Abstract
Objectives
To gain insight into early mechanisms of aortic widening, we examined associations between the diameter of the abdominal aorta (AD) and cardiovascular disease (CVD) risk factors and biomarkers, as well as measures of subclinical atherosclerosis, in a multi-ethnic population.
Design
Cross-sectional cohort
Methods
A total of 1926 participants (mean age 62, 50% women) underwent chest and abdomen scanning by computed tomography, ultrasound of the carotid arteries, and CVD risk factor assessment. AD was measured 5 cm above and at the bifurcation.
Results
In a model containing traditional CVD risk factors, biomarkers and ethnicity, only age (standardized β=0.97), male sex (β=1.88), body surface area (standardized β=0.92), current smoking (β=0.42), D-dimer levels (β=0.19) and hypertension (β=0.53) were independently and significantly associated with increasing AD (in mm) at the bifurcation; use of cholesterol-lowering medications predicted smaller AD (β=-0.70) (P<.01 for all). These findings were similar for AD 5 cm above the bifurcation with one exception: compared to Caucasian-Americans, Americans of Chinese, African and Hispanic descent had significantly smaller AD 5 cm above the bifurcation (β's= -0.59, -0.49, and -0.52, respectively, all P<.01), whereas AD at the bifurcation did not differ by ethnicity. Physical activity, alcohol consumption, diabetes and levels of IL-6, CRP and homocysteine were not independently associated with AD. Higher aortic and coronary artery calcium burden, but not common carotid artery intima-media thickness, were independently, but modestly (β=0.11 to 0.19), associated with larger AD.
Conclusions
Incremental widening of the aortic diameter shared some, but not all, risk factors for occlusive vascular disease.
Keywords: aorta, aneurysm, atherosclerosis, ethnicity, epidemiology
Introduction
There is controversy as to whether the predominant etiology of abdominal aortic aneurysm (AAA) is arteriosclerosis (the process leading to muscular thickening of the arterial wall) (1) or atherosclerosis (deposition of lipid into and accompanied by inflammation of the intima-media complex) (1,2). For instance, traditional risk factors are differentially associated with AAA compared to CHD (3) and aortic diameter (AD) is inconsistently related to aortic calcification (4-6). Studies attempting to discern which process is dominant are difficult since there is an overlap in the risk factors for these two conditions. Some have concluded that risk factors for occlusive arterial disease such as hypertension and lipids may not be associated with AAA (7,8).
Studies that allow for more sensitive detection of atherosclerosis in the extracoronary vasculature and its association with abdominal AD, an early marker of aneurysmal development, provide the opportunity to expand the current literature on this issue. The Multi-Ethnic Study of Atherosclerosis (MESA) provides a unique opportunity to do so in a population of younger adults without AAA, while considering potential ethnic differences. To gain insight into the mechanisms underlying widening of the aortic diameter, we examined the magnitude and significance of the associations of abdominal AD at, and 5 cm above, the bifurcation with coronary and aortic calcification, carotid atherosclerosis, as well as CVD risk factors and biomarkers.
Methods
Study participants
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective cohort study investigating subclinical atherosclerosis in 6814 individuals aged 45-84 years who were free of clinically manifest CVD at baseline (9). Participants of non-Hispanic white, Chinese-American, African-American, or Hispanic-American origin were originally recruited between July 2000 and August 2002 from 6 U.S. field centers.
This report includes the random sample of MESA subjects who participated in the MESA Abdominal Aortic Calcium Study (MESA-AACS). MESA-ACCS participants were recruited during follow-up visits between August 2002 and September 2005 from five MESA field centers: Chicago, IL; Forsyth County, NC; Los Angeles County, CA; New York, New York; and St. Paul, MN. Of 2202 MESA subjects recruited, 2172 agreed to participate, and 1968 satisfied MESA-ACCS eligibility criteria including age and ethnicity subsampling from the MESA, postmenopausal status, and no recent diagnostic abdominal computed tomography (CT). This study includes the 970 men and 956 postmenopausal women who had AD data available at the four anatomic locations of interest. Signed informed consent was obtained for all participants, and institutional review board approval was obtained from all participating institutions.
Risk factor assessment
Standardized questionnaires at the baseline MESA examination were used to obtain participant information on demographics, current prescription medication usage, medical history, smoking history, alcohol consumption, and physical activity. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared; body surface area (BSA) as 0.20247 × (height (m0.725)) × (weight (kg0.425)). Blood pressure was measured 3 times in the seated position with a Dinamap model Pro 100 automated oscillometric sphygmomanometer, and the average of the last 2 measurements was used.
Blood samples obtained after a 12-h fast were used for measurement of total cholesterol, high density lipoprotein (HDL) cholesterol, triglycerides, glucose, C-reactive protein (CRP), interleukin-6 (IL-6), fibrinogen, D-dimer, factor VIII and homocysteine by previously described methods (10). Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg or current use of antihypertensive medication. Diabetes was defined as fasting plasma glucose >126 mg/dL, reported physician diagnosis of diabetes, or use of hypoglycemic medications. Individuals with a total to HDL cholesterol ratio >5 or who reported use of a medication to treat high cholesterol were classified as dyslipidemic.
