Abstract
Decoy receptors bind with TNF related apoptosis inducing ligands (TRAIL) but do not contain the cytoplasmic domains necessary to transduce apoptotic signals. We hypothesized that decoy receptors may confer neuronal protection against lethal ischemia after ischemic preconditioning (IPC). Mixed cortical neurons were exposed to IPC one day prior to TRAIL treatment or lethal ischemia. IPC increased decoy receptor but reduced death receptor expression compared to lethal ischemia. IPC-induced increase in decoy receptor expression was reduced by prior treatment with CAPE, a nuclear factor-kappa B inhibitor (NFκB). Expression of decoy molecules, dependent on NFκB, may mediate neuronal survival induced by IPC.
Keywords: stroke, ischemia
Introduction
Cerebral ischemia is the endpoint of multiple disease states including stroke, cardiac arrest, and traumatic brain injury. Neuronal ischemic preconditioning (IPC) is an endogenous neuroprotective mechanism by which a sublethal insult confers temporary protection against subsequent lethal ischemia (Wegener et al. 2004). The neuronal ischemic tolerance conferred by IPC requires gene transcription and protein synthesis, with the responsible genes representing attractive therapeutic targets, but not, as of yet, clearly identified.
Tumor necrosis factor (TNF) related apoptosis inducing ligand (TRAIL), also named Apo2 ligand, is a member of the TNF superfamily. Although originally recognized for its ability to produce selective growth inhibition of tumors, TRAIL-mediated death of non-transformed cells suggest a potential role for TRAIL in non-cancer-related pathology (Jo et al. 2000). Two primary TRAIL receptors, death receptor-4 (DR4) and death receptor-5 (DR5), have been identified. Upon activation by the TRAIL, the death receptors recruit Fas associated death domain (FADD), which results in caspase activation and subsequent apoptosis. While TRAIL production has not been demonstrated in the normal human brain, DR4 and DR5 expression has been documented in human neurons and TRAIL receptor expression patterns are markedly modulated in disease (Aktas et al. 2007). Furthermore, TRAIL has been detected in neuropathology such as human brain tumors, Alzheimer's disease, and HIV-encephalopathy. In addition, TRAIL-mediated apoptosis has been documented in a rodent model of ischemia, suggesting a role for TRAIL in stroke.
In addition to the primary receptors, two additional TRAIL receptors have been identified: decoy receptors 1 and 2 (DcR1 and DcR2). Unlike DR4 and DR5, DcR1 and DcR2 contain mutated or absent intracellular death domains, rendering them inactive with regard to apoptotic signaling. Because DcR1 and DcR2 can bind TRAIL but do not induce apoptosis, they have been proposed to serve as `decoys' that compete with death receptors for TRAIL binding and ultimately inhibit apoptosis (Pan et al. 1997). The primary interest in decoy molecules comes from their expression in certain tumors, where they competitively inhibit the parent receptors, assist tumor cells in evading immune system destruction, and confer resistance to certain chemotherapeutic agents. In addition, DcR1 and DcR2 have been demonstrated in oligodendrocytes and neurons of normal adult brains (Cannella et al. 2007).
We hypothesized a potential role for decoy receptors in neuronal IPC. In the present study, we demonstrate that DcR1 and DcR2 are upregulated by IPC in neurons, that this expression is independent of the primary TRAIL receptors DR4 and DR5, and that decoy receptor expression in IPC is dependent on nuclear factor κB (NFκB). With a potential endogenous neuroprotective role, the decoy receptors may represent novel therapeutic targets for diseases involving cerebral ischemia.
Methods
Neuronal ischemic preconditioning model
Neonatal rat neurons were isolated using a papain dissociation kit (Worthington Biochemical Corp., Lakewood, NJ, USA) and cultured in supplemented neurobasal media. Ischemia was produced by complete oxygen-glucose deprivation (OGD). Primary neurons were subjected to 20 min oxygen-glucose deprivation (OGD) as the IPC stimulus. After 24 h recovery, neurons were exposed to either 60 min OGD or TRAIL (50μg/ml, determined from dose-dependent study, data not shown) as the lethal insult.
