Summary
Due to the increasing prevalence of nosocomial and community-acquired antibiotic resistant Staphylococcus aureus (SA), understanding the determinants of SA nasal carriage has become a major imperative. Previous research has revealed many host and bacterial factors that contribute to SA nasal carriage. To assess bacterial factors that facilitate nasal carriage, we compared the exoproteome of a nasal carrier strain of SA to a genetically similar non-carrier strain. Additionally, the carrier strain biofilm exoproteome was also compared against its planktonic counterpart. Using high throughput proteomics, it was observed that the carrier strain of SA secretes a greater number of proteins that may promote successful colonization of the human nose, including cell attachment and immunoevasive proteins, than the non-carrier strain. Similarly, SA carrier strain biofilm exoproteome contains a greater number of immunoevasive proteins than its planktonic counterpart. Analysis of the most abundant immunoevasive proteins revealed that Staphylococcal protein A was present at significantly higher levels in carrier than in non-carrier strains of SA, suggesting an association with nasal carriage. While further analyses of specific differences between carrier and non-carrier strains of SA are required, many of the differentially expressed proteins identified can be considered to be putative determinants of nasal carriage.
Keywords: Innate immunity, bacteria/bacterial immunity, comparative proteomics, nasal colonization, exoproteome
Introduction
Staphylococcus aureus is one of the most common causes of community-acquired and nosocomial infections throughout the world.1 These infections have become even more pertinent with the global spread of community-acquired Methicillin-resistant SA (CA-MRSA)2 and the emergence of Vancomycin-resistant SA (VRSA).3 In the US alone, the mortality rate from SA infections surpasses those attributed to HIV/AIDS.2 Many community-acquired and nosocomial SA infections are disseminated through nasal carriage, which occurs in approximately one quarter of the population,1 thus identifying determinants of nasal carriage is a priority for successful amelioration of this condition.
Although nasal carrier strains of SA have evolved diverse strategies to ensure their survival and carriage in nasal passages, colonization can also be attributed to amenable hosts that carry SA persistently or intermittently.4-6 In an immunologically robust host, nasal secretions contain a plethora of defensive antibacterial proteins and peptides such as lysozyme, lactoferrin, secretory leukoprotease inhibitor (SLPI), cathelicidins, α-defensins and β-defensins including human β-defensin 3 (HBD-3), the most effective of the β-defensins against SA infections.4,7-9 However, a key determinant for the nasal carriage of SA is the failure of these secretions to prevent colonization. Carrier strains, and not non-carrier strains of SA, have been reported to persist and replicate within nasal fluids from carrier donors and on the surface of organotypic nasal epithelia, indicating that carriers have factors that aid in nasal colonization of SA.10,11 Likewise, investigations by several groups have indicated that colonization of the human nose by SA is influenced by bacterial determinants including sortase A (SrtA),12,13 clumping factor B (ClfB),14,15 tagX,16 and enterotoxins.17-19 However, this may not represent the complete repertoire of bacterial factors necessary for nasal colonization in humans.20,21
Our previous studies on a nasal carrier strain of SA indicate that it possesses several colonization advantages in comparison to its genetically similar non-carrier counterpart.11 These advantages included downregulation of host HBD-2, HBD-3 and interleukin-1 (IL-1), along with the ability to form biofilms.11,22 Biofilm-producing strains of SA exhibit higher survival rates against not only antibiotic drugs, but also against natural AMPs present in the host’s nasal mucosa.22,23 Interestingly, the formation of protective biofilms was not evident in the non-carrier strain.22 Collectively, these findings led us to postulate that the origin of factors, which facilitated nasal colonization by SA carrier strains, are present at the primary interface between the host and the pathogen, namely the bacterial surface and freely secreted proteins collectively termed the exoproteome.
Using high throughput gel-free proteomics in concert with 2D-PAGE, we determined the repertoire of proteins contained within the exoproteome of a successful nasal carrier strain of SA in comparison to its genetically similar non-carrier counterpart. Analysis of these differences revealed putative determinants of nasal carriage. For the first time, we also compared the exoproteome of the biofilm form of the carrier strain of SA with its planktonic counterpart to assess its contribution to successful nasal carriage. Exoproteome analysis by 2D-PAGE revealed a marked difference in the distribution of proteins between carrier and non-carrier SA strains. Subsequent isobaric tagging for relative and absolute quantification (iTRAQ)24 confirmed these findings, revealing that the carrier strain of SA expressed a greater number of proteins involved in cell attachment and immunoevasion than the non-carrier strain. On closer examination of the most abundant immunomodulatory proteins, we found that Staphylococcal protein A (SPA), known to be involved in SA virulence and biofilm formation,25-27 was secreted in significantly greater amounts by SA nasal carrier strains compared to the non-carrier strain. This may indicate a relationship between SPA and the carriage status of SA. By comparing the exoproteomes between a successful carrier strain of SA and a non-carrier strain, we have identified individual proteins and functional classes of proteins that may determine the nasal carriage status of SA.
Materials and Methods
Bacterial strains and culture conditions
SA strain D30 that has been extensively characterized,5,11,21 was originally isolated from the anterior nares of a healthy donor, and served as the carrier strain for the experiments herein. SA strain 930918-3, (from Ian Holder, Shriners Burn Hospital, Cincinnati, Ohio, USA), which is genetically similar to the carrier strain D30, and has been extensively characterized,21 served as the non-carrier strain of SA in these experiments. Additionally, persistent SA carrier strains D20, D98, D39 and intermittent carrier strain D37 were used in this study.5 SA was cultivated on Tryptic Soy Agar (TSA; Bacto™, Becton, Dickinson and Company, MD, USA) and subcultured in Trypticase Soy Broth (TSB; Bacto™, Becton, Dickinson and Company, MD, USA), from which stocks were prepared. For all experiments, snap-frozen (-80°C) stocks of SA were thawed rapidly and cultured at 37°C. Levels of inocula were estimated by measuring the absorbance of a washed bacterial suspension in PBS (Mediatech Inc., VA, USA) at 625 nm. An OD at 625 nm of 0.1 approximated 2.0 × 107 CFU/ml. Inocula were quantitated by spreading 10μl aliquots of the liquid culture on TSA and enumerating CFU following 18 hrs incubation at 37°C.11,22
Preparation of carrier strain biofilms
SA carrier strain snap-frozen culture was incubated in TSB media and allowed to grow until stationary phase (6-8 h). The stationary culture was transferred to flasks containing 50 ml TSB at a 1:100 dilution and incubated without shaking at 25°C for 3 days to promote biofilm formation. Biofilms were subsequently washed three times with 0.85% NaCl and incubated overnight in ambient PBS. The supernatant was then centrifuged and used for exoproteome purification.22
Preparation of protein extracts and 2D-PAGE
2D-PAGE was used to analyze exoproteomes from the carrier strain D30 and non-carrier strain 930918-3. Based on the growth kinetics of our SA strains, a modified approach from Dreisbach and colleagues was utilized for SA exoproteome preparation.28 Briefly, one liter of bacterial culture was grown for 24 hrs at 250 rpm, 37°C, centrifuged and resuspended in 40 ml of PBS. Protease and phosphatase inhibitors (Halt™ protease and phosphatase single-use inhibitor, Pierce, IL, USA) were added to the culture and shaken overnight and the resulting supernatant was collected and filter (0.2μm) sterilized. The extract was further purified by a C18 SepPak (Waters Corporation, MA, USA) column according to the manufacturer’s instructions, except that the final elution was in 80% acetonitrile/ 20% HPLC grade water. The sample was dehydrated to 10μl and diluted with 100μl of HPLC grade water. The total protein concentration of the sample was estimated using Micro BCA™ Protein Assay Kit (Thermo Scientific, Pierce, IL, USA) according to the manufacturer’s instructions.29-32 Approximately 400μg of total protein was subjected to 2D-PAGE analysis as per the manufacturer’s protocol (BioRad, CA, USA). The sample was prepared in rehydration sample buffer I (8 M urea, 2% CHAPS, 50 mM DTT (dithiothreitol) 1x ReadyStrip buffer 0.1–0.4% (w/v) Bio-Lyte ampholytes) and absorbed onto a ReadyStrip IPG gel strip, pH 4-7 (BioRad, CA, USA). The strip was then subjected to isoelectric focusing (IEF) at 50 VμA per strip, initially at 250V for 15 minutes and then ramping to 10,000V in 3 hours followed by an additional focusing for 12–16 hrs at 20°C until 60,000 volt-hours was reached. Strips were rinsed in glycine gel running buffer and soaked in Equilibration Buffer I (6 M urea, 0.375 M Tris, pH 8.8, 2% SDS, 20% glycerol, 2% (w/v) DTT) for 5 minutes followed by Equilibration Buffer II (6 M urea, 0.375 M Tris, pH 8.8, 2% SDS, 20% glycerol, and 2.5% (w/v) iodoacetamide) for 5 minutes. The gel strips were then rinsed in SDS gel buffer and resolved by a 2nd dimension using a precast BioRad Ready Gel Tris-HCl, 4–20% linear gradient at 40 mA for 5 hrs. The gels were subsequently stained using silver nitrate.
