Abstract
Two experiments examined the effect of 5 days of passive exposure to ethanol (or water) on later self-infusion of ethanol or water via surgically implanted intragastric catheters in mouse genotypes previously shown to drink high (C57BL/6J, HAP2) or low (DBA/2J, LAP2) amounts of ethanol in home-cage continuous access two-bottle choice procedures. Intragastric ethanol self-infusion was affected by both genotype and a history of passive ethanol exposure, with greater intakes in the high drinking genotypes and in groups that received passive exposure to ethanol. Passive ethanol exposure also increased preference for the flavor that signaled ethanol infusion (S+), eliminating genetic differences in this measure. The increases in ethanol intake and S+ preference induced by ethanol exposure might have been mediated jointly by development of tolerance to aversive post-absorptive ethanol effects and negative reinforcement due to alleviation of withdrawal. Bout analyses indicated that ethanol exposure increased ethanol self-infusion by increasing the total number of daily bouts rather than by increasing bout size. These analyses also showed that DBA/2J mice infused larger ethanol bouts and a greater percentage of their total intakes in large bouts than C57BL/6J mice. Overall, these studies suggest that the intragastric self-infusion procedure is a potentially useful new tool for studying genetic and environmental influences on excessive ethanol intake and preference in mice.
Keywords: ethanol, self-administration, tolerance, dependence, C57BL/6J, DBA/2J, inbred mice, HAP2, LAP2, selectively bred mice
Introduction
The contribution of genotype to individual differences in oral ethanol intake and preference in rodents is well established (Cunningham & Phillips, 2003) and significant progress has been made toward identification of genes that influence these phenotypes (e.g., Crabbe et al., 2006; Mulligan et al., 2006). However, progress toward understanding the genetic and neurobiological mechanisms underlying excessive ethanol intake has been severely hampered because most rodent strains avoid ethanol when concurrently offered water in their home cage (e.g., Belknap et al., 1993; McClearn & Rodgers, 1959; Yoneyama et al., 2008). A notable exception is the inbred C57BL/6 (B6) mouse strain, which shows a strong preference for 10% ethanol over water and consumes relatively large amounts of ethanol per day (> 10 g/kg; op. cit.). Consequently, the B6 strain is disproportionately represented in ethanol research, participating in more than half of all studies involving inbred mice over the last two decades1. B6 mice stand in stark contrast to DBA/2 (D2) mice, prototypical teetotalers that drink very little ethanol when given a choice (Crabbe, 2002).
Selective breeding has also been used successfully to create lines of rats and mice that differ substantially in ethanol intake measured in two-bottle home cage drinking procedures (Crabbe et al., 2010). For example, High Alcohol Preferring (HAP) and Low Alcohol Preferring (LAP) mouse lines were selected from a genetically heterogeneous population (HS/Ibg) on the basis of average ethanol intake during a 4-week continuous access two-bottle choice drinking procedure (Grahame et al., 1999). After many generations of selection, non-deprived HAP mice drink substantial amounts of ethanol (~20 g/kg/day) whereas LAP mice consume very little (~1 g/kg/day; Oberlin et al., 2010).
While such differences in oral ethanol intake and preference are sometimes assumed to reflect genetic differences in sensitivity to rewarding post-absorptive pharmacological effects of ethanol, several other possibilities exist, including differences in sensitivity to aversive orosensory effects such as a bad taste or burning sensation (Belknap et al., 1977, 1978) or to aversive post-absorptive effects (Cunningham et al., 2009). In an attempt to better understand the determinants of high ethanol intake in mice, the present studies used a procedure in which B6 and D2 mice (Experiment 1) or HAP2 and LAP2 mice (Experiment 2) were able to self-administer ethanol (or water) directly into the stomach via a surgically implanted catheter, thus minimizing aversive orosensory effects (Deutsch & Cannis, 1980; Fidler et al., 2006). Further, because ethanol intake in rats and mice is often enhanced by chronic ethanol exposure (e.g., Becker & Lopez, 2004; Camarini & Hodge, 2004; Deutsch & Cannis, 1980; Fidler et al., 2006, 2009; Roberts et al., 1996), we also examined the effect of passive exposure to ethanol (or water) on later ethanol self-infusion. In addition to inducing dependence, this manipulation was expected to reduce the potential impact of post-absorptive aversive effects by inducing tolerance. Although similar procedures have been used previously in rats (Fidler et al., 2006, 2009), these studies are the first to examine the intragastric (IG) ethanol self-infusion procedure in mice.
Materials and Methods
Subjects
Adult male mice ranging between 8–17 weeks old were used. DBA/2J (D2) mice were shipped from the Jackson Laboratory (Bar Harbor, ME). Most of the C57BL/6J (B6) mice were also shipped from the Jackson Laboratory, but six B6 mice (three per group) were bred locally from B6 parents that were within three generations of breeders obtained from the Jackson Laboratory.
HAP2 and LAP2 breeding pairs (n = 10/line) were shipped from the Alcohol Research Center at Indiana University-Purdue University at Indianapolis. These animals were from the 32nd selected generation of the second replicate of mice selectively bred for high (HAP2) or low (LAP2) alcohol intake/preference. Ethanol naïve offspring from consecutive matings of these breeder pairs served as subjects (one to four litters from each family).
After weaning or shipment from the Jackson Laboratory, mice were group housed with same sex mice in shoebox cages in a Thoren rack (2–4 mice/cage). The colony was maintained at 20–24°C on a normal 12 hr light/dark cycle (lights on at 0700). Mice had free access to Rodent Diet 5001 and water except during the night before surgery when food was removed from the cages. Wet food was provided as needed during the first few days of recovery from surgery. After surgery, mice were housed individually in shoebox cages until the experiment began. During experiments, mice were housed in the experimental chambers (see below), except for approximately 1 hr per day during chamber maintenance.
Due to limitations on equipment, each experiment was conducted using several consecutive cohorts of B6 and D2 mice (Experiment 1) or HAP2 and LAP2 mice (Experiment 2). Within each cohort, similar numbers of mice were assigned to each of the two treatment groups. Approximately equal numbers of siblings from locally bred mice were assigned to each group. All procedures were conducted in accordance with The National Institute of Health (NIH) “Principles of Laboratory Animal Care.” The Oregon Health & Science University IACUC approved the protocol.
Apparatus
Experimental chambers made from clear acrylic and aluminum (20 × 20 × 22.5 cm high) were enclosed in individual laminated sound-attenuating enclosures (61 × 40.6 × 55.9 cm high) equipped with ventilating fans. Each chamber was fitted with two retractable sipper tubes (ENV-252M, Med Associates Inc., St. Albans VT) mounted on an aluminum end panel 9.5 cm apart and 3.5 cm above the floor. The sipper tubes were connected to lickometers (ENV-250B, Med Associates Inc.) interfaced to a computer that stored lick and infusion totals automatically every 5 min using LabVIEW™ software (National Instruments, Austin TX). The IG catheter back mount was attached to a connector assembly that consisted of a length of polyethylene tubing with a threaded connector encased in a metal spring covering (C313CS, Plastics One Inc., Roanoke VA). The connector assembly was connected to a 22 ga. fluid swivel (375/22, Instech Solomon, Plymouth Meeting PA) mounted on a counter-balanced lever arm (SMCLA, Instech Solomon). A short piece of Tygon tubing (U-95609-18, Cole-Parmer Instrument Co., Vernon Hills IL) connected the swivel to a Y-connector (STCY-22-10, Small Parts, Miami Lakes FL). Two fluid lines (Tygon tubing) attached the Y-connector to 12 ml syringes with blunt 22 ga. needles mounted on two syringe pumps (Model A or Model R-E Razel Scientific Instruments Inc., St. Albans VT) fitted with either 0.083 RPM (0.031 ml/min) or 0.33 RPM (0.129 ml/min) motors, depending on the experimental phase (see below).
Procedure
The experimental phases and treatments are summarized in Table 1.
Table 1.
Sequence of experimental treatments
| Phase (Treatment) | Duration (days) |
|---|---|
| Surgery | 1 |
| Recovery | 6–10 |
| Habituation | 3 |
| Passive Infusions (EtOH or water) | 5 |
| No-Choice Self-Infusion (S+ → EtOH) | 2 |
| Choice Self-Infusion (S+ → EtOH; S− → water) | 4 |
Surgery
The catheter was constructed from Silastic™ silicone tubing, (0.51 mm i.d. × 0.94 mm o.d.; Dow Corning Corp., Midland MI). A knob was created on one end by slipping a short piece of larger silicone tubing (1.02 mm i.d. × 2.16 mm o.d.) over one end and fixing it in place with adhesive (Medical Adhesive A, Dow Corning Corp.). A piece of knitted polypropylene mesh (Bard Mesh, Davol Inc., Cranston RI) was also attached to the catheter near the knob end. The catheters and back mounts (313-000BM-10/SPC, Plastics One Inc.) were sterilized prior to surgery by immersion in a glutaraldehyde solution (Cidex Plus 28-day solution, Johnson & Johnson, Langhorne PA) overnight and then rinsed inside and out with sterile water.
