIL are acutely decreased in the early hours after acute myocardial infarction and tend to increase in the following days,11,12 suggesting that TRAIL reduction might represent an acute and transient consequence of plaque complication or myocardial ischemia, in our present sample baseline soluble TRAIL levels was only moderately lower in individuals with prevalent CVD compared to those without CVD. This finding is in good agreement with a previous cross-sectional analysis in which patients with angiographically documented coronary artery disease had a borderline reduction of soluble TRAIL levels compared to healthy controls.10 Furthermore, since our sample is composed of community-dwelling individuals with stable cardiac conditions, this sampling characteristic might provide an additional explanation for the modest reduction of TRAIL levels observed in patients with CVD in this study, as compared to those reported in patients with acute or decompensated conditions.11,12
Although we are aware that a cause and effect relationship cannot be established in observational studies, there are several potential mechanisms by which elevated TRAIL levels might reduce the risk of death in patients affected by cardiovascular disease. First, recombinant TRAIL showed a protective role against progression of atherosclerosis in the apoE-null mice model,9 contributing to plaque stabilization by preserving endothelial cell coverage, decreasing the number of infiltrating macrophages and increasing the number of vascular smooth muscle cells, as confirmed in vitro studies.25 These findings are particularly noteworthy since patients with unstable angina or acute myocardial infarction are usually affected by a more active atherosclerotic disease with vulnerable coronary plaques.26,27 Second, taking into account that human endothelial cell from different vascular beds express detectable amount of all transmembrane TRAIL receptors,28,29 soluble TRAIL might ameliorate endothelial dysfunction by inducing endothelial cell proliferation,29 by increasing the release of nitric oxide,6 and by counteracting TNF-α-induced leukocyte adhesion to endothelial cells.8 Third, in vitro studies have shown that soluble recombinant TRAIL induces apoptotic cell death of neutrophils,30,31 which play an important role in the pathogenesis of atherosclerosis.32
After adjustment for age and other potential confounders, low levels of soluble TRAIL were not associated with the likelihood of mortality over the follow-up in participant free of CVD at baseline. At least two not mutually explanations might justify the differential prognostic effect of TRAIL in patients with or without CVD. First, it is possible that six years of follow-up might be an insufficient time frame to detect a protective effect of higher TRAIL levels in patients without clinical manifestation of atherosclerosis and other CVD conditions. Second, it is conceivable that soluble TRAIL might have different roles in the subsequent phases of the natural history of atherosclerosis. From this point of view, our data might suggest that TRAIL would play a more important role in the evolution and complication of pre-existing pathological conditions, rather than in the onset and pathogenesis of the diseases itself.
Our analysis is based on a double approach for taking into account the baseline presence and severity of cardiovascular disease,32 by adjusting for measure of disease presence in multivariate analyses and by excluding participants who had died during the first year of follow-up. The results of both analyses indicated an independent relationship of TRAIL level with the risk of death in individuals with prevalent CVD. From this point of view, our findings suggest that soluble TRAIL assessment does not simply capture information related to the severity of the underlying cardiac disease and that its prognostic value is not restricted to patients with active cardiac disease and limited life expectancy. Nevertheless, a residual confounding effect of baseline disease status cannot be completely ruled out. Indeed, other measures of cardiac performance and myocardial damage or dysfunction that were not available for this study, including but not limited to, ejection fraction determination, objective exercise tolerance testing, B-type natriuretic peptide could have provided more precise and appropriate information.
These findings might have important clinical implications. Soluble TRAIL is helpful in identifying a subgroup of older CVD patients with high risk of death over a period of six years and therefore routine TRAIL assessment might provide additional prognostic information in clinical practice. Finally, from a therapeutic perspective, the potential anti-inflammatory and anti-atherosclerotic activity of soluble recombinant TRAIL33 might open future pharmacological options for secondary prevention of atherosclerotic cardiovascular diseases.
Acknowledgments
The InCHIANTI study baseline (1998–2000) was supported as a “targeted project” (ICS110.1/RF97.71) by the Italian Ministry of Health and in part by the U.S. National Institute on Aging (Contracts: 263 MD 9164 and 263 MD 821336); the InCHIANTI Follow-up 1 (2001–2003) was funded by the U.S. National Institute on Aging (Contracts: N.1-AG-1-1 and N.1-AG-1-2111); the InCHIANTI Follow-ups 2 and 3 studies (2004–2010) were financed by the U.S. National Institute on Aging (Contract: N01-AG-5-0002); supported in part by the Intramural research program of the National Institute on Aging, National Institutes of Health, Baltimore, Maryland.
