Abstract
Objective
We examined the long-term outcome of participants in the Treatment of SSRI-Resistant Depression in Adolescents (TORDIA) study, a randomized trial of 334 adolescents (aged 12-18 years) with DSM-IV-defined major depression disorder initially resistant to selective serotonin reuptake inhibitor (SSRI) treatment who were and subsequently treated for 12 weeks with another SSRI, venlafaxine, another SSRI + cognitive behavioral therapy (CBT), or venlafaxine + CBT. Responders then continued with the same treatment through week 24, while non-responders were given open treatment.
Method
For the current study, patients were reassessed 48 (N=116) and 72 (N=130) weeks from intake. Data were gathered from February 2011 to February 2007. Standardized diagnostic interviews and measures of depression, suicidal ideation, related psychopathology and level of functioning were periodically administered. Remission was defined as ≥ 3 weeks with ≤ 1 clinically significant symptom and no associated functional impairment (score of 1 on the adolescent version of the Longitudinal Interval Follow-Up Evaluation [A-LIFE], and relapse as ≥ 2 weeks with probable or definite depressive disorder (score of 3 or 4 on the A-LIFE). Mixed effects regression models were applied to estimate remission, relapse, and functional recovery.
Results
By 72 weeks, an estimated 61.1% of the randomized youths had reached remission. Randomly assigned treatment (first 12 weeks) did not influence remission rate or time to remission, but the group assigned to SSRI's had a more rapid decline in self-reported depressive symptoms and suicidal ideation than those assigned to venlafaxine (p<.05). Participants with more severe depression, greater dysfunction, and alcohol/drug use at baseline were less likely to remit. The depressive symptom trajectory of the remitters diverged from that of non-remitters by the first 6 weeks of treatment (p<.001). Of the 130 participants in remission at week 24, 25.4% relapsed in the subsequent year.
Conclusions
While most adolescents achieved remission, more than one-third did not, and one-fourth of remitted patients experienced a relapse. More effective interventions are needed for patients who do not show robust improvement early in treatment.
Keywords: depression, adolescents, treatment, resistance
After 6 months of first-step treatment, the rate of clinical remission of a unipolar major depressive episode is around 55%.1 With continuous treatment, most adolescents reach remission by 9 months, but about 40%, even if improved, have residual symptoms.1 Further observational evaluation up to one year indicates that remission is in most cases maintained.2 Less is known, however, about the long-term course of adolescents whose depression had proven resistant to an initial treatment with antidepressant medication.
The Treatment of SSRI-Resistant Depression in Adolescents study (TORDIA) was designed to examine the effects of second step interventions for adolescents whose depression had not responded to an adequate trial of selective serotonin reuptake inhibitor (SSRI).3 Participants were randomized to either: (1) switch to another SSRI; (2) switch to venlafaxine; (3) switch to another SSRI + cognitive behavior therapy (CBT); or (4) switch to venlafaxine + CBT. After 12 weeks, the response rate was superior in those who received CBT and either medication switch (54.8%) compared to those who received a medication switch alone (40.5%), but there were no differences between the two medication strategies. Indices of greater severity predicted a poorer overall response, and there were both positive (number of comorbid disorders) and negative moderators (history of abuse) of combination treatment's efficacy.4 Although there were few differences in the effects of venlafaxine vs. SSRI, participants with high entry levels of suicidal ideation were more likely to experience self-harm (suicidal events or episodes of non-suicidal self-harm) if they were treated with venlafaxine.5
Responders at week 12 continued in the same treatment through week 24, while non-responders were given open treatment. By 24 weeks, the cumulative remission rate was 38.9%, and the strongest predictors of lack of remission were lower rate of response during the first 6 weeks, a diagnosis of dysthymia, high baseline depression, family conflict, and higher drug and alcohol use at the end of acute treatment.6 Among non-responders by 12 weeks, subsequent remission was predicted by having received, during the first 12 weeks, either an augmentation of antidepressant treatment with a mood stabilizer, or, for those not been previously treated with CBT, the addition of psychotherapy.6
After the end of the 24-week treatment, participants were discharged from the study to community care and naturalistically assessed at weeks 48 and 72. We now report the clinical outcomes of these participants with the aim of describing the 72-week trajectory of depression, estimating the rate of remission, relapse, and functional recovery, and examining possible predictors of distal outcome. Because of the history of resistance to first-step SSRI treatment, we expected a lower remission rate than previously reported after first-step treatment.2 Based on the superiority of the CBT/medication combination over medication monotherapy at week 12,3 we hypothesized that remission rate would be higher, and relapse rate lower, among patients originally randomized to combined treatment, and that this effect would be moderated by history of abuse (CBT less effective) and by the number of comorbidities (CBT more effective).4 Additional hypotheses were that randomization to venlafaxine would be associated with a greater remission rate,7 but also with more suicidal ideation,5 and that lack of remission, or relapse after achieving remission, would be predicted by higher baseline levels of depression, suicidal ideation, hopelessness, drug and alcohol use, and family conflict.4 Finally, we examined whether remitters had shown a more rapid symptomatic improvement in the first 6 weeks of treatment than non-remitters.
Methods
Design and Participants
The design, methods, and results of TORDIA at 12 and 24 weeks have been reported in detail.3-6, 8 Briefly, TORDIA randomized 334 participants, with age ranging between 12 and 18 years (mean 15.9 years), 70% female, with a unipolar major depression of moderate severity (median duration of the current episode was 17 months at study entry) that had been unresponsive to one adequate trial of an SSRI. The race/ethnicity of the sample was: White 83%, Hispanic 5.4%, Biracial 4.8%, Black 3.3%, Asian/Pacific Islander 1.8%, Native American/Alaskan Native 0.6%, and Other 1.2%. Previous SSRI non-response was determined based on documented treatment with an SSRI for at least 8 weeks, of which the last 4 consisted at a daily dose of at least 40 mg of fluoxetine or its equivalent.