The surgical procedure was similar to that described elsewhere (Cunningham et al., 2002a). Each mouse was fully anesthethized with isoflurane gas (5% loading dose, 1.5–3% maintenance) and placed on the operating table on top of several layers of paper towel on an isothermal pad (Braintree Scientific, Braintree MA). A dab of puralube was put on each eye and the mouse was injected with 1.0 ml s.c. saline (divided among 4 injection sites) prior to being shaved. The stomach was externalized through an incision on the animal’s left side caudal to the rib cage. The knob end of the catheter was inserted through a puncture in the stomach wall and secured to the stomach by a purse-string suture and two stitches through the polypropylene mesh. A small amount of sterile water was infused into the catheter to ensure that the catheter was patent and that there was no leak from the stomach. The stomach was returned to the cavity and the incision through the muscle and peritoneum was sutured. The catheter was threaded subcutaneously to a small incision on the back just posterior to the scapulae. The back mount was inserted through the same incision and made to emerge through a hole anterior to the incision. Once in place, the back mount was flushed with sterile water. The catheter was trimmed, attached to the back mount and secured with a single suture through the mesh on the back mount. The skin incisions were sutured, the back mount capped (303DCFT/1, Plastics One Inc.) and anesthesia was terminated. Mice were allowed 6–10 days to recover before the start of the experiment. During this time, mice were infused once per day with at least 0.2 ml of sterile water.
Habituation Phase
At the beginning of habituation, mice were weighed, manually infused with 0.2 ml of sterile water, and placed into the chambers with free access to food and two bottles of 0.2 % (w/v) saccharin (Sigma-Aldrich, St. Louis MO) in tap water. Mice were attached to the fluid tether, but no infusions were given in the chambers during this phase. The right and left bottles were available during alternate 30-min periods. This bottle alternation was intended to ensure that mice encountered both bottles and to reduce the formation of side preferences. The position of the first bottle available alternated over the 3 days of habituation in a counterbalanced manner across mice. Sipper tubes were fully inserted into the chambers on the first day to increase the likelihood of drinking. However, the tubes were gradually retracted over days until they were flush with the chamber wall in order to increase the reliability of lickometer counts and to avoid inadvertent contacts. Mice received manual infusions of sterile water via the IG catheter when they were removed from the chambers for daily maintenance.
Passive Infusion Phase
Mice from each genotype were randomly assigned to control (water) and experimental (EtOH) groups. The groups differed only in whether they were infused with sterile water (control groups) or with 10% v/v ethanol in sterile water (EtOH groups) during this 5-day phase. The mice had access to food and two bottles of water, which were both available continuously throughout the passive phase. Each mouse was weighed, infused with at least 0.2 ml of sterile water, put into the chamber and attached to a tether pre-loaded with the appropriate solution. On the first day, mice received three passive infusions of 10% (v/v) ethanol (3 g/kg/infusion) in sterile water (EtOH groups) or an equivalent volume of sterile water (control groups). Infusions were delivered at a rate of 0.031 ml/min with infusions starting at 340-min (5.67 h) intervals. These infusions occurred approximately at the onset of the dark cycle (1840–2045), in the middle of the dark cycle (0020–0205) and near the end of the dark cycle (0600–0745). The duration of the infusion (i.e., total infusion volume) was varied for each animal to individually control ethanol dose based on body weight. Ethanol dose (or water volume) increased by 0.5 g/kg/infusion on each subsequent day. The inter-infusion interval (5.67 h) was selected on the basis of our previous studies, which demonstrated the ability of this dosing schedule to increase later IG self-infusion in rats (Fidler et al., 2009). Based on the estimated rate of ethanol metabolism in mice (Marshall & Owens, 1955), the first or second dose of the day might not have been completely eliminated before the next dose was administered. However, the long ethanol-free recovery period after the third daily infusion (~12 h) should have allowed complete elimination of ethanol before the first infusion on the next day. Most mice received three infusions per day during this phase. However, one LAP2 mouse failed to lick on the 4th day of passive ethanol exposure and the first infusion was eliminated on the 5th day to allow more recovery time.
The average blood ethanol concentrations (BECs) in tail blood samples taken from separate groups of B6 and D2 mice 5 min after the last passive ethanol infusion were 3.1 and 3.3 mg/ml, respectively (Fidler et al., unpublished). Limited availability of mice precluded a similar assessment of BECs in HAP-2 and LAP-2 mice. Previous research has shown no line difference in peak BECs after repeated IP ethanol injections in HAP1/LAP1 mice (Chester et al., 2001) or in the initial BECs induced by a high dose (4 g/kg) injection in HAP2/LAP2 mice (Chester & Barrenha, 2007), although the latter study also reported slower elimination of ethanol in LAP2 mice during acute withdrawal. Thus, interpretation of our findings is limited by the possibility of genetic differences in BEC despite exposure to an identical schedule of ethanol infusions.
Intoxication was visually assessed 3 h (Exp. 1) or 2 h (Exp. 2) after the start of the third infusion each day by placing each mouse in the center of a plastic arena (28 × 28 × 13 cm) and observing its behavior for 2 min. Mice were given a composite intoxication rating which was the sum of four separate scales: leg splay (0–5), wobbling (0–4), nose down (0–1) and belly down (0–1), with higher numbers indicating greater impairment. This procedure was adapted from one previously described by Metten et al. (2004). At the end of the daily session, approximately 10 h (Exp. 1) or 7.5 h (Exp. 2) after the start of the last infusion, withdrawal was assessed on a scale of 0–7 (0 = no convulsion) using handling induced convulsions (HICs; see Metten & Crabbe, 2005). The time intervals for these assessments were changed for Experiment 2 because the ratings in Experiment 1 yielded little evidence of either intoxication or withdrawal. When mice were removed from the chambers to assess HICs, chambers were cleaned and food, drinking bottles and pump reservoirs were replenished. Mice were also weighed and infused with at least 0.2 ml of sterile water before they were returned to the apparatus.
No-Choice Self-Infusion Phase
All mice from the EtOH and control groups were treated identically during this phase. After withdrawal assessment and chamber cleaning on the final passive infusion day, mice were returned to the apparatus with only one drinking tube available (S+), which contained 0.05% (w/v) grape or cherry Kool-Aid (Kraft Foods, Rye Brook, NY) and 0.2% (w/v) saccharin in tap water. The S+ tube was in the location (right or left) that had been preferred by each mouse during the habituation and passive phases (as determined by licks and intake). Infusions of 20% (v/v) ethanol in sterile water were contingent upon drinking the S+ solution. Similar to previous IG studies in rats (Deutsch & Cannis, 1980; Fidler et al., 2006, 2009), ethanol concentration during self-infusion was increased to 20% to offset dilution in the stomach by the orally consumed S+ fluid. Every 10th lick was followed by a 4-sec infusion (0.129 ml/min) of ethanol up to a maximum limit of 1.5 g/kg/30 min. Each infusion was approximately 0.05–0.07 g/kg for mice weighing between 20–30 g. S+ continued to be available after the maximum number of infusions had been delivered, but no further infusions were made until cumulative ethanol intake during the most recent 30 min period fell below the dose limit. The 2 no-choice days were included to ensure that all of mice encountered the contingency between S+ and ethanol.
Choice Self-Infusion Phase
All mice from the EtOH and control groups were treated identically during the choice phase. This 4-day phase was identical to the no-choice phase except that a second drinking tube containing the other Kool-Aid flavor (S−) was available. Licks on the S− tube were paired with infusions of sterile water using the same infusion rate and limits as the S+ tube. Flavor assignment was counterbalanced within each group. S+ remained in the same position it had occupied during the no-choice phase.
Blood-Ethanol Concentrations
Tail blood samples (20 μl) were collected during only one session of the choice self-infusion phase within a 14 h period that overlapped the dark portion of the light/dark cycle. Rather than sample all mice at an arbitrary time point, we adopted a strategy that was intended to identify peak BECs after periods of high drinking. More specifically, blood samples were taken 5 min after a mouse reached or closely approached the dose limit (1.5 g/kg/30 min). Blood samples were not obtained from mice that failed to approach the dose limit during the 14-h sample window. In Experiment 1, most samples were taken on the 4th choice day, but a few samples were obtained during a later choice session. Since choice self-infusion continued past the 4th day for only a few cohorts, behavioral data after the 4th day are not reported here. Because blood sampling on the 4th choice day potentially interfered with ethanol self-infusion, we collected all samples for Experiment 2 on a later choice self-infusion day for which no behavioral data are reported. Each blood sample was added to 50 μl of chilled ZnSO4 and stored on ice. Next, 50 μl of 0.3 M Ba(OH)2 and 300 μl of distilled water were added to each sample. The samples were vortexed and then centrifuged at 12,000 rpm for 5 min. The supernatant was removed and analyzed by gas chromatography (Rustay & Crabbe, 2004).