CT scanning
Participants underwent CT scanning of the chest and abdomen to ascertain the presence and extent of coronary artery calcium and abdominal aorta calcium. Coronary scans were performed in duplicate for accuracy with cardiac-gated electron-beam scanners at 3 field centers (Imatron C-150; Imatron, Inc., San Francisco, California) or with a prospectively electrocardiogram-triggered scan acquisition at 50% of the RR interval with multidetector scanners at the remaining 3 centers (New York, Forsyth County, and St. Paul field centers). Details of the MESA CT scanning and quality assurance procedures have been reported (11).
Image analysis for calcium
Scans were read centrally by the MESA CT Reading Center at the Los Angeles Biomedical Research Institute (Torrance, California). After scanning, images were reconstructed in a 35-cm field of view with 5-mm slice thickness. All scan scores were brightness adjusted with a standard phantom. Calcium was scored in an 8-cm segment of the distal abdominal aorta ending at the aortic bifurcation. Calcification was identified as a plaque of ≥1 mm2 with a density of >130 HU and quantified using the Agatston scoring method (12).
Image analysis for Aortic Diameter
Images from the abdominal CT scans were retrospectively interrogated to determine the diameter of the abdominal aorta using computer software (Osiris 4.19, University of Geneva, Geneva, Switzerland). Measures were conducted at 5 cm proximal to the aortic bifurcation and at the slice just above the aortic bifurcation. An adjustable-size electronic caliper in the shape of a circle was used to measure the diameter (d) by fitting the caliper around the circumference (C) of the adventitia of the aorta. The computer then calculated the diameter from the circumference measurement using the equation, d=C/π. Each location was measured three times by a single reader who was unaware of subject characteristics; the average of these measurements was used in the analysis; the intraclass correlation was 0.93, average difference between measurements was 4.0%.
Carotid ultrasonography
Images of the right and left common carotid arteries (CCA) were captured, including images of the near and far walls, by trained personnel using high-resolution B-mode ultrasound. A Logiq 700 ultrasound machine (GE Medical Systems, Waukesha, Wisconsin) was used at all centers. All studies were recorded on optical disk and super VHS videotape and sent weekly to a central ultrasound reading center located at Tufts Medical Center, Tufts University. The high-resolution images of the CCAs were analyzed to calculate near- and far-wall IMT, lumen diameter, and vessel width at each arterial site using a specially designed computer program (13). Intima-media thickness (IMT) of the CCA was defined as the mean of all available maximum wall thicknesses across both left and right sides.
Statistical analysis
Descriptive statistics of the study cohort were summarized by means, medians, or frequencies as appropriate. Analysis of covariance was used to calculate the adjusted mean value or prevalence of the subject characteristics by sex-specific quartile of AD. Multivariable linear regression was used to determine the simultaneous significance of associations between AD and the CVD risk factors, as well as the presence and extent of atherosclerosis in distinct vascular beds. The vascular calcium variables were normalized by transformation using log10 of (calcium score +1). Multivariable linear regression results are presented per sex-specific standard deviation (SD) increase in each vascular calcium and IMT measurement. There was no significant collinearity (conservatively defined as variance inflation factor >5 or tolerance <0.2). Following the recommendation of Rothman (14), no adjustment was made for multiple comparisons; rather exact P-values for two-sided tests are shown; p ≤0.05 was considered statistically significant. Analyses were conducted using SPSSv15 (SPSS Inc, Chicago, IL).
Results
Characteristics of the 1926 participants (49.6% women) are presented in Table 1. The men and women ranged in age from 44 to 84 years; 41% were classified as overweight and 31% as obese. The median calcium scores in the coronary arteries and abdominal aortic artery were 0 and 235, respectively, and the median CCA IMT was 0.85 mm; 50.3% had any coronary artery calcium, 71.5% had abdominal aortic artery calcium. The mean (SD) aortic diameter (AD) was 18.8 (3.0) mm at 5 cm proximal to the aortic bifurcation and 19.0 (3.0) mm just above the aortic bifurcation. AD was significantly larger (P<.001) in men than women at both sites independent of age, BSA (or height) and ethnicity.