Participants were randomized to receive a different SSRI or venlafaxine, with or without CBT, for 12 weeks of acute treatment and 12 other weeks of continuation. CBT consisted of 12 weekly sessions followed by up to 6 booster sessions over the subsequent 12 weeks, using techniques of behavior activation, cognitive restructuring, problem-solving, social skills training, and emotion regulation.3 Participants assigned to CBT received an average of 8.3 acute sessions (SD=3.6) and 2.8 booster session (SD=2.8). Medication was administered under double-blind conditions for the first 12 weeks, after which responders continued blinded treatment and non responders entered clinically indicated open-label treatment, which could consist of a higher medication dose, a switch to another medication, augmentation with another medication, CBT, or other psychotherapy. After the end of the 24-week treatment, participants were naturalistically treated in the community and reassessed at weeks 48 and 72. For some participants who entered the study toward the end of TORDIA, the follow-up was truncated before the week-72 assessment. Overall, of the 334 randomized participants, 316 were eligible for assessment at week 48, and 293 at week 72.
The protocol of TORDIA, including the naturalistic follow-up, was approved by the institution review boards of the six participating clinical sites (University of Pittsburgh, University of Texas-Southwestern Medical Center, University of California-Los Angeles, University of Texas-Galveston, Brown University, and Kaiser Permanente-Portland).
Assessments
Diagnostic status was assessed with the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime (K-SADS-PL), which generates DSM-IV diagnoses.9 The K-SADS-PL was re-administered at study entry and at 12, 24, and 72 weeks. Before starting treatment in TORDIA and at 6, 12, 24, 48, and 72 weeks afterwards, independent clinical evaluators, who were blind to random assignment, rated depressive symptoms on the Children's Depression Rating Scale-Revised (CDRS-R)10 and the adolescent version of the Longitudinal Interval Follow-up Evaluation (A-LIFE)11; overall severity of illness and improvement on the Clinical Global Impressions Severity (CGI-S) and Improvement (CGI-I) scales;12 and level of functioning on the Children's Global Assessment Scale.13 At the same time-points, participants completed the Beck Depression Inventory (BDI),14 Hopelessness Scale (BHS),15 Screen for Anxiety-related Emotional Disorders,16 Conflict Behavior Questionnaire (CBQ),17 and the Suicidal Ideation Questionnaire for Adolescents (SIQ-Jr).18
Outcomes
Response was defined as a CGI-I score of 2 or less (i.e., much or very much improved) and a decrease of at least 50% in the CDRS-R over the pre-randomization baseline. Remission was defined as at least three consecutive weeks with no more than 1 clinically significant symptom and no associated functional impairment, as rated as a 1 on the A-LIFE.. Participants who were remitted at week 24 were assessed for relapse, defined as at least two consecutive weeks with probable or definite depressive disorder (i.e., a score of 3 or 4 on the A-LIFE). Functional recovery was defined as a CGAS score above 70. Secondary outcomes were continuous measures of interview-rated depressive symptoms (CDRS-R) and self-reported measures of depression (BDI) and suicidal ideation (SIQ-Jr).
Data Analyses
The rates of and times to remission were compared across treatment groups using the χ2 and Kaplan-Meier statistics, respectively. The baseline, 12, and 24 week demographic, clinical, and treatment characteristics of remitters and non-remitters were compared using standard univariate statistics. The most parsimonious set of predictors of time to remission were identified using Cox regression. Mantel-Cox log rank test and Cox proportional hazards survival analyses were conducted on time to remission.19 A similar approach was taken for to the analyses with relapse as an outcome. For assessment of the differential impact of treatments on continuous outcomes, such as depressive symptoms or suicidal ideation, mixed effects regression models with repeated measures were used.
Data were analyzed with both the last observation carried forward (LOCF) and multiple imputation assuming missing at random data.20 As these analyses yielded similar results, the LOCF analyses are here presented. Possible demographic and clinical predictors of remission, relapse, or functional recovery were examined using χ2, Fisher's exact or Student's t tests, as appropriate. Receiver operator curves (ROC) were plotted for selected significant predictor variables in order to examine the relationship between sensitivity and 1-specificity as a function of chosen cut-offs. Given the exploratory nature of these analyses, alpha was set at ≤0.05, without attempts to correct for multiple comparisons. Analyses were conducted using SPSS 17.0 and STATA 9.2.21,22
Results
Sample retention
Of the eligible participants, 56.3% (178 out of 316) were assessed at week 48, and 56.0% (164 out of 293) at week 72. Compared to the participants who were assessed, those not retained for assessment had more severe illness (CGI-S, 4.6 ± SD 0.7 vs. 4.4 ±0.6, t=3.44, df=331, p=.001), greater functional impairment (CGAS, 49.6 ±8.4 vs. 51.3 ±7.1, t=-1.94, df=329, p=.05), and higher rates of comorbid conduct or oppositional disorder (14.4% vs. 7.1%, χ21=4.72, p=.03) at study entry. Those not retained also had lower rates of treatment response and remission at 12 weeks (38.1% vs. 54.0%, χ21=8.17, p=.004; 9.0% vs. 23.5%, χ21=11.67, p=.001) and at 24 weeks (41.8% vs. 65.0%, χ21=17.52, p<.001; 27.3% vs. 48.0%, χ21=13.81, p=.001).