Protein digestion, labeling with iTRAQ reagents and On-Line 2D NanoLC-MS/MS
Approximately 120 μg of the exoproteome sample from the planktonic SA carrier strain D30, the planktonic non-carrier strain 930918-3 and the carrier strain D30 biofilm were subjected to overnight acetone precipitation at −20°C. Samples were resuspended in dissolution buffer, alkylated, trypsin-digested at 37°C overnight and labeled with iTRAQ 4plex Reagents kit as per the manufacturer’s instruction (AB Sciex Inc., CA, USA). iTRAQ tags were applied as follows: iTRAQ 114 = Carrier strain D30 biofilm, iTRAQ 115 = planktonic non-carrier strain 930919-3, iTRAQ 116 = planktonic carrier strain D30, iTRAQ 117 = planktonic carrier strain D30. The four iTRAQ samples were mixed, lyophilized, resuspended in Strong Cation Exchange (SCX) solvent, and subjected to LC-MS/MS analysis of each peak fraction by QSTAR ESI quadrupole time-of-flight tandem MS system (Applied Biosystems, CA, USA).24,33,34 Complete iTRAQ analyses were performed three independent times for each sample tested.
iTRAQ Data Analysis
Raw MS data processing, protein identification, quantification and subsequent statistical analyses were performed using the Paragon™ algorithm35 of ProteinPilot version 2.0.1 software (AB Sciex Inc., CA, USA). Comprehensive searches against the National Center for Biotechnology Information (NCBI) bacterial database with biological modifications and amino acid substitutions were performed to identify the proteins. Additionally, parameters such as cysteine alkylation by MMTS, iTRAQ modification of N-terminal peptide residues, modifications of lysine and tyrosine residues were considered during the analyses, as well as other default parameters were considered by the software. Further classification and analyses of the identified proteins were performed using the ProGroup™ algorithm. This algorithm enabled confident protein identification using the least set of identified peptides based on total protein scores that is generated from peptide confidence scores. Scores higher than a 95% confidence level were used for identifying proteins. Mean, Standard Deviation (S.D) and p values generated by the ProGroup algorithm were used for relative protein quantification between the samples. iTRAQ fold-ratio >1.2 and <0.8 combined with a p value < 0.05 was used to determine differential protein expression between the samples. These cutoff values for variation in the expression, recommended by the Paragon™ algorithm, are a widely accepted fold-ratio.34,36-42 Proteins identified with one distinct contributing peptide were subjected to manual validation by the assessment and confirmation of their MS/MS spectra (Supplemental Fig S1).24,33,35,43
Anti-SPA ELISA
The expression of protein A (SPA) was quantified using the Assay Designs™ protein A Enzyme Immunometric Assay (EIA) kit (Enzo Life Sciences International, PA, USA) as per the manufacturer’s instructions with minor modifications. Briefly, exoproteome samples from 1L of stationary phase cultures (~2.0 × 1010 CFU/ml) of nasal carrier, non-carrier and epidemic strains were prepared as mentioned previously and equal volumes (25μl) at dilutions 1/1×105, 1/2 ×105, 1/4 ×105 and 1/16 ×105 were subjected to assay analysis. The concentration of SPA was calculated against standards according to manufacturer’s instructions and represented as μg/ml of total protein. A one-tailed Student’s t-test was used to measure the significance of SPA expression between SA strains.
Results
Comparative analyses of SA exoproteomes reveal differences in the distribution pattern of proteins between nasal carrier and non-carrier strains
Since the first interface between host and pathogen is the bacterial exoproteome, we hypothesized that a comparison between a carrier and a genetically similar non-carrier SA strain exoproteome would reveal key differences that could be important in nasal colonization of SA. To screen initially for these differences we used 2D-PAGE and observed significant differences in the distribution of secreted proteins between nasal carrier and non-carrier strains as typified by the distribution of MS/MS-verified SPA, (49.5 kDa). Additionally, multiple isoforms of SPA were exclusively detected in the carrier strain (Fig 1).
FIG 1. Comparative exoproteome analysis of carrier (D30) and non-carrier (930918-3) strains of SA reveals significant differences in protein distribution.
The distribution pattern of the exoproteomes of genetically similar strains of SA differed from the carrier to the non-carrier strain as evidenced by the distribution of Staphylococcal protein A (SPA) 49.5 KDa (circled). SPA spots were confirmed by ELISA and Mass spectrometry.
Although 2D-PAGE analysis displayed a majority of the proteins in carrier (D30) and non-carrier (930918-3) exoproteomes and their distribution, a more thorough quantitative approach was required in order to identify low abundance and hydrophobic proteins. To this end, we employed a gel-free system known as iTRAQ coupled with mass spectrometry to provide quantitative as well as qualitative analysis of the differences between the carrier and non-carrier strain of SA.
iTRAQ-coupled LC-MS/MS analyses of identified a total of 488 proteins in the aggregate SA exoproteome
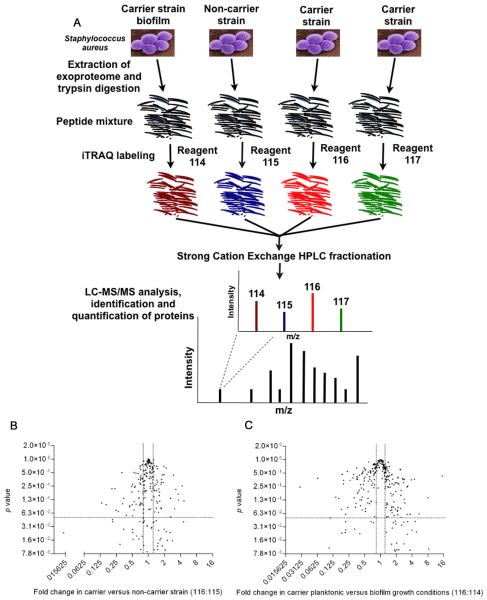
An experimental workflow for the analysis of SA exoproteomes by iTRAQ is described in Fig 2A. Briefly, purified proteins were subjected to trypsin-digestion and labeled with iTRAQ reagents. Peptides from SA carrier strain (D30) exoproteome were labeled with iTRAQ reagents 116 and 117 whilst peptides from the non-carrier strain (930918-3) and carrier strain (D30) biofilm exoproteomes were labeled with iTRAQ reagents 115 and 114, respectively. These iTRAQ-labeled samples were then mixed, lyophilized and fractionated using Strong Cation Exchange (SCX) chromatography. Subsequently, SCX fractions were subjected to LC-MS/MS analysis (Fig 2A). The entire set of experiments from the collection of the exoproteome to LC-MS/MS analysis was performed 3 independent times (N=3). LC-MS/MS analysis of 21 SCX fractions identified a total of 488 proteins (95% confidence, unused score >1.3) from 5970 distinct peptides (supplemental Table S2 and S3). In addition, the identified proteins’ expression levels were significantly different between carrier strain, non-carrier strain and carrier strain biofilm (p<0.05) conditions as depicted by the volcano plot illustration of iTRAQ data (Fig 2B and 2C).
FIG 2. Integrated experimental workflow for the analysis of SA exoproteome by iTRAQ and volcano plot illustration of iTRAQ data.
(A) The exoproteome of the carrier strain, the non-carrier strain and a carrier strain biofilm (growth media free) were extracted, purified and concentrated with the aid of C18 solid phase extraction cartridges. Proteins were subject to trypsin-digestion and labeled with iTRAQ reagents. Digests from the carrier strain (D30) exoproteome were labeled with iTRAQ reagents 116 and 117 whilst digests from the non-carrier strain (930918-3) exoproteome and carrier strain (D30) biofilm were labeled with iTRAQ reagents115 and 114 respectively. Labeled samples were mixed, lyophilized and fractionated using Strong Cation Exchange (SCX) chromatography. The HPLC fractions were analyzed by LC-MS/MS on a QSTAR ESI quadruple time-of-flight tandem MS system. The entire set of experiments from the collection of the exoproteome to LC MS/MS analysis was performed 3 times (N=3) and the raw MS/MS data were collated together for further analysis. (B) Protein expression fold change in SA carrier strain as compared to non-carrier strain. (C) Protein expression fold change in SA carrier strain under planktonic and biofilm growth conditions. Fold differences of protein expression are plotted against their respective p values. All proteins identified by at least one peptide with greater than 95% confidence are represented here. Horizontal dashed lines identify fold changes with p values of 0.05. Vertical dashed lines delineate a 2-fold change in the ratio of protein expression.
Representative iTRAQ ion spectra and MS/MS spectra showing protein identification and quantification of selected proteins from carrier and non-carrier strains are illustrated in Fig 3. Fig 3A reveals representative MS/MS and iTRAQ ion spectra of a uniquely identified peptide (99% confidence) from immunoglobulin G binding protein A precursor. iTRAQ analysis revealed a >2.2 fold increase in expression of this protein between carrier D30 (iTRAQ label 116) and non-carrier 930918-3 (iTRAQ label 115) strains. Fig 3B and Fig 3C illustrate the iTRAQ ion spectra and MS/MS spectra for the proteins ABC transporter substrate-binding protein and autolysin revealing lower and equal expression levels, respectively, between carrier strain D30 (iTRAQ label 116) and non-carrier strain 930918-3 (iTRAQ label 115). The corresponding amino acid sequences of the peptides depicted in Fig 3 are given in supplemental Table S1.
FIG 3. Representative iTRAQ MS/MS spectra showing protein identification in carrier and non-carrier strains from selected proteins.
Representative MS/MS spectra of uniquely identified peptides (confidence, 99%) from (A) Immunoglobulin G binding protein A precursor (B) ABC transporter, substrate-binding protein and (C) Autolysin are represented here. The inset reveals the iTRAQ reagent peaks for relative quantitation in the SA strains. Illustrations for higher, lower and equal expression levels between carrier strain D30 (iTRAQ label 116) and non-carrier strain 930918-3 (iTRAQ label 115) have been provided.