Dependent Variables
The primary dependent variable was mean ethanol intake (g/kg/day) during each experimental phase. We also examined the pattern of ethanol intake by calculating the mean number and mean size of ethanol bouts during the no-choice and choice phases. Data were collected in 5-min time bins and pooled across all days within each phase. A bout was defined as the ethanol intake in consecutive 5-min periods without a break greater than 5-min.
Results
All data were excluded when mice were removed due to poor health or when there were catheter problems, equipment failures or experimenter errors that affected access to ethanol. In a few cases, however, these problems did not occur until the choice self-infusion phase and data from earlier phases were included in analyses. In Experiment 1, the number of mice completing all phases of the experiment in each group were as follows: B6 experimental = 16; B6 control = 14; D2 experimental = 18; D2 control = 16. In Experiment 2, the group sizes were: HAP2 experimental = 21; HAP2 control = 20; LAP2 experimental = 20; LAP2 control = 18. Data from each experiment were analyzed separately. However, because the experimental designs and procedures were similar, the results from both experiments are presented in parallel for each phase.
Separate two-way (Group x Genotype) analyses of variance (ANOVAs) were applied to the data from each phase, reflecting our a priori interest in understanding treatment and genotype effects under the unique experimental conditions within each phase. Repeated measures ANOVAs that compared the no-choice and choice phases (Group x Genotype x Phase) were also conducted to examine the impact of introducing a second drinking tube (S−) on ethanol intake, bout number and bout size. These secondary analyses focused primarily on interpreting significant interactions with phase. The alpha level for all analyses was set at 0.05. P-values for post-hoc comparisons between genotypes or groups were Bonferroni-corrected for multiple comparisons.
Ethanol Intake
Statistical analyses of ethanol intakes yielded no significant differences between locally bred B6 mice and B6 mice obtained from the Jackson Laboratory. Moreover, exclusion of the locally bred mice from our analyses did not alter any of our conclusions. To simplify presentation, source of the B6 mice has been ignored in all analyses reported below.
Passive Phase
As planned, the passively infused ethanol dose increased across days in both experiments (data not shown). In Experiment 1, the mean cumulative ethanol doses were 60.3 ± 0.3 and 60.4 ± 0.2 g/kg for B6 and D2 mice, respectively. In Experiment 2, the mean cumulative ethanol doses were 60.3 ± 0.1 and 59.9 ± 0.4 g/kg for HAP2 and LAP2 mice, respectively. There was no significant difference between genotypes in total ethanol exposure in either experiment.
No-Choice Phase
Experiment 1
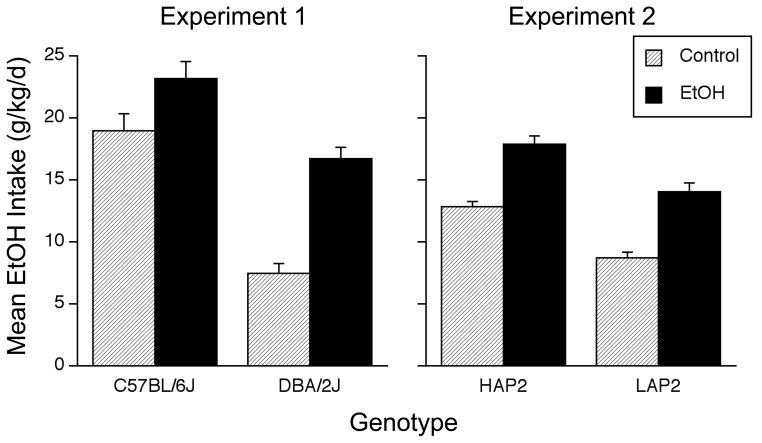
The left-hand panel of Figure 1 depicts mean daily ethanol intake (g/kg/d) for all groups during the no-choice phase. As can be seen, passive ethanol exposure increased self-infusion in both strains. B6 mice generally infused more ethanol than D2 mice, but passive ethanol exposure produced a greater increase (relative to control mice) in D2 mice (difference between EtOH and control groups = +9.3 g/kg/d) than in B6 mice (difference between EtOH and control groups = +4.2 g/kg/d). A Group x Genotype ANOVA revealed significant main effects of Group [F(1,62) = 36.8, p < .0001] and Genotype [F(1,62) = 64.8, p < .0001], and a significant interaction [F(1,62) = 5.1, p < .03]. Follow-up comparisons confirmed that the EtOH group within each strain infused more ethanol than the Control group; B6 mice within each treatment condition also infused more ethanol than D2 mice [all Bonferroni-corrected p’s < .05].
Figure 1.
Mean (+SEM) daily ethanol intakes (g/kg/day) during the no-choice phase when only the S+ flavor (paired with infusion of ethanol) was available. The left panel shows intakes by C57BL/6J (B6) mice and DBA/2J (D2) mice (Experiment 1) and the right panel shows intakes by HAP2 and LAP2 mice (Experiment 2). Mice had previously received passive exposure to water (shaded bars) or EtOH (black bars). The Experiment 1 ANOVA yielded significant effects of Group, Genotype and their interaction. Post-hoc tests showed that the EtOH group infused more ethanol than the Control group for both strains and that B6 mice infused more ethanol than D2 mice in both groups. The Experiment 2 ANOVA yielded significant effects of Group and Genotype, but no interaction. The number of mice per group was 15–21 (see text for details).
Experiment 2
As in the case of the inbred strains, passive ethanol exposure increased self-infusion in both of the selectively bred lines (Figure 1, right panel). Moreover, as predicted by the selection phenotype, HAP2 mice generally infused more ethanol than LAP2 mice. These observations were supported by a Group x Genotype ANOVA that yielded significant main effects of Group [F(1,77) = 81.3, p < .0001] and Genotype [F(1,77) = 47.1, p < .0001], but no interaction. The line difference suggests there is a positive genetic correlation between home cage two-bottle ethanol drinking and no-choice ethanol intake in the IG self-infusion model, implying an overlap in the genes that influence these phenotypes. However, the genetic basis for this relationship must be considered provisional until it is replicated in an independent set of selectively bred lines (Crabbe et al., 1990).
Choice Phase
Experiment 1
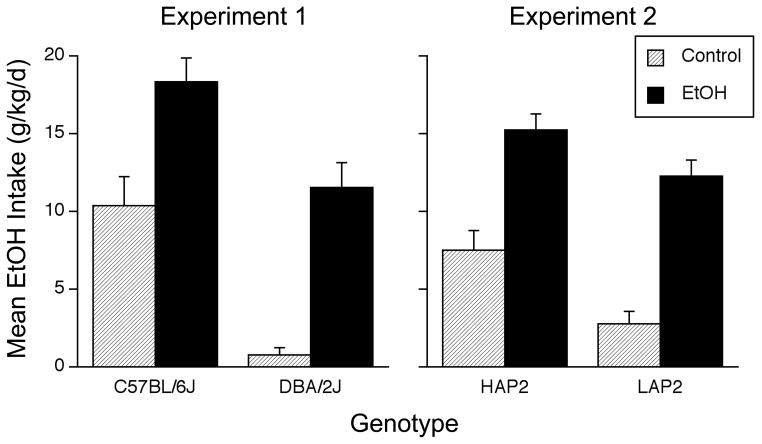
Mean daily ethanol intakes for all groups during the choice phase are shown in Figure 2 (left panel). Although ethanol intake was generally reduced by the availability of a second drinking solution (compared to the no-choice phase), the EtOH groups continued to infuse more ethanol than the Control groups in both strains. B6 mice also continued to infuse more ethanol than D2 mice. A Group x Genotype ANOVA supported these observations, yielding significant main effects of Group [F(1,60) = 40.9, p < .0001] and Genotype [F(1,60) = 31.5, p < .0001], but no interaction. A three-way (Group x Genotype x Phase) repeated measures ANOVA that compared intakes during the choice and no-choice phases showed a significant main effect of phase [F(1,60) = 128.0, p < .0001], reflecting the general reduction in ethanol intake during the choice phase. This analysis also revealed a significant Group x Phase interaction [F(1,60) = 4.9, p < .04], reflecting a larger decrease in Control groups (−7.4 g/kg/d) compared to EtOH groups (−5.0 g/kg/d). However, these analyses yielded no significant interactions with genotype, indicating that the decreases in intake were similar in each strain. Overall, these data suggest that introduction of the S− tube had a greater detrimental effect on ethanol intake in control group mice than in EtOH group mice.
Figure 2.