Treatment during the naturalistic follow-up phase
At weeks 48 and 72, 83.1% (148/178) and 70.1% (115/164) of the participants, respectively, reported being in treatment with an antidepressant medication. Patients tended to stay on the medication to which they were initially randomized, both for venlafaxine (58.0%) and SSRI's (62.9%), with 23% in both treatment arms having been switched to the alternative agent. As expected in a community treated sample, treatment was heterogeneous, and about 8% of the participants received a mood stabilizer and/or an antidepressants other than venlafaxine or an SSRI. At the same time points, 43.8% (78/178) and 47.6% (78/164), respectively, of the assessed participants reported receiving psychotherapy. Psychotherapy was in general eclectic, involving a mix of family and individual treatment. Participants who had been randomized to CBT did not differ from those randomized to medication only with respect to psychotherapy utilization during the naturalistic follow-up phase.
Remission
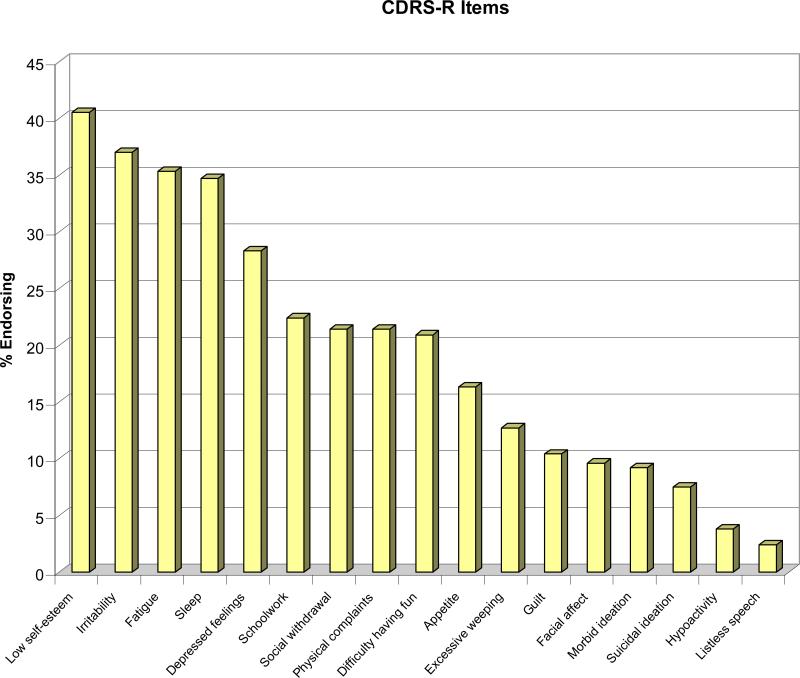
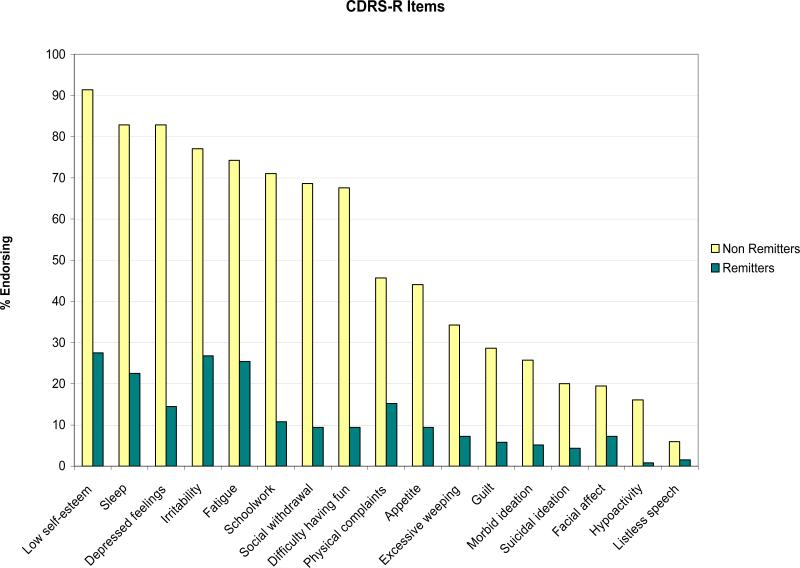
The cumulative rate of remission, which was 17.7% by week 12 and 38.9% by week 24, increased to 50.0% by week 48 and 61.1% by week 72 (Table 1). Median time to remission was 25 weeks (95% CI: 20.7-29.1). Most (72.3%) of the participants assessed at week 72 had at least one residual symptom of depression, such as irritability, fatigue and low self-esteem, and especially those who had not achieved remission (Figure 1). At the same time-point, a third of the youths reported irritability and feelings of disappointment or self-blame on the BDI, and 40% had irritability/anger on the KSADS. Of the participants assessed at week 72, 11% met DSM-IV criteria for MDD. The number of depression symptoms still experienced by participants who didn't meet full criteria for major depression ranged from 0 to 5 (median=0). No participant met criteria for manic episode during the 72 week period. Four patients displayed symptoms of possible hypomania (two patients during the 24 week treatment phase and two during the subsequent naturalistic follow-up).
Table 1.
