Abstract
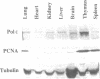
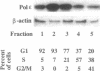
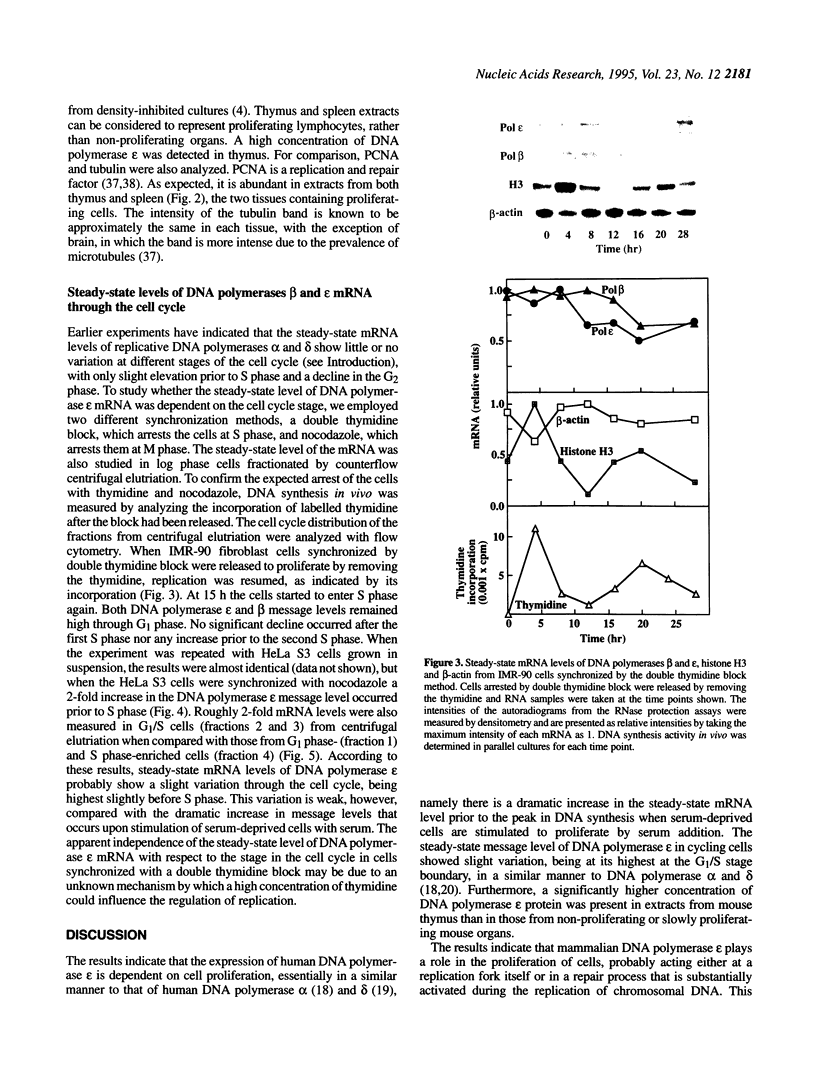
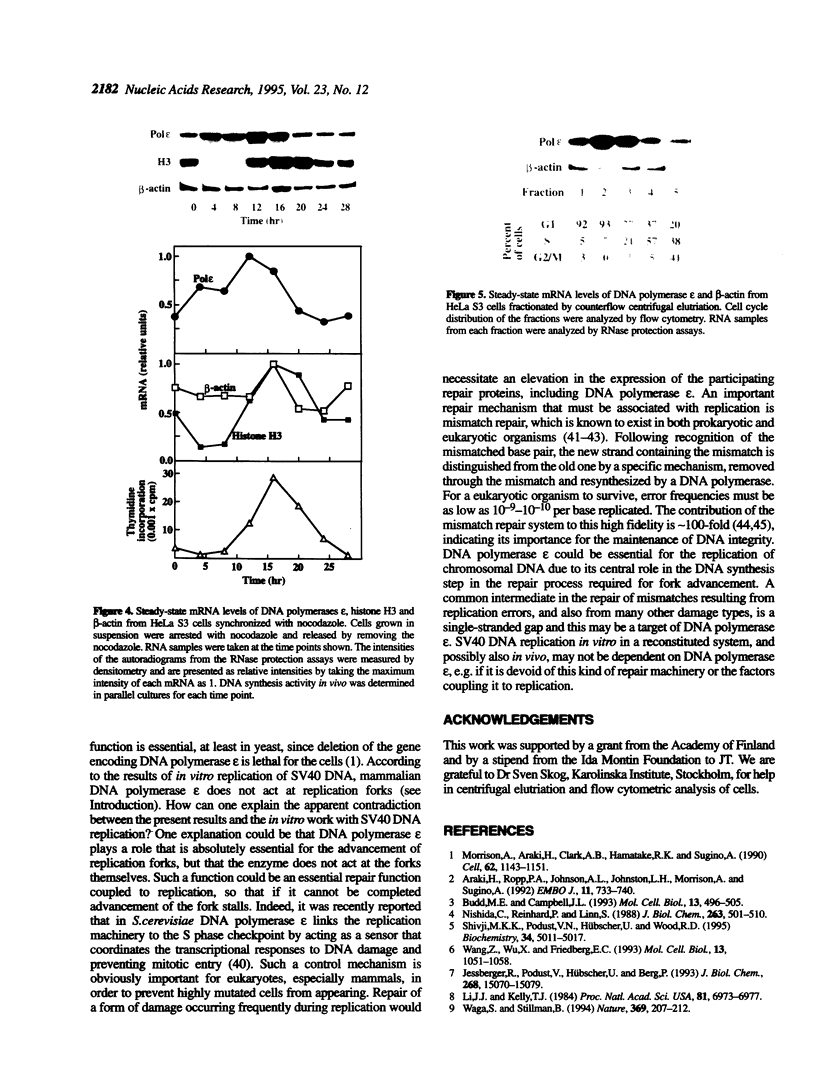
In order to shed light on the role of mammalian DNA polymerase epsilon we studied the expression of mRNA for the human enzyme during cell proliferation and during the cell cycle. Steady-state levels of mRNA encoding DNA polymerase epsilon were elevated dramatically when quiescent (G0) cells were stimulated to proliferate (G1/S) in a similar manner to those of DNA polymerase alpha. Message levels of DNA polymerase beta were unchanged in similar experiments. The concentration of immunoreactive DNA polymerase epsilon was also much higher in extracts from proliferating tissues than in those from non-proliferating or slowly proliferating tissues. The level of DNA polymerase epsilon mRNA in actively cycling cells synchronized with nocodazole and in cells fractionated by counterflow centrifugal elutriation showed weaker variation, being at its highest at the G1/S stage boundary. The results presented strongly suggest that mammalian DNA polymerase epsilon is involved in the replication of chromosomal DNA and/or in a repair process that may be substantially activated during the replication of chromosomal DNA. A hypothetical role for DNA polymerase epsilon in a repair process coupled to replication is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almendral J. M., Huebsch D., Blundell P. A., Macdonald-Bravo H., Bravo R. Cloning and sequence of the human nuclear protein cyclin: homology with DNA-binding proteins. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1575–1579. doi: 10.1073/pnas.84.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki H., Ropp P. A., Johnson A. L., Johnston L. H., Morrison A., Sugino A. DNA polymerase II, the probable homolog of mammalian DNA polymerase epsilon, replicates chromosomal DNA in the yeast Saccharomyces cerevisiae. EMBO J. 1992 Feb;11(2):733–740. doi: 10.1002/j.1460-2075.1992.tb05106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard J. M., Piechaczyk M., Dani C., Chambard J. C., Franchi A., Pouyssegur J., Jeanteur P. c-myc gene is transcribed at high rate in G0-arrested fibroblasts and is post-transcriptionally regulated in response to growth factors. Nature. 1985 Oct 3;317(6036):443–445. doi: 10.1038/317443a0. [DOI] [PubMed] [Google Scholar]
