Abstract
Relations between a modifiable psychosocial factor, self-efficacy (SE), and behavioral and neural indices of self-regulation, including post-error behavior, the error-related negativity (ERN), and error positivity (Pe) were examined in young adults during a flanker task emphasizing either accuracy or speed. SE was predicted to be associated with larger ERN and Pe amplitudes, as well as greater post-error behavioral performance during task conditions emphasizing accuracy, but not speed. Results showed that higher SE was associated with greater post-error response accuracy during the accuracy condition, but not the speed condition, and higher SE was related with greater ERN amplitudes across instruction conditions. Further, ERN amplitude mediated the relationship between SE and post-error response accuracy in the accuracy condition. These findings emphasize the role of motivation and incentive on the self-regulatory system and suggest that SE is beneficially related to self-regulatory processes and outcomes.
Keywords: Self-efficacy (SE), Self-regulation, Error-Related Negativity (ERN), Event-Related Brain Potentials (ERPs)
1. Introduction
1.1 Self-efficacy
Self-efficacy (SE) is the primary variable of interest in social cognitive theory (Bandura, 1986, 1997). Social cognitive theory specifies four core features of human agency: “intentionality, forethought, self-reactiveness, and self-reflectiveness” (Bandura and Locke, 2003, p. 97). In this framework, SE works within a dual control system that operates both as a proactive agent to institute higher levels of functioning as well as a reactive agent to reduce discrepant outcomes (Bandura, 1991, 2001). Specifically, SE reflects individuals’ judgments in their capabilities to successfully execute courses of action (Bandura, 1977) and is theorized to influence effort expenditure and perseverance under failure and aversive stimuli (Bandura, 1986), with more efficacious individuals expending more effort and persevering longer than less efficacious individuals. SE has been positively associated with work-related performance (Stajkovic and Luthans, 1998) as well as cognitive performance (Berry and West, 1993; Bouffard-Bourchard, 1990; Lachman and Jelalian, 1984). Further, SE is quite malleable and can be altered through attainments based on mastery experiences, social modeling, and social persuasion (Bandura, 1986) and experimentally induced SE has been positively associated with cognitive task performance (Bouffard-Bouchard, 1990). Finally, SE plays an important role in achievement and self-regulatory adjustments during the completion of challenging tasks or task conditions (Bandura and Cervone, 1983; Cervone and Peake, 1986).
Given the flexibility of SE and its sensitivity to experience, it is important to consider potential ongoing adjustments to both SE and task performance. Specifically, as one engages a task, self-feedback and mastery experiences are obtained. Thus, more information is available to adjust both SE and related self-regulatory processes in order to perform the task in accord with one’s intentions. Accordingly, the relations between SE and indices of self-regulation may be adjusted as well. One interpretation of the relation between SE and self-regulation during task execution is that during the initial completion of a task, individuals may still be learning how to engage the task and developing their self-regulatory capabilities (Zimmerman and Kitsantas, 1997). This would lead them to focus more on process goals during task execution. Process goals focus on strategies needed to execute the task (e.g., ensure hands are placed properly on the response buttons; visually focus on the target stimulus). During later performances, individuals would have better developed experience-based self-regulatory skills with the task and would be able focus more on outcome goals. Outcome goals involve the final outcome of task execution (e.g., make the correct response).
An alternative interpretation suggests that process and outcome distinctions may not be warranted for self-regulatory processing in a relatively simple speeded-response task. As such, the goal of self-regulation (improved performance) would remain consistent (outcome goal-oriented) and relations between SE and all indices of self-regulation would be similar across task experiences. In sum, greater experience would uniformly enhance perceptions of SE, self-regulatory function, and subsequent task performance (Bandura and Wood, 1989).
1.2 Indices of self-regulation
To assess the nature of the relationship between SE and self-regulation across task experience, the measurement of self-regulatory processes and outcomes are required during the completion of a speeded-response task. Assessments of self-regulatory outcomes have come largely from examining post-error behavior; specifically post-error response accuracy and post-error response time (RT; Rabbitt, 1966). These behavioral measures reflect the outcome of self-regulation and provide evidence for the overall implementation and effectiveness of self-regulation. The assessment of self-regulatory processes in the field of psychophysiology has come largely from the investigation of the error-related negativity (ERN; Gehring et al., 1993; or Ne; Falkenstein et al., 1991; Falkenstein et al., 2000) and error positivity (Pe; Falkenstein et al., 2000).
The ERN is a negative-going component of the response-locked event-related brain potential (ERP) with a fronto-central maximum and peaks around 50–100 ms after an incorrect response. The ERN is typically identified as either a reinforcement learning index of error detection (Holroyd and Coles, 2002) or an early indicator of response conflict in association with erroneous task performance (Yeung et al., 2004). Specifically, the ERN is believed to index the self-regulatory detection of behavioral conflict or an error during task execution. This detection process appears to exist outside of conscious awareness (Nieuwenhuis et al., 2001) and an array of variables and factors appear to influence ERN amplitude and this detection process. These include psychological factors such as neuroticism (Pailing and Segalowitz, 2004a), negative affect (Hajcak et al., 2004; Luu et al., 2000), depression (Chiu and Deldin, 2007), and motivation and incentive (Hajcak et al., 2005; Pailing and Segalowitz, 2004a). Given the strong, positive relationships SE has with motivation (Bandura, 1977, 1986, 1993) and neuroticism (Judge et al., 2002), a relation between SE and ERN fits well with the established state and trait influences on the ERN and self-regulatory processing.
Additionally, task instructions stressing accuracy over speed (Gehring et al., 1993) have been associated with enhanced ERN amplitudes, suggesting motivational factors associated with an increased salience of errors under accuracy instructions (Gehring et al., 1993; Hajcak et al., 2005), a greater certainty of error commission during more careful task completion (Pailing and Segalowitz, 2004b), or an increase in attentional focus on the target stimulus leading to a more rapid upsurge in post-error activation of the correct response (Yeung et al., 2004) may influence this component. Finally, ERN amplitude has been found to be smaller for older, compared to younger, adults (Band and Kok, 2000; Nieuwenhuis et al., 2002; Themanson et al., 2006; Themanson et al., 2008). This difference is believed to reflect an age-related degradation in self-regulatory processing, which is consistent with the notion that cognitive health peaks during young adulthood (Salthouse and Davis, 2006) and older adults exhibit deficits in executive control processes (Kramer et al., 1999; West, 1996).