The nasal carrier strain of SA expresses a greater number of proteins implicated in colonization than its non-carrier counterpart
The proteins identified in the exoproteomes were categorized with reference to their potential contribution to nasal carriage as conceived by Burian and colleagues.44 These included proteins involved in metabolism, protein synthesis and trafficking, stress, pathogenesis and immunomodulation, cell adhesion, cell division and cycle, transport and unknown functions (supplemental Table S1). iTRAQ analyses revealed that the carrier strain of SA expressed a markedly different repertoire of proteins in comparison to the non-carrier strain (Fig 4A and 4B). We observed that 131 proteins were differentially expressed between carrier and non-carrier strain exoproteomes. Of these 131 proteins, 66 were expressed in higher amounts in the carrier strain of SA compared to the non-carrier strain, and 25 of these proteins exhibited a >2-fold increase in expression levels (see Table 1 for a partial list of proteins and supplemental Table S2 for the complete list).
FIG 4. The nasal carrier strain of SA expresses a greater number of adhesion/ binding proteins in its exoproteome than its non-carrier counterpart.
A comparison of SA exoproteomes using iTRAQ has revealed that (A) the nasal carrier strain of SA (D30) expresses a different repertoire of proteins than (B) a genetically similar non-carrier strain (930918-3). (C) Percentage of proteins that were significantly up-regulated in that category in comparison to the other tested strain. The carrier strain of SA contains a greater proportion of proteins related to binding and adhesion but less stress proteins. Proteins were identified with high confidence (p <0.05) in three independent experiments (N=3).
TABLE 1.
Proteins from the exoproteome of the nasal carrier strain of SA and the non-carrier strains are assigned into functional categories as identified and quantified by iTRAQ
| Accession No. |
Protein name | Number of peptidesa |
Fold ratiob |
|||
|---|---|---|---|---|---|---|
| carrier/ non-carrier |
p value | Planktonic carrier/ Biofilm carrier |
p value |
|||
| Stress related proteins | ||||||
| gi|87162409 | CsbD-like superfamily | 34 | 0.4565 c | 0 | 1.3708 d | 0.0058 |
| gi|87162159 | Hypothetical protein SAUSA300_1582, similar to CsbD- like family protein |
13 | 0.6747 | 0 | 2.0123 | 0.0135 |
| gi|87162200 | Alkyl hydroperoxide reductase subunit C | 27 | 1.1686 d | 0.0002 | 1.6239 | 0.0128 |
| gi|87161642 | Alkyl hydroperoxide reductase subunit F | 1 | 0.4971 | 0.0957 | 0.3723 | 0.188 |
| gi|87160786 | Hypothetical protein SAUSA300_1652, putative universal stress protein |
24 | 0.862 | 0.0006 | 0.8978 | 0.2323 |
| gi|87162087 | Universal stress protein family | 18 | 2.9027 | 0.0004 | 3.0664 | 0 |
| gi|70726220e | Hypothetical protein SH1219, putative universal stress protein |
7 | ||||
| gi|894289 | Alkaline shock protein 23 (ASP23) | 20 | 0.5771 | 0 | 1.1024 | 0.6569 |
| gi|87160079 | Peptide methionine sulfoxide reductase regulator (MsrR) | 10 | 0.8648 | 0.1957 | 1.7545 | 0.0863 |
| gi|87161086 | Methionine-R-sulfoxide reductase | 1 | 1.238 | 0.8192 | 15.0177 | 0.4261 |
| gi|87161236 | Thioredoxin* | 12 | 1.3978 | 0.0003 | 0.7305 | 0.0387 |
| gi|87161001 | Thioredoxin-disulfide reductase | 9 | 0.792 | 0.169 | 1.1776 | 0.6298 |
| gi|87161687 | Thiol peroxidase | 8 | 0.756 | 0 | 1.4239 | 0.2404 |
| gi|21282513 | Hypothetical protein MW0784, similar to thioredoxin-fold containing protein family |
5 | 0.8983 | 0.0001 | 1.2352 | 0.3626 |
| gi|87160477 | Putative thioredoxin | 5 | 1.218 | 0.2208 | 1.2503 | 0.6608 |
| gi|87160405 | Hypothetical protein SAUSA300_1909, similar to thioredoxin family of proteins |
4 | 0.987 | 0.9858 | 0.7007 | 0.628 |
| gi|87160511 | Catalase | 5 | 0.5021 | 0.027 | 4.7999 | 0.0241 |
| gi|87161707 | Superoxide dismutase (Mn/Fe family) | 5 | 0.9286 | 0.4111 | 0.7623 | 0.7832 |
| gi|88195790 | Putative ferritin | 4 | 0.7199 | 0.5798 | 0.4058 | 0.1265 |
| gi|87162273 | Osmc/Ohr family protein | 1 | 2.1538 | 0.6357 | 2.8528 | 0.3618 |
| gi|87160980e | Hypothetical protein SAUSA300_0725, similar to putative Oxidoreductase |
1 | 0.5794 | 1.3866 | ||
| Pathogenesis and immunomodulatory proteins | ||||||
| gi|87160749 | Cell surface elastin binding protein | 200 | 1.7517 d | 0 | 1.3029 | 0.0961 |
| gi|133853458 | Immunoglobulin G binding protein A precursor | 138 | 2.2282 | 0 | 1.0756 | 0.3757 |
| gi|56749001 | Immunodominant staphylococcal antigen A precursor | 57 | 1.4061 | 0 | 0.9476 | 0.656 |
| gi|15926764 | Penicillin-binding protein 1 | 43 | 1.3495 | 0.0004 | 1.4429 | 0.0048 |
| gi|87162077 | Penicillin binding protein 2 | 32 | 0.6419 | 0.0001 | 0.6655 | 0.1167 |
| gi|87161157 | Penicillin-binding protein 4 | 6 | 1.6283 | 0.1152 | 2.7796 | 0.013 |
| gi|70726765 | Beta-lactamase | 10 | 0.0849 | 0 | 0.1207 | 0.0022 |
| gi|87161577 | Cold shock protein, CSD family | 36 | 0.7651 c | 0 | 0.7602 | 0.0001 |
| gi|87160015 | Staphylococcal tandem lipoprotein | 14 | 0.4398 | 0.0002 | 1.1702 | 0.4995 |
| gi|47169194e | Chain A, staphylococcal protein A, B-domain, Y15W mutant, Nmr, 25 structures |
12 | 0.9803 | |||
| gi|87160380 | Alpha-hemolysin precursor | 10 | 2.6865 | 0 | 1.1949 | 0.4484 |
| gi|87160982 | Leukocidin/hemolysin toxin family protein | 10 | 0.3131 | 0 | 0.7128 | 0.2953 |
| gi|87161881 | Antibacterial protein | 7 | 0.5416 | 0 | 0.8559 | 0.5626 |
| gi|15927581 | Hypothetical protein SA1813 similar to leukocidin-like protein 2 |
5 | 0.3607 | 0 | 1.1987 | 0.519 |
| gi|87162162 | Hypothetical protein SAUSA300_1018 similar to SCP/PR1-like extracellular protein |
8 | 1.0669 | 0.5321 | 0.6697 | 0.3322 |
| gi|87160365 | Antibacterial protein | 4 | 0.9121 | 0.1981 | 0.2771 | 0 |
| gi|87162347 | Hypothetical protein SAUSA300_2164 similar to extracelluar adherence protein |
4 | 0.3271 | 0.1009 | 0.1106 | 0.0494 |
| gi|87160217 | Secretory Antigen Precursor (SsaA) | 4 | 0.9951 | 0.9764 | 0.5127 | 0.069 |
| gi|88194063 | Hypothetical protein SAOUHSC_00257 similar to EsxA virulent protein |
4 | 0.384 | 0.0557 | 2.158 | 0.2965 |
| gi|68565538 | Protein esaA | 2 | 0.2372 | 0.2204 | 0.197 | 0.2859 |
| gi|87160905e | Hypothetical protein SAUSA300_0282 similar to virulence protein EssB |
1 | 0.2942 | 0.4179 | ||
| gi|87162375 | Hypothetical protein SAUSA300_1323 similar to conserved virulence factor C |
2 | 0.809 | 0.5491 | 0.1985 | 0.3431 |
| gi|87160520 | Acetyltransferase family protein in Oat A family | 3 | 2.0482 | 0.0094 | 0.8063 | 0.7857 |
| gi|87161173 | Teicoplanin Resistance Associated Membrane Protein (TcaA) |
3 | 0.8209 | 0.9069 | 1.4697 | 0.7023 |
| gi|88195687 | Hypothetical protein SAOUHSC_01999 similar to peroxiredoxin Q/BCP |
3 | 2.9885 | 0.0002 | 1.9644 | 0.0359 |
| gi|87162379e | Ferredoxin | 1 | 1.1786 | 1.8553 | ||
| gi|87161897 | IgG-binding protein SBI | 2 | 3.6719 | 0.1219 | 5.4157 | 0.0616 |
| gi|87160565e | Immunodominant antigen B | 1 | 29.6467 | 2.0875 | ||
| gi|62391257e | Secreted penicillin binding protein [Corynebacterium glutamicum ATCC 13032] |
1 | 1.6876 | 2.2857 | ||
| Cell adhesion proteins | ||||||
| gi|87160939 | Cell wall surface anchor family protein | 57 | 3.6116 d | 0 | 1.2777 | 0.0838 |
| gi|151222604 | Hypothetical protein NWMN_2392 similar to cell wall surface anchor family protein |
40 | 0.0911 c | 0 | 0.2444 | 0 |
| gi|87162315 | Putative cell wall binding lipoprotein | 16 | 1.2742 | 0.1446 | 1.1145 | 0.7887 |
| gi|87162026 | Autolysin | 31 | 1.0742 | 0.6135 | 0.7695 | 0.2717 |
| gi|87160697 | D-alanine-activating enzyme/D-alanine-D-alanyl protein (dltD) |
18 | 1.2272 | 0.2515 | 1.9912 | 0.0001 |
| gi|87160121 | D-alanine-activating enzyme/D-alanine-D-alanyl protein (dltC) |
1 | 0.6864 | 0.533 | 16.1543 | |
| gi|61213890 | 77 kda outer membrane protein precursor | 11 | 0.2627 | 0 | 0.6572 | 0.1167 |