- Budd M. E., Campbell J. L. DNA polymerases delta and epsilon are required for chromosomal replication in Saccharomyces cerevisiae. Mol Cell Biol. 1993 Jan;13(1):496–505. doi: 10.1128/mcb.13.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael E. P., Roome J. M., Wahl A. F. Binding of a sequence-specific single-stranded DNA-binding factor to the simian virus 40 core origin inverted repeat domain is cell cycle regulated. Mol Cell Biol. 1993 Jan;13(1):408–420. doi: 10.1128/mcb.13.1.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro J., Heiden T., Wang N., Tribukait B. Preparation of cell nuclei from fresh tissues for high-quality DNA flow cytometry. Cytometry. 1993 Oct;14(7):793–804. doi: 10.1002/cyto.990140712. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coppock D. L., Pardee A. B. Control of thymidine kinase mRNA during the cell cycle. Mol Cell Biol. 1987 Aug;7(8):2925–2932. doi: 10.1128/mcb.7.8.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echols H., Goodman M. F. Fidelity mechanisms in DNA replication. Annu Rev Biochem. 1991;60:477–511. doi: 10.1146/annurev.bi.60.070191.002401. [DOI] [PubMed] [Google Scholar]
- Fang W. H., Modrich P. Human strand-specific mismatch repair occurs by a bidirectional mechanism similar to that of the bacterial reaction. J Biol Chem. 1993 Jun 5;268(16):11838–11844. [PubMed] [Google Scholar]
- Fornace A. J., Jr, Zmudzka B., Hollander M. C., Wilson S. H. Induction of beta-polymerase mRNA by DNA-damaging agents in Chinese hamster ovary cells. Mol Cell Biol. 1989 Feb;9(2):851–853. doi: 10.1128/mcb.9.2.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. D., Milsted A., Schloss J. A., Starger J., Yerna M. J. Cytoplasmic fibers in mammalian cells: cytoskeletal and contractile elements. Annu Rev Physiol. 1979;41:703–722. doi: 10.1146/annurev.ph.41.030179.003415. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Hamatake R. K., Hasegawa H., Clark A. B., Bebenek K., Kunkel T. A., Sugino A. Purification and characterization of DNA polymerase II from the yeast Saccharomyces cerevisiae. Identification of the catalytic core and a possible holoenzyme form of the enzyme. J Biol Chem. 1990 Mar 5;265(7):4072–4083. [PubMed] [Google Scholar]
- Jessberger R., Podust V., Hübscher U., Berg P. A mammalian protein complex that repairs double-strand breaks and deletions by recombination. J Biol Chem. 1993 Jul 15;268(20):15070–15079. [PubMed] [Google Scholar]
- Kesti T., Frantti H., Syväoja J. E. Molecular cloning of the cDNA for the catalytic subunit of human DNA polymerase epsilon. J Biol Chem. 1993 May 15;268(14):10238–10245. [PubMed] [Google Scholar]
- Kesti T., Syväoja J. E. Identification and tryptic cleavage of the catalytic core of HeLa and calf thymus DNA polymerase epsilon. J Biol Chem. 1991 Apr 5;266(10):6336–6341. [PubMed] [Google Scholar]
- Lee S. H., Pan Z. Q., Kwong A. D., Burgers P. M., Hurwitz J. Synthesis of DNA by DNA polymerase epsilon in vitro. J Biol Chem. 1991 Nov 25;266(33):22707–22717. [PubMed] [Google Scholar]
- Li J. J., Kelly T. J. Simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6973–6977. doi: 10.1073/pnas.81.22.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrich P. Methyl-directed DNA mismatch correction. J Biol Chem. 1989 Apr 25;264(12):6597–6600. [PubMed] [Google Scholar]