Table 1. Characteristics of the study population.
| Characteristic | Value (N=1926) |
|---|---|
| Age, mean (SD) years | 62.1 (9.8) |
| BMI, mean (SD) kg/m2 | 28.1 (5.1) |
| BSA, mean (SD) m2 | |
| Female Sex, No. (%) | 956 (49.6) |
| Ethnicity, No. (%) | |
| Caucasian | 775 (40.2) |
| Chinese-American | 254 (13.2) |
| African-American | 405 (21.0) |
| Hispanic-American | 492 (25.5) |
| Smoker Former, No. (%) | 704 (36.6) |
| Smoker Current, No. (%) | 247 (12.8) |
| Alcohol Former, No. (%) | 434 (22.6) |
| Alcohol Current, No. (%) | 1090 (56.8) |
| Physical activity, Mets/wk | 3454 (3902) |
| Dyslipidemia, No. (%) | 676 (35.1) |
| Hypertension, No. (%) | 874 (45.4) |
| Diabetes Mellitus, No. (%) | 218 (11.4) |
| Family History CHD, No. (%) | 779 (43.5) |
| Calcium, No. (%>0) | |
| Coronary | 969 (50.3) |
| Descending aorta | 1377 (71.5) |
| Carotid Intima-Media Thickness | |
| CCA, median (IQR) mm | 0.85 (0.24) |
CHD, coronary heart disease; IQR, interquartile range; CCA, common carotid artery
Mean age- and sex-adjusted values for traditional CVD risk factors, biomarkers and measures of subclinical atherosclerosis by quartile of AD are shown in Tables 2. There were significant linear trends for increasing age, BMI, BSA, height and smoking and alcohol consumption prevalence across AD quartiles at each site (P <.01 for all). Abdominal AD was not related to lipid levels or to the prevalence of dyslipidemia or diabetes. Blood pressure measures increased linearly with AD at the bifurcation (P ≤.01), with less robust associations for AD 5 cm above the bifurcation. Levels of IL-6, CRP and D-dimer were positively related to AD at both sites (P <.01 for all). Homocysteine and fibrinogen associations were only marginally significant, whereas factor VIII was not related to AD. The prevalence of calcium in the abdominal aorta and coronary arteries and IMT of the CCA increased across quartiles of AD at each site (P<.05).
Table 2.
Population characteristics by sex-specific regional AD quartile.
| AD site: | 5 cm above Bifurcation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | P value | Q1 | Q2 | Q3 | Q4 | P value | |
| range women (mm) | (12-16) | (17-17) | (18-18) | (19-30) | (11-16) | (17-17) | (18-19) | (20-33) | ||
| range men (mm) | (13-18) | (19-19) | (20-21) | (22-41) | (13-18) | (19-19) | (20-21) | (22-38) | ||
| sample size | 594 | 381 | 487 | 464 | 593 | 327 | 569 | 437 | ||
| Characteristic | ||||||||||
| Age, yrs | 59.8 | 60.8 | 62.3 | 65.9 | <0.01 | 58.9 | 60.5 | 62.7 | 66.7 | <0.01 |
| BMI, kg/m2 | 26.8 | 27.9 | 28.6 | 29.6 | <0.01 | 26.5 | 27.6 | 28.5 | 30.2 | <0.01 |
| BSA, m2 | 1.79 | 1.84 | 1.90 | 1.96 | <0.01 | 1.78 | 1.85 | 1.90 | 1.97 | <0.01 |
| Height, cm | 164.2 | 165.4 | 168.3 | 170.0 | <0.01 | 164.4 | 166.3 | 167.9 | 169.4 | <0.01 |
| Smoker past, % | 33.0 | 31.8 | 38.1 | 43.5 | <0.01 | 35.1 | 35.6 | 37.5 | 38.1 | 0.73 |
| Smoker current, % | 8.9 | 10.3 | 14.3 | 18.5 | <0.01 | 10.6 | 11.9 | 13.0 | 16.3 | 0.07 |
| Alcohol past, % | 23.4 | 21.2 | 20.8 | 24.8 | 0.65 | 23.6 | 21.7 | 19.9 | 25.5 | 0.66 |
| Alcohol current, % | 48.8 | 58.1 | 60.6 | 61.2 | <0.01 | 52.6 | 54.2 | 59.1 | 60.4 | <0.01 |
| Physical activity, Mets/wk | 3461 | 3492 | 3287 | 3591 | 0.78 | 3381 | 3558 | 3434 | 3502 | 0.73 |
| TC/HDL ratio | 4.12 | 4.12 | 4.08 | 4.14 | 0.85 | 4.15 | 4.04 | 4.13 | 4.14 | 0.78 |
| Dyslipidemia, % | 35.7 | 37.0 | 35.0 | 32.5 | 0.24 | 37.7 | 35.4 | 35.3 | 30.8 | 0.04 |
| Lipid-lowering med use, % | 18.3 | 16.7 | 15.7 | 13.1 | 0.08 | 17.2 | 18.0 | 17.0 | 11.8 | 0.06 |
| Diabetes mellitus, % | 11.9 | 11.4 | 11.1 | 10.8 | 0.57 | 10.5 | 10.4 | 12.6 | 11.7 | 0.40 |
| SBP, mmHg | 125.9 | 127.6 | 126.6 | 127.8 | 0.23 | 126.1 | 125.8 | 126.1 | 129.9 | <0.01 |
| DBP, mmHg | 71.5 | 72.7 | 72.7 | 73.4 | <0.01 | 71.3 | 71.4 | 72.3 | 75.1 | <0.01 |
| Hypertension, % | 43.5 | 42.1 | 44.1 | 51.6 | <0.01 | 40.9 | 38.6 | 45.3 | 56.5 | <0.01 |
| CRP, mg/L | 1.71 | 1.64 | 1.98 | 2.29 | <0.01 | 1.71 | 1.88 | 1.87 | 2.19 | <0.01 |
| IL-6, pg/ml | 1.11 | 1.14 | 1.19 | 1.35 | <0.01 | 1.11 | 1.14 | 1.16 | 1.38 | <0.01 |
| D-dimer, μg/ml | 0.20 | 0.23 | 0.21 | 0.24 | <0.01 | 0.20 | 0.23 | 0.20 | 0.26 | <0.01 |