| gi|81781509 | UPF0365 protein SAV1573 | 8 | 1.8364 | 0.0005 | 2.9173 | 0.0019 |
| gi|87160285 | Rod shape-determining protein MreC | 8 | 0.8103 | 0.0767 | 2.9577 | 0 |
| gi|87160775 | N-acetylmuramoyl-L-alanine amidase | 8 | 1.5402 | 0.0651 | 2.0519 | 0.0096 |
| gi|87161887 | N-acetylmuramoyl-L-alanine amidase domain protein | 7 | 1.893 | 0.3018 | 1.3838 | 0.4234 |
| gi|88196468 | Putative sortase | 5 | 2.0829 | 0.001 | 1.2683 | 0.5337 |
| gi|87161790 | 5′-nucleotidase family protein | 3 | 3.6218 | 0.1664 | 1.5688 | 0.1773 |
| gi|87160715 | Fmt protein | 2 | 1.1273 | 0.8064 | 1.1849 | 0.6709 |
| gi|81673756 | Phosphoglucosamine mutase | 1 | 0.7583 | 0.739 | 3.3119 | 0.2556 |
| gi|87160798 | Serine-aspartate repeat family protein (SdrH) | 1 | 1.49 | 0.0115 | 1.3251 | 0.4213 |
| gi|116694144e | Flp pilus assembly protein TadC | 1 | 1.3693 | 2.585 | ||
| gi|81781921 | Extracellular matrix protein-binding protein EMP precursor |
1 | 0.5809 | 0.4841 | 1.4533 | 0.5086 |
| gi|91211353e | AsmA suppressor of OmpF assembly mutants | 1 | ||||
| Transport proteins | ||||||
| gi|87162197 | Amino acid ABC transporter, amino acid-binding protein | 34 | 0.9535 | 0.4842 | 1.3001 | 0.0518 |
| gi|87162140 | Oligopeptide ABC transporter, substrate-binding protein |
30 | 1.1547 d | 0.0301 | 1.3918 | 0.0533 |
| gi|87161352 | ABC transporter, substrate-binding protein | 12 | 0.2931 | 0.0126 | 0.4137 | 0.092 |
| gi|21282147 | Hypothetical protein MW0418 similar to ABC transporter, substrate-binding protein |
6 | 1.0242 | 0.8594 | 1.2602 | 0.507 |
| gi|87161864 | ABC transporter, substrate-binding protein | 3 | 2.8266 | 0.0151 | 2.1079 | 0.0742 |
| gi|87160588 | Molybdenum ABC transporter, molybdenum-binding protein (ModA) |
17 | 1.5245 | 0.0005 | 1.8056 | 0.0558 |
| gi|87161641 | Amino acid ABC transporter, permease/substrate-binding protein |
7 | 6.1167 | 0 | 2.5162 | 0.0341 |
| gi|21284120 | Oligopeptide transporter putative substrate binding domain |
6 | 2.4913 | 0.0004 | 1.9632 | 0.1937 |
| gi|87161764 | Putative iron compound ABC transporter, iron compound- binding protein |
5 | 1.4063 | 0.0805 | 0.7301 | 0.561 |
| gi|87160849 | Iron compound ABC transporter, iron compound-binding protein |
4 | 0.5869 | 0.0986 | 0.6344 | 0.5184 |
| gi|87161518 | Glycine betaine/carnitine/choline ABC transporter | 4 | 0.9366 | 0.6998 | 1.0605 | 0.8404 |
| gi|87162224 | Osmoprotectant ABC transporter, permease | 2 | 0.6537 | 3.9979 | ||
| gi|126355053e | ABC transporter-related protein [Pseudomonas putida GB-1] |
1 | 37.5193 | 6.929 | 0.0699 | |
| gi|149194563e | ABC transporter-related protein [Caminibacter mediatlanticus TB-2] |
1 | 1.6215 | 0.0083 | ||
| gi|87162212e | Amino acid ABC transporter, ATP-binding protein | 1 | 5.1042 | 0.571 | ||
| gi|87161315 | Hypothetical protein SAUSA300_2378 similar to potassium efflux protein kefA |
21 | 1.4953 | 0 | 1.6492 | 0.0873 |
| gi|87160965 | Phosphocarrier protein (HPr) | 11 | 1.1099 | 0.0026 | 0.4598 | 0 |
| gi|87162382 | PTS system, glucose-specific IIA component | 11 | 1.0668 | 0.4928 | 2.9434 | 0.004 |
| gi|87162442 | Transferrin receptor | 8 | 3.331 | 0 | 3.5596 | 0.0002 |
| gi|87160279 | AcrB/AcrD/AcrF family protein | 7 | 1.4716 | 0.1045 | 1.0728 | 0.9096 |
| gi|87162284 | Putative ferrichrome ABC transporter | 1 | 0.2468 | 0.0722 | 0.5068 | 0.6105 |
| gi|87161389 | Putative iron compound ABC transporter, iron compound- binding protein |
1 | 2.6052 | 0.134 | 1.4787 | 0.3341 |
| gi|87160515 | Protein-export membrane protein SecF | 6 | 1.4937 | 0.0097 | 0.7504 | 0.4761 |
| gi|87160369 | Hypothetical protein SAUSA300_0833 | 3 | 0.5373 | 0.5051 | 0.4642 | 0.5479 |
| gi|15925912 | RGD-containing lipoprotein | 3 | 1.2004 | 0.4097 | 1.0422 | 0.9709 |
| gi|87161142 | Ferric hydroxamate receptor | 3 | 1.0332 | 0.8931 | 0.4321 | 0.2314 |
| gi|87160674 | Putative lipoprotein | 41 | 0.2959 c | 0 | 0.7197 | 0.0011 |
| gi|87161872 | Putative lipoprotein | 2 | 4.2798 | 0.2519 | 2.4403 | 0.1588 |
| gi|87160414 | Multidrug resistance protein A, drug resistance transporter |
1 | 0.6934 | 0.2928 | 0.7756 | 0.7943 |
| gi|23005821e | COG1131: ABC-type multidrug transport system, ATPase component |
1 | 0.6174 | |||
| gi|149201149 | Nitrate transport ATP-binding subunits C and D | 1 | 1.5776 | 7.9917 | 0.1401 | |
| gi|151575108 | Outer membrane efflux protein | 1 | 1.1986 | 0.2951 | 3.8451 | 0.3525 |
| gi|87161139 | Iron transport associated domain protein | 1 | 1.6828 | 0.1596 | ||
| gi|149910101 | Hypothetical transport protein [Moritella sp. PE36] | 1 | 2.2325 | 0 | 4.2379 | 0 |
| gi|35211526e | gll0963 [Gloeobacter violaceus PCC 7421] | 1 | ||||
| gi|127512243e | Efflux transporter, RND family, MFP subunit | 1 | ||||
| gi|146301866e | RND efflux system, outer membrane lipoprotein, NodT family |
1 | 0.9695 | 0.7073 | 0.4187 | |
| gi|17131745e | all2652 | 1 | 1.7839 | 0.8275 | ||
| gi|87162344 | Phosphonate ABC transporter, phosphonate-binding protein |
1 | 1.3653 | 0.3305 | 6.3883 | 0.0356 |
| gi|51595518 | Molybdenum transport regulatory (repressor) protein (ModE) |
1 | 1.3022 | 0.3562 | 1.1322 | 0.9636 |
| gi|152936446e | Flagellar motor switch protein fliG | 1 | ||||
The list contains quantitative information of the proteins (including the number of peptides) from the iTRAQ data set. These proteins have met the criteria (i.e., unused prot score >2.0, change in expression levels of at least 1.2-fold p-value <0.05 and EF <1.4 for all ratios) as defined in the Experimental Procedures.
Relative change in protein levels between secretomes
italicized numbers= down-regulation.
bold numbers =up-regulation.
iTRAQ identified unique proteins not quantified.
Denotes a protein with multiple functions.
i.) CELL ADHESION
Many adhesive proteins such as clumping factor B (ClfB), fibronectin-binding protein A (FnBPA), and iron-regulated surface determinant A (IsdA) are involved in binding of the bacteria to host matrix molecules and hence are thought to play key roles in nasal colonization of SA.45 Our exoproteomic analyses revealed that additional adhesive proteins might play critical roles in colonizing the human nasal mucosa (Table 1). We observed that the SA carrier exoproteome contained a markedly larger number of adhesive proteins than the non-carrier strain (Fig 4C). One of the major proteins involved in host attachment, the cell wall surface anchor family protein (SasD), demonstrated nearly 4-fold higher expression in the carrier compared to the non-carrier strain (Table 1). iTRAQ analysis also detected significantly higher levels of two proteins in the carrier strain that have previously been implicated in nasal colonization: serine-aspartate repeat family protein (SrdH) and sortase (Srt) (Table 1). SasD and SrdH are two cell wall-anchored proteins containing host attachment domains (MSCRAMMs).46,47 These MSCRAMM proteins are covalently anchored to the host adhesive matrix molecules via the LPXTG motif recognized by sortase.13 SasD contains a slightly modified LPXAG anchor to host matrix molecules.
ii.) IMMUNOMODULATORY PROTEINS AND TOXINS
Immunomodulatory proteins play critical roles in SA infection of the host and subsequent nasal carriage.48 Based on a comprehensive literature survey of the functions of these proteins, our results indicated that the carrier strains of SA contained a greater number of proteins which downregulate immunity, whereas the non-carrier strains contained a greater number of proteins which upregulate host immunity. Some of the most noticeable immunomodulatory proteins detected in greater abundance in the carrier strain were SPA and surface elastin binding protein (EbpS) (Table I). On the other hand, penicillin binding protein (PBP) 2, and cold shock protein (Csp) that makes SA susceptible to antibiotics and antimicrobial peptide human cathepsin G respectively,49,50 were found in higher levels in the non-carrier strain.