- Morris G. F., Mathews M. B. Regulation of proliferating cell nuclear antigen during the cell cycle. J Biol Chem. 1989 Aug 15;264(23):13856–13864. [PubMed] [Google Scholar]
- Morrison A., Araki H., Clark A. B., Hamatake R. K., Sugino A. A third essential DNA polymerase in S. cerevisiae. Cell. 1990 Sep 21;62(6):1143–1151. doi: 10.1016/0092-8674(90)90391-q. [DOI] [PubMed] [Google Scholar]
- Morrison A., Johnson A. L., Johnston L. H., Sugino A. Pathway correcting DNA replication errors in Saccharomyces cerevisiae. EMBO J. 1993 Apr;12(4):1467–1473. doi: 10.1002/j.1460-2075.1993.tb05790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasheuer H. P., Moore A., Wahl A. F., Wang T. S. Cell cycle-dependent phosphorylation of human DNA polymerase alpha. J Biol Chem. 1991 Apr 25;266(12):7893–7903. [PubMed] [Google Scholar]
- Nishida C., Reinhard P., Linn S. DNA repair synthesis in human fibroblasts requires DNA polymerase delta. J Biol Chem. 1988 Jan 5;263(1):501–510. [PubMed] [Google Scholar]
- Pearson B. E., Nasheuer H. P., Wang T. S. Human DNA polymerase alpha gene: sequences controlling expression in cycling and serum-stimulated cells. Mol Cell Biol. 1991 Apr;11(4):2081–2095. doi: 10.1128/mcb.11.4.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prelich G., Kostura M., Marshak D. R., Mathews M. B., Stillman B. The cell-cycle regulated proliferating cell nuclear antigen is required for SV40 DNA replication in vitro. Nature. 1987 Apr 2;326(6112):471–475. doi: 10.1038/326471a0. [DOI] [PubMed] [Google Scholar]
- Schaaper R. M. Base selection, proofreading, and mismatch repair during DNA replication in Escherichia coli. J Biol Chem. 1993 Nov 15;268(32):23762–23765. [PubMed] [Google Scholar]
- SenGupta D. N., Zmudzka B. Z., Kumar P., Cobianchi F., Skowronski J., Wilson S. H. Sequence of human DNA polymerase beta mRNA obtained through cDNA cloning. Biochem Biophys Res Commun. 1986 Apr 14;136(1):341–347. doi: 10.1016/0006-291x(86)90916-2. [DOI] [PubMed] [Google Scholar]
- Shivji K. K., Kenny M. K., Wood R. D. Proliferating cell nuclear antigen is required for DNA excision repair. Cell. 1992 Apr 17;69(2):367–374. doi: 10.1016/0092-8674(92)90416-a. [DOI] [PubMed] [Google Scholar]
- Shivji M. K., Podust V. N., Hübscher U., Wood R. D. Nucleotide excision repair DNA synthesis by DNA polymerase epsilon in the presence of PCNA, RFC, and RPA. Biochemistry. 1995 Apr 18;34(15):5011–5017. doi: 10.1021/bi00015a012. [DOI] [PubMed] [Google Scholar]
- Skog S., Tribukait B., Sundius G. Energy metabolism and ATP turnover time during the cell cycle of Ehrlich ascites tumour cells. Exp Cell Res. 1982 Sep;141(1):23–29. doi: 10.1016/0014-4827(82)90063-5. [DOI] [PubMed] [Google Scholar]