| Fibrinogen, g/L | 3.37 | 3.35 | 3.34 | 3.40 | 0.46 | 3.34 | 3.33 | 3.36 | 3.43 | 0.05 |
| Homocysteine, μmol/L | 8.85 | 8.85 | 8.88 | 9.12 | 0.08 | 9.0 | 8.68 | 8.8 | 9.28 | 0.05 |
| Factor VIII, % | 161.5 | 156.8 | 158.6 | 158.8 | 0.64 | 163.4 | 155.5 | 154.4 | 161.9 | 0.67 |
| Family Hx CHD, % | 39.3 | 43.7 | 43.4 | 49.1 | <0.01 | 40.6 | 45.1 | 42.8 | 47.4 | 0.10 |
| CCA IMT (mm) | 0.858 | 0.864 | 0.869 | 0.891 | <0.01 | 0.862 | 0.859 | 0.874 | 0.885 | 0.02 |
| Calcium (>0), % | ||||||||||
| Coronary | 47.3 | 51.2 | 47.6 | 56.1 | 0.01 | 46.2 | 51.9 | 51.2 | 53.4 | 0.03 |
| Descending aorta | 68.1 | 72.0 | 70.6 | 76.3 | <0.01 | 67.9 | 72.0 | 73.1 | 73.8 | 0.03 |
Values are age and sex-adjusted, p-values are for linear trend
BSA, body surface area; TC, total cholesterol; Hx, history; SBP, systolic blood pressure; DBP, diastolic blood pressure CCA IMT, common carotid artery intima-media thickness
We next conducted multivariable linear regression analysis to determine which of the risk factors were independently associated with AD (Table 3). Because the age and sex-adjusted correlation of BSA with AD was stronger than that for BMI or height at both sites (respective partial correlations 0.30, 0.16, and 0.27 with AD 5 cm above the bifurcation and 0.35, 0.25, and 0.24 with AD at the bifurcation), BSA was used as a measure of body size for these analyses. In the multivariate models, age, male sex, BSA, and past and current smoking were independently associated with larger AD at both sites. Use of lipid-lowering medications predicted smaller diameter at both sites (P <.01), whereas hypertension was associated with larger AD only at the bifurcation. Compared to Caucasians, African-Americans and Hispanics had smaller AD on average 5 cm proximal to the bifurcation adjusting for all other covariates; AD just above the bifurcation did not vary significantly by ethnicity. The only biomarker independently related to AD was D-dimer (P<.01). The multivariate models explained 33 and 37% of the variance in AD, with sex, age and BSA explaining 90% or more of this variance.
Table 3. Multivariable linear regression between traditional cardiovascular risk factors and AD.
| Aortic site: | 5 cm above Bifurcation | Bifurcation | ||
|---|---|---|---|---|
| Independent Variable* | β | p-value | β | p-value |
| Age (10 yrs) | 0.72 | <.01 | 0.97 | <.01 |
| Sex (male vs female) | 2.23 | <.01 | 1.88 | <.01 |
| Ethnicity (vs Caucasian) | ||||
| Chinese-American | -0.59 | <.01 | 0.14 | .43 |
| African-American | -0.49 | <.01 | 0.25 | .19 |
| Hispanic | -0.52 | <.01 | 0.13 | .30 |
| Body surface area (1 SD) | 0.68 | <.01 | 0.92 | <.01 |
| Diabetes (yes vs no) | -0.17 | .30 | -0.22 | .33 |
| Hypertension (yes vs no) | 0.18 | .12 | 0.53 | <.01 |
| Lipid-lowering meds (yes vs no) | -0.33 | .03 | -0.70 | <.01 |
| Smoker former | 0.39 | <.01 | -0.01 | .83 |
| Smoker current | 1.24 | <.01 | 0.42 | .03 |
| Alcohol current | 0.03 | .79 | 0.22 | .11 |
| Physical activity (1 SD) | -0.01 | .83 | 0.02 | .83 |
| CRP (1 SD) | 0.01 | .87 | -0.12 | .08 |
| IL-6 (1 SD) | 0.02 | .82 | 0.08 | .26 |
| D-dimer (1 SD) | 0.17 | <.01 | 0.19 | <.01 |
| Homocysteine (1 SD) | -0.05 | .37 | -0.05 | .44 |
| MESA study site | 0.02 | .52 | 0.03 | .43 |
| Model R2 | .37 | .33 | ||
SD, sex-specific standard deviation
All variables in the same model
Linear regression coefficients; units are mm
Figure 1 presents results of multivariable linear regression analyses for AD where the measure of subclinical atherosclerosis for each vascular bed was separately added to a model containing all the risk factors from Table 3; it shows the increment in AD attributable to the presence of each subclinical measure. A 1-SD increase in aortic calcium score was significantly (P<.01) associated with 0.16-mm larger AD 5 cm proximal to the bifurcation and non-significantly (P=.17) with 0.11 mm larger AD just above the bifurcation. A SD increase in coronary calcium scores was associated with 0.19-mm larger AD 5 cm proximal and just above the bifurcation (P <.01 for both). IMT of the common carotid artery was not associated with increased AD. These results were not materially changed in models excluding the 22 individuals with AD ≥30 mm (data not shown).