The sponsors played no part in the study design; collection, analysis, or interpretation of data; the writing of the report; or the decision to submit the article for publication.
Footnotes
Relationship with industry: none declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zauli G, Secchiero P. The role of the TRAIL/TRAIL receptors system in hematopoiesis and endothelial cell biology. Cytokine Growth Factor Rev. 2006;17:245–57. doi: 10.1016/j.cytogfr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Almasan A, Ashkenazi A. Apo2L/TRAIL: apoptosis signaling, biology, and potential for cancer therapy. Cytokine Growth Factor Rev. 2003;14:337–48. doi: 10.1016/s1359-6101(03)00029-7. [DOI] [PubMed] [Google Scholar]
- 3.Li JH, Kirkiles-Smith NC, McNiff JM, Pober JS. TRAIL induces apoptosis and inflammatory gene expression in human endothelial cells. J Immunol. 2003;171:1526–33. doi: 10.4049/jimmunol.171.3.1526. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien LA, Richardson MA, Mehrbod SF, et al. Activated protein C decreases tumor necrosis factor related apoptosis-inducing ligand by an EPCR- independent mechanism involving Egr-1/Erk-1/2 activation. Arterioscler Thromb Vasc Biol. 2007;27:2634–41. doi: 10.1161/ATVBAHA.107.153734. [DOI] [PubMed] [Google Scholar]
- 5.Simoncini S, Njock MS, Robert S, et al. TRAIL/Apo2L mediates the release of procoagulant endothelial microparticles induced by thrombin in vitro: a potential mechanism linking inflammation and coagulation. Circ Res. 2009;104:943–51. doi: 10.1161/CIRCRESAHA.108.183285. [DOI] [PubMed] [Google Scholar]
- 6.Zauli G, Pandolfi A, Gonelli A, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) sequentially upregulates nitric oxide and prostanoid production in primary human endothelial cells. Circ Res. 2003;92:732–40. doi: 10.1161/01.RES.0000067928.83455.9C. [DOI] [PubMed] [Google Scholar]
- 7.Alladina SJ, Song JH, Davidge ST, Hao C, Easton AS. TRAIL-induced apoptosis in human vascular endothelium is regulated by phosphatidylinositol 3-kinase/Akt through the short form of cellular FLIP and Bcl-2. J Vasc Res. 2005;42:337–47. doi: 10.1159/000086599. [DOI] [PubMed] [Google Scholar]
- 8.Secchiero P, Corallini F, di Iasio MG, Gonelli A, Barbarotto E, Zauli G. TRAIL counteracts the proadhesive activity of inflammatory cytokines in endothelial cells by down-modulating CCL8 and CXCL10 chemokine expression and release. Blood. 2005;105:3413–9. doi: 10.1182/blood-2004-10-4111. [DOI] [PubMed] [Google Scholar]
- 9.Secchiero P, Candido R, Corallini F, et al. Systemic tumor necrosis factor-related apoptosis-inducing ligand delivery shows antiatherosclerotic activity in apolipoprotein E-null diabetic mice. Circulation. 2006;114:1522–30. doi: 10.1161/CIRCULATIONAHA.106.643841. [DOI] [PubMed] [Google Scholar]
- 10.Schoppet M, Sattler AM, Schaefer JR, Hofbauer LC. Osteoprotegerin (OPG) and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) levels in atherosclerosis. Atherosclerosis. 2006;184:446–7. doi: 10.1016/j.atherosclerosis.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 11.Secchiero P, Corallini F, Ceconi C, et al. Potential prognostic significance of decreased serum levels of TRAIL after acute myocardial infarction. PLoS ONE. 2009;4:e4442. doi: 10.1371/journal.pone.0004442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niessner A, Hohensinner PJ, Rychli K, et al. Prognostic value of apoptosis markers in advanced heart failure patients. Eur Heart J. 2009;30:789–96. doi: 10.1093/eurheartj/ehp004. [DOI] [PubMed] [Google Scholar]
- 13.Michowitz Y, Goldstein E, Roth A, et al. The involvement of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in atherosclerosis. Journal of the American College of Cardiology. 2005;45:1018–24. doi: 10.1016/j.jacc.2004.12.065. [DOI] [PubMed] [Google Scholar]
- 14.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–9. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 15.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–25. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 16.Kaaks R, Riboli E. Validation and calibration of dietary intake measurements in the EPIC project: methodological considerations. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol. 1997;26:S15–S25. doi: 10.1093/ije/26.suppl_1.s15. [DOI] [PubMed] [Google Scholar]