The Pe is a positive-going component observed in response-locked ERP averages of error responses. It is maximal over centro-parietal recording sites and peaks after the ERN (about 300 ms following an incorrect response). The Pe has been described as an emotional reaction to the commission of an error (Falkenstein et al., 2000; van Veen and Carter, 2002), a post-response evaluation of an error (Davies et al., 2001; Falkenstein et al., 1990), or the allocation of attention toward an error following error commission (Mathewson et al., 2005). More specifically, Davies et al. (2001) found strong correlations between Pe and P3 amplitude, suggesting that the Pe could be a P3-like self-regulatory response to the internal detection of an error, with the error response being the salient stimulus to which attention is allocated.
To date, one study has investigated the relationship between SE and neural and behavioral indices of self-regulation (Themanson et al., 2008). This study examined high- and low-SE older participants during the execution of a modified flanker task under speed and accuracy instruction conditions. Results indicated that SE was related to ERN amplitude following performance errors and SE moderated a positive linear relationship between ERN amplitude and post-error response accuracy, with a relationship present in the high-SE group, but not the low-SE group. These findings were evidenced under accuracy instructions, but not speed instructions, suggesting that under circumstances where errors are more salient and participants have more motivational incentive to detect and correct their errors, SE is associated with enhanced functioning of self-regulatory processes above and beyond the influence of task parameters (Themanson et al., 2008). However, this study focused specifically on an older adult population and performed a median split on SE scores to create high- and low-SE groups, which limits the findings and implications of the study. Aging has been associated with the decline of higher-order cognitive processes (West, 1996), including self-regulatory processes (Band and Kok, 2000; Nieuwenhuis et al., 2002; Themanson et al., 2006). Thus, findings of an effect in older adults may not be generalizable as more room exists to find such cognitive effects in older adults compared to younger adults, who are at their peak cognitive health (Salthouse and Davis, 2006). Additionally, SE is a continuous variable that is best examined along a continuum to avoid artificial or arbitrary groupings of disparate individuals (Bandura, 2006). Finally, this study did not examine the dynamics of the relation between SE and self-regulatory processes across the participants’ task experiences. This leaves the nature of SE/self-regulation relationship undefined over the course of task execution and does not address whether the association between SE and self-regulation is uniform over experience or dynamically shifts as task experience grows.
1.3 Present study
In the present study, we investigated the relationship between SE and self-regulatory processes in conjunction with task instructions emphasizing either speed or accuracy. We examined SE as a continuous variable in a sample of healthy young adults to extend our previous investigation of SE effects on self-regulation (Themanson et al., 2008). We measured ERN, Pe, and post-error behavior (accuracy, RT) while participants made responses during a modified Eriksen flanker task (Eriksen and Eriksen, 1974). Further, we examined the order of instruction conditions to determine whether the relations between SE and self-regulatory processes would be sensitive to the amount of experience gained by the participants.
We predicted positive relationships for SE with ERN and Pe amplitudes, as well as post-error response accuracy and response slowing, with higher SE associated with greater ERN, Pe, post-error accuracy, and post-error slowing in the accuracy condition, but not the speed condition, extending our previous findings (Themanson et al., 2008). No relationships were predicted to exist between SE and ERPs on correct trials (CRN, correct trial Pe) in either instruction condition as self-regulatory processes are not implemented to the same degree following correct task execution. These findings would suggest greater levels of self-regulatory processing for more efficacious individuals when task instructions emphasized the salience of errors (Gehring et al., 1993; Hajcak et al., 2005; Themanson et al., 2008). That is, greater SE might heighten self-regulatory adjustments following error commission to improve subsequent task performance, especially when errors are more meaningful and aversive to the individual, corroborating the influence of SE on self-reactive processes described by social cognitive theory (Bandura and Locke, 2003). Further, we predicted that SE would linearly moderate the relationship between ERN and post-error task performance, extending our previous finding in older adults, with high-SE participants exhibiting a stronger relationship between the ERN and post-error performance measures. Finally, we examined the influence of prior task experience on the relation between SE and self-regulation in the accuracy instruction condition: If the relations between SE and ERN, Pe, and post-error behavior are differentially sensitive to levels of task experience; this may suggest that the goal orientation of self-regulation is actively developing from process goals to outcome goals across participants’ engagement in the task (Zimmerman and Kitsantas, 1997). This difference in process or outcome orientation would be evidenced through accuracy-first participants showing relatively stronger relationships with the ERN and Pe, indices of the process of detecting and evaluating errors, and weaker relations with post-error accuracy, an outcome index of self-regulation, when compared to accuracy-second participants. If all aspects of self-regulation are similarly influenced by task experience, the goal orientation of self-regulation is consistent for the duration of task engagement and experience should strengthen the relations between SE and all indices of self-regulation.
2. Material and methods
2.1 Participants
Seventy-two healthy adults (18–25 years) were recruited from undergraduate kinesiology courses at the University of Illinois at Urbana-Champaign. Participants received extra course credit in exchange for their participation. Participants (n = 5) with fewer than six errors in each task condition (i.e., accuracy, speed) were discarded from the analyses (Olvet and Hajcak, 2009; Pontifex et al., 2010) as were participants (n = 4) who did not perform above 50% accuracy in each task condition, resulting in a sample size of 63 participants (38 females, 25 males). The study was approved by the Institutional Review Board of the University of Illinois at Urbana-Champaign.
2.2 Behavioral Task
Participants completed a modified version of the Eriksen flanker task (Eriksen and Eriksen, 1974) utilizing symbols that were either congruent (<<<<< or >>>>>), or incongruent (>><>> or <<><<) to the central target stimulus. The central target symbol pointing to the right (“>”) required a right-handed response and the central target symbol pointing to the left (“<”) required a left-handed response. Thus, on congruent trials, the flanking symbols were identical to the target and did not activate the incorrect response. Alternatively, on incongruent trials, the flanking symbols pointed in the opposite direction of the central target symbol, which was designed to elicit activation of the incorrect response and lead to an increased number of performance errors and response delays. Participants viewed a series of white stimuli on a black background presented focally on a computer monitor at a distance of 1 m and each array of five arrows subtended 13.5° of the horizontal visual angle and 3.4° of the vertical visual angle when presented on the computer monitor. Stimuli were 4 cm in height and were presented for 80 ms with an inter-trial interval (ITI) varying between either 1000, 1200, or 1400 ms for each trial. The trials were grouped into two task blocks, with a brief rest period between each block. One block was conducted under the instruction to respond as accurately as possible (i.e., accuracy instruction) and the other was conducted under the instruction to respond as quickly as possible (i.e., speed instruction). Each block contained 600 trials. Congruent and incongruent trials were equiprobable and randomly ordered within each task block. Finally, the two blocks were counterbalanced across participants.