Figure 1.
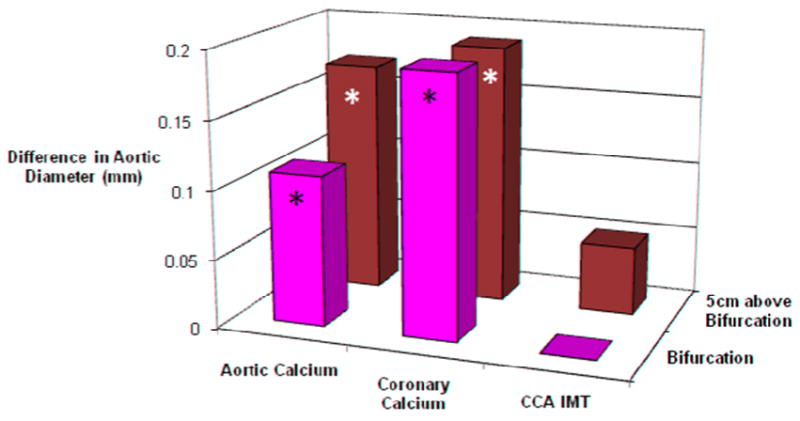
Results of linear regression analyses showing the difference in aortic diameter 5 cm above the aortic bifurcation (BIF) and just above the BIF per 1 SD increase in aortic artery calcium score, coronary artery calcium score, and common carotid artery intima-media thickness (CCA IMT). Values are adjusted for age, sex, ethnicity, BSA, alcohol, smoking, diabetes, hypertension, D-dimer, and use of lipid-lowering medications; * indicates P<.01.
Abdominal diameter was also assessed inferior to the renal artery and 10 cm above the bifurcation. Notably, multivariate associations with risk factors at both of these sites were similar to those reported above including significant associations of lipid-lowering medications and non-Caucasian ethnicity with lower AD, and hypertension and increasing aortic calcium with larger AD (all P<0.01) (data not shown).
Discussion
In this cross-sectional study of nearly 2000 MESA participants, the diameter of the abdominal aorta at four distinct locations was significantly and positively associated with age, male sex, body size, and smoking independent of ethnicity and the other traditional cardiovascular risk factors; use of lipid-lowering medications was associated with smaller AD. Americans of Chinese, African and Hispanic descent had smaller AD than Caucasian-Americans 5 cm above the birfurcation even after adjusting for differences in body size and other covariates. Increments of aortic diameter were modestly associated with measures of subclinical atherosclerosis including calcified atherosclerosis in the abdominal aorta and coronary arteries, independent of associated risk factors. Taken together, these results suggest a link between atherosclerotic risk factors, as well as subclinical atherosclerotic disease, and enlargement of the abdominal aortic diameter.
The primary positive predictors of AD in this study (age, male sex and larger body size) agree with prior findings in nearly 70,000 patients from 15 Veteran Affairs (VA) medical centers (15) and a study of 504 patients undergoing whole body CT (16). The 2-3 mm sex difference in aortic diameter in the MESA cohort was comparable to earlier reports (16-18), and persisted after adjusting for larger male body size and other covariates. Unlike the studies above, we also found significant increments in AD associated with smoking and hypertension that were not explained by the other risk factors. The relative risk of aneurysm in current smokers is at least three times that of non-smokers (19) and smoking constitutes a significant risk factor for aneurysm enlargement (20). If risk factors for larger AD are similar to those for AAA, our results suggest that smoking may be involved early in the pathogenesis of aneurysm formation. In most prior studies, hypertension had only a weak or no association with AAA (21). We found a moderately strong positive association of hypertension with AD at the bifurcation, despite the fact that 71% of individuals classified as hypertensive were taking anti-hypertensive medications, which might be expected to reduce any association.