Immunoevasive proteins including cell surface elastin binding protein (EbpS), SPA, PBP1, immunodominant staphylococcal antigen A precursor and α-hemolysin precursor identified in our study have also been independently observed in other studies.28,43,51-53 However, their roles with relevance to nasal colonization have not been elucidated. Notably, we observed that the expression levels of immunomodulatory toxins such as α-hemolysin precursor that are responsible for bacterial invasion of the host, were elevated in the carrier strain. Interestingly, leukocidin/hemolysin toxin family protein that helps in the formation of pores during SA pathogenesis of host was dominant in the non-carrier strain (Table 1).
iii.) TRANSPORT PROTEINS
S. aureus carrier strains can resist the host’s antimicrobial agents by active efflux of the agents using translocation machineries.54 This phenomenon is mirrored in our results in which the nasal carrier strain produced substantially more transport proteins compared to its non-carrier counterpart (Fig 4C, Table 1). These included several proteins from the ATP-binding cassette (ABC) transporter super family and one from the common protein translocation machinery, Sec translocon (Table 1). Sec translocon proteins are implicated in bacterial pathogenesis and in the secretion of virulence proteins.53 We observed that a key member of this translocon, protein-export membrane protein SecF that is part of the bifunctional translocase SecDF, was found in higher levels in the carrier strain indicating the likely involvement of these transport proteins in nasal colonization.
iv.) STRESS
S. aureus, when subjected to osmotic shock, heat, oxidative stress, starvation, alkaline shock etc, triggers a stress response.55 Our iTRAQ data revealed that the carrier strain of SA secreted fewer stress response proteins in comparison to its non-carrier counterpart. Specifically, stress proteins such as CsbD-like superfamily and alkaline shock protein 23 (ASP23) were expressed in lower levels in the carrier strain (Table I). However, not all stress proteins were down-regulated in the carrier strain. Some stress proteins that are normally expressed when SA is exposed to environmental stress such as superoxide dismtase (SOD) and thioredoxin-disulphide reductase were expressed at similar levels by both strains (Table I).
The SA nasal carrier biofilm exoproteome contains greater number of stress and immunoevasion proteins than its planktonic counterpart
We previously reported that the carrier strain of SA (D30) adopted a biofilm mode of growth under ambient laboratory conditions.22 We hypothesized that biofilm formation may facilitate nasal carriage, given the importance of this form of growth to colonization and defensive capabilities of SA.56 We therefore compared the biofilm exoproteome of a carrier strain of SA with its planktonic counterpart. To our knowledge, this was the first time such a comparison has been reported for the respective exoproteomes. We observed that 84 proteins were differentially expressed between planktonic and biofilm growth forms of the carrier strain of SA. Of the 84 proteins, 46 were expressed at higher amounts in the biofilm exoproteome compared to its planktonic counterpart and 35 exhibited >2-fold expression (See Table 1 for partial list of proteins and supplemental Table S3 for the complete list).
It was observed that the exoproteome of the biofilm form of the nasal carrier strain of SA was markedly different in terms of stress and immunomodulatory proteins compared to its planktonic counterpart (Fig 5A and 5B). Specifically, iTRAQ analyses revealed that the carrier strain biofilm had 4-fold fewer adhesive proteins as compared to the planktonic form (Fig 5C). Specifically, D-alanine-activating enzyme (DltD) protein, involved in D-alanylation of wall techoic acid (WTA) during cell wall synthesis, was significantly down-regulated in the biofilm exoproteome.57 Another protein involved in cell wall peptidoglycan synthesis, N-acetylmuramoyl-L-alanine amidase, was also significantly down-regulated in the biofilm form, perhaps indicating a lower cell wall turnover in the SA carrier strain when it adapts a biofilm mode of growth.
FIG 5. The biofilm growth form of nasal carrier strain of SA, in comparison to its planktonic counterpart contains marked differences in its exoproteome related to stress and immunoevasion.
The expression of proteins from the exoproteome of (A) the planktonic form of SA nasal carrier strain and (B) the biofilm form of the SA nasal carrier strain differ significantly in (C) proteins pertaining to stress, adhesion, immunomodulation and transport. Proteins were identified with high confidence (p <0.05) in three independent experiments (N=3).
Adhesive proteins, together with immunomodulatory proteins, enable biofilms to resist the action of antibiotics and other antimicrobial agents.58,59 Not surprisingly, our iTRAQ analyses revealed that the biofilm growth form of the carrier strain of SA secreted more immunomodulatory proteins than the planktonic form (Fig 5C). Curiously, penicillin binding proteins (PBPs), including PBP1 and PBP4 that enable SA to resist β-lactam antibiotics, were expressed in lower levels in the biofilm growth mode. Another mechanism that allows bacterial biofilms to evade antimicrobial agents is the use of efflux pumps and transporters.60 Unexpectedly, the carrier strain biofilm secreted 3-fold fewer number of transport proteins compared to the planktonic form (Fig 5C).
Although the number of immunomodulatory proteins was only marginally greater in the carrier strain than the non-carrier strain based on the iTRAQ findings, these results did not concur with other analyses we conducted. We had observed that the resistance of the carrier strain to innate immune defense molecules (Reactive Oxygen Intermediates) is twice as much as the non-carrier strain (data not shown). Additionally, the resistance of biofilms to innate host molecules is 10-15 times greater than the planktonic variety (data not shown). Perhaps, the effectiveness of immune evasion by SA is not determined by the number of immunomodulatory proteins, but by the potency and quantity of individual proteins. To this end, we further analyzed one of the most abundant immunological SA proteins, Staphylococcal Protein A (SPA).
SPA from SA carrier strains is found in higher concentrations than the non-carrier strain
SPA which can be found to be either cell-wall associated or secreted, is well known for its binding, immunological, and biofilm promoting properties.48,26,25 Not surprisingly, we noticed throughout the SA exoproteomic analyses that levels of SPA were consistently higher in the carrier strain than the non-carrier strain (Fig 1). Using an SPA ELISA, we confirmed that the carrier strain had nearly 8-fold higher SPA levels than the non-carrier strain (Fig 6). This was verified in several other persistent carrier strains such as (D20, D39 and D98) and an intermittent carrier strain (D37). The levels of SPA were much higher in persistent carriers and comparatively lower in the intermittent carrier, although all carriers had significantly higher SPA levels compared to the non-carrier strain (Fig 6). These results suggest a possible correlation between increased SPA levels and nasal carriage.
FIG 6. Immunomodulatory Staphylococcal protein A (SPA) is significantly up regulated in nasal carrier strains of SA compared to its non-carrier counterpart.
The expression of SPA was measured in the exoproteomes of one non-carrier and 4 persistent carrier strains (PC) D20 (**p =0.001), D30 (**p =0.006), D39 (**p =0.007), D98 (**p =0.009) and 1 intermittent carrier (IC) D37 (**p =0.008), strain using anti-SPA ELISA and compared to non-carrier strain 930918-3. Equal volumes of total exoproteome from nasal carrier and non-carrier strains were used for ELISA in three independent experiments (N=3) and the result was expressed as percentage of SPA concentration to total protein concentration.
Peptide mass fingerprinting of SPA from the carrier strain revealed that one of the isoforms of SPA was 49.5 kDa. Subsequent MS/MS and in silico analyses revealed that the protein contains three IgG binding domains as found in most species of Staphylococcus and also a cell wall localization motif LysM.61 This motif is responsible for SPA interaction with cell wall components of the bacteria but is not consistently present in all species of SA.56,61 Furthermore, MS/MS analysis also revealed the repeated occurrence of the LysM motif in other proteins such as cell wall binding autolysin, and cell surface elastin binding protein (data not shown). This repeated occurrence of LysM motif in important cell attachment proteins may indicate its possible involvement in SA nasal colonization.
Discussion
Through a comprehensive analysis of the exoproteomes of a nasal carrier strain of SA and a genetically similar non-carrier strain, we have endeavored to analyze proteins or groups of proteins, which may contribute to nasal carriage. In tandem, we compared the exoproteomes of biofilm and planktonic carrier SA cultures in order to evaluate possible determinants of nasal carriage. Interestingly, our results indicated that the exoproteome of the carrier strain of SA (D30) contains a greater number of proteins related to adhesion, protein transport and immunoevasion, and fewer stress proteins than its genetically similar non-carrier counterpart (930918-3). Similarly, an analysis of the exoproteome of the biofilm growth form of SA carrier strain revealed a greater number of immunoevasive proteins in comparison to its planktonic counterpart but fewer stress, adherence, and transport proteins.