2.3 Self-efficacy Assessment
Two measures were constructed to assess SE for task performance under conditions that stress either accuracy or speed (McAuley et al., 2005). These measures followed the format recommended by Bandura (1977) for construction of efficacy measures and were composed of 10 items in each scale, which reflected beliefs relative to the accurate completion of successively more trials on the flanker task. In the context of SE for accuracy, participants were asked to report their degree of confidence in completing trials as accurately as possible. The first item on the scale was “I believe that I am able to accurately complete 10 out of 100 trials without regard for speed.” Each item on the scale increased by 10 trial increments so that the last item examined beliefs relative to completing 100 out of 100 trials. Each item was scored on a Likert scale from 0% (“not at all confident”) to 100% (“highly confident”). Responses to all 10 items were summed and divided by the total number of items resulting in an accuracy efficacy score with a possible range from 0 to 100. The measure of SE for speed was constructed and scored in the same manner and reflected items worded to emphasize the speed of performance by utilizing the following wording “I believe that I am able to accurately complete x out of 100 trials as fast as possible.” Both measures had high internal consistency, α for accuracy = .95, α for speed = .94, and have been utilized in previous research (Themanson et al., 2008).
2.4 Neural Assessment
The electroencephalogram (EEG) was recorded from 64 sintered Ag-AgCl electrodes embedded in an elastic cap, arranged in an extended 10–20 system montage with a ground electrode (AFz) on the forehead. The sites were referenced online to a midline electrode placed at the midpoint between Cz and CPz. Vertical and horizontal bipolar electrooculographic activity (EOG) was recorded to monitor eye movements using sintered Ag-AgCl electrodes placed above and below the right orbit and near the outer canthus of each eye. Impedances were kept below 10 kΩ for all electrodes. A Neuroscan Synamps2 bioamplifier (Neuro Inc., El Paso, TX), with a 24 bit A/D converter and +/− 200 millivolt (mV) input range, was used to continuously digitize (500 Hz sampling rate), amplify (gain of 10), and filter (70 Hz low-pass filter, including a 60 Hz notch filter) the raw EEG signal in DC mode (763 µV/bit resolution). EEG activity was recorded using Neuroscan Scan software (v 4.3.1). Stimulus presentation, timing, and measurement of behavioral response time and accuracy were controlled by Neuroscan Stim (v 2.0) software.
Offline neural processing of the response-locked components included: eye blink correction using a spatial filter (Compumedics Neuroscan, 2003), re-referencing to average mastoids, creation of response-locked epochs (−400 to 1000 ms relative to behavioral response), baseline removal (100 ms time window that runs from −100 ms to 0 ms prior to the response; Yeung et al., 2004), low-pass filtering (15 Hz; 24dB/octave), and artifact rejection (epochs with signal that exceeded ± 75µV were rejected). The spatial filter is a multi-step procedure that generates an average eye blink, utilizes a spatial singular value decomposition based on principal component analysis (PCA) to extract the first component and covariance values, and then uses those covariance values to develop a filter that is specifically sensitive to eye blinks. Average ERP waveforms for correct trials were matched to error trial waveforms on response time and number of trials to protect against differential artifacts from any stimulus-related activity (Coles et al., 2001). Matching involved selecting individual correct trials for each participant, without replacement, that matched the response time for each of the error trials for that individual. This procedure removes any differences that may exist in the timing of processing due to differences in response latency for correct and error trials (Falkenstein et al., 2001; Mathewson et al., 2005; Yeung et al., 2004) and results in an equal number of matched-correct trials and error trials for each individual to compare differences across accuracy conditions (Themanson and Hillman, 2006; Themanson et al., 2008). ERN amplitude was quantified as the average amplitude between 0–100 ms post-response at FCz and Pe amplitude was quantified as the average amplitude between 200–500 ms post-response at Pz in the average waveforms of error trials, with the CRN and Pe on correct trials derived in the same fashion in average waveforms from matched-correct trials.
2.5 Task Performance
Behavioral data were collected on response time (i.e., time in ms from the presentation of the stimulus) and response accuracy (i.e., number of correct and error responses) for all trials across task blocks. Errors of omission (non-responses to task stimuli) were categorized as incorrect responses for calculations of response accuracy, but these trials were not included in the creation of ERP waveforms due to the lack of a behavioral response. The mean number of error trials included in the ERP waveforms in the accuracy condition was 36 (range = 6 – 153, SD = 28.0). In the speed condition, the mean number of error trials was 64 (range = 13 – 192, SD = 33.2). Multiple average response latencies were calculated for each participant (Themanson et al., 2008). Specifically, these latencies were calculated for: 1) correct trials, 2) error trials, 3) matched-correct trials (the subset of correct trials matched to specific error trials based on RT), 4) correct trials following an error trial (post-error RT), and 5) correct trials following a matchedcorrect trial (post-matched-correct RT). Each participant’s post-error RT was compared to his or her post-matched-correct RT due to the consistent finding that average error RT is faster than average correct RT (Mathewson et al., 2005; Ridderinkhof et al., 2004; Yeung et al., 2004) and thus accounts for any effects of RT slowing that are present simply because error RT generally tends to be faster than correct RT (Themanson and Hillman, 2006; Themanson et al., 2008).
2.6 Procedure
After providing informed consent, participants completed: a health history and demographics questionnaire and the Edinburgh handedness inventory (Oldfield, 1971). Participants visited the laboratory to have their behavioral and neural measures collected during the completion of a modified Eriksen flanker task (Eriksen and Eriksen, 1974). Participants were first seated in a comfortable chair in front of a computer screen and prepared for neural measurement in accordance with the guidelines of the Society for Psychophysiological Research (Picton et al., 2000). After acceptable EEG signals were observed, the lights were dimmed, participants were given task instructions (speed: “respond as quickly as possible after seeing the stimulus”; or accuracy: “respond as accurately as possible after seeing the stimulus”), and 20 practice trials were administered. Following the practice trials, participants completed the relevant SE measure to assess expectations relative to subsequent performance on the cognitive task. After the completion of the first task condition, the other task condition (speed or accuracy) was completed. The protocol for this task condition was identical to the first, with participants receiving appropriate task instructions, completing 20 practice trials, and then completing the relevant SE questionnaire prior to the task. Following the completion of the last block, participants were briefed on the purpose of the experiment. This session lasted approximately 120 minutes.