Mean (+SEM) daily ethanol intakes (g/kg/day) during the choice phase in which mice could choose between drinking the S+ flavor (paired with infusion of ethanol) or the S− flavor (paired with infusion of water). The left panel shows intakes by C57BL/6J mice and DBA/2J mice (Experiment 1) and the right panel shows intakes by HAP2 and LAP2 mice (Experiment 2). Mice had previously received passive exposure to water (shaded bars) or EtOH (black bars). ANOVAs for each experiment revealed significant effects of Group and Genotype, but no interaction. The number of mice per group was 14–21 (see text for details).
Experiment 2
Results during the choice phase of Experiment 2 were similar to those in Experiment 1 (Figure 2, right panel). That is, the EtOH groups from both genotypes continued to infuse more ethanol than the Control groups. Also, HAP2 mice continued to infuse more ethanol than LAP2 mice. A Group x Genotype ANOVA revealed only significant main effects of Group [F(1,75) = 61.6, p < .0001] and Genotype [F(1,75) = 12.3, p < .002]. The line difference suggests a provisional genetic correlation between home cage ethanol drinking and choice phase IG self-infusion. As in Experiment 1, ethanol intake during the choice phase was generally reduced by the introduction of a second drinking tube. This conclusion was supported by a three-way (Group x Genotype x Phase) ANOVA that compared intakes during the choice and no-choice phases [main effect of phase: F(1,75) = 68.7, p < .0001]. This analysis also indicated a significant Group x Phase interaction [F(1,75) = 13.0, p < .002], reflecting a larger decrease in Control groups (−5.6 g/kg/d) compared to EtOH groups (−2.2 g/kg/d). However, these analyses showed no interactions with genotype, indicating that the size of the decrease did not differ across lines.
Ethanol Bouts
Number of bouts
Experiment 1
Bout information for each group during the self-infusion phases is shown in Tables 2 and 3. In general, the analyses for mean number of ethanol bouts/day (Table 2, top panel) paralleled those for mean ethanol intake, suggesting that passive ethanol exposure enhanced later self-infusion of ethanol by increasing the number of daily bouts. Also, the increases in the EtOH group compared to the control group were greater for D2 mice than for B6 mice. These observations were generally supported by separate two-way (Group x Genotype) ANOVAs that yielded significant main effects of Group [no-choice: F(1,62) = 32.1; choice: F(1,60) = 28.0; p’s < .0001] and Genotype [no-choice: F(1,62) = 53.9; choice: F(1,60) = 68.3; p’s < .0001] during both phases. There was also a significant interaction during the no-choice phase, reflecting the greater impact of passive exposure on D2 mice [F(1,62) = 5.2, p < .03]; the interaction was not significant during the choice phase (p > .15). Post-hoc two-group comparisons during the no-choice phase confirmed that the EtOH group had significantly more bouts than the Control group in both strains and that B6 mice had more bouts than D2 mice in both treatment groups (all Bonferroni-corrected p’s < .001).
Table 2.
Mean (± SEM) number of ethanol bouts per day
| Experiment 1ef | No-Choice Phaseab | Choice Phasea | ||
| Group | n | bouts/day | n | bouts/day |
| B6 Control | 15 | 32.33 ± 2.34c | 14 | 28.54 ± 4.35 |
| B6 EtOH | 16 | 38.50 ± 1.73cd | 16 | 39.40 ± 2.53 |
| D2 Control | 17 | 14.77 ± 1.28c | 16 | 1.48 ± 0.97 |
| D2 EtOH | 18 | 29.28 ± 1.88cd | 18 | 20.22 ± 2.71 |
| Experiment 2e | No-Choice Phasea | Choice Phasea | ||
| Group | n | bouts/day | n | bouts/day |
| HAP2 Control | 20 | 25.75 ± 1.27 | 20 | 17.28 ± 2.51 |
| HAP2 EtOH | 21 | 32.10 ± 1.26 | 21 | 35.63 ± 1.73 |
| LAP2 Control | 20 | 18.50 ± 1.15 | 18 | 6.08 ± 1.53 |
| LAP2 EtOH | 20 | 29.68 ± 1.43 | 20 | 30.70 ± 2.03 |
Main effects of Group and Genotype (p’s < .0001)
Group × Genotype interaction (p < .03)
Differs from same group for other genotype (p’s < .001)
Differs from control group for same genotype (p’s < .001)
Group × Phase interaction (p < .05); see text
Genotype × Phase interaction (p < .0001); see text
Table 3.
Mean (± SEM) ethanol bout size (excludes mice with no ethanol bouts)
| Experiment 1b | No-Choice Phase | Choice Phasea | ||
| Group | n | g/kg/bout | n | g/kg/bout |
| B6 Control | 15 | 0.62 ± 0.05 | 13 | 0.36 ± 0.04 |
| B6 EtOH | 16 | 0.61 ± 0.03 | 16 | 0.46 ± 0.02 |
| D2 Control | 17 | 0.51 ± 0.04 | 6 | 0.57 ± 0.08 |
| D2 EtOH | 18 | 0.60 ± 0.04 | 17 | 0.57 ± 0.05 |
| Experiment 2d | No-Choice Phasec | Choice Phase | ||
| Group | n | g/kg/bout | n | g/kg/bout |
| HAP2 Control | 20 | 0.51 ± 0.02 | 20 | 0.41 ± 0.04 |
| HAP2 EtOH | 21 | 0.57 ± 0.02 | 21 | 0.43 ± 0.03 |
| LAP2 Control | 20 | 0.49 ± 0.03 | 18 | 0.52 ± 0.07 |
| LAP2 EtOH | 20 | 0.48 ± 0.02 | 20 | 0.40 ± 0.02 |
Main effect of Genotype (p < .003)
Genotype × Phase interaction (p < .002); see text
Main effect of Genotype (p < .02)
Genotype × Phase interaction (p < .02); see text
As in the case of ethanol intake, a secondary analysis that included Phase as a factor yielded a significant Group x Phase interaction [F(1,60) = 4.3, p < .05], reflecting a larger decrease between phases in Control-group bouts (−9.1 bouts/d) compared to EtOH group bouts (−4.4 bouts/d). Follow-up tests showed that both groups had significantly fewer bouts during the choice phase than during the no-choice phase. The Genotype x Phase interaction was also significant [F(1,60) = 19.2, p < .0001]. Follow-up comparisons indicated a significant reduction in D2 bouts (−11.2 bouts/d), but no significant change in B6 bouts (−1.3 bouts/d). Thus, introduction of the S− tube had a greater impact on bout frequency in D2 and control-group mice.
Experiment 2
As in Experiment 1, passive ethanol exposure enhanced later self-infusion of ethanol by increasing the number of bouts/day in the selectively bred lines (Table 2, bottom panel). There was also a trend for a greater increase (compared to the control group) in LAP2 mice than in HAP2 mice. These conclusions were supported by separate two-way (Group x Genotype) ANOVAs that showed significant main effects of Group [no-choice: F(1,77) = 46.6, p < .0001; choice: F(1,75) = 114.7, p < .0001] and Genotype [no-choice: F(1,77) = 14.2, p < .0001; choice: F(1,75) = 16.1, p < .0001] during both phases. The interaction for the no-choice phase fell short of the criterion for significance [F(1,77) = 3.5, p = .06], but suggested a potentially greater impact of passive ethanol exposure in LAP2 mice. The interaction was not significant during the choice phase (p > .12).
The secondary analysis revealed a significant Group x Phase interaction [F(1,75) = 44.2, p < .05], reflecting a large decrease between phases in the number of Control-group bouts (−10.4 bouts/d) compared to a small increase in EtOH-group bouts (+2.3 bouts/d). Post-hoc tests showed that the decrease in control group bouts was significant, but the increase in EtOH group bouts was not. There were no significant interactions between phase and genotype. Thus, addition of the S− tube affected bout number only in the control groups.
Bout size
Experiment 1
In contrast to its effect on bout number, passive ethanol exposure did not affect mean bout size (g/kg/bout) during subsequent self-infusion of ethanol (Table 3, top panel). This conclusion was supported by the absence of a main effect of Group on bout size during either phase. Of greater interest, D2 mice had significantly larger bouts than B6 mice during the choice phase [Genotype Main effect: F(1,48) = 10.1, p < .003], although both strains showed bouts of similar size during the no-choice phase [p > .14]. The Group x Genotype interaction was not significant during either phase.
In contrast to the analyses of ethanol intake and bout number, the secondary analysis of bout size did not show a significant Group x Phase interaction (p > .15), indicating that EtOH and control groups had similar bout sizes during the no-choice and choice phases. However, there was significant Genotype x Phase interaction [F(1,60) = 11.6, p < .002] that was due to a larger decrease between phases in B6 bout size (−0.18 g/kg/bout) compared to D2 bout size (−0.03 g/kg/bout). Post-hoc tests showed that introduction of the S− tube significantly reduced bout size only in B6 mice, not in D2 mice.