Other researchers have examined various aspects of the SA proteome, with reference to proteins that promote adherence,45,62 immunoevasive proteins,48,63 total proteome,52 secretome,51 exoproteome64 and surfacome28 in addition to mRNA levels during nasal colonization.65,44 However, our studies have focused on the difference between proteins in the exoproteome of a carrier strain of SA and a non-carrier strain in order to elucidate putative determinants that might influence SA’s ability to colonize nasal mucosa.
Several proteomics studies have indicated that SA proteins present in the extracellular milieu such as secreted and cell surface exposed proteins are highly diverse.53,66,28 However, no correlation between nasal carriage and heterogeneity of these proteins was discussed. In contrast, our study not only identified similar heterogeneity in cell surface and secreted proteins, but also deduced a marked difference in profile of cell surface and secreted proteins between nasal carrier and non-carrier strains.
The adherence of the SA carrier strain to host cells is an integral process in nasal carriage. Although several adhesive proteins have already been identified as important factors for nasal carriage,15,45,16 our exoproteome analysis has revealed that the carrier strain of SA contains twice as many adhesive proteins as a non-carrier strain. The results have highlighted the importance of SasD and SrdH, which have hitherto not been considered important in nasal carriage. Together with high expression levels of Srt, this seems to be indicative of high processing levels of membrane bound proteins, which is an important indicator in nasal carriage as considered by Burian and colleagues.44
In contrast, SA carrier biofilm exoproteome secreted fewer adhesive proteins compared to the planktonic exoproteome. This may be expected as adhesion is already established once the carrier has formed a biofilm and subsequently the main function of the bacteria is survival and possibly ongoing detachment to aid in biofilm dissemination. Specifically, the adhesive D-alanine-activating enzyme (DltD) (responsible for D-alanylation of WTA during cell wall synthesis) was detected in the biofilm exoproteome.57 It has been noted that inactivating the dlt operon in SA leads to increased susceptibility of SA to antimicrobial peptides including defensins and protegrins.67 Perhaps the presence of dlt operon in SA biofilms confers a multifunctional role of resistance to antimicrobial peptides, adhesion and biofilm formation.
Together with adhesion, immunomodulation is one of the key factors in the nasal carriage of SA, since it allows for long-term survival of the bacteria in the host.48,62 Previously, we observed the ability of the carrier strain of SA (D30) to down-regulate defensins and pathogen receptors,11 indicating the involvement of bacterial immunomodulatory proteins in SA nasal carriage. In support of this concept, the present results revealed that the nasal carrier isolates of SA contain a greater number of proteins that downregulate host immunity, whereas the non-carrier isolates contain a greater number of proteins that upregulate host immunity. This result is verified in Burian and colleagues’ observations on nasal colonization.44 A large proportion of these immunomodulatory proteins have also been corroborated in the surfacomes of the SA COL, Newman, RN6390 and USA300.28 Additionally, our proteomics approach revealed that the carrier strain biofilm revealed a similar trend with greater number of immunomodulatory proteins being found in the biofilm when contrasted with the planktonic growth form.
The high abundance of SPA, a versatile immunoevasive and binding molecule, may be linked to the carrier strains’ overall immunoevasive strategy. Interestingly, differential SPA expression patterns even within persistent and intermittent carriers could advantageously be used as a diagnostic tool to differentiate them. Certainly, greater amounts of surface bound molecules with high levels of sortase could be associated with the observation that proteins involved in protein modification, secretion and trafficking are found in greater numbers in the carrier strain of SA in comparison to the non-carrier strain.
The rates of cell wall turnover found in Staphylococcus in general are quite high.68 This constant turnover of the cell wall provides ample decoy material for SA to engage host innate defenses and is proportional to successful colonization of the human nasal passages.44 This strategy can be seen in other pathogen/host dynamics such as Schistosoma spp and is referred to as sloughing.69
Recently, researchers discovered that low concentrations of host chemokines, including CXCL9 and CXCL10, induce the release of SPA, while high concentrations of chemokines can also be antibacterial.70,71 This echoes findings from our previous research on the effects of the cytokine IL-1α. We demonstrated that the carrier strain of SA downregulated the production of IL-1α during infection and IL-1α decreased the growth of SA carrier strain on nasal epithelial cells.22 At this stage we have not assessed if the cytokine would also induce SPA. Since we detected multiple isoforms of SPA in the carrier strain of SA but not in the non-carrier, we suspect that it may be posttranslationally modified in sequential stages such as the glycosylation patterns observed in the Golgi apparatus.72 Glycosylation of exoproteins such as SPA might play crucial roles in bacterial pathogenesis and immunoevasion.73 We hypothesize that SPA is sequentially glycosylated in nasal carrier strains of SA and may be linked to nasal carriage.
One of the more surprising observations from our carrier strain exoproteome analysis was the low expression of stress proteins in comparison to the non-carrier strain. Perhaps, as observed by other authors, nasal colonization does not constitute a full blown infection but rather a persistent sub-clinical infection.5,44 Interestingly, transcript analysis of some key stress proteins also revealed that stress response stimuli are absent in the nasal milieu.44
It has been postulated that SA develops resistance to nasal fluids by decreasing the uptake of antimicrobials or actively translocating them from the cell,54 and several bacterial transport proteins play vital roles in these processes. A greater number of transport proteins in the exoproteome of nasal carrier strain in comparison to the non-carrier strain could make it more resistant to antimicrobial agents in the nasal milieu. Specifically, several proteins from the ATP-binding cassette (ABC) transporter super family and the protein-export membrane protein SecF (part of the bifunctional translocase SecDF) were found in much higher levels in the carrier strain. Further investigation into the implications of these results and the role of these transport proteins in the nasal carriage of SA is currently being pursued.
Increasing evidence suggests that the phenomenon of SA nasal carriage is a complex host-pathogen interaction for which the bacteria has evolved numerous immunoevasion strategies for successful colonization of the host.5,11,22 Our current exoproteomic study suggests that the SA nasal carrier strain is able to adapt itself to the human nasal passages by secreting a distinct repertoire of proteins in comparison to the non-carrier strain. Indeed, we have elicited the identities of several underreported proteins, which may be important in the nasal carriage of SA. Additionally, while it is not known if every carrier strain of SA adopts a biofilm mode of growth in the nose, it has become increasingly apparent that a biofilm mode of growth may be more representative of the in vivo condition.74,75 This comparison can be a foundation for future studies to identify fully the state of SA in the nose and its contributing factors. In conclusion, this exoproteome analysis has elucidated important strategies adopted by the SA carrier strain in its dissemination through nasal carriage that further augments our understanding of nasal carriage of SA.
Supplementary Material
Acknowledgements
These studies were supported by grant AI060753 (to A.M.C.) from the National Institutes of Health. The authors would like to thank Colleen R. Eade, Matthew P. Wood and Julie A. Martellini for their helpful discussions during the preparation of this manuscript.
Abbreviations
- SA
Staphylococcus aureus
- MRSA
Methicillin resistant Staphylococcus aureus
- HBD-3
Human β-defensin 3
- 2D-PAGE
Two-dimensional gel electrophoresis
- AMPs
Antimicrobial peptides
- ITRAQ
Isobaric tags for relative and absolute quantitation
- ACN
Acetonitrile
- SCX
Strong cation exchange
- MMTS
Methyl methane-thiosulphonate
- EF
Error factor
- 2D-LC
Two-dimensional liquid chromatography
- TSB
Tryptic Soy Broth
- SPA
Staphylococcal protein A
Reference
- 1.Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, Nouwen JL. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5(12):751–62. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 2.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. Jama. 2007;298(15):1763–71. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 3.Kuehnert MJ, Kruszon-Moran D, Hill HA, McQuillan G, McAllister SK, Fosheim G, McDougal LK, Chaitram J, Jensen B, Fridkin SK, Killgore G, Tenover FC. Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001-2002. J Infect Dis. 2006;193(2):172–9. doi: 10.1086/499632. [DOI] [PubMed] [Google Scholar]