Inverse correlates of AD have also been identified in the literature. The presence of diabetes was inversely related to the diameter of the abdominal aorta in three studies (16,21,22). In the present study, AD did not vary by diabetes, dyslipidemia, cholesterol, or the total to HDL cholesterol ratio at any of the four aortic segments examined. However, use of lipid-lowering medications, 95% of which were statins, was a strong independent predictor of smaller AD; the reduction in AD associated with statin use was equivalent to more than a 5 year reduction in age. Possible explanations for statin benefit may include suppression of matrix metalloproteinase (MMP), down regulation of proinflammatory gene expression, and preservation of medial elastin and smooth muscle cells (23,24). Moreover, there is some clinical evidence that statin therapy improves prognosis for individuals with small AAA. In two observational studies including 280 AAA patients followed for 2 to 3 years, the pooled growth rate was 3 mm less per year in those who were taking statins compared with those not taking statins (25).
To our knowledge, this is the first population-based study of the epidemiology of aortic diameter to include a large proportion of ethnic minorities. In the VA study by Lederle et al (15), black race was associated with a small (0.1 mm), but significant, increase in infrarenal AD. This contrasts with our finding of 0.4 to 0.5 mm smaller AD in African-Americans compared to Caucasians at three of the four aortic segments studied. We tested whether this difference might be due to the fact that the VA population was almost exclusively (97%) male. In analyses stratified by sex, AD was significantly smaller among both male and female African-Americans in MESA compared to their Caucasian counterparts. The lower AD observed in Chinese and Hispanic Americans is in line with reports of a much lower prevalence of AAA among British citizens of Asian descent compared to Caucasians (26,27) and fewer surgical procedures to repair aortic aneurysms in Hispanics than Caucasians (28,29). These ethnic differences provide additional evidence that AAA disproportionately affects Caucasians. That they are independent of body size as well as novel and traditional CVD risk factors suggests a role for either unmeasured or ethnicity-related genetic factors in determining AD.
Of the six cardiovascular biomarkers considered in this analysis, only D-dimer levels were associated with aortic diameter independent of the major risk factors. Levels of D-dimer are higher in individuals with small (30) and large (31,32) abdominal aneurysms and are thought to be a result of the release of fibrin degradation products during continual remodeling of intraluminal thrombus (33), a key pathological element in aneurysmal expansion. Higher D-dimer levels in individuals with larger AD is evidence of increased fibrinolytic activity and implicates chronic fibrin turnover early in the pathogenesis of AAA.
Some limitations of the present study should be noted. Gomes and colleagues have shown that CT is a valid method to evaluate AD and is more accurate than ultrasonography (34). Nonetheless, there is a small probability of residual error in the measurement of AD by EBCT scanners due to motion artifact, although mechanisms aimed at reducing this error were employed and the aorta moves little compared to the coronaries. Another limitation is the absence of data on family history of AAA, a major AAA risk factor (3,22). This study is also limited by the nature of the population. Participation in MESA was restricted to individuals who were free of clinically manifest CVD at baseline. Thus, this sample may not be fully generalizable to the general population which includes persons with known atherosclerotic disease.
An unresolved question is the extent to which aortic aneurysms are a manifestation of atherosclerosis or a result of distinct pathogenic processes. Although 31-90% of patients with established AAA are reported to have coronary artery disease (35), the fact that the prevalence of aortic aneurysm has not changed or is even increasing at the same time that incidence and mortality rates from coronary artery disease are decreasing (36,37) argues against a common pathway. So does the much stronger relative risk of current smoking for AAA (3 to 6-fold) compared to CHD (1 to 2-fold) (19) and the inverse association of diabetes with diagnosed AAA in some studies (38). The positive association of AD with subclinical atherosclerotic calcification observed in this and a prior study (16) adds to the evidence that dilatation of the AD may be another component of systemic vascular disease, but it does not prove an etiologic role for atherosclerotic processes. In our study, incremental widening of the AD shared some, but not all, risk factors for occlusive vascular disease, and much of the variance in AD remained unexplained. Thus, while this study provides additional evidence that the pathophysiology of aortic aneurysms may depend in part on atherosclerotic processes, other mechanisms seem likely.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions.
Funding Sources: This research was supported by grant HL72403 and contracts N01-HC-95159 through N01-HC-95165 from the National Heart, Lung, and Blood Institute, Bethesda, Maryland. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. GAL is supported by American Heart Association Award 0930073N.