Cell death and decoy and death receptor analysis
Cell death was assayed 24 h following lethal ischemia using trypan blue staining and immunoblot analysis of apoptosis markers. Immunoblot and immunofluorescence analysis were performed to assess changes in decoy and death receptor expression. Primary antibodies included; Bad, CAD, Cleaved caspase-3 (Santa Cruz Biotechnology Inc., CA), DcR1, DcR2, DR4 and DR5 (QED Bioscience Inc., CA). For immunofluorescence, images were captured with an Axiophoto microscopic system.
Experimental groups
The ability of the IPC stimulus to produce neuroprotection was assessed by comparing cell survival and pro-apoptotic protein expression following a lethal insult (60 min OGD or TRAIL) in preconditioned versus naïve neurons. The role of NFκB in neuronal IPC was assessed by comparing cell survival in neurons undergoing pretreatment with caffeic acid phenyl ester (CAPE; 200ng/mL), which is a potent and specific NFκB inhibitor. To assess for a potential role in neuronal IPC, decoy receptor expression (DcR1 and DcR2) was compared to that of the primary TRAIL receptors (DR4 and DR5) before and after a lethal insult in naïve neurons, IPC neurons, and neurons undergoing the IPC stimulus following pretreatment with CAPE.
Statistical analysis
One-way analysis of variance with Bonferroni's multiple comparison post-test analyses were performed using GraphPad Prism 4.0 software. Data are reported as mean±SEM with at least n=4 for each group. Significance was assumed for a p-value <0.05.
Results
IPC protects against TRAIL-induced neuronal apoptosis in vitro
Increased cell death was observed in primary neurons following TRAIL administration (Fig 1A). Increased survival and decreased expression of pro-apoptotic proteins (Bad, CAD and cleaved caspase-3) were observed in neurons undergoing IPC prior to TRAIL administration (Fig 1B and 1C). Neuronal protection offered by IPC correlates with the increase in decoy receptor expression (Fig 1B and 1D). These results suggest that IPC is protective against TRAIL-induced neuronal apoptosis in vitro.
Figure 1. IPC protects apoptotic neuronal death by increasing amount of decoy receptor expression.
Primary neurons underwent ischemic preconditioning (IPC) in the presence or absence of TRAIL (50μg/mL). A) Trypan blue analysis of neuronal death. Representative Western blot (B) of apoptotic proteins (Bad, caspase activated DNase (CAD) and cleaved caspase-3 (Cl-Csp3)) and TRAIL receptors (DcR1, DcR2, DR4, and DR5) in neuronal whole cell lysate. Densitometry assessment of apoptotic (C), and TRAIL receptor (D) proteins. In C) * represents p<0.05 vs Ctrl, IPC+No TRAIL, and IPC+TRAIL. In D) * represents p<0.05 vs Ctrl; @ - p<0.05 vs Ctrl, IPC+No TRAIL, and IPC+TRAIL.
IPC-mediated neuroprotection and decoy receptor expression is mediated via NF| B
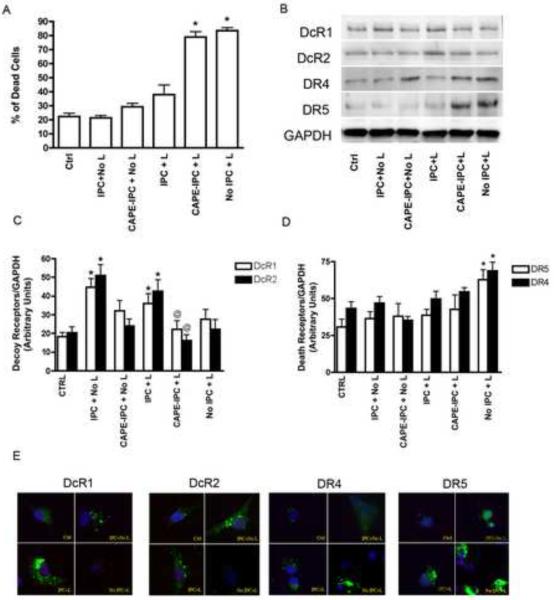
IPC also protected neurons from simulated lethal ischemia-induced injury (Fig 2A). Cells treated with CAPE prior to undergoing IPC were not protected against lethal ischemia. Increased expression of DcR1 and DcR2 was observed in IPC versus naïve neurons before and after lethal ischemia (Figs 2C, and 2E). This increase in expression was abolished in neurons pre-treated with CAPE prior to the IPC stimulus (2B). Expression of DR4 and DR5 did not change substantially following the IPC stimulus, with or without pre-treatment with CAPE, but increased following lethal ischemia alone (Fig 2B, 2D). Immunofluorescence microscopy also confirmed the increase in DcR1 and DcR2 but not DR4 and DR5 following IPC and an increase in DR4 and DR5 following lethal ischemia in naïve neurons (Fig 2E). These results suggest that IPC-mediated protection and subsequent induction of decoy receptors is sensitive to NF| B inhibition.