- 4.van Belkum A, Melles DC, Nouwen J, van Leeuwen WB, van Wamel W, Vos MC, Wertheim HF, Verbrugh HA. Co-evolutionary aspects of human colonisation and infection by Staphylococcus aureus. Infect Genet Evol. 2009;9(1):32–47. doi: 10.1016/j.meegid.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Cole AM, Tahk S, Oren A, Yoshioka D, Kim YH, Park A, Ganz T. Determinants of Staphylococcus aureus nasal carriage. Clin Diagn Lab Immunol. 2001;8(6):1064–9. doi: 10.1128/CDLI.8.6.1064-1069.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Belkum A, Emonts M, Wertheim H, de Jongh C, Nouwen J, Bartels H, Cole A, Cole A, Hermans P, Boelens H, Toom NL, Snijders S, Verbrugh H, van Leeuwen W. The role of human innate immune factors in nasal colonization by Staphylococcus aureus. Microbes Infect. 2007;9(12-13):1471–7. doi: 10.1016/j.micinf.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Cole AM, Kim YH, Tahk S, Hong T, Weis P, Waring AJ, Ganz T. Calcitermin, a novel antimicrobial peptide isolated from human airway secretions. FEBS Lett. 2001;504(1-2):5–10. doi: 10.1016/s0014-5793(01)02731-4. [DOI] [PubMed] [Google Scholar]
- 8.Cole AM, Liao HI, Stuchlik O, Tilan J, Pohl J, Ganz T. Cationic polypeptides are required for antibacterial activity of human airway fluid. J Immunol. 2002;169(12):6985–91. doi: 10.4049/jimmunol.169.12.6985. [DOI] [PubMed] [Google Scholar]
- 9.Midorikawa K, Ouhara K, Komatsuzawa H, Kawai T, Yamada S, Fujiwara T, Yamazaki K, Sayama K, Taubman MA, Kurihara H, Hashimoto K, Sugai M. Staphylococcus aureus susceptibility to innate antimicrobial peptides, beta-defensins and CAP18, expressed by human keratinocytes. Infect Immun. 2003;71(7):3730–9. doi: 10.1128/IAI.71.7.3730-3739.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole AM, Dewan P, Ganz T. Innate antimicrobial activity of nasal secretions. Infect Immun. 1999;67(7):3267–75. doi: 10.1128/iai.67.7.3267-3275.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quinn GA, Cole AM. Suppression of innate immunity by a nasal carriage strain of Staphylococcus aureus increases its colonization on nasal epithelium. Immunology. 2007;122(1):80–9. doi: 10.1111/j.1365-2567.2007.02615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferry T, Perpoint T, Vandenesch F, Etienne J. Virulence determinants in Staphylococcus aureus and their involvement in clinical syndromes. Curr Infect Dis Rep. 2005;7(6):420–8. doi: 10.1007/s11908-005-0043-8. [DOI] [PubMed] [Google Scholar]
- 13.Weidenmaier C, Kokai-Kun JF, Kulauzovic E, Kohler T, Thumm G, Stoll H, Gotz F, Peschel A. Differential roles of sortase-anchored surface proteins and wall teichoic acid in Staphylococcus aureus nasal colonization. Int J Med Microbiol. 2008;298(5-6):505–13. doi: 10.1016/j.ijmm.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Schaffer AC, Solinga RM, Cocchiaro J, Portoles M, Kiser KB, Risley A, Randall SM, Valtulina V, Speziale P, Walsh E, Foster T, Lee JC. Immunization with Staphylococcus aureus clumping factor B, a major determinant in nasal carriage, reduces nasal colonization in a murine model. Infect Immun. 2006;74(4):2145–53. doi: 10.1128/IAI.74.4.2145-2153.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wertheim HF, Walsh E, Choudhurry R, Melles DC, Boelens HA, Miajlovic H, Verbrugh HA, Foster T, van Belkum A. Key role for clumping factor B in Staphylococcus aureus nasal colonization of humans. PLoS Med. 2008;5(1):e17. doi: 10.1371/journal.pmed.0050017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weidenmaier C, Kokai-Kun JF, Kristian SA, Chanturiya T, Kalbacher H, Gross M, Nicholson G, Neumeister B, Mond JJ, Peschel A. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat Med. 2004;10(3):243–5. doi: 10.1038/nm991. [DOI] [PubMed] [Google Scholar]
- 17.Nashev D, Toshkova K, Bizeva L, Akineden O, Lammler C, Zschock M. Distribution of enterotoxin genes among carriage- and infection-associated isolates of Staphylococcus aureus. Lett Appl Microbiol. 2007;45(6):681–5. doi: 10.1111/j.1472-765X.2007.02254.x. [DOI] [PubMed] [Google Scholar]
- 18.Lindsay JA, Moore CE, Day NP, Peacock SJ, Witney AA, Stabler RA, Husain SE, Butcher PD, Hinds J. Microarrays reveal that each of the ten dominant lineages of Staphylococcus aureus has a unique combination of surface-associated and regulatory genes. J Bacteriol. 2006;188(2):669–76. doi: 10.1128/JB.188.2.669-676.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becker K, Friedrich AW, Peters G, von Eiff C. Systematic survey on the prevalence of genes coding for staphylococcal enterotoxins SElM, SElO, and SElN. Mol Nutr Food Res. 2004;48(7):488–95. doi: 10.1002/mnfr.200400044. [DOI] [PubMed] [Google Scholar]
- 20.Miller M, Cook HA, Furuya EY, Bhat M, Lee MH, Vavagiakis P, Visintainer P, Vasquez G, Larson E, Lowy FD. Staphylococcus aureus in the community: colonization versus infection. PLoS One. 2009;4(8):e6708. doi: 10.1371/journal.pone.0006708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sivaraman K, Venkataraman N, Tsai J, Dewell S, Cole AM. Genome sequencing and analysis reveals possible determinants of Staphylococcus aureus nasal carriage. BMC Genomics. 2008;9:433. doi: 10.1186/1471-2164-9-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quinn GA, Tarwater PM, Cole AM. Subversion of interleukin-1-mediated host defence by a nasal carrier strain of Staphylococcus aureus. Immunology. 2009;128(1 Suppl):e222–9. doi: 10.1111/j.1365-2567.2008.02952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kostenko V, Ceri H, Martinuzzi RJ. Increased tolerance of Staphylococcus aureus to vancomycin in viscous media. FEMS Immunol Med Microbiol. 2007;51(2):277–88. doi: 10.1111/j.1574-695X.2007.00300.x. [DOI] [PubMed] [Google Scholar]
- 24.Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Daniels S, Purkayastha S, Juhasz P, Martin S, Bartlet-Jones M, He F, Jacobson A, Pappin DJ. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics. 2004;3(12):1154–69. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- 25.Merino N, Toledo-Arana A, Vergara-Irigaray M, Valle J, Solano C, Calvo E, Lopez JA, Foster TJ, Penades JR, Lasa I. Protein A-mediated multicellular behavior in Staphylococcus aureus. J Bacteriol. 2009;191(3):832–43. doi: 10.1128/JB.01222-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forsgren A. Significance of protein a production by staphylococci. Infect Immun. 1970;2(5):672–3. doi: 10.1128/iai.2.5.672-673.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomez MI, Lee A, Reddy B, Muir A, Soong G, Pitt A, Cheung A, Prince A. Staphylococcus aureus protein A induces airway epithelial inflammatory responses by activating TNFR1. Nat Med. 2004;10(8):842–8. doi: 10.1038/nm1079. [DOI] [PubMed] [Google Scholar]
- 28.Dreisbach A, Hempel K, Buist G, Hecker M, Becher D, van Dijl JM. Profiling the surfacome of Staphylococcus aureus. Proteomics. 10(17):3082–96. doi: 10.1002/pmic.201000062. [DOI] [PubMed] [Google Scholar]
- 29.Brown RE, Jarvis KL, Hyland KJ. Protein measurement using bicinchoninic acid: elimination of interfering substances. Anal Biochem. 1989;180(1):136–9. doi: 10.1016/0003-2697(89)90101-2. [DOI] [PubMed] [Google Scholar]
- 30.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 31.Kessler RJ, Fanestil DD. Interference by lipids in the determination of protein using bicinchoninic acid. Anal Biochem. 1986;159(1):138–42. doi: 10.1016/0003-2697(86)90318-0. [DOI] [PubMed] [Google Scholar]
- 32.Wiechelman KJ, Braun RD, Fitzpatrick JD. Investigation of the bicinchoninic acid protein assay: identification of the groups responsible for color formation. Anal Biochem. 1988;175(1):231–7. doi: 10.1016/0003-2697(88)90383-1. [DOI] [PubMed] [Google Scholar]
- 33.Zhu M, Dai S, McClung S, Yan X, Chen S. Functional differentiation of Brassica napus guard cells and mesophyll cells revealed by comparative proteomics. Mol Cell Proteomics. 2009;8(4):752–66. doi: 10.1074/mcp.M800343-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Unwin RD, Pierce A, Watson RB, Sternberg DW, Whetton AD. Quantitative proteomic analysis using isobaric protein tags enables rapid comparison of changes in transcript and protein levels in transformed cells. Mol Cell Proteomics. 2005;4(7):924–35. doi: 10.1074/mcp.M400193-MCP200. [DOI] [PubMed] [Google Scholar]
- 35.Shilov IV, Seymour SL, Patel AA, Loboda A, Tang WH, Keating SP, Hunter CL, Nuwaysir LM, Schaeffer DA. The Paragon Algorithm, a next generation search engine that uses sequence temperature values and feature probabilities to identify peptides from tandem mass spectra. Mol Cell Proteomics. 2007;6(9):1638–55. doi: 10.1074/mcp.T600050-MCP200. [DOI] [PubMed] [Google Scholar]
- 36.Griffiths SD, Burthem J, Unwin RD, Holyoake TL, Melo JV, Lucas GS, Whetton AD. The use of isobaric tag peptide labeling (iTRAQ) and mass spectrometry to examine rare, primitive hematopoietic cells from patients with chronic myeloid leukemia. Mol Biotechnol. 2007;36(2):81–9. doi: 10.1007/s12033-007-0005-5. [DOI] [PubMed] [Google Scholar]
- 37.Donowitz M, Singh S, Salahuddin FF, Hogema BM, Chen Y, Gucek M, Cole RN, Ham A, Zachos NC, Kovbasnjuk O, Lapierre LA, Broere N, Goldenring J, deJonge H, Li X. Proteome of murine jejunal brush border membrane vesicles. J Proteome Res. 2007;6(10):4068–79. doi: 10.1021/pr0701761. [DOI] [PubMed] [Google Scholar]