Remission (A-LIFE) over Time Through 72 Weeksa
| Week | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Randomization Statusb | 12 N (%) | 24 N (%) | 48 N (%) | 72 N (%) | ||||||||
| N assessed | LOCF | Observed | N assessed | LOCF | Observed | N assessed | LOCF | Observed | N assessed | LOCF | Observed | |
| SSRI (N=168) | 144 | 29 (17.3) | 29 (20.1) | 126 | 64 (38.1) | 58 (46.8) | 87 | 74 (46.0) | 51 (58.6) | 81 | 86 (58.1) | 62 (76.5) |
| Venlafaxine (N=166) | 143 | 30 (18.1) | 30 (21.0) | 135 | 66 (40.0) | 65 (48.2) | 91 | 84 (54.2) | 65 (71.4) | 83 | 93 (64.1) | 68 (81.9) |
| Med Only (N=168) | 147 | 28 (16.7) | 28 (19.1) | 135 | 69 (41.1) | 63 (46.7) | 90 | 84 (53.2) | 62 (68.9) | 84 | 93 (63.3) | 69 (82.1) |
| Med + CBT (N=166) | 140 | 31 (18.7) | 31 (22.1) | 126 | 61 (36.7) | 60 (48.4) | 88 | 74 (46.8) | 54 (61.4) | 80 | 86 (58.9) | 61 (76.3) |
| All groups (N=334) | 287 | 59 (17.7) | 59 (20.6) | 261 | 130 (38.9) | 123 (47.5) | 178 | 158 (50.0) | 116 (65.2) | 164 | 179 (61.1) | 130 (79.3) |
Both observed and cumulative with last observation carried forward (LOCF)
Randomly determined treatment during the first 12 weeks. SSRI: selective serotonin reuptake inhibitor. Med: antidepressant monotherapy. No statistically significant differences between venlafaxine and SSRI, or between CBT and medication monotherapy.
cIncludes only participants eligible for the week-48 interview (N=316).
dIncludes only participants eligible for the week-72 interview (N=293).
Figure 1.
I – Residual Symptoms at Week 72 Assessment – Overall Samplea
aPercentage of participants assessed at 72 weeks (N=173) endorsing (score of 3 or above) specific symptoms of the Children's Depression Rating Scale-Revised (CDRS-R).
II - Residual Symptoms at Week 72 Assessment – Remitters and Non-Remittersb
bPercentage of participants assessed at 72 weeks who had reached remission (N=138) or not (N=35) in TORDIA, endorsing (score of 3 or above) specific symptoms of the Children's Depression Rating Scale-Revised (CDRS-R). Remission is defined as at least three consecutive weeks without clinically significant depressive symptoms, corresponding to a score of 1 on the A-LIFE.
Moderators and predictors of remission
Random treatment assignment did not influence the rate of remission or time to remission, but combined treatment was less likely to result in remission than monotherapy if there was a history of abuse (OR= 0.35, 95% CI .12-.98; p=.046). Continuing to receive an antidepressant was associated with higher remission rates than having discontinued it at week 48 (64.8% vs. 25.0%, χ21=9.72, p=0.002), but not at week 72 (78.8% vs. 76.5%, Fisher's exact test, p=0.76). No baseline predictor of continuing antidepressant medication at week 72 was identified.
Remission was predicted by a number of baseline (pre-randomization) variables, including lower CDRS-R, BDI, BHS, and CBQ scores, shorter duration of depressive episode, lower CGI-S, higher CGAS scores, less non-suicidal self-harm behavior and less drug and alcohol abuse. Other pre-randomization variables, such as age, gender, race, family income, comorbidity with anxiety or behavior disorders, or history of abuse or suicide attempts, were not associated with the likelihood of achieving remission by week 72. Using the most parsimonious set of predictors (i.e., BDI, CGI-S, drug and alcohol abuse, and duration of current depressive episode), 66.1% of the participants were correctly classified as remitters or non-remitters (Hosmer and Lemeshow χ28=8.68, p=0.37). Receiver operator analyses of the CDRS, CGI-S, CGAS, BDI, BHS, and duration of depressive episode were conducted, but did not reveal cut-off scores with sufficient sensitivity/specificity ratios for clinical utility.
Relapse
Of the 130 participants in remission by week 24, 33 (25.4%) relapsed in the following 48 weeks. The relapse rate and time to relapse of the participants who had received the combination of CBT plus medication in the first 12 weeks were not statistically different from that observed in the participants who were randomized to medication monotherapy (31.2% vs. 20.3% (χ21=2.02, p=0.16), and 47 weeks vs. 41 weeks (z=1.39, p=0.17, respectively). Outcomes were similar for those who received CBT versus those who didn’t, either for random assignment (20.3% vs. 31.1%, χ21=2.02, p=0.16; 47 weeks vs. 41 weeks, z=1.39, p=0.16) or subsequent addition (27.5% vs. 22.0%, χ21=0.49, p=0.48; 45 weeks vs. 44 weeks, z=0.82, p=0.41). Randomization to venlafaxine versus SSRI did not influence relapse rate (22.7% vs. 28.1%, χ21=0.50, p=0.48), or time to relapse (47 weeks vs. 43 weeks, z=-0.82, p=0.41).
Of 21demographic and clinical variables were examined as possible predictors of relapse, only non-White race was associated (p≤0.05) with higher relapse rate. No statistically significant differences in the rate of medication continuation were found between non-White and White participants at week 48 (88.5% vs. 91.4%) or at week 72 (94.7% vs. 84.3%).
Level of functioning
The decline in depressive symptoms, as measured on the CDRS-R, was correlated with an increasing level of functioning, measured on the CGAS (r=-0.66, p<0.001). Remission status was associated with achieving functional recovery (defined as a CGAS >70) at week 48 and week 72 (kappa coefficients 0.45 (95% CI: 0.36-0.55)-0.58 (95% CI: 0.49-0.67), p<0.001).