Figure 2. IPC induced expression of decoy receptors inhibited by NFkB inhibition.
Primary neurons underwent IPC with and without NFκB inhibition. A) Trypan blue analysis of neuronal death. * Represents p<0.05 vs IPC+L. B) Representative immunoblot of decoy (DcR1 and DcR2) and death receptor (DR4 and DR5) expression in whole cell lysates from primary neurons. C) and D) Densitometry analysis of decoy and death receptor expression, respectively. In C) * represents p<0.05 vs control, and @ represents p<0.05 vs IPC+L. In D) * represents p<0.05 vs Ctrl, IPC+No L, and IPC+L. E) Immunohistochemistry analysis shows expression of DcR1, DcR2, DR3, and DR4. IPC, ischemic preconditioning; L, lethal ischemia; CAPE, caffeic acid phenyl ester.
Discussion
Neuronal IPC provides robust neuroprotection against subsequent lethal insults. Here we used an in vitro model to demonstrate a potential role for decoy receptors as mediators of IPC. Sublethal ischemia appears to alter the ratio of decoy receptors to death receptors to allow competitive inhibition of apoptosis. In addition, the expression pattern of decoy receptors was also abolished by pre-treatment with CAPE, suggesting that NFκB plays a critical role in the gene expression induced by IPC.
The existence of decoy molecules in the TNF-receptor family is a relatively recent discovery, and little is known about their physiologic role. The presence of decoy molecules in certain tumors is associated with an increased likelihood of metastasis and decreased susceptibility to certain chemotherapeutic agents (Chang et al. 2008). Decoy molecules have not been studied in the setting of ischemia but possess several attributes that make them attractive candidates for mediating IPC. First, their expression confers protection via competitive inhibition of TRAIL. Thus, upregulation of decoy receptors alone could theoretically prevent caspase 8 activation via extrinsic apoptotic pathways, even in the presence of pro-apoptotic factors. Second, the TNF-receptor family appears to play a role in multiple neurodegenerative diseases (Aktas et al. 2005). Although decoy receptors are expressed by neurons, potential role for decoy receptors has not been studied in IPC. The present study represents the first investigation of a potential neuroprotective role for decoy receptors in cerebral ischemia.
Induction of TRAIL and other death ligands have been reported in the setting of ischemia and HIV infection, with their suppression producing neuroprotection (Martin-Villalba et al. 1999). Although the precise role of TRAIL receptors in mediating neuronal cell death is not investigated in this paper, several potential mechanisms can be hypothesized. DcR1 may prevent DR5-associated death-inducing signaling complex (DISC) assembly by binding TRAIL without transduction of pro-apoptotic signals (Merino et al. 2006). Clancy et al. proposed DcR2 as a regulatory receptor forming a ligand-independent, death-inhibitory complex (Clancy et al. 2005). The pre-ligand assembly domain (PLAD) between DcR2 and DR5 permits formation of mixed rather than homotrimeric complexes, thus preventing activation of DR5. Conversely, Merino et al. demonstrated that DcR2 facilitates DR5-mediated DISC formation but prevents initiation of caspase activation within the DISC (Merino et al. 2006).