Footnotes
Conflict of Interest Statement: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sinha S, Frishman WH. Matrix metalloproteinases and abdominal aortic aneurysms: a potential therapeutic target. J Clin Pharmacol. 1998;38:1077–88. [PubMed] [Google Scholar]
- 2.Xu C, Zarins CK, Glagov S. Aneurysmal and occlusive atherosclerosis of the human abdominal aorta. J Vasc Surg. 2001;33:91–6. doi: 10.1067/mva.2001.109744. [DOI] [PubMed] [Google Scholar]
- 3.Wanhainen A, Bergqvist D, Boman K, Nilsson TK, Rutegard J, Bjorck M. Risk factors associated with abdominal aortic aneurysm: a population-based study with historical and current data. J Vasc Surg. 2005;41:390–6. doi: 10.1016/j.jvs.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Matsushita M, Nishikimi N, Sakurai T, Nimura Y. Infrarenal aortic dilatation less than 4 cm is not unusual in patients with aortoiliac occlusive disease. Int Angiol. 2002;21:222–7. [PubMed] [Google Scholar]
- 5.Paivansalo MJ, Merikanto J, Jerkkola T, et al. Effect of hypertension and risk factors on diameters of abdominal aorta and common iliac and femoral arteries in middle-aged hypertensive and control subjects: a cross-sectional systematic study with duplex ultrasound. Atherosclerosis. 2000;153:99–106. doi: 10.1016/s0021-9150(00)00374-9. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds HR, Tunick PA, Kort S, et al. Abdominal aortic aneurysms and thoracic aortic atheromas. J Am Soc Echocardiogr. 2001;14:1127–31. doi: 10.1067/mje.2001.113814. [DOI] [PubMed] [Google Scholar]
- 7.Brady AR, Thompson SG, Fowkes FG, Greenhalgh RM, Powell JT. Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation. 2004;110:16–21. doi: 10.1161/01.CIR.0000133279.07468.9F. [DOI] [PubMed] [Google Scholar]
- 8.Lindholt JS, Heegaard NH, Vammen S, Fasting H, Henneberg EW, Heickendorff L. Smoking, but not lipids, lipoprotein(a) and antibodies against oxidised LDL, is correlated to the expansion of abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2001;21:51–6. doi: 10.1053/ejvs.2000.1262. [DOI] [PubMed] [Google Scholar]
- 9.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 10.Roy B, Diez-Roux AV, Seeman T, Ranjit N, Shea S, Cushman M. Association of optimism and pessimism with inflammation and hemostasis in the Multi-Ethnic Study of Atherosclerosis (MESA) Psychosom Med. 72:134–40. doi: 10.1097/PSY.0b013e3181cb981b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 12.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 13.O'Leary DH, Polak JF, Wolfson SK, Jr, et al. Use of sonography to evaluate carotid atherosclerosis in the elderly. The Cardiovascular Health Study. CHS Collaborative Research Group Stroke. 1991;22:1155–63. doi: 10.1161/01.str.22.9.1155. [DOI] [PubMed] [Google Scholar]
- 14.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990:43–6. [PubMed] [Google Scholar]
- 15.Lederle FA, Johnson GR, Wilson SE, et al. Relationship of age, gender, race, and body size to infrarenal aortic diameter. The Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Investigators. J Vasc Surg. 1997;26:595–601. doi: 10.1016/s0741-5214(97)70057-0. [DOI] [PubMed] [Google Scholar]
- 16.Allison MA, Kwan K, DiTomasso D, Wright CM, Criqui MH. The epidemiology of abdominal aortic diameter. J Vasc Surg. 2008;48:121–7. doi: 10.1016/j.jvs.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 17.Pearce WH, Slaughter MS, LeMaire S, et al. Aortic diameter as a function of age, gender, and body surface area. Surgery. 1993;114:691–7. [PubMed] [Google Scholar]
- 18.Pedersen OM, Aslaksen A, Vik-Mo H. Ultrasound measurement of the luminal diameter of the abdominal aorta and iliac arteries in patients without vascular disease. J Vasc Surg. 1993;17:596–601. doi: 10.1067/mva.1993.39525. [DOI] [PubMed] [Google Scholar]
- 19.Lederle FA, Nelson DB, Joseph AM. Smokers' relative risk for aortic aneurysm compared with other smoking-related diseases: a systematic review. J Vasc Surg. 2003;38:329–34. doi: 10.1016/s0741-5214(03)00136-8. [DOI] [PubMed] [Google Scholar]
- 20.MacSweeney ST, Ellis M, Worrell PC, Greenhalgh RM, Powell JT. Smoking and growth rate of small abdominal aortic aneurysms. Lancet. 1994;344:651–2. doi: 10.1016/s0140-6736(94)92087-7. [DOI] [PubMed] [Google Scholar]