Experiment 2
Mean bout size was not affected by passive ethanol exposure in the selectively bred lines (Table 3, bottom panel) as reflected by a non-significant group effect during both phases [both F’s < 1.4]. However, HAP2 mice had larger bouts than LAP2 mice during the no-choice phase [Genotype Main effect: F(1,77) = 6.1, p < .02]. There was no line difference in bout size during the choice phase and the Group x Genotype interaction was not significant in either phase.
The secondary analysis showed a non-significant Group x Phase interaction, although there was a trend (p = .06) in the direction of a greater decrease in bout size across phases in the EtOH groups. As in Experiment 1, there was a significant Genotype x Phase interaction [F(1,75) = 6.6, p < .02], reflecting a significant decrease in bout size for the high intake genotype (HAP-2: −0.12 g/kg/bout), but not for the low intake genotype (LAP-2: −0.03 g/kg/bout).
Correlations between ethanol intakes and bout number/size
Pearson correlations calculated separately on the pooled data from each experiment were generally largest for the positive correlations between ethanol intake and bout number within each self-infusion phase (r’s ≥ 0.76, p < .0001). There were also significant but smaller positive correlations between intake and bout size during the no-choice phase in each study (r’s = 0.41–0.43, p < .0001), but not during the choice phase (r’s ≤ 0.24, p > .08). As one might expect, mean intake, bout number and bout size during the no-choice phase were each positively correlated with the same dependent variable during the choice phase (r’s ≥ 0.43, p < .0001). Finally, there were small negative correlations between bout number and size within each phase, but this relationship was significant only during the no-choice phase in Experiment 2 (r = −0.24, p < .05).
Percent of choice intake explained by different sized bouts
Although bout frequency and size are useful indices, these dependent variables offer an incomplete picture of ethanol intake patterns because they do not address the relative contributions by bouts that are more (or less) likely to induce pharmacological effects. Therefore, we also examined intake patterns using a dependent variable based on the proportion of total choice-phase ethanol intake that occurred in different sized bouts (Fidler et al., 2006, 2009). Because the 30-min self-infusion dose limit usually constrained the maximum bout size to 1.5 g/kg, we simply divided the range of possible bout sizes into thirds (small: < 0.5 g/kg; medium: 0.5–0.99 g/kg; large: ≥ 1.0 g/kg) and calculated the percentage of total choice phase intake that was attributable to bouts in each category (Table 4). Thus, this dependent variable offers a means of normalizing intake pattern information against total intake. In cases where groups or genotypes show a greater contribution by large bouts, one might infer a more important role for pharmacological effect in determining intake. Previously, rats passively exposed to IG ethanol later self-infused a larger proportion of their total ethanol intake in large bouts (compared to water control rats), whereas control rats consumed a larger proportion of intake in small bouts (Fidler et al., 2006).
Table 4.
Mean (± SEM) percentage of total choice phase ethanol intake that occurred in small, medium or large bouts (excludes mice with no ethanol bouts)
| Experiment 1a | ||||
| Group | n | Small (< 0.5 g/kg)b | Medium (0.5–0.99 g/kg) | Large (≥ 1.0 g/kg)b |
| B6 Control | 13 | 53.4 ± 7.2 | 37.4 ± 5.2 | 9.2 ± 3.3 |
| B6 EtOH | 16 | 35.2 ± 3.3 | 40.1 ± 2.2 | 24.7 ± 2.6 |
| D2 Control | 6 | 28.5 ± 14.9 | 37.3 ± 15.3 | 34.2 ± 11.8 |
| D2 EtOH | 17 | 27.7 ± 6.1 | 33.2 ± 3.5 | 39.1 ± 5.7 |
| Experiment 2c | ||||
| Group | n | Small (< 0.5 g/kg) | Medium (0.5–0.99 g/kg) | Large (≥1.0 g/kg) |
| HAP2 Control | 20 | 41.8 ± 5.2 | 30.3 ± 3.1 | 27.9 ± 4.8 |
| HAP2 EtOH | 21 | 41.4 ± 3.8 | 32.6 ± 2.5 | 26.0 ± 2.9 |
| LAP2 Control | 18 | 36.1 ± 7.2 | 27.0 ± 6.5 | 36.9 ± 7.9 |
| LAP2 EtOH | 20 | 44.3 ± 3.9 | 36.7 ± 2.1 | 19.0 ± 2.9 |
Genotype × Bout Size interaction (p < .005)
Main effect of Genotype (p < .03)
Main effect of Bout Size (p < .005)
Experiment 1
The proportions of total choice-phase ethanol intake that occurred in small, medium and large bouts are shown in Table 4 (top panel). A three-way ANOVA (Group x Genotype x Bout Size) yielded only one significant effect, the interaction between Genotype x Bout Size [F(2,96) = 5.9, p < .005]. Follow-up ANOVAs at each bout size showed that B6 mice infused a higher percentage of their total ethanol intake in small bouts than D2 mice [F(1,48) = 5.0, p < .03]. In contrast, D2 mice infused a higher percentage of their ethanol intake in large bouts than B6 mice [F(1,48) = 12.6, p < .001]. These analyses yielded no significant Group effects, although there was a trend for EtOH group mice to infuse a higher percentage of their choice phase intake in large bouts relative to control group mice [F(1,48) =3.4, .05 < p < .08].
Experiment 2
The percentages of total ethanol intake that occurred in small, medium or large bouts during the choice phase are shown in Table 4 (bottom panel). Three-way ANOVA (Group x Genotype x Bout Size) yielded only a significant main effect of bout size [F(2,150) = 6.0, p < .005], reflecting the smaller contribution of larger bouts to total intake. There were no significant effects involving group or genotype.
S+ Preference
Experiment 1
The daily percentages of total licks on the S+ tube were averaged across the four choice days for each mouse to determine S+ preferences for each group (Figure 3, left panel). Although B6 control mice showed higher preferences than D2 control mice, their overall S+ preference was still below 50%. Passive ethanol exposure increased preference in both strains and eliminated the strain difference. A two-way (Group x Genotype) ANOVA supported these observations, yielding significant main effects of Group [F(1,60) = 34.3, p < .0001] and Genotype [F(1,60) = 10.6, p < .002], as well as a significant interaction [F(1,60) = 7.2, p < .01]. Follow-up comparisons indicated that the D2 EtOH group had a significantly higher S+ preference than the D2 Control group [Bonferroni-corrected p < .001], but that the B6 EtOH and B6 Control groups did not differ. Moreover, although B6 control mice had significantly higher S+ preferences than D2 control mice [Bonferroni-corrected p < .001], passive ethanol exposure eliminated the strain difference. One group t-tests were used to determine whether the percent of S+ licking differed from 50%. These analyses indicated a significant S+ aversion in the D2 control group [t(15) = −21.6, p < .0001] and a significant S+ preference in the B6 EtOH group [t(15) = 2.7, p < .02]. The other groups showed neither preference nor aversion.
Figure 3.
Mean (+SEM) preference for the ethanol-paired flavor (S+) during the choice phase. Preference (expressed as a percentage of total licks) was calculated for each mouse by averaging preference ratios calculated separately for each choice day using the following formula: [(S+ Licks)/(S+ Licks) + (S− Licks)] * 100. The left panel shows intakes by C57BL/6J (B6) mice and DBA/2J (D2) mice (Experiment 1) and the right panel shows intakes by HAP2 and LAP2 mice (Experiment 2). Mice had previously received passive exposure to water (shaded bars) or EtOH (black bars). The Experiment 1 ANOVA yielded significant effects of Group, Genotype and their interaction. Post-hoc tests supported the following conclusions: B6 EtOH = B6 Control; D2 EtOH > D2 Control; B6 Control > D2 Control; B6 EtOH = D2 EtOH = 50%; B6 EtOH > 50%; D2 Control < 50%. The Experiment 2 ANOVA showed only a significant effect of Group. Post-hoc t-tests supported the following conclusions: HAP2 Control = 50%; LAP 2 Control < 50%; both EtOH groups > 50%. The number of mice per group was 14–21 (see text for details).
Experiment 2
In this study, S+ preference was influenced more by passive ethanol exposure than by genotype. As shown in Figure 3 (right panel), passive ethanol exposure increased S+ preference, but the line difference was small and occurred primarily in the control groups. Two-way (Group x Genotype) ANOVA confirmed these conclusions, showing a significant Group effect [F(1,75) = 58.9, p < .0001], but no effect of Genotype or interaction. One group t-tests indicated significant S+ preferences in both EtOH groups [HAP2: t(20) = 4.6, p < .0001; LAP2: t(19) = 5.411, p < .0001] and a significant S+ aversion in LAP2 control mice [t(17) = 4.5, p < .0001]. The HAP2 control mice showed neither preference nor aversion. The absence of a line difference in S+ preference suggests there is no genetic correlation between home cage two-bottle ethanol drinking and preference for the ethanol-paired flavor (S+) in the IG self-infusion model.