Depression Symptom Trajectory
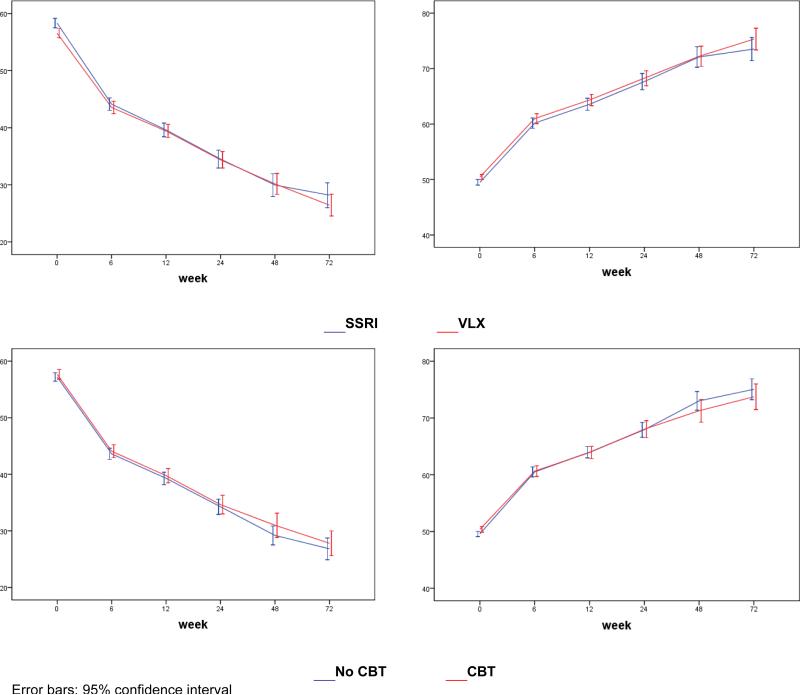
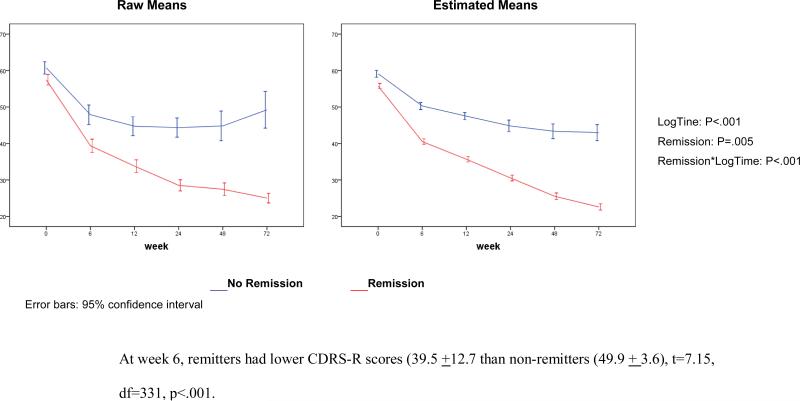
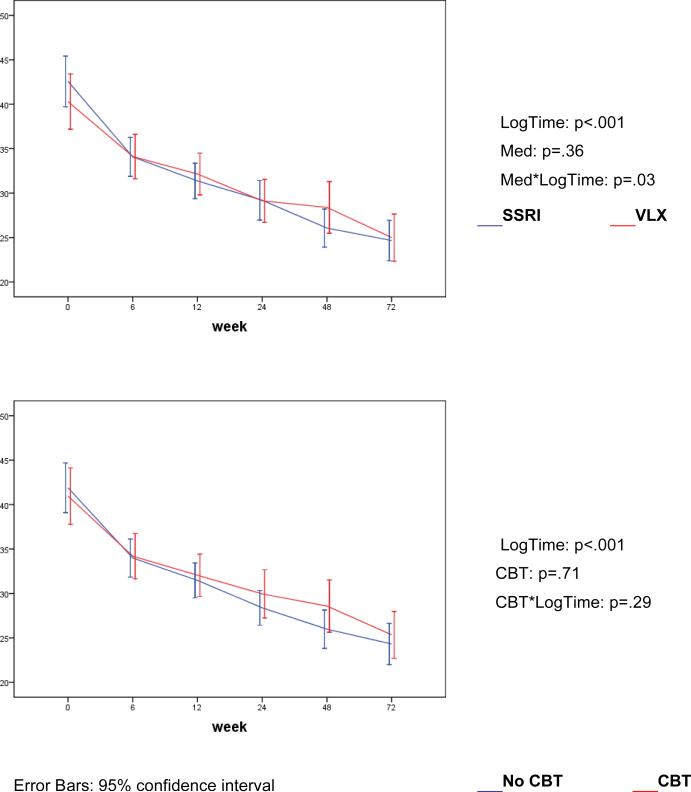
The severity of depression symptoms, as assessed with the CDRS-R total score, continued to decrease throughout the 72 weeks, with no effect from original randomization (Fig. 2). Remitters and non-remitters had different trajectories of CDRS-R scores, which diverged from each other early in treatment (Figure 3). The percent change on the CDRS-R by 6 weeks was 43.6% (SD=29.7) in those who remitted and 23.6% (SD=30.5) in those who did not (t=-6.00, df=332, p<.001). BDI scores also declined with time (LogTime: p<0.001), with no difference between the randomized CBT vs. non-CBT conditions (CBT*LogTime: p=0.77), but with greater improvement in the patients randomized to SSRI rather than venlafaxine (Med*Log-Time: p=.03).
Figure 2.
Trajectories of CDRS (left) and in CGAS (right) (estimated means)
Figure 3.