- Syvaoja J., Linn S. Characterization of a large form of DNA polymerase delta from HeLa cells that is insensitive to proliferating cell nuclear antigen. J Biol Chem. 1989 Feb 15;264(5):2489–2497. [PubMed] [Google Scholar]
- Szpirer J., Pedeutour F., Kesti T., Riviere M., Syväoja J. E., Turc-Carel C., Szpirer C. Localization of the gene for DNA polymerase epsilon (POLE) to human chromosome 12q24.3 and rat chromosome 12 by somatic cell hybrid panels and fluorescence in situ hybridization. Genomics. 1994 Mar 15;20(2):223–226. doi: 10.1006/geno.1994.1156. [DOI] [PubMed] [Google Scholar]
- Uitto L., Halleen J., Hentunen T., Höyhtyä M., Syväoja J. E. Structural relationship between DNA polymerases epsilon and epsilon* and their occurrence in eukaryotic cells. Nucleic Acids Res. 1995 Jan 25;23(2):244–247. doi: 10.1093/nar/23.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waga S., Stillman B. Anatomy of a DNA replication fork revealed by reconstitution of SV40 DNA replication in vitro. Nature. 1994 May 19;369(6477):207–212. doi: 10.1038/369207a0. [DOI] [PubMed] [Google Scholar]
- Wahl A. F., Geis A. M., Spain B. H., Wong S. W., Korn D., Wang T. S. Gene expression of human DNA polymerase alpha during cell proliferation and the cell cycle. Mol Cell Biol. 1988 Nov;8(11):5016–5025. doi: 10.1128/mcb.8.11.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Wu X., Friedberg E. C. DNA repair synthesis during base excision repair in vitro is catalyzed by DNA polymerase epsilon and is influenced by DNA polymerases alpha and delta in Saccharomyces cerevisiae. Mol Cell Biol. 1993 Feb;13(2):1051–1058. doi: 10.1128/mcb.13.2.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. W., Wahl A. F., Yuan P. M., Arai N., Pearson B. E., Arai K., Korn D., Hunkapiller M. W., Wang T. S. Human DNA polymerase alpha gene expression is cell proliferation dependent and its primary structure is similar to both prokaryotic and eukaryotic replicative DNA polymerases. EMBO J. 1988 Jan;7(1):37–47. doi: 10.1002/j.1460-2075.1988.tb02781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. L., Chang L. S., Zhang P., Hao H., Zhu L., Toomey N. L., Lee M. Y. Molecular cloning of the cDNA for the catalytic subunit of human DNA polymerase delta. Nucleic Acids Res. 1992 Feb 25;20(4):735–745. doi: 10.1093/nar/20.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X. R., Hao H., Jiang Y., Lee M. Y. Regulation of human DNA polymerase delta during the cell cycle. J Biol Chem. 1994 Sep 30;269(39):24027–24033. [PubMed] [Google Scholar]
- Zmudzka B. Z., Fornace A., Collins J., Wilson S. H. Characterization of DNA polymerase beta mRNA: cell-cycle and growth response in cultured human cells. Nucleic Acids Res. 1988 Oct 25;16(20):9587–9596. doi: 10.1093/nar/16.20.9587. [DOI] [PMC free article] [PubMed] [Google Scholar]