DR4 and DR5 activate kinase pathways by promoting the association of a secondary signaling complex following DISC assembly. This secondary complex retains the DISC components, FADD and caspase 8, but recruits several other factors (RIP1, TRAF2, and NEMO) involved in kinase activation. Overexpression of Fas-associated death domain-like interleukin-1-b-converting enzyme inhibitory protein (FLIP), a caspase 8 inhibitor, was shown to be sufficient to rescue neurons from apoptosis during ischemia (Taoufik et al. 2007). This data suggests that downstream manipulation of death receptor signaling is also able to reduce neuronal death suggesting that this is an important pathway to target for protecting neurons. Our approach focuses uniquely on upstream decoy receptors to limit DISC formation.
The neuroprotective effect of IPC also appears to involve upregulation of NFκB pathways. The C-terminal element of DcR2 has signaling capacity similar to that of DR4 and DR5 with respect to NFκB activation (Degli-Esposti et al. 1997) but is unable to induce apoptosis. Thus, increased expression of decoy receptors by IPC may activate a secondary complex-linked survival kinase pathway. Studies implicating NFκB in TRAIL induced apoptosis are few in number and conflicting. NFκB expression is shown to be involved in protection against TRAIL (Travert et al. 2008), but a functional NFκB binding site is located in the promoter region of DR4 (Mendoza et al. 2008). NFκB has been reported to enhance TRAIL induced apoptosis through intronic regulation of DR5 (Chen et al. 2008). DcR2 expression can be induced by transcription factors such as hypoxia inducible factor (Pei et al. 2010). In relation to our finding with NFκB, it appears various transcription factors, regulated by hypoxia, may be involved in decoy receptor expression. In the present study, NFκB inhibition did not show any alteration in death receptor expression but resulted in decreased decoy receptor expression in IPC. It is possible that the downstream signaling potential of NFκB is stimulus and compartmentation dependent allowing for activation of diverse cellular effects in neurons.
With their initial discovery, decoy receptors were thought to be inactive mutant forms of the death receptors. It is therefore possible that a common regulatory mechanism was retained, with concurrent upregulation of the primary TRAIL receptors neutralizing any neuroprotective effect of an increase in decoy receptor expression. Thus, the differential expression patterns for decoy versus death receptors is an important observation, supporting the presence of separate mechanisms regulating their relative expression. Future investigations are needed to elucidate the mechanisms by which decoy receptor expression is regulated and apoptotic signaling is modulated. In addition to serving as competitive inhibitors of TRAIL, it is possible that DcR1 and DcR2 interact directly with death receptors or somehow disrupt downstream pro-apoptotic pathways (Merino et al. 2006).
Conclusions
Neuronal IPC provides robust neuroprotection against potentially lethal insults. The IPC response appears to involve NFκB activation, as inhibition with CAPE abolishes the expression pattern of decoy receptor afforded by sublethal ischemia. Here we demonstrate upregulation of decoy but not death receptors following an IPC stimulus and this upregulation does not occur following pre-treatment with CAPE, an inhibitor of NFκB, prior to the IPC stimulus. These data suggest a potential role for decoy receptors in mediating neuronal IPC and offer a novel therapeutic target for stroke.
Acknowledgements
Funding Supported by grants from the National Institutes of Health (NS047570 to PMP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aktas O, Schulze-Topphoff U, Zipp F. The role of TRAIL/TRAIL receptors in central nervous system pathology. Front Biosci. 2007;12:2912–21. doi: 10.2741/2281. [DOI] [PubMed] [Google Scholar]