Trajectories of Depression Symptoms (CDRS-R) by Remission Status at Week 72
Suicidality
Suicidal ideation was systematically measured on the SIQ-Jr, and continued to decline throughout the 72 weeks of observation (Figure 4). The SIQ-Jr mean score at 72 weeks was about 25, which is below the threshold for clinically significant suicidal ideation (i.e., ≥31). Having received CBT in the first 12 weeks did not influence SIQ-Jr scores. Having been treated with venlafaxine was associated with higher SIQ-Jr scores (trajectory of SIQ-Jr scores over time, medication*Logtime interaction p=0.03). Four participants reported having attempted suicide, at week 39, 40, 41, and 42, respectively, and other 3 having had clinically significant suicidal ideation, in the year following the end of the 6-month TORDIA treatment. All the four youths who attempted suicide had an SIQ-Jr score above 31.
Figure 4.
Suicidal Ideation Questionnaire-Adolescent Version (SIQ-Jr) (estimated means)
Discussion
This naturalistic, observational, systematic, follow-up study of the TORDIA sample offers a view on the long-term outcome of adolescents with major depression, who, after being unresponsive to an adequate course of SSRI treatment, were subsequently treated with an alternative antidepressant (another SSRI or venlafaxine) with or without CBT. The conclusions are limited by the lack of experimental control beyond the first 12 weeks. The data, however, provide descriptive information about a number of clinically relevant outcomes, such as remission, relapse, functional recovery and presence of residual symptoms, which were obtained in a well characterized sample of adolescents with treatment resistant major depressive disorder.
Overall, depressive symptoms continued to decrease after the end of the 24-week TORDIA treatment through week 72, with 61.1% achieving remission by 72 weeks, comparable to the 63% achieved by week 72 in the Treatment of Adolescent Depression Study (TADS),2 and the 12 months rate of remission after second-step treatment in adult depression (61.8%).23 However, residual symptoms of depression, especially irritability, were common. The decrease in depressive symptoms was associated with better functioning, as shown by the negative correlation between CDRS-R and CGAS (r=-0.66), consistent with previous studies showing that improvement in functioning is mediated by the treatment effect on depressive symptoms.24 Functional recovery was tightly linked to achieving remission, thus providing further evidence of the clinical validity of remission as main target of treatment.
Initial treatment assignment over the first 12 weeks did not predict remission or time to remission. While the lack of longer-term impact of CBT was consistent with findings from other studies, it also could be attributable to the relatively low dose of CBT, and also to the large proportion of participants initially assigned to medication monotherapy who later received psychotherapy as part of open treatment. A history of abuse did predict a lower rate of remission among those exposed to combination therapy, which indicates that not only is abuse a moderator of short-term response to CBT as has been previously reported, but may have longer-term effects as well.4 At week 48, but not by week 72, youths who had continued taking antidepressant medication had higher rates of remission than participants who had discontinued it, which is consistent with experimental studies showing the benefit of continuation pharmacotherapy during the first year of treatment.25 Baseline duration and severity of depression and of alcohol or substance use also predicted poorer outcome in the long term,
Consistent with recent reports,26 the trajectories of depressive symptoms for remitters and non-remitters diverged from each other during the first few weeks of treatment (Figure 3). These findings should give impetus to developing methods for early identification of treatment non-response so that patients who are unlikely to reach remission on a particular treatment can be offered alternative, potentially more effective interventions at the earliest possible point in time. While there was no effect of treatment assignment on the long-term trajectory of interview-rated depressive symptoms, patients randomized to an SSRI showed greater improvement in their self-reported depressive symptoms than did those assigned to venlafaxine, contrary to the stated hypothesis.
Suicidal ideation declined in parallel with the decrease in general depressive symptom scores (Figure 4). The rate of decline in suicidal ideation was greater in those initially assigned to an SSRI than in those assigned to venlafaxine, which is consistent with our previous report.5 Also, meta-analyses of psychopharmacological trials in adolescent depression, venlafaxine was the only drug that showed a statistically significant higher rate of suicidal events than placebo,27 and in analyses of population-based databases venlafaxine use had the highest risk of suicide compared with other antidepressants.28 Hence, albeit within the limitations of naturalistic follow-up, the risk-benefit ratio for adolescent depression may favor SSRI's over venlafaxine. By the end of the 24-week TORDIA treatment, the mean SIQ-Jr score was below the threshold for clinically significant suicide risk, and remained so throughout the subsequent 48 weeks of observation. Despite this favorable trend, four participants attempted suicide during the 48-week period, thus indicating the continuous risk for suicide behavior in this psychopathology. All these four adolescents had SIQ-Jr scores indicative of elevated suicidal risk at previous assessments.
Among the youths who reached remission by the end of TORDIA, the relapse rate was 25.4% in one-year period, which appear comparable to that observed among treated non-SSRI resistant adolescents.29 The finding that non-White race, independent from family income, was associated with higher risk for relapse highlights the importance of understanding and reducing ethnic disparities in depressive outcomes. While this suggests the influence of cultural factors on the long-term treatment response for depression, it does not appear to be mediated by differences in antidepressant use. We previously found that non-White TORDIA participants showed a less vigorous response to CBT at 12 weeks.4
This study has some important limitations, among which the lack of long-term experimental control is especially noteworthy. In fact, although TORDIA was a randomized trial for the first 12 weeks, participants were then treated as clinically indicated for another 12 weeks and then discharged to community care. Ethical concerns and practical considerations would have prevented doing otherwise. Thus, the study is primarily a descriptive naturalistic observation of a carefully diagnosed and characterized sample of adolescents with major depression. Evaluation of the effects of CBT is limited by the dose actually received by the participants, which consisted, on average, of 8.3 sessions in the first 12 weeks, followed by a mean of 2.8 booster session in the following weeks. This dose might have been too low to produce long-lasting effects. Another limitation is the attrition during the post-treatment follow-up and the fact that the participants available for assessment through 72 weeks were, on average, less severely depressed and more likely to have improved during treatment than those who did not return for follow-up visits. These sample characteristics can be considered a bias towards more favorable conclusions about long-term outcome. However, the results with the last observation carried forward approach were convergent with analyses with imputed values, suggesting that a retention bias is not a main explanation of these findings. These limitations notwithstanding, the study presents informative data on the distal outcome of one of the largest clinical samples of adolescents with major depressive disorder.