- 17.Guralnik JMFL, Simonsick EM, Kasper JD, Lafferty ME, editors. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability. Bethesda: National Institute on Aging; 1995. [Google Scholar]
- 18.Newman AB, Siscovick DS, Manolio TA, et al. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group. Circulation. 1993;88:837–45. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 19.McDermott MM, Guralnik JM, Corsi A, et al. Patterns of inflammation associated with peripheral arterial disease: the InCHIANTI study. American Heart Journal. 2005;150:276–81. doi: 10.1016/j.ahj.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 20.Fried LP, Kronmal RA, Newman AB, et al. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. J Am Med Ass. 1998;279:585–92. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 21.Volpato S, Leveille SG, Corti MC, Harris TB, Guralnik JM. The value of serum albumin and high-density lipoprotein cholesterol in defining mortality risk in older persons with low serum cholesterol. J Am Geriatr Soc. 2001;49:1142–47. doi: 10.1046/j.1532-5415.2001.49229.x. [DOI] [PubMed] [Google Scholar]
- 22.Volpato S, Guralnik JM, Ferrucci L, et al. Cardiovascular disease, interleukin-6, and risk of mortality in older women: the women’s health and aging study. Circulation. 2001;103:947–53. doi: 10.1161/01.cir.103.7.947. [DOI] [PubMed] [Google Scholar]
- 23.Blaum CS, Volpato S, Cappola AR, et al. Diabetes, hyperglycaemia and mortality in disabled older women: The Women’s Health and Ageing Study I. Diabet Med. 2005;22:543–50. doi: 10.1111/j.1464-5491.2005.01457.x. [DOI] [PubMed] [Google Scholar]
- 24.Liabeuf S, Barreto DV, Barreto FC, et al. The circulating soluble TRAIL is a negative marker for inflammation inversely associated with the mortality risk in chronic kidney disease patients. Nephrol Dial Transplant. 2010;25:2596–602. doi: 10.1093/ndt/gfq042. [DOI] [PubMed] [Google Scholar]
- 25.Secchiero P, Zerbinati C, Rimondi E, et al. TRAIL promotes the survival, migration and proliferation of vascular smooth muscle cells. Cell Mol Life Sci. 2004;61:1965–74. doi: 10.1007/s00018-004-4197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Wal AC, Becker AE, van der Loos CM, Das PK. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation. 1994;89:36–44. doi: 10.1161/01.cir.89.1.36. [DOI] [PubMed] [Google Scholar]
- 27.Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation. 2001;104:365–72. doi: 10.1161/01.cir.104.3.365. [DOI] [PubMed] [Google Scholar]
- 28.Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277:815–8. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- 29.Secchiero P, Gonelli A, Carnevale E, Milani D, Pandolfi A, Zella D, Zauli G. TRAIL promotes the survival and proliferation of primary human vascular endothelial cells by activating the Akt and ERK pathways. Circulation. 2003;107:2250–6. doi: 10.1161/01.CIR.0000062702.60708.C4. [DOI] [PubMed] [Google Scholar]
- 30.Renshaw SA, Parmar JS, Singleton V, Rowe SJ, Dockrell DH, Dower SK, Bingle CD, Chilvers ER, White MK. Acceleration of human neutrophil apoptosis by TRAIL. J Immunol. 2003;170:1027–33. doi: 10.4049/jimmunol.170.2.1027. [DOI] [PubMed] [Google Scholar]
- 31.Lum JJ, Bren G, McClure R, Badley AD. Elimination of senescent neutrophils by TNF-related apoptosis-inducing [corrected] ligand. J Immunol. 2005;175:1232–8. doi: 10.4049/jimmunol.175.2.1232. [DOI] [PubMed] [Google Scholar]
- 32.Shimada K. Immune system and atherosclerotic disease: heterogeneity of leukocyte subsets participating in the pathogenesis of atherosclerosis. Circ J. 2009;73:994–1001. doi: 10.1253/circj.cj-09-0277. [DOI] [PubMed] [Google Scholar]
- 33.Mori K, Ikari Y, Jono S, et al. Association of serum TRAIL level with coronary artery disease. Thrombosis Research. 2010;125:322–5. doi: 10.1016/j.thromres.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 34.Satoh D, Inami N, Shimazu T, et al. Soluble TRAIL prevents RANTES-dependent restenosis after percutaneous coronary intervention in patients with coronary artery disease. J Thromb Thrombolysis. 2010;29:471–6. doi: 10.1007/s11239-009-0364-9. [DOI] [PubMed] [Google Scholar]