Intoxication
As noted earlier, little intoxication was seen 3 h after the third daily infusion each day during the passive phase in the first experiment. In a subsequent series of studies involving the B6 and D2 strains (to be reported separately), observations made at the 2 h time point confirmed that the same ethanol exposure regimen induced a similar level of intoxication in both strains. Data pooled across three independent experiments (n = 37–40 in each strain x group combination) showed mean daily intoxication ratings of 1.2 ± 0.2 and 1.4 ± 0.2 in B6 and D2 EtOH group mice, respectively. These ratings were statistically identical and higher than those recorded from B6 or D2 water control groups (mean ratings < 0.1). Thus, the failure to observe intoxication in the experiment reported here is most likely due to selection of an inappropriate time window for observation.
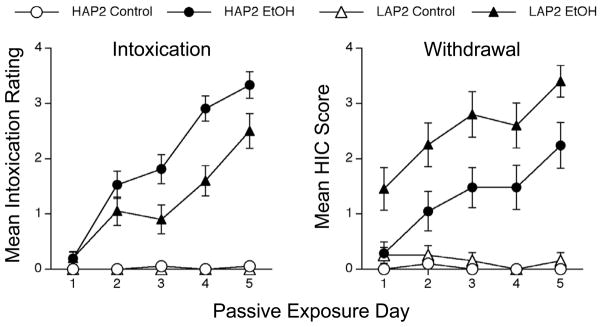
The mean daily intoxication ratings (taken at the 2 h time point) for each group in Experiment 2 are shown in Figure 4 (left panel). As can be seen, intoxication ratings increased in both lines as we increased ethanol dose over the 5 passive infusion days. Although both EtOH groups showed similar intoxication on the first day, HAP2 EtOH mice showed greater intoxication than LAP2 EtOH mice on subsequent days. As expected, the control groups in both lines showed no intoxication (and were excluded from statistical analysis). A two-way (Genotype x Day) repeated measures ANOVA yielded significant main effects of Genotype [F(1,39) = 8.3, p < .007] and Day [F(4,156) = 62.5, p < .0001], and a significant interaction [F(4,156) = 3.6, p < .01]. Separate group comparisons indicated that the line difference was significant on each of the last three passive infusion days (p’s < .05). Thus, these data suggest a provisional negative genetic correlation between ethanol drinking and sensitivity to this particular measure of intoxication. Although previous research has shown no differences between HAP1 and LAP1 mice in activity after exposure to low (activating) ethanol doses or high (depressant) ethanol doses in HAP1/LAP1 mice (Grahame et al., 2000), line differences in ataxia have not been directly examined in any of the HAP/LAP replicate lines.
Figure 4.
Mean (+SEM) intoxication ratings (left panel) and withdrawal HIC scores (right panel) for HAP2 (circles) and LAP2 (triangles) mice in Experiment 2. Mice had previously received passive exposure to water (open symbols) or EtOH (closed symbols). The ANOVA for intoxication ratings revealed significant effects of Genotype, Day and their interaction. Post-hoc tests indicated a Genotype difference between the EtOH groups on Days 3–5. The ANOVA for HIC scores yielded significant effects of Genotype and Day, but no interaction. The number of mice per group was 20–21.
Withdrawal
Since HIC ratings were generally near zero in Experiment 1, they are not reported here. However, in the later B6/D2 studies mentioned above (to be reported separately), exposure to the same ethanol regimen was effective in inducing significant signs of withdrawal at the 7.5-h time point in D2 mice (mean daily HIC score = 2.1 ± 0.2), but not in B6 mice (mean < 0.1). Mean HIC scores in control mice from both strains were less than 0.1. Thus, the failure to observe withdrawal in the experiment reported here is most likely due to selection of an inappropriate time window for observation. The B6-D2 strain difference in withdrawal sensitivity is quite consistent with previously reported data (e.g., Crabbe, 1998).
Figure 4 (right panel) depicts withdrawal data for Experiment 2. Like intoxication, HIC scores increased as ethanol dose was increased across days of passive exposure. Although HIC scores in the control groups from both lines were negligible (and were therefore excluded from analysis), the LAP2 EtOH group consistently showed higher HIC scores than the HAP2 EtOH group throughout the passive exposure phase. These observations were supported by a Genotype x Day ANOVA that revealed significant main effects of Genotype [F(1,39) = 15.1, p < .0001] and Day [F(4,156) = 9.3, p < .0001], but no interaction. Thus, these data (as well as the B6/D2 data) are generally consistent with the previously reported negative genetic correlation between strain means for ethanol drinking and sensitivity to ethanol withdrawal (Metten et al., 1998). These data offer one of the first demonstrations of this relationship in the HAP2/LAP2 selected lines.
To determine whether there was also a phenotypic relationship between withdrawal experienced during the passive phase and later self-infusion of ethanol, HIC scores for each HAP2 and LAP2 mouse were summed over the 5 days of passive exposure and correlated with mean intakes during later self-infusion. These analyses yielded significant negative correlations both for the no-choice (r = −0.55, n = 41, p < .001) and choice (r = −0.35, n = 41, p < .05) phases, reflecting lower ethanol intakes in mice that experienced greater ethanol withdrawal. In contrast, there was no phenotypic correlation between withdrawal and S+ preference in these mice (r = −0.04, n = 40). These data provide the first demonstration of a phenotypic relationship between withdrawal severity and IG ethanol intake in mice.
BECs
Since the range of ethanol intakes during the 35-min period before each sample was constrained by our strategy for taking samples (see Methods), BECs were correlated with intakes recorded during the 60 min before the sample for each mouse (Figure 5). Across both experiments combined, there was a significant positive correlation between BEC and 60-min ethanol intake (r = +0.49, n = 37, p < 0.002).
Figure 5.
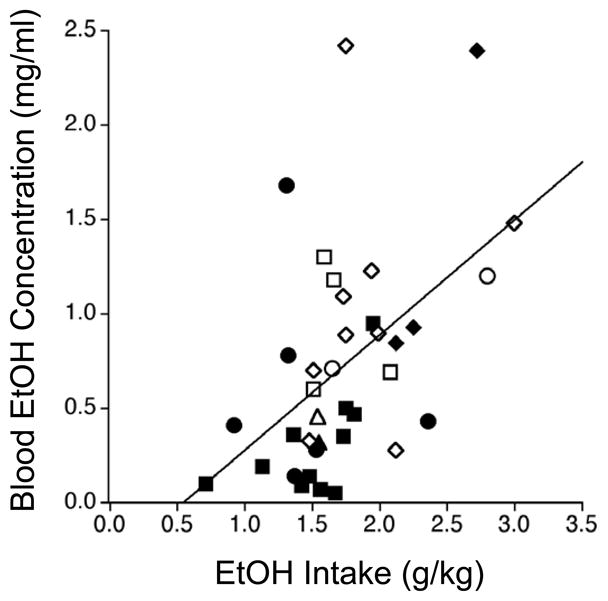
Scatter plot showing the relationship between BEC (mg/ml) and choice ethanol-infusion intake (g/kg) during the 60 min before each sample was taken from mice at or near the 30-min self-infusion dose limit (see Methods for additional details). Squares = B6; Circles = D2; Diamonds = HAP-2; Triangles = LAP-2. Open symbols depict mice assigned to the Control groups; closed symbols depict mice assigned to the EtOH groups. The curve shows the least fit linear regression (r = +0.49, n = 37, p < 0.002).
Discussion
These studies offer the first demonstrations of IG self-infusion of ethanol in mice and a more comprehensive examination of the combined effects of genotype and chronic ethanol exposure on self-administration than any published report. They show that IG ethanol self-infusion in mice is affected by both genotype and passive ethanol exposure. Genetic differences in IG ethanol intakes in control mice were generally consistent with those reported in drinking studies (B6 > D2, HAP2 > LAP2), although the difference between HAP2 and LAP2 intakes was much smaller than reported for oral intakes (Oberlin et al., 2010). Our studies also showed that ethanol intake and S+ preference were significantly increased by pre-exposure to ethanol in all genotypes, an effect that might be mediated by dependence, withdrawal, tolerance or some combination of these processes. Although ethanol pre-exposure did not eliminate genetic differences in IG intake, the difference was reduced during the no-choice phase in Experiment 1 (B6 vs. D2). Moreover, ethanol pre-exposure completely eliminated genetic differences in preference for the flavor that signaled delivery of IG ethanol infusions. Finally, the bout analyses underscore the importance of examining ethanol intake patterns when interpreting effects of genotype and prior ethanol exposure, revealing an unexpected finding of larger ethanol bouts in the prototypical teetotaler strain, D2.
B6 versus D2 mice
Consistent with the previously reported difference in oral ethanol intake (e.g., McClearn & Rodgers, 1959), B6 control mice self-infused more ethanol than D2 control mice during both self-infusion phases. B6 control mice were also less averse to the S+ during the choice phase than D2 control mice. The mean daily intakes for control groups (Fig. 2, left panel) were similar to intakes previously measured in these strains during a home cage drinking procedure (Belknap et al., 1993). The fact that D2 control intakes during the choice phase remained low in the IG procedure, despite removal of ethanol’s pre-absorptive effects, suggests that aversive post-absorptive ethanol effects probably contribute to avoidance of oral ethanol by D2 mice (Cunningham et al., 2009).