In conclusion, in examining the course of a unipolar major depression episode in adolescents initially unresponsive to antidepressant treatment and then treated with an alternative antidepressant, alone or in combination with CBT, we found that most adolescents eventually achieved remission. This rate of remission, however, was not substantially lower than that reported after first-step treatment, suggesting that youths with initial treatment resistance retain considerable potential of achieving remission with further treatment. However, a substantial proportion either never remitted or relapsed. Since lack of remission was predicted by more severe depression and the persistence of depressive symptoms during the early phases of treatment, clinicians and researchers should focus their efforts on those patients who do not achieve clinically significant improvement in the first six weeks of treatment. Treatment strategies that accelerate response during the initial phase of treatment may hold promise for improving the long-term trajectory for adolescent depression.
Acknowledgments
Funding/Support: Funded by the National Institute of Mental Health through grants U01MH61835 (University of Pittsburgh, P.I.: D. Brent), U01 MH61856 (University of Texas at Galveston, P.I.: K. Wagner), U01MH 61864 (University of California at Los Angeles, P.I.: J. Asarnow), U01MH61869 (Kaiser Foundation, Portland, Oregon, P.I.: G. Clarke), U01MH61958 (University of Texas at Dallas, P.I.: G. Emslie), and U01MH62014 (Brown University, P.I.: M. Keller).
The opinions and assertions contained in this report are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of Health and Human Services, the National Institutes of Health, or the National Institute of Mental Health.
Footnotes
Financial Disclosures (relative to the last 12 months): Dr. Emslie receives research support from the National Institute of Mental Health, Biobehavioral Diagnostics Inc., Forest Laboratories, Shire, and Somerset; and is a consultant for Biobehavioral Diagnostics, Inc., Eli Lilly, Forest Laboratories, Inc., Pfizer Inc., Shire, Validus Pharmaceuticals, and Wyeth Pharmaceuticals. Dr. Wagner receives research support from the National Institute of Mental Health. Dr. Asarnow has consulted on cognitive behavior therapy, depression treatment quality improvement, and on an unrestricted grant from Pfizer; had unrestricted funding from Philip Morris; and a family member has funding from Bristol-Myers Squibb and has consulted for Roche, Novartis, Sanofil-Adventis, and Janssen. Dr. Keller has reported research support from Pfizer; is a consultant with Abbott, CENEREX, Cephalon, Cypress Bioscience, Cyberonics, Forest Laboratories, Janssen, JDS, Medtronic, Organon, Novartis, Pfizer, Roche, Solvay, and Wyeth; and has served on advisory boards for Abbott Laboratories, Bristol-Myers Squibb, CENEREX, Cyberonics, Cypress Bioscience, Forest Laboratories, Janssen, Neuronetics, Novartis, Organon, and Pfizer. Dr. Birmaher receives research support from the National Institute of Mental Health; has participated in forums sponsored by Shire Pharmaceuticals (provided training for an investigators meeting) and Forest Laboratories, Inc. (Advisory Board); and receives royalties from Random House, Inc. and Lippincotte Williams & Wilkins. Dr. McCracken has received research support from Eli Lilly, McNeil, Bristol-Myers Squibb, and Shire; and is a consultant for Shire, Eli Lilly, McNeil, Pfizer, Janssen, Johnson & Johnson, Novartis, and Wyeth. The other authors have no relevant financial relationships.
References
- 1.Kennard B, Silva SG, Tonev S, Rohde P, Hughes JL, Vitiello B, Kratochvil CJ, Curry JF, Emslie GJ, Reinecke M, March J. Remission and recovery in the Treatment for Adolescents with Depression Study (TADS): acute and long-term outcomes. J Am Acad Child Adolesc Psychiatry. 2009;48:186–195. doi: 10.1097/CHI.0b013e31819176f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.TADS Team The Treatment for Adolescents With Depression Study (TADS): outcomes over 1 year of naturalistic follow-up. Am J Psychiatry. 2009;166:1141–1149. doi: 10.1176/appi.ajp.2009.08111620. [DOI] [PubMed] [Google Scholar]
- 3.Brent D, Emslie G, Clarke G, Wagner K, Asarnow J, Keller M, Vitiello B, Ritz L, Satish Iyengar S, Abebe K, Birmaher B, Ryan N, Kennard B, Hughes C, DeBar L, McCracken J, Strober M, Suddath R, Spirito A, Leonard H, Porta G, Onorato M, Zelazny J. The Treatment of Adolescents with SSRI-Resistant Depression (TORDIA). A comparison of switch to venlafaxine or to another SSRI, with or without additional cognitive behavioral therapy. JAMA. 2008;299:901–13. doi: 10.1001/jama.299.8.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asarnow JR, Emslie G, Clarke G, Wagner KD, Spirito A, Vitiello B, Iyengar S, Shamseddeen W, Ritz L, Birmaher B, Ryan N, Kennard B, Mayes T, Debar L, McCracken J, Strober M, Suddath R, Leonard H, Porta G, Keller M, Brent D. Treatment of Selective Serotonin Reuptake Inhibitor-Resistant-Depression in Adolescents: predictors and moderators of Treatment Response. J Am Acad Child Adolesc Psychiatry. 