- 21.Golledge J, Muller J, Daugherty A, Norman P. Abdominal aortic aneurysm: pathogenesis and implications for management. Arterioscler Thromb Vasc Biol. 2006;26:2605–13. doi: 10.1161/01.ATV.0000245819.32762.cb. [DOI] [PubMed] [Google Scholar]
- 22.Lederle FA, Johnson GR, Wilson SE, et al. The aneurysm detection and management study screening program: validation cohort and final results. Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators. Arch Intern Med. 2000;160:1425–30. doi: 10.1001/archinte.160.10.1425. [DOI] [PubMed] [Google Scholar]
- 23.Kalyanasundaram A, Elmore JR, Manazer JR, et al. Simvastatin suppresses experimental aortic aneurysm expansion. J Vasc Surg. 2006;43:117–24. doi: 10.1016/j.jvs.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Steinmetz EF, Buckley C, Shames ML, et al. Treatment with simvastatin suppresses the development of experimental abdominal aortic aneurysms in normal and hypercholesterolemic mice. Ann Surg. 2005;241:92–101. doi: 10.1097/01.sla.0000150258.36236.e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guessous I, Periard D, Lorenzetti D, Cornuz J, Ghali WA. The efficacy of pharmacotherapy for decreasing the expansion rate of abdominal aortic aneurysms: a systematic review and meta-analysis. PLoS One. 2008;3:e1895. doi: 10.1371/journal.pone.0001895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salem MK, Rayt HS, Hussey G, et al. Should Asian men be included in abdominal aortic aneurysm screening programmes? Eur J Vasc Endovasc Surg. 2009;38:748–9. doi: 10.1016/j.ejvs.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 27.Spark JI, Baker JL, Vowden P, Wilkinson D. Epidemiology of abdominal aortic aneurysms in the Asian community. Br J Surg. 2001;88:382–4. doi: 10.1046/j.1365-2168.2001.01709.x. [DOI] [PubMed] [Google Scholar]
- 28.Morrissey NJ, Giacovelli J, Egorova N, et al. Disparities in the treatment and outcomes of vascular disease in Hispanic patients. J Vasc Surg. 2007;46:971–8. doi: 10.1016/j.jvs.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogel TR, Cantor JC, Dombrovskiy VY, Haser PB, Graham AM. AAA repair: sociodemographic disparities in management and outcomes. Vasc Endovascular Surg. 2008;42:555–60. doi: 10.1177/1538574408321786. [DOI] [PubMed] [Google Scholar]
- 30.Parry DJ, Al-Barjas HS, Chappell L, Rashid T, Ariens RA, Scott DJ. Haemostatic and fibrinolytic factors in men with a small abdominal aortic aneurysm. Br J Surg. 2009;96:870–7. doi: 10.1002/bjs.6632. [DOI] [PubMed] [Google Scholar]
- 31.Yamazumi K, Ojiro M, Okumura H, Aikou T. An activated state of blood coagulation and fibrinolysis in patients with abdominal aortic aneurysm. Am J Surg. 1998;175:297–301. doi: 10.1016/s0002-9610(98)00014-2. [DOI] [PubMed] [Google Scholar]
- 32.Nomura F, Ihara A, Yoshitatsu M, Tamura K, Katayama A, Ihara K. Relationship between coagulation cascade, cytokine, adhesion molecule and aortic aneurysm. Eur J Cardiothorac Surg. 2003;23:1034–8. doi: 10.1016/s1010-7940(03)00156-8. discussion 1038-9. [DOI] [PubMed] [Google Scholar]
- 33.Stenbaek J, Kalin B, Swedenborg J. Growth of thrombus may be a better predictor of rupture than diameter in patients with abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2000;20:466–9. doi: 10.1053/ejvs.2000.1217. [DOI] [PubMed] [Google Scholar]
- 34.Gomes MN, Hakkal HG, Schellinger D. Ultrasonography and CT scanning: a comparative study of abdominal aortic aneurysms. Comput Tomogr. 1978;2:99–109. doi: 10.1016/0363-8235(78)90007-8. [DOI] [PubMed] [Google Scholar]
- 35.Van Kuijk JP, Flu WJ, Dunckelgrun M, Bax JJ, Poldermans D. Coronary artery disease in patients with abdominal aortic aneurysm: a review article. J Cardiovasc Surg (Torino) 2009;50:93–107. [PubMed] [Google Scholar]
- 36.Melton LJ, 3rd, Bickerstaff LK, Hollier LH, et al. Changing incidence of abdominal aortic aneurysms: a population-based study. Am J Epidemiol. 1984;120:379–86. doi: 10.1093/oxfordjournals.aje.a113902. [DOI] [PubMed] [Google Scholar]
- 37.Best VA, Price JF, Fowkes FG. Persistent increase in the incidence of abdominal aortic aneurysm in Scotland, 1981-2000. Br J Surg. 2003;90:1510–5. doi: 10.1002/bjs.4342. [DOI] [PubMed] [Google Scholar]
- 38.Alcorn HG, Wolfson SK, Jr, Sutton-Tyrrell K, Kuller LH, O'Leary D. Risk factors for abdominal aortic aneurysms in older adults enrolled in The Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1996;16:963–70. doi: 10.1161/01.atv.16.8.963. [DOI] [PubMed] [Google Scholar]