- Aktas O, Smorodchenko A, Brocke S, Infante-Duarte C, Schulze Topphoff U, Vogt J, Prozorovski T, Meier S, Osmanova V, Pohl E, Bechmann I, Nitsch R, Zipp F. Neuronal damage in autoimmune neuroinflammation mediated by the death ligand TRAIL. Neuron. 2005;46:421–32. doi: 10.1016/j.neuron.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Cannella B, Gaupp S, Omari KM, Raine CS. Multiple sclerosis: death receptor expression and oligodendrocyte apoptosis in established lesions. J Neuroimmunol. 2007;188:128–37. doi: 10.1016/j.jneuroim.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PM, Chen PM, Hsieh SL, Tzeng CH, Liu JH, Chiou TJ, Wang WS, Yen CC, Gau JP, Yang MH. Expression of a soluble decoy receptor 3 in patients with diffuse large B-cell lymphoma predicts clinical outcome. Int J Oncol. 2008;33:549–54. [PubMed] [Google Scholar]
- Chen JJ, Chou CW, Chang YF, Chen CC. Proteasome inhibitors enhance TRAIL-induced apoptosis through the intronic regulation of DR5: involvement of NF-kappa B and reactive oxygen species-mediated p53 activation. J Immunol. 2008;180:8030–9. doi: 10.4049/jimmunol.180.12.8030. [DOI] [PubMed] [Google Scholar]
- Clancy L, Mruk K, Archer K, Woelfel M, Mongkolsapaya J, Screaton G, Lenardo MJ, Chan FK. Preligand assembly domain-mediated ligand-independent association between TRAIL receptor 4 (TR4) and TR2 regulates TRAIL-induced apoptosis. Proc Natl Acad Sci. 2005;102:18099–104. doi: 10.1073/pnas.0507329102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degli-Esposti MA, Dougall WC, Smolak PJ, Waugh JY, Smith CA, Goodwin RG. The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity. 1997;7:813–20. doi: 10.1016/s1074-7613(00)80399-4. [DOI] [PubMed] [Google Scholar]
- Jo M, Kim TH, Seol DW, Esplen JE, Dorko K, Billiar TR, Strom SC. Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat Med. 2000;6:564–7. doi: 10.1038/75045. [DOI] [PubMed] [Google Scholar]
- Martin-Villalba A, Herr I, Jeremias I, Hahne M, Brandt R, Vogel J, Schenkel J, Herdegen T, Debatin KM. CD95 ligand (Fas-L/APO-1L) and tumor necrosis factor-related apoptosis-inducing ligand mediate ischemia-induced apoptosis in neurons. J Neurosci. 1999;19:3809–17. doi: 10.1523/JNEUROSCI.19-10-03809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza FJ, Ishdorj G, Hu X, Gibson SB. Death receptor-4 (DR4) expression is regulated by transcription factor NF-kappaB in response to etoposide treatment. Apoptosis. 2008;13:756–70. doi: 10.1007/s10495-008-0210-0. [DOI] [PubMed] [Google Scholar]
- Merino D, Lalaoui N, Morizot A, Schneider P, Solary E, Micheau O. Differential inhibition of TRAIL-mediated DR5-DISC formation by decoy receptors 1 and 2. Mol Cell Biol. 2006;26:7046–55. doi: 10.1128/MCB.00520-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277:815–8. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- Pei GT, Wu CW, Lin WW. Hypoxia-induced decoy receptor 2 gene expression is regulated via a hypoxia-inducible factor 1alpha-mediated mechanism. Biochem Biophys Res Commun. 391:1274–9. doi: 10.1016/j.bbrc.2009.12.058. [DOI] [PubMed] [Google Scholar]
- Taoufik E, Valable S, Muller GJ, Roberts ML, Divoux D, Tinel A, Voulgari-Kokota A, Tseveleki V, Altruda F, Lassmann H, Petit E, Probert L. FLIP(L) protects neurons against in vivo ischemia and in vitro glucose deprivation-induced cell death. J Neurosci. 2007;27:6633–46. doi: 10.1523/JNEUROSCI.1091-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travert M, Ame-Thomas P, Pangault C, Morizot A, Micheau O, Semana G, Lamy T, Fest T, Tarte K, Guillaudeux T. CD40 ligand protects from TRAIL-induced apoptosis in follicular lymphomas through NF-kappaB activation and up-regulation of c-FLIP and Bcl-xL. J Immunol. 2008;181:1001–11. doi: 10.4049/jimmunol.181.2.1001. [DOI] [PubMed] [Google Scholar]
- Wegener S, Gottschalk B, Jovanovic V, Knab R, Fiebach JB, Schellinger PD, Kucinski T, Jungehulsing GJ, Brunecker P, Muller B, Banasik A, Amberger N, Wernecke KD, Siebler M, Rother J, Villringer A, Weih M. Transient ischemic attacks before ischemic stroke: preconditioning the human brain? A multicenter magnetic resonance imaging study. Stroke. 2004;35:616–21. doi: 10.1161/01.STR.0000115767.17923.6A. [DOI] [PubMed] [Google Scholar]