2009;48:330–339. doi: 10.1097/CHI.0b013e3181977476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brent DA, Emslie GJ, Clarke GN, Asarnow J, Spirito A, Ritz L, Vitiello B, Iyengar S, Birmaher B, Ryan ND, Zelazny J, Onorato M, Kennard B, Mayes TL, Debar LL, McCracken JT, Strober M, Suddath R, Leonard H, Porta G, Keller MB. Predictors of spontaneous and systematically assessed suicidal adverse events in the Treatment of SSRI-Resistant Depression in Adolescents (TORDIA) study. Am J Psychiatry. 2009;166:418–426. doi: 10.1176/appi.ajp.2008.08070976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emslie G, Mayes T, Porta G, Vitiello B, Clarke G, Wagner KD, Asarnow JR, Spirito A, Birmaher B, Ryan N, Kennard B, DeBar L, McCracken JT, Strober M, Onorato M, Zelazny J, Keller M, Iyengar S, Brent DA. Treatment of Resistant Depression in Adolescents (TORDIA): week 24 outcomes. Am J Psychiatry. 2010 doi: 10.1176/appi.ajp.2010.09040552. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nemeroff CB, Entsuah R, Benattia I, Demitrack M, Sloan DM, Thase ME. Comprehensive analysis of remission (COMPARE) with venlafaxine versus SSRIs. Biol Psychiatry. 2008;63:424–434. doi: 10.1016/j.biopsych.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 8.Spirito A, Abebe KZ, Iyengar S, Brent D, Vitiello B, Clarke G, Wagner KD, Asarnow J, Emslie G, Keller M. Sources of site differences in the efficacy of a multi-site clinical trial: the Treatment of SSRI Resistant Depression in Adolescents. J Consult Clinical Psychology. 2009;77:439–450. doi: 10.1037/a0014834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Poznanski EO, Mokros HB. Manual for the Children's Depression Rating Scale-Revised, Manual. Western Psychological Services; Los Angeles, LA: 1996. [Google Scholar]
- 11.Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, Andreasen NC. The longitudinal interval follow-up evaluation: a comprehensive method for assessing outcome in prospective longitudianl studies. Arch Gen Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- 12.Guy W. ECDEU Assessment Manual for the Psychopharmacology. 2nd ed. Dept. of Health and Human Services; Washington, DC: 1976. pp. 91–338. Publication No. (ADM) [Google Scholar]
- 13.Shaffer D, Gould MS, Brasic J, et al. A children's global assessment scale (CGAS). Arch Gen Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 14.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 15.Beck A, Kovacs M, Weissman A. Assessment of suicidal intention: the Scale for Suicidal Ideation. J Cons Clin Psychol. 1979;47:343–352. doi: 10.1037//0022-006x.47.2.343. [DOI] [PubMed] [Google Scholar]
- 16.Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, Neer SM. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36:545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Robin AL, Weiss JG. Criterion-related validity of behavioral and self-report measures of problem-solving communication skills in distressed and non-distressed parent-adolescent dyads. Behav Assess. 1980;2:339–352. [Google Scholar]
- 18.Reynolds WM. Professional Manual for the Suicidal Ideation Questionnaire. Psychological Assessment Resources, Inc.; Lutz, FL: 1987. [Google Scholar]
- 19.Little RJA, Rubin DB. 2nd edition Wiley; New York: 2002. Statistical Analysis with Missing Data. [Google Scholar]
- 20.Hosmer DW, Lemeshow S. Applied Survival Analysis. Wiley; New York: 1999. [Google Scholar]
- 21.SPSS for Windows . Re. 16.0.2. SPSS Inc.; Chicago, IL: 2007. [Google Scholar]
- 22.Stata Statistical Software . Release 9.2. StataCorp LP; College Station, TX: 2005. [Google Scholar]
- 23.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 24.Vitiello B, Rohde P, Silva SG, Wells KC, Casat C, Waslick BD, Simons A, Reinecke MA, Weller EB, Kratochvil CJ, Walkup J, Pathak S, Robins M, March JS, the TADS Team Effects of treatment on level of functioning, global health, and quality of life in depressed adolescents. J Am Acad Child Adolesc Psychiatry. 2006;45:1419–1426. doi: 10.1097/01.chi.0000242229.52646.6e. Ph.D. [DOI] [PubMed] [Google Scholar]
- 25.Emslie GJ, Kennard BD, Mayes TL, Nightingale-Teresi J, Carmody T, Hughes CW, Rush AJ, Tao R, Rintelmann JW. Fluoxetine versus placebo in preventing relapse of major depression in children and adolescents. Am J Psychiatry. 2008;165:459–467. doi: 10.1176/appi.ajp.2007.07091453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tao R, Emslie G, Mayes T, Nakonezny P, Kennard B, Hughes C. Early prediction of acute antidepressant treatment response and remission in pediatric major depressive disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:71–72. doi: 10.1097/CHI.0b013e318190043e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bridge JA, Iyengar S, Salary CB, Barbe RP, Birmaher B, Pincus HA, Ren L, Brent DA. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297:1683–1696. doi: 10.1001/jama.297.15.1683. [DOI] [PubMed] [Google Scholar]
- 28.Tiihonen J, Lönnqvist J, Wahlbeck K, Klaukka T, Tanskanen A, Haukka J. Antidepressants and the risk of suicide, attempted suicide, and overall mortality in a nationwide cohort. Arch Gen Psychiatry. 2006;63:1358–1367. doi: 10.1001/archpsyc.63.12.1358. [DOI] [PubMed] [Google Scholar]
- 29.TADS Team The Treatment for Adolescents with Depression Study (TADS): long-term effectiveness and safety outcomes. Arch Gen Psychiatry. 2007;64:1132–1144. doi: 10.1001/archpsyc.64.10.1132. [DOI] [PubMed] [Google Scholar]