2.7 Statistical Analyses
ERN, Pe, post-error RT, and post-error accuracy were analyzed separately using omnibus 2 (condition: speed, accuracy) × 2 (accuracy: error, correct) × 2 (order: accuracy-first, accuracy-second) × 2 (sex: male, female) mixed-model ANCOVAs with SE entered as a covariate to better establish the relation between SE and these indices of self-regulation. Bonferroni-corrected ANCOVAs, ANOVAs, paired-samples t tests, and zero-order bivariate correlations were performed for follow-up testing. The alpha level was set at p ≤ .05 for each individual analysis and all analyses included every participant in the final sample (n = 63). In instances where SE exhibited a significant interaction with a well-established effect (i.e., accuracy, condition), rendering that effect non-significant in the ANCOVA, follow-up 2 (condition) × 2 (accuracy) × 2 (order) × 2 (sex) mixed-model ANOVAs were conducted to verify that the data conformed to the expected condition and accuracy effects for that dependent measure. Finally, simple linear regression analyses (Baron and Kenny, 1986) were conducted to determine if SE moderated the relationship between ERN and post-error behavior and to determine if the relation between SE and indices of self-regulation was sensitive to one’s level of task experience. Additionally, regression analyses were conducted to examine the potential mediating effect of ERN on the predicted relationships between SE and post-error behavior. Since the focus of this study was on SE, only those follow-up analyses that are related to SE are included in the text.
3. Results
3. 1 Self-efficacy
In the accuracy condition (accuracy-SE), the mean (± SD) SE score was 64.7 (± 15.2) with scores ranging from 31 to 96 on a scale with a possible range of 0 to 100. In the speed condition, the mean SE score (speed-SE) was 61.1 (± 16.9) with scores ranging from 29 to 100. The correlation between the two SE measures was significant, r = .71, p < .001, suggesting the two measures were related, but not identical. A paired-samples t test indicated a significant difference in SE across task conditions, t(62) = 2.6, p = .01, with higher SE reported in the accuracy condition compared to the speed condition.
3. 2 Behavioral Task Performance
A 2 (condition) × 2 (order) mixed-model ANOVA was conducted for both response accuracy (% correct) and response time (RT) to verify that these data conformed to the expected effects. Both analyses revealed significant condition effects as individuals performed significantly more accurately (F(1, 61) = 71.9, p < .001, partial η2 = .54) and more slowly (F(1, 61) = 164.2, p < .001, partial η2 = .73) under instructions stressing accuracy (mean ± SD = 87% correct ± 8.6; 390 ms ± 41.2) compared to speed (mean ± SD = 79% correct ± 9.3; 350 ms ± 39.2). No order effects were observed. Bonferroni-corrected paired-samples t tests conducted in both the speed and accuracy conditions verified the expected differences in error and correct RT (Falkenstein et al., 2001; Mathewson et al., 2005; Rabbitt, 1966; Yeung et al., 2004), with errors being significantly faster than correct trials in both the accuracy condition (t(62) = 18.7, p < .001; error-RT mean ± SD = 321 ms ± 43.4; correct-RT mean ± SD = 389 ms ± 41.2) and the speed condition (t(62) = 20.8, p < .001; error-RT mean ± SD = 293 ms ± 35.3; correct-RT mean ± SD = 350 ms ± 39.2).
3. 3 Corrective Behavioral Actions
3.3.1 Post-error RT
The omnibus 2 (condition) × 2 (accuracy) × 2 (order) × 2 (sex) ANCOVA analysis revealed a main effect for sex, F(1, 58) = 10.9, p = .002, partial η2 = .16, with faster post-error RT for males compared to females. Additionally, the expected significant condition, F(1, 58) = 11.0, p = .002, partial η2 = .16, and accuracy, F(1, 58) = 9.5, p = .003, partial η2 = .14, effects were present, suggesting slower post-error RTs in the accuracy condition and greater RT slowing following errors, respectively. These main effects were modified by a significant two-way condition × order interaction, F(1, 58) = 9.3, p = .003, partial η2 = .14, as well as a three-way condition × accuracy × order interaction, F(1, 58) = 19.3, p < .001, partial η2 = .25. Table 1 provides post-error RT and post-error accuracy means (SD) by condition and accuracy for each task order. No significant main or interaction effects for SE were present in the analysis.
Table 1.
Post-error RT and Post-error Accuracy Means (SD) by Task Condition (Accuracy, Speed) and Accuracy (Post-Error, Post-Matched-Correct) for Each Task Order (Accuracy-first, Accuracy-second).
| Accuracy-First | Accuracy-Second | |||
|---|---|---|---|---|
| Variable | Accuracy Condition |
Speed Condition |
Accuracy Condition |
Speed Condition |
| P-E RT | 398.3 (51.1) | 352.0 (38.6) | 382.6 (41.0) | 378.3 (44.0) |
| PMC RT | 363.9 (39.8) | 327.7 (39.2) | 369.0 (41.2) | 335.6 (39.5) |
| P-E PC | 90.1 (7.8) | 82.0 (10.7) | 89.2 (9.3) | 80.2 (9.4) |
| PMC PC | 85.5 (8.6) | 77.9 (9.0) | 85.4 (9.3) | 74.3 (10.7) |
Note. P-E = post-error; PMC = post-matched-correct; RT = response time; PC = percentage correct (response accuracy).
3.3.2 Post-error accuracy
The omnibus 2 (condition) × 2 (accuracy) × 2 (order) × 2 (sex) ANCOVA revealed the expected significant accuracy main effect, F(1, 58) = 5.4, p = .024, partial η2 = .09, suggesting greater post-error accuracy following errors (see Table 1) as well as a significant main effect for SE, F(1, 58) = 11.9, p = .001, partial η2 = .17, suggesting greater SE is associated with greater post-error accuracy. These main effects were modified by a significant two-way condition × accuracy interaction, F(1, 58) = 5.5, p = .022, partial η2 = .09, as well as a three-way condition × accuracy × SE interaction, F(1, 58) = 5.6, p = .021, partial η2 = .09.
Decomposition of the three-way interaction into simple accuracy ANCOVAs for each condition revealed significant accuracy main effects for both conditions, with similar effects in both the accuracy condition, F(1, 26) = 17.5, p < .001, partial η2 = .40, and speed condition, F(1, 21) = 16.0, p = .001, partial η2 = .43. Zero-order bivariate correlations between SE and post-error behavior revealed the largest correlation between SE and post-error accuracy in the accuracy condition, r = .30, p = .02, with a significant relationship between SE and post-matched-correct accuracy in the accuracy condition as well, r = .25, p < .05. In the speed condition, SE was not significantly correlated with post-error accuracy, r = .12, p = .32, but was significantly correlated with post-matched-correct accuracy, r = .26, p = .04, suggesting SE is differentially related with post-error self-regulation across task instruction conditions, but no differences exist across task conditions in relation to post-correct processes. Finally, though the omnibus ANCOVA did not reveal the expected condition main effect, a follow-up ANOVA without SE included as a covariate did reveal the condition main effect, F(1, 59) = 69.1, p < .001, partial η2 = .54, with greater post-error accuracy in the accuracy condition compared to the speed condition. Further, the accuracy main effect was much larger, F(1, 59) = 39.4, p < .001, partial η2 = .40, when SE was removed from the model.