Passive ethanol exposure enhanced later ethanol intake in both EtOH groups (compared to the control groups) during both self-infusion phases, but the impact of passive exposure was greater in D2 than in B6 mice during the no-choice phase. Moreover, ethanol pre-exposure increased S+ preference and eliminated the strain difference in preference. The high ethanol intakes in D2 mice are especially remarkable given their long-standing reputation as teetotalers in drinking procedures. These findings are generally consistent with data showing that repeated intraperitoneal injections of ethanol enhanced oral ethanol intake and preference of B6 and D2 mice and eliminated the strain difference in 5% ethanol intake (Camarini & Hodge, 2004). The reduced difference between B6 and D2 mice in the IG procedure is also consistent with findings from an intravenous ethanol self-administration study that showed a diminishing strain difference as cumulative exposure to ethanol increased across training days (Grahame & Cunningham, 1997).
Bout analyses showed that the effect of passive exposure on later ethanol intake was mediated by an increase in the number of bouts per day rather than by an increase in bout size. Mirroring the genotype effects on total ethanol intake, the increase in number of bouts in EtOH group mice (compared to control mice) was larger in D2 than in B6 mice during no-choice self-infusion. These analyses also showed that, despite having a lower total ethanol intake, D2 mice had larger bouts than B6 mice during the choice phase. Moreover, whereas B6 mice infused a higher proportion of their total intake as small bouts compared to D2 mice, D2 mice infused a higher proportion of their total intake as large bouts. Overall, these data suggest that B6 mice are “sippers” whereas D2 mice are “gulpers.”
HAP2 versus LAP2 mice
Mice selectively bred for high (HAP2) and low (LAP2) ethanol intake differed in ethanol self-infusion during both phases, suggesting potential overlap in the genes that influence home-cage 2-bottle choice drinking and IG self-infusion. That conclusion is generally consistent with a study that showed differences in IG ethanol intake between rat lines selectively bred to prefer (P) or avoid (NP) ethanol (Waller et al., 1984). However, the magnitudes of the line difference in daily intake and preference for control groups during the IG choice phase were considerably smaller than those reported in drinking studies. For example, male HAP2 and LAP2 mice (Generation 33) drank about 20 and 1 g/kg/day, respectively, with corresponding preferences of 84% and 6% (Oberlin et al., 2010) as compared to intakes of 7.5 and 2.8 g/kg/day (Fig. 2) with S+ preferences of 37% and 21% (Fig. 3). These discrepancies suggest that selective breeding for ethanol drinking has created line differences in sensitivity to pre-absorptive effects of ethanol such as its taste in addition to producing a difference in sensitivity to ethanol’s post-absorptive effects. When these pre-absorptive effects are removed by the IG self-infusion procedure, magnitude of the line difference is substantially reduced.
As for inbred strains, passive ethanol exposure increased daily ethanol intake and S+ preference in both lines. In fact, this manipulation eliminated the line difference and established a true preference (i.e., > 50%) for S+. Also, similar to the inbred strains, ethanol exposure increased intake by increasing the number of bouts per day rather than by changing bout size. HAP2 mice had more bouts than LAP2 mice in both phases, but HAP2 bout size was larger only during the no-choice phase. In contrast to the finding in inbred strains, ethanol exposure produced no group or line differences in the percentages of intake infused in small, medium or large bouts. In general, both lines were more like B6 mice (sippers), infusing more of their total intake in small sized bouts than in large sized bouts.
Mechanisms Underlying Effects of Genotype and Passive Ethanol Exposure
Individual differences in ethanol drinking are most commonly attributed to a combination of variables that includes sensitivity to ethanol’s pre-absorptive effects (e.g., taste, odor), calories and post-absorptive pharmacological effects, as well as effects that are related to previous experience with ethanol (e.g., sensitization, tolerance, dependence, withdrawal). Since our IG procedure removed ethanol’s usual pre-absorptive effects, ethanol’s calories and/or pharmacological effects were presumably the primary determinants of ethanol intake and S+ preference in control mice. Although the effects of ethanol exposure generally implicated ethanol’s pharmacological effects, nothing in our data eliminates a possible contribution of ethanol’s calories to the genetic differences in intake or S+ preference. Future studies should address whether these genetic differences are specific to ethanol by comparing IG self-infusion of non-pharmacological high calorie reinforcers such as sucrose or maltodextrin in the same genotypes (Sclafani & Glendinning, 2003; Sclafani et al., 2010).
Repeated ethanol exposure might increase later ethanol self-infusion through several possible mechanisms, including: (a) sensitization to ethanol reward, (b) tolerance to post-absorptive aversive effects, and (c) negative reinforcement based on alleviation of withdrawal. A possible role for sensitization is generally consistent with reports of sensitization to ethanol’s locomotor activating effects in B6 and D2 mice (Lessov et al., 2001) and in HAP and LAP mice (Chester et al., 2001). However, a regimen of passive ethanol exposure that induces locomotor sensitization does not consistently increase later ethanol drinking (Lessov et al., 2001) or ethanol reward as indexed by conditioned place preference (Cunningham et al., 2002b). The literature offers better support for the suggestion that development of tolerance to ethanol’s post-absorptive aversive effects during passive exposure might contribute to later enhancement of ethanol self-infusion. First, there is substantial evidence for a negative genetic correlation between ethanol drinking and sensitivity to ethanol’s ability to induce a conditioned taste aversion (CTA; Cunningham et al., 2009). Thus, the lower intakes of D2 and LAP2 control mice during the choice phase (compared to B6 or HAP2 control mice) could be due to conditioning of an aversion to S+. Second, because ethanol pre-exposure has been shown to reduce the later development of ethanol-induced CTA (Risinger & Cunningham, 1995), the increase in ethanol intake and preference after ethanol exposure could be attributed, in part, to induction of tolerance to whatever post-absorptive ethanol effects underlie CTA.
Although insensitivity or tolerance to aversive effects might explain the absence of an aversion to S+ (i.e., preference = 50%), they do not readily explain the development of an absolute preference for S+ (Deutsch & Walton, 1977). One possibility is that self-infusion of ethanol was reinforced by alleviation of an aversive state induced by ethanol withdrawal (Deutsch & Walton, 1977; Deutsch & Cannis, 1980). In other words, the greater intake of the EtOH groups compared to the control groups might have been jointly determined by reduced sensitivity to post-absorptive aversive effects and negative reinforcement due to withdrawal alleviation.
Overall, these studies illustrate the value of using the IG self-infusion model to examine effects of genotype and ethanol pre-exposure on ethanol intake. Although removal of ethanol’s usual pre-absorptive effects did not eliminate genetic differences in ethanol intake predicted from drinking studies, the smaller IG intake difference between HAP2 and LAP2 mice suggests that a portion of the larger drinking difference between those lines is likely due to selection for sensitivity to pre-absorptive ethanol effects. In contrast, removal of ethanol’s pre-absorptive effects did not appear to affect magnitude of the expected ethanol intake difference between B6 and D2 mice. Of particular interest, ethanol pre-exposure produced a significant increase in later ethanol self-infusion in all four genotypes, including the two genotypes that typically avoid ethanol in drinking studies (D2, LAP2). No other rodent model of ethanol self-administration has shown such generality across genotype in the effect of chronic ethanol exposure. Although the exact mechanisms underlying increased ethanol intake are unknown (and might differ between genotypes), these data strongly encourage further studies with this new tool for studying genetic and environmental influences on excessive ethanol intake and preference in mice.
Acknowledgments
This research was supported by a grant from the National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism (U01-AA13479-INIA Project). We thank Nicholas Grahame, Lawrence Lumeng and the Indiana Alcohol Research Center at Indiana University-Purdue University (Indianapolis, IN) for their generosity is providing HAP2 and LAP2 breeding pairs (R24-AA015512). We also thank the Portland Alcohol Research Center Core for assistance with BEC analysis (P60-AA10760).
Footnotes
Based on the ratio (b/a) of the number of articles retrieved from PubMed® on September 10, 2010 using the following two searches: (a) (“1990”[Publication Date] : “2010”[Publication Date]) AND ((mice, inbred strains [MH]) AND (ethanol [MH])), (b) (“1990”[Publication Date] : “2010”[Publication Date]) AND ((mice, inbred C57BL [MH]) AND (ethanol [MH])). This search indicated that C57BL mice were used in 55.7% of the articles indexed under “ethanol” and “inbred strains” since 1990. Similar searches indicated that the next most frequently used inbred strains were BALBc and DBA, appearing in 12.8% and 9.8% of such articles, respectively.