- 38.Guo Y, Singleton PA, Rowshan A, Gucek M, Cole RN, Graham DR, Van Eyk JE, Garcia JG. Quantitative proteomics analysis of human endothelial cell membrane rafts: evidence of MARCKS and MRP regulation in the sphingosine 1-phosphate-induced barrier enhancement. Mol Cell Proteomics. 2007;6(4):689–96. doi: 10.1074/mcp.M600398-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kassie F, Anderson LB, Higgins L, Pan Y, Matise I, Negia M, Upadhyaya P, Wang M, Hecht SS. Chemopreventive agents modulate the protein expression profile of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone plus benzo[a]pyrene-induced lung tumors in A/J mice. Carcinogenesis. 2008;29(3):610–9. doi: 10.1093/carcin/bgn014. [DOI] [PubMed] [Google Scholar]
- 40.Graham RL, Sharma MK, Ternan NG, Weatherly DB, Tarleton RL, McMullan G. A semi-quantitative GeLC-MS analysis of temporal proteome expression in the emerging nosocomial pathogen Ochrobactrum anthropi. Genome Biol. 2007;8(6):R110. doi: 10.1186/gb-2007-8-6-r110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin B, Brenneman R, Becker KG, Gucek M, Cole RN, Maudsley S. iTRAQ analysis of complex proteome alterations in 3xTgAD Alzheimer’s mice: understanding the interface between physiology and disease. PLoS One. 2008;3(7):e2750. doi: 10.1371/journal.pone.0002750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keshamouni VG, Jagtap P, Michailidis G, Strahler JR, Kuick R, Reka AK, Papoulias P, Krishnapuram R, Srirangam A, Standiford TJ, Andrews PC, Omenn GS. Temporal quantitative proteomics by iTRAQ 2D-LC-MS/MS and corresponding mRNA expression analysis identify post-transcriptional modulation of actin-cytoskeleton regulators during TGF-beta-Induced epithelial-mesenchymal transition. J Proteome Res. 2009;8(1):35–47. doi: 10.1021/pr8006478. [DOI] [PubMed] [Google Scholar]
- 43.Drummelsmith J, Winstall E, Bergeron MG, Poirier GG, Ouellette M. Comparative proteomics analyses reveal a potential biomarker for the detection of vancomycin-intermediate Staphylococcus aureus strains. J Proteome Res. 2007;6(12):4690–702. doi: 10.1021/pr070521m. [DOI] [PubMed] [Google Scholar]
- 44.Burian M, Wolz C, Goerke C. Regulatory adaptation of Staphylococcus aureus during nasal colonization of humans. PLoS One. 5(4):e10040. doi: 10.1371/journal.pone.0010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corrigan RM, Miajlovic H, Foster TJ. Surface proteins that promote adherence of Staphylococcus aureus to human desquamated nasal epithelial cells. BMC Microbiol. 2009;9:22. doi: 10.1186/1471-2180-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patti JM, Allen BL, McGavin MJ, Hook M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 47.Foster TJ, Hook M. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 1998;6(12):484–8. doi: 10.1016/s0966-842x(98)01400-0. [DOI] [PubMed] [Google Scholar]
- 48.Foster TJ. Immune evasion by staphylococci. Nat Rev Microbiol. 2005;3(12):948–58. doi: 10.1038/nrmicro1289. [DOI] [PubMed] [Google Scholar]
- 49.Leski TA, Tomasz A. Role of penicillin-binding protein 2 (PBP2) in the antibiotic susceptibility and cell wall cross-linking of Staphylococcus aureus: evidence for the cooperative functioning of PBP2, PBP4, and PBP2A. J Bacteriol. 2005;187(5):1815–24. doi: 10.1128/JB.187.5.1815-1824.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katzif S, Danavall D, Bowers S, Balthazar JT, Shafer WM. The major cold shock gene, cspA, is involved in the susceptibility of Staphylococcus aureus to an antimicrobial peptide of human cathepsin G. Infect Immun. 2003;71(8):4304–12. doi: 10.1128/IAI.71.8.4304-4312.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ravipaty S, Reilly JP. Comprehensive characterization of methicillin-resistant Staphylococcus aureus subsp. aureus COL secretome by two-dimensional liquid chromatography and mass spectrometry. Mol Cell Proteomics. 9(9):1898–919. doi: 10.1074/mcp.M900494-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kohler C, Wolff S, Albrecht D, Fuchs S, Becher D, Buttner K, Engelmann S, Hecker M. Proteome analyses of Staphylococcus aureus in growing and non-growing cells: a physiological approach. Int J Med Microbiol. 2005;295(8):547–65. doi: 10.1016/j.ijmm.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 53.Sibbald MJ, Ziebandt AK, Engelmann S, Hecker M, de Jong A, Harmsen HJ, Raangs GC, Stokroos I, Arends JP, Dubois JY, van Dijl JM. Mapping the pathways to staphylococcal pathogenesis by comparative secretomics. Microbiol Mol Biol Rev. 2006;70(3):755–88. doi: 10.1128/MMBR.00008-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hassan KA, Skurray RA, Brown MH. Active export proteins mediating drug resistance in staphylococci. J Mol Microbiol Biotechnol. 2007;12(3-4):180–96. doi: 10.1159/000099640. [DOI] [PubMed] [Google Scholar]
- 55.Clements MO, Foster SJ. Stress resistance in Staphylococcus aureus. Trends Microbiol. 1999;7(11):458–62. doi: 10.1016/s0966-842x(99)01607-8. [DOI] [PubMed] [Google Scholar]
- 56.Andre G, Leenhouts K, Hols P, Dufrene YF. Detection and localization of single LysM-peptidoglycan interactions. J Bacteriol. 2008;190(21):7079–86. doi: 10.1128/JB.00519-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perego M, Glaser P, Minutello A, Strauch MA, Leopold K, Fischer W. Incorporation of D-alanine into lipoteichoic acid and wall teichoic acid in Bacillus subtilis. Identification of genes and regulation. J Biol Chem. 1995;270(26):15598–606. doi: 10.1074/jbc.270.26.15598. [DOI] [PubMed] [Google Scholar]
- 58.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2(2):95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 59.Hall-Stoodley L, Stoodley P. Evolving concepts in biofilm infections. Cell Microbiol. 2009;11(7):1034–43. doi: 10.1111/j.1462-5822.2009.01323.x. [DOI] [PubMed] [Google Scholar]
- 60.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358(9276):135–8. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 61.Buist G, Steen A, Kok J, Kuipers OP. LysM, a widely distributed protein motif for binding to (peptido)glycans. Mol Microbiol. 2008;68(4):838–47. doi: 10.1111/j.1365-2958.2008.06211.x. [DOI] [PubMed] [Google Scholar]
- 62.Foster TJ. Colonization and infection of the human host by staphylococci: adhesion, survival and immune evasion. Vet Dermatol. 2009;20(5-6):456–70. doi: 10.1111/j.1365-3164.2009.00825.x. [DOI] [PubMed] [Google Scholar]
- 63.Rooijakkers SH, van Kessel KP, van Strijp JA. Staphylococcal innate immune evasion. Trends Microbiol. 2005;13(12):596–601. doi: 10.1016/j.tim.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 64.Burlak C, Hammer CH, Robinson MA, Whitney AR, McGavin MJ, Kreiswirth BN, Deleo FR. Global analysis of community-associated methicillin-resistant Staphylococcus aureus exoproteins reveals molecules produced in vitro and during infection. Cell Microbiol. 2007;9(5):1172–90. doi: 10.1111/j.1462-5822.2006.00858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burian M, Rautenberg M, Kohler T, Fritz M, Krismer B, Unger C, Hoffmann WH, Peschel A, Wolz C, Goerke C. Temporal expression of adhesion factors and activity of global regulators during establishment of Staphylococcus aureus nasal colonization. J Infect Dis. 201(9):1414–21. doi: 10.1086/651619. [DOI] [PubMed] [Google Scholar]
- 66.Jones RC, Deck J, Edmondson RD, Hart ME. Relative quantitative comparisons of the extracellular protein profiles of Staphylococcus aureus UAMS-1 and its sarA, agr, and sarA agr regulatory mutants using one-dimensional polyacrylamide gel electrophoresis and nanocapillary liquid chromatography coupled with tandem mass spectrometry. J Bacteriol. 2008;190(15):5265–78. doi: 10.1128/JB.00383-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peschel A, Otto M, Jack RW, Kalbacher H, Jung G, Gotz F. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem. 1999;274(13):8405–10. doi: 10.1074/jbc.274.13.8405. [DOI] [PubMed] [Google Scholar]
- 68.Blumel P, Uecker W, Giesbrecht P. Zero order kinetics of cell wall turnover in Staphylococcus aureus. Arch Microbiol. 1979;121(2):103–10. doi: 10.1007/BF00689972. [DOI] [PubMed] [Google Scholar]
- 69.Pearce EJ, Sher A. Mechanisms of immune evasion in schistosomiasis. Contrib Microbiol Immunol. 1987;8:219–32. [PubMed] [Google Scholar]
- 70.Cole AM, Ganz T, Liese AM, Burdick MD, Liu L, Strieter RM. Cutting edge: IFN-inducible ELR- CXC chemokines display defensin-like antimicrobial activity. J Immunol. 2001;167(2):623–7. doi: 10.4049/jimmunol.167.2.623. [DOI] [PubMed] [Google Scholar]
- 71.Yung SC, Parenti D, Keiser J, Murphy PM. Host chemokines bind to Staphylococcus aureus and stimulate protein A release. J Biol Chem. doi: 10.1074/jbc.M110.195180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rivinoja A, Hassinen A, Kokkonen N, Kauppila A, Kellokumpu S. Elevated Golgi pH impairs terminal N-glycosylation by inducing mislocalization of Golgi glycosyltransferases. J Cell Physiol. 2009;220(1):144–54. doi: 10.1002/jcp.21744. [DOI] [PubMed] [Google Scholar]
- 73.Schmidt MA, Riley LW, Benz I. Sweet new world: glycoproteins in bacterial pathogens. Trends Microbiol. 2003;11(12):554–61. doi: 10.1016/j.tim.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 74.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318–22. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 75.Boles BR, Horswill AR. Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog. 2008;4(4):e1000052. doi: 10.1371/journal.ppat.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.