3. 4 ERN
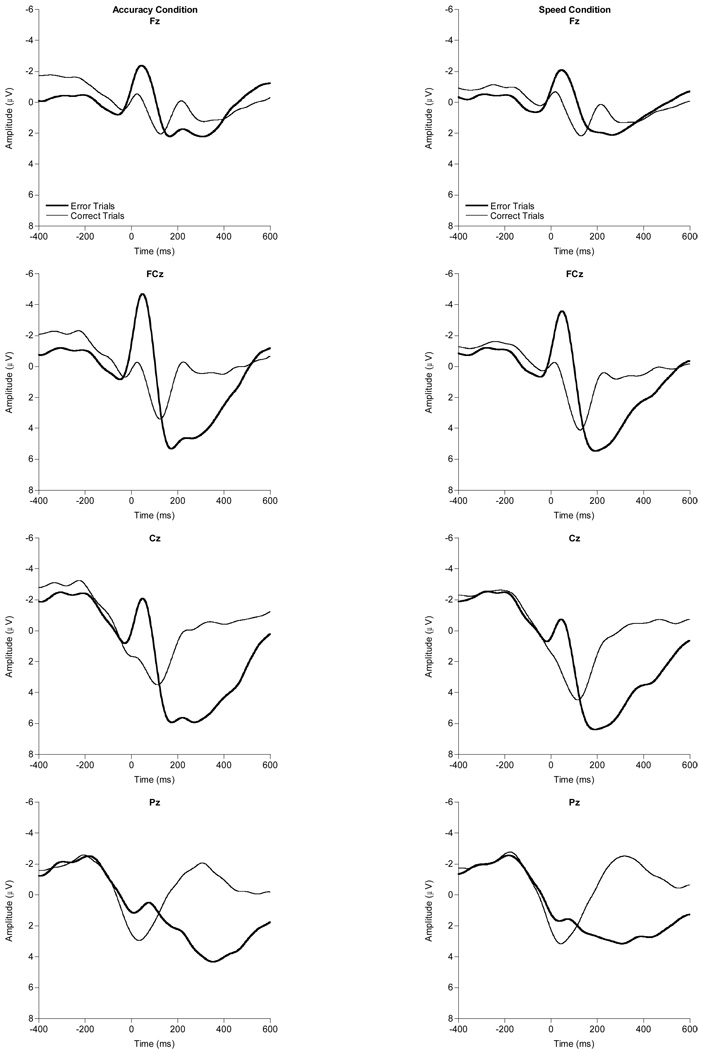
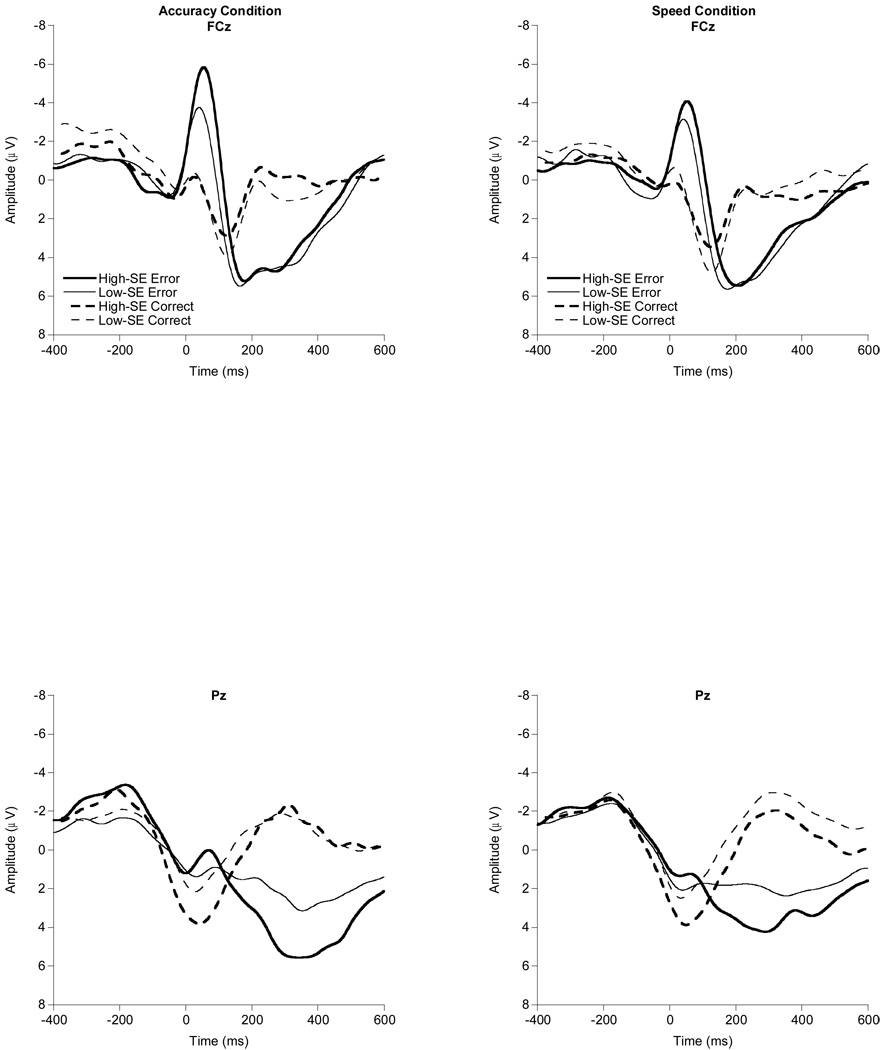
Figure 1 provides grand-averaged response-locked waveforms by instruction condition (accuracy, speed) and response accuracy (error, correct). The omnibus 2 (condition) × 2 (accuracy) × 2 (order) × 2 (sex) ANCOVA revealed significant SE, F(1, 58) = 5.1, p = .03, partial η2 = .08, and sex, F(1, 58) = 5.6, p = .02, partial η2 = .09, main effects, suggesting larger ERN amplitudes for individuals with greater-SE and for females, respectively. These main effects were modified by a significant two-way accuracy × SE interaction, F(1, 58) = 5.1, p = . 03, partial η2 = .08, as well as a three-way accuracy × sex × order interaction, F(1, 58) = 7.3, p = .009, partial η2 = .11. Decomposition of the accuracy × SE interaction into zero-order bivariate correlations between SE, ERN, and CRN revealed that SE is significantly correlated with ERN, r = −.26, p = .04, but not with CRN, r = −.07, p = .57, with greater SE associated with larger (more negative) ERN amplitude across instruction conditions. Figure 2 provides grand-averaged response-locked waveforms highlighting SE influences at the FCz and Pz electrode sites. A median split was performed for SE to facilitate the display of these waveforms. Finally, though the omnibus ANCOVA did not reveal the expected condition and accuracy main effect, a follow-up ANOVA without the SE covariate did reveal both the condition, F(1, 59) = 20.4, p < .001, partial η2 = .26, and accuracy main effects, F(1, 59) = 74.6, p < .001, partial η2 = .56, with larger amplitudes in the accuracy instruction condition compared to the speed condition and in relation to error trials compared to correct trials, respectively1.