References
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Belknap ND, Berg JH, Coleman R. Preabsorptive vs postabsorptive control of ethanol intake in C57BL/6J and DBA/2J mice. Behavior Genetics. 1977;7:413–425. doi: 10.1007/BF01066776. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Coleman RR, Foster K. Alcohol consumption and sensory threshold differences between C57BL/6J and DBA/2J mice. Physiological Psychology. 1978;6:71–74. [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Camarini R, Hodge CW. Ethanol preexposure increases ethanol self-administration in C57BL/6J and DBA/2J mice. Pharmacol Biochem Behav. 2004;79:623–632. doi: 10.1016/j.pbb.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Chester JA, Barrenha GD. Acoustic startle at baseline and during acute alcohol withdrawal in replicate mouse lines selectively bred for high or low alcohol preference. Alcohol Clin Exp Res. 2007;31:1633–1644. doi: 10.1111/j.1530-0277.2007.00462.x. [DOI] [PubMed] [Google Scholar]
- Chester JA, Grahame NJ, Li T-K, Lumeng L, Froehlich JC. Effects of acamprosate on sensitization to the locomotor-stimulant effects of alcohol in mice selectively bred for high and low alcohol preference. Behavioural Pharmacology. 2001;12:535–543. doi: 10.1097/00008877-200111000-00015. [DOI] [PubMed] [Google Scholar]
- Crabbe JC. Provisional mapping of quantitative trait loci for chronic ethanol withdrawal severity in BXD recombinant inbred mice. Journal of Pharmacology and Experimental Therapeutics. 1998;286:263–271. [PubMed] [Google Scholar]
- Crabbe JC. Alcohol and genetics: new models. Am J Med Genet. 2002;114:969–974. doi: 10.1002/ajmg.b.10984. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Belknap JK. The Complexity of Alcohol Drinking: Studies in Rodent Genetic Models. Behav Genet. 2010 doi: 10.1007/s10519-010-9371-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Harris RA, Arends MA, Koob GF. Alcohol-related genes: contributions from studies with genetically engineered mice. Addict Biol. 2006;11:195–269. doi: 10.1111/j.1369-1600.2006.00038.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Kosobud A, Belknap JK. Estimation of genetic correlation: interpretation of experiments using selectively bred and inbred animals. 1990. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Clemans JM, Fidler TL. Injection timing determines whether intragastric ethanol produces conditioned place preference or aversion in mice. Pharmacol Biochem Behav. 2002a;72:659–668. doi: 10.1016/s0091-3057(02)00734-7. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Genetic influences on conditioned taste aversion. In: Reilly S, Schachtman TR, editors. Conditioned taste aversion: Behavioral and neural processes. Oxford University Press; New York: 2009. pp. 387–421. [Google Scholar]
- Cunningham CL, Phillips TJ. Genetic basis of ethanol reward. In: Maldonado R, editor. Molecular biology of drug addiction. Humana Press, Inc; Totowa, NJ: 2003. pp. 263–294. [Google Scholar]
- Cunningham CL, Tull LE, Rindal KE, Meyer PJ. Distal and proximal pre-exposure to ethanol in the place conditioning task: tolerance to aversive effect, sensitization to activating effect, but no change in rewarding effect. Psychopharmacology (Berl) 2002b;160:414–424. doi: 10.1007/s00213-001-0990-1. [DOI] [PubMed] [Google Scholar]
- Deutsch JA, Cannis JT. Rapid induction of voluntary alcohol choice in rats. Behavioral and Neural Biology. 1980;30:292–298. doi: 10.1016/s0163-1047(80)91174-7. [DOI] [PubMed] [Google Scholar]
- Deutsch JA, Walton NY. A rat alcoholism model in a free choice situation. Behavioral Biology. 1977;19:349–360. doi: 10.1016/s0091-6773(77)91712-6. [DOI] [PubMed] [Google Scholar]
- Fidler TL, Clews TW, Cunningham CL. Reestablishing an intragastric ethanol self-infusion model in rats. Alcohol Clin Exp Res. 2006;30:414–428. doi: 10.1111/j.1530-0277.2006.00046.x. [DOI] [PubMed] [Google Scholar]
- Fidler TL, Oberlin BG, Struthers AM, Cunningham CL. Schedule of passive ethanol exposure affects subsequent intragastric ethanol self-infusion. Alcohol Clin Exp Res. 2009;33:1909–1923. doi: 10.1111/j.1530-0277.2009.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahame N, Cunningham C. Intravenous ethanol self-administration in C57BL/6J and DBA/2J mice. Alcohol Clin Exp Res. 1997;21:56–62. [PubMed] [Google Scholar]
- Grahame NJ, Rodd-Henricks K, Li TK, Lumeng L. Ethanol locomotor sensitization, but not tolerance correlates with selection for alcohol preference in high-and low-alcohol preferring mice. Psychopharmacology (Berl) 2000;151:252–260. doi: 10.1007/s002130000388. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Li TK, Lumeng L. Selective breeding for high and low alcohol preference in mice. Behav Genet. 1999;29:47–57. doi: 10.1023/a:1021489922751. [DOI] [PubMed] [Google Scholar]
- Lessov CN, Palmer AA, Quick EA, Phillips TJ. Voluntary ethanol drinking in C57BL/6J and DBA/2J mice before and after sensitization to the locomotor stimulant effects of ethanol. Psychopharmacology (Berl) 2001;155:91–99. doi: 10.1007/s002130100699. [DOI] [PubMed] [Google Scholar]
- Marshall EK, Jr, Owens AH., Jr Rate of metabolism of ethyl alcohol in the mouse. Proc Soc Exp Biol Med. 1955;89:573–576. doi: 10.3181/00379727-89-21879. [DOI] [PubMed] [Google Scholar]
- McClearn GE, Rodgers DA. Differences in alcohol preference among inbred strains of mice. Quarterly Journal of Studies on Alcohol. 1959;20:691–695. [Google Scholar]
- Metten P, Best KL, Cameron AJ, Saultz AB, Zuraw JM, Yu CH, Wahlsten D, Crabbe JC. Observer-rated ataxia: rating scales for assessment of genetic differences in ethanol-induced intoxication in mice. J Appl Physiol. 2004;97:360–368. doi: 10.1152/japplphysiol.00086.2004. [DOI] [PubMed] [Google Scholar]
- Metten P, Crabbe JC. Alcohol withdrawal severity in inbred mouse (Mus musculus) strains. Behav Neurosci. 2005;119:911–925. doi: 10.1037/0735-7044.119.4.911. [DOI] [PubMed] [Google Scholar]
- Metten P, Phillips TJ, Crabbe JC, Tarantino LM, McClearn GE, Plomin R, Erwin VG, Belknap JK. High genetic susceptibility to ethanol withdrawal predicts low ethanol consumption. Mamm Genome. 1998;9:983–990. doi: 10.1007/s003359900911. [DOI] [PubMed] [Google Scholar]
- Mulligan MK, Ponomarev I, Hitzemann RJ, Belknap JK, Tabakoff B, Harris RA, Crabbe JC, Blednov YA, Grahame NJ, Phillips TJ, Finn DA, Hoffman PL, Iyer VR, Koob GF, Bergeson SE. Toward understanding the genetics of alcohol drinking through transcriptome meta-analysis. Proc Natl Acad Sci U S A. 2006;103:6368–6373. doi: 10.1073/pnas.0510188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin B, Best C, Matson L, Henderson A, Grahame N. Derivation and characterization of replicate High- and Low-Alcohol Preferring lines of mice and a high-drinking crossed HAP line. Behav Genet. 2010 doi: 10.1007/s10519-010-9394-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risinger F, Cunningham C. Genetic differences in ethanol-induced conditioned taste aversion after ethanol preexposure. Alcohol. 1995;12:535–539. doi: 10.1016/0741-8329(95)00040-2. [DOI] [PubMed] [Google Scholar]
- Roberts A, Cole M, Koob G. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Rustay NR, Crabbe JC. Genetic analysis of rapid tolerance to ethanol’s incoordinating effects in mice: inbred strains and artificial selection. Behav Genet. 2004;34:441–451. doi: 10.1023/B:BEGE.0000023649.60539.dd. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Glass DS, Margolskee RF, Glendinning JI. Gut T1R3 sweet taste receptors do not mediate sucrose-conditioned flavor preferences in mice. Am J Physiol Regul Integr Comp Physiol. doi: 10.1152/ajpregu.00495.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Glendinning JI. Flavor preferences conditioned in C57BL/6 mice by intragastric carbohydrate self-infusion. Physiol Behav. 2003;79:783–788. doi: 10.1016/s0031-9384(03)00174-4. [DOI] [PubMed] [Google Scholar]
- Waller MB, McBride WJ, Gatto GJ, Lumeng L, Li TK. Intragastric self-infusion of ethanol by ethanol-preferring and -nonpreferring lines of rats. Science. 1984;225:78–80. doi: 10.1126/science.6539502. [DOI] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]