Figure 1.
Grand averaged response-locked waveforms for the accuracy and speed conditions on error and correct trials at the Fz, FCz, Cz, and Pz electrode sites.
Figure 2.
Grand averaged response-locked waveforms for SE influences on error and correct trials in the accuracy and speed conditions at the FCz and Pz electrode sites. A median split was performed for SE to facilitate the display of these waveforms.
3.5 ERN and post-error accuracy
3.5.1 SE moderation analysis
Zero-order correlations revealed a significant relationship between ERN and post-error accuracy, r = −.41, p = .001, with larger (more negative) ERN amplitude associated with greater post-error accuracy across instruction conditions. Since the relationship between SE and ERN did not interact with instruction condition for the ERN (i.e., the relationship was not specific to the accuracy instruction condition, but existed across instruction conditions), analyses examining SE as a moderator of the ERN and post-error accuracy relationship are only appropriate across task conditions. Thus, to determine if SE moderated the relation between ERN and post-error accuracy, a linear multiple regression was conducted regressing post-error accuracy on SE, ERN, and the product of SE and ERN across task conditions (Baron and Kenny, 1986). Results indicated that SE did not moderate the relationship as the product variable (SE × ERN), was not significant (t(59) = 1.2, p = .24), suggesting a linear moderation relationship was not present.
Previous research on this topic utilized a median-split to group participants by SE and showed a moderation effect (Themanson et al., 2008), suggesting the moderating effect of SE may be best summarized as a step function (Baron and Kenny, 1986) and is not appropriately examined in a linear model. To assess this possibility, a post-hoc median split was performed on SE to examine the potential existence of a non-linear moderation relationship (Baron and Kenny, 1986). Simple linear regression analyses were conducted separately for both the high- and low-SE groups to determine whether SE moderated the relationship between ERN and post-error accuracy (Themanson et al., 2008), with post-error accuracy regressed on ERN amplitude. The analyses revealed a significant relation between ERN and post-error accuracy in the high-SE group, r = −.46, F(1, 29) = 6.3, p = .01, with larger ERN associated with greater post-error accuracy. However, no significant relationship was present in the low-SE group, r = −.34, F(1, 30) = 3.6, p = .07. A Fisher’s Z-transformation was calculated to compare the ERN and post-error accuracy correlations across levels of SE. The results showed a non-significant difference in the relationships between ERN and post-error accuracy across SE groups, z = .54, p = .59, suggesting no difference in the relation between ERN and post-error accuracy in the high-SE participants compared to the low-SE participants. These findings do not support those from previous research on older adults (Themanson et al., 2008) as SE did not moderate the relation between ERN amplitude and post-error accuracy.
3.5.2 Mediation analysis
Previous analyses demonstrated a significant positive relationship between SE and post-error accuracy, but only in the accuracy instruction condition. Further, SE was significantly related with ERN across task conditions, with a significant correlation evidenced in the accuracy condition, r = −.31, p = .01. Moreover, ERN was significantly related with post-error accuracy across task conditions, with a significant relationship in the accuracy condition, r = −.41, p = .001. Therefore, it is possible that ERN amplitude mediates the exhibited SE effect on post-error accuracy in the accuracy instruction condition. To formally test for ERN mediation of the relationship between SE and post-error accuracy, three linear regression analyses were performed to test for ERN mediation (Baron and Kenny, 1986). As expected, the first and second regression analyses revealed that SE affected ERN amplitude, t(61) = 2.6, p = .01, and post-error accuracy, t(61) = 2.4, p = .02, respectively, in the accuracy instruction condition. The third analysis, which regressed post-error accuracy on both SE and ERN amplitude, revealed a significant effect of ERN amplitude on post-error accuracy, t(60) = 3.0, p = .004, and a smaller, non-significant, effect of SE on post-error accuracy, t(60) = 1.2, p = .23 (see Table 2). A Sobel (1982) test was conducted to assess the significance of the mediating variable (ERN) on the strength of the relation between SE and post-error accuracy. The test revealed a significant effect, z = 2.01, p = .04, suggesting ERN does mediate, in part, the relation between SE and post-error accuracy in the accuracy instruction condition as the relation between SE and post-error accuracy was significantly weaker with the inclusion of ERN in the regression model.
Table 2.
Summary of Regression Analyses to Test ERN Mediation of Relation Between SE and Post-Error Accuracy in the Accuracy Instruction Condition: Regressing Post-Error Accuracy on SE (top), regression ERN amplitude on SE (middle), and regression Post-Error Accuracy on both SE and ERN amplitude (bottom).
| Post-Error Accuracy | |||
|---|---|---|---|
| Variable | B | SE B | β |
| SE | .16 | .08 | .30* |
| ERN Amplitude | |||
| Variable | B | SE B | β |
| SE | −.08 | .03 | −.31* |
| Post-Error Accuracy | |||
| Variable | B | SE B | β |
| SE | .09 | .08 | .15 |
| ERN | −.88 | .07 | −.37** |
Note. SE = self-efficacy; ERN = error-related negativity
p < .05.
p < .01.
3.6 Pe
The omnibus 2 (condition) × 2 (accuracy) × 2 (order) × 2 (sex) ANCOVA revealed a significant main effect for SE, F(1, 58) = 9.2, p = .004, partial η2 = .14, suggesting greater SE is associated with larger Pe amplitude regardless of task condition or response accuracy, as well as a significant three-way condition × accuracy × order interaction, F(1, 58) = 16.5, p < .001, partial η2 = .22, and a significant four-way condition × accuracy × order ×sex interaction, F(1, 58) = 7.9, p = .007, partial η2 = .12. Though the omnibus ANCOVA did not reveal the expected condition and accuracy main effect, a follow-up ANOVA without the SE covariate did reveal the accuracy main effect, F(1, 59) = 60.4, p < .001, partial η2 = .51, with larger amplitudes for error trials compared to correct trials (see Figure 1).
3.7 Order/Experience Effects on SE and Self-Regulation
To determine if prior task experience had an effect on the relation between SE and indices of self-regulation under accuracy instructions, we computed bivariate zero-order correlations between SE, ERN, Pe, and post-error accuracy separately for each task order (accuracy-first, accuracy-second). Correlations revealed that accuracy-first participants exhibited smaller, but still significant, correlations between SE and ERN, r = −.36, p = .04, and SE and post-error accuracy, r = .29, p = .08, compared to accuracy-second participants who gained task experience prior to engaging in the accuracy condition (SE with ERN: r = −.24, p = .16; SE with post-error accuracy: r = .35, p = .05). Fisher’s Z-transformations were calculated to compare the ERN and post-error accuracy correlations across task orders. The results showed no significant difference in the relationships between SE and ERN across task orders, z = .72, p = .47, or between SE and post-error accuracy across task orders, z = .36, p = .72. These findings suggest that the relations between SE and indices of self-regulation do not strengthen with task experience and different indices of self-regulation are not differentially sensitive to one’s relative level of task experience.
4. Discussion
The present data confirm that beneficial relationships exist between SE and both behavioral and neural indices of self-regulation. SE was associated with greater post-error response accuracy during accuracy instruction conditions, corroborating previous evidence relating SE to self-regulatory processes (Themanson et al., 2008) and extending the literature to include young adults using a more robust continuous assessment of SE. Additionally, support is provided for social cognitive theory, which details SE as a self-regulatory agent for improving goal-directed behavior (Bandura, 2001; Bandura and Locke, 2003) as SE was associated with ERN amplitude, a neural index of self-regulatory processes intended to improve desired behavioral outcomes, across instruction conditions. Further, findings suggest that ERN amplitude mediated the relationship between SE and post-error response accuracy in the accuracy instruction condition, such that the activation of self-regulatory processes indexed by ERN amplitude help explain the improved performance following errors in the accuracy instruction condition for those individuals with greater SE. Finally, findings showed the association between motivational influences (SE, accuracy instructions) and indices of self-regulation remains constant as one gains experience in task engagement, with no strengthening of the relationships or modulatory differences among those indices due to increased task experience, suggesting no variation in goal orientation (process, outcome) over the course of participation and no improvements in self-regulatory processes associated with SE.
Researchers have examined the beneficial relationship between SE and cognitive behavior (Berry and West, 1993; Bouffard-Bourchard, 1990; Lachman and Jelalian, 1984; Stajkovic and Luthans, 1998) and have detailed SE influences on effort and motivation following substandard performance (Bandura and Cervone, 1983; Bandura, and Locke, 2003). However, there has been little focus on the relationship between SE and behavioral outcomes following self-regulatory processing and how the impact of SE may be implemented through patterns of neural activation (Themanson et al., 2008). Fundamentally, post-error adaptations in behavior are made to improve subsequent performance. Further, the degree to which one is able to accurately perform following behavioral errors is a direct indicator of one’s ability to beneficially monitor and modify actions to meet desired and intended outcomes. The current findings indicate that SE may be one factor related with improved post-error corrective adjustments, with the greatest beneficial influence when performance accuracy is most salient (Gehring et al., 1993) or when the individual is more motivated (Hajcak et al., 2005) or has more incentive (Pailing and Segalowitz, 2004a) to perform accurately. This result is also consistent with social cognitive theory, which details SE as a positive influence on motivation, effort, and perseverance (Bandura, 1991, 1993). Thus, greater SE may lead to greater levels of motivation, incentive, or effort to perform accurately under task instructions emphasizing accuracy, above and beyond the influence of task parameters, and this enhanced motivation is associated with an enhanced ability to self-regulate following errors to improve performance.
In addition to post-error accuracy, a relationship exists between SE and ERN amplitude across task conditions, with more efficacious individuals exhibiting greater (more negative) ERN amplitudes. This finding extends the post-error accuracy finding described above to include neural measures of self-regulation, though the neural effect was evidenced across task instruction conditions. This does not corroborate previous evidence relating SE to ERN amplitude in older adults, which showed a relationship only under accuracy instructions (Themanson et al., 2008). This indicates that while greater SE heightens the detection of errors (Holroyd and Coles, 2002) or behavioral conflict (Yeung et al., 2004) during tasks emphasizing the importance of accurate task execution in young adults, the beneficial impact of SE on self-regulatory processes indexed by the ERN generalizes to different task parameters. Further, the relation between SE and ERN provides support for the reinforcement learning (R-L) theory of ERN (Holroyd and Coles, 2002). The R-L theory suggests that ERN amplitude is sensitive to performance expectancy, such that lower expectations and poorer performance are associated with smaller (less negative) ERN amplitudes and higher expectations and better performance are associated with larger (more negative) ERN amplitudes. In the present study, higher SE expectations were indeed associated with both improved post-error performance, in accord with social cognitive theory (Bandura, 1986), and larger ERN amplitudes, which result from higher expectations as predicted by R-L theory (Holroyd and Coles, 2002). Finally, no effects of SE were present with the CRN, suggesting the SE effect is specific to neural indices of error-related self-regulation. This finding is consistent with Hajcak et al. (2005), who reported a similar pattern of results in relation to motivational influences on the ERN and suggested that a “functional differentiation” (Hajcak et al., 2005, p. 159) may exist between correct and error trials in terms of ACC activation and self-regulation.
Moreover, the relationship between SE and post-error accuracy in the accuracy condition was mediated by ERN amplitude, such that self-regulatory processes indexed by ERN amplitude help explain the improved post-error performance of individuals with greater SE. Importantly, in addition to SE, larger (more negative) ERN amplitudes were also associated with greater response accuracy following error commission, which is consistent with fMRI research showing ACC activation during errors and task conditions that elicit response conflict predicted the recruitment of additional prefrontal neural structures believed to be crucial for the implementation of control on subsequent trials (Garavan et al., 2002; Kerns et al., 2004). Our findings in older adults (Themanson et al., 2008) suggested that the relationship between ERN and post-error accuracy was moderated by SE, with a tighter coupling of the neural self-regulatory processes and post-error behavioral improvements in the higher-SE participants. However, the current finding in young adults provides more information on how SE, ERN, and post-error accuracy are related. Notably, the intervening effects SE has on ERN and ERN has on post-error accuracy provide one important mechanism through which SE is related with compensatory post-error adjustments in behavior.
Given the relation between SE and indices of self-regulation, and the modifiable nature of SE through a variety of means, including mastery experiences (Bandura, 1986), one could hypothesize that SE and its relations with process- and outcome-oriented self-regulatory goals develops over the course of task experience (Zimmerman and Kitsantas, 1997). Alternatively, the goal orientation of self-regulation may not change or develop in relation to task experience at all. Instead, the relation between SE and all indices of self-regulation may strengthen with greater task experience (Bandura and Wood, 1989). However, our findings indicate that the relations between SE and all indices of self-regulation in the accuracy condition remained consistent with task experience; they did not change/develop (Zimmerman and Kitsantas, 1997) or strengthen (Bandura and Wood, 1989); suggesting the association between SE and self-regulation was not enhanced, or influenced in any way, by greater task experience.
Upon initial inspection, the adaptive relations larger ERN amplitudes has with SE and post-error performance (present study; Themanson et al., 2008), stress regulation (Compton et al. 2008), and academic performance (Hirsh and Inslicht, 2010), may seem paradoxical with research detailing maladaptive associations between larger ERN and psychological processes like obsessive-compulsive disorder (OCD; Gehring et al., 2000), anxiety (Hajcak et al., 2003), depression (Chiu and Deldin, 2007; Holmes and Pizzagalli, 2008) and negative affect (Hajcak et al., 2004; Luu et al., 2000; see Olvet and Hajcak, 2008 for a review). However, these findings should not be interpreted to indicate that the self-regulatory system itself is functionally adaptive or maladaptive. Rather, these findings should be used to detail the psychological and emotional context surrounding the use or misuse of the self-regulatory system. As theorized and modeled by both Yeung et al. (2004) and Holroyd and Coles (2002), the ERN is part of a cognitive selfregulatory system. Its function is related with one’s ability to detect and correct mistaken behavioral interactions/executions following erroneous task performance. Given that functionality, larger ERN can be both adaptive and maladaptive as both the self-regulatory system and the implementation of that system can be imprecise, misguided, and imperfect. When running smoothly, in an appropriately sensitive and controlled manner and existing in a well-adjusted and healthy individual, enhanced neural signaling of error detection and the recruitment of additional cognitive control to alleviate subsequent mistakes is an adaptive process. However, when the self-regulatory system is not calibrated appropriately due to psychological or emotional disruptions affecting the system or the individual in general, the system does not behave in the most efficient or healthy manner. This can lead to “hyperactive or hypoactive errorprocessing,” (Olvet and Hajcak, 2008, p. 1349) and the inefficiency, miscalibration, or misuse of the self-regulatory system can be viewed as an indicator of the larger unhealthy and maladaptive state of the individual (e.g., internalizing disorders and/or externalizing disorder; Olvet and Hajcak, 2008).
Limitations
Although we report on interesting relationships among SE, behavioral performance, and neural indices of self-regulation, there are a number of limitations to the present study. Although our analyses were able to determine the extent to which SE was independently associated with post-error behavior and ERN amplitude, it is important to clarify that no causal relationships or temporal models are being proposed. The cross-sectional nature of the study, as well as the lack of random assignment to levels of SE, limits the strength of the findings because the effects may be attributable to other factors. Additionally, only one relatively simple cognitive task was utilized in the current investigation. Future research should implement an array of cognitive measures with greater levels of complexity to more completely assess the relationships between SE, indices of self-regulation, and the potential development of self-regulatory processes across task experiences.
Conclusions
Overall, our findings support SE, a modifiable psychosocial construct, as an important correlate of self-regulation and post-error adjustments in behavior. As predicted by social cognitive theory (Bandura, 1986, 1997), SE expectations were associated with higher levels of reactive evaluation and behavioral adaptations utilized to improve performance following error commission. These benefits were evident in both neural (ERN) and behavioral (post-error accuracy) measures of self-regulation, with a more general relationship between SE and ERN across task instruction conditions and a more specific relation between SE and post-error accuracy only under task conditions emphasizing the accuracy of performance, suggesting the influence of SE interacts with task constraints (Themanson et al., 2008). Further, the relationship between SE and post-error response accuracy in the accuracy condition was mediated by ERN amplitude, suggesting the improved post-error performance of individuals with greater SE may be explained by the self-regulatory processes underlying ERN activation. Whether the manipulation of SE beliefs differentially alters the functioning of an individual’s self-regulatory system and the quality of their interactions with the environment remains to be determined. Future efforts might consider employing true experimental designs in which SE is manipulated to determine how these task-specific improvements in behavior and post-error cognitive modifications might subsequently enhance overall cognitive health and well-being.
Research highlights.
Self-efficacy is positively related with greater post-error accuracy.
Self-efficacy is positively related with greater ERN amplitude.
ERN mediates the relationship between self-efficacy and post-error accuracy.
Acknowledgements
This research was supported by grants from the National Institute of Mental Health (F31 MH076463) and Illinois Wesleyan University to Jason Themanson and the National Institute on Aging (RO1 AG021188) to Charles Hillman.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All ERN analyses were also conducted at Cz. The pattern of findings did not differ from those reported in the text at FCz. These analyses were not included in the text to clarify the presentation of the data.
References
- Band GPH, Kok A. Age effects on response monitoring in a mental-rotation task. Biol. Psychol. 2005;51:201–221. doi: 10.1016/s0301-0511(99)00038-1. [DOI] [PubMed] [Google Scholar]
- Bandura A. Self-efficacy: Toward a unifying theory of behavioral change. Psychol. Rev. 1977;84:191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- Bandura A. Social foundations of thought and action: A social cognitive theory. Englewood Cliffs, NJ: Prentice-Hall; 1986. [Google Scholar]
- Bandura A. Self-regulation of motivation through anticipatory and self-regulatory mechanisms. In: Dienstbier RA, editor. Perspectives on motivation: Nebraska symposium on motivation. Vol. 38. Lincoln: University of Nebraska Press; 1991. pp. 69–164. [PubMed] [Google Scholar]
- Bandura A. Perceived self-efficacy in cognitive development and functioning. Educ. Psychol. 1993;28:117–148. [Google Scholar]
- Bandura A. Self-efficacy: The exercise of control. New York: Freeman; 1997. [Google Scholar]
- Bandura A. Social cognitive theory: An agentic perspective. Annu. Rev. Psychol. 2001;52:1–26. doi: 10.1146/annurev.psych.52.1.1. [DOI] [PubMed] [Google Scholar]
- Bandura A. Guide for constructing self-efficacy scales. In: Pajares F, Urdan T, editors. Self-efficacy beliefs of adolescents. Vol. 5. Charlotte, NC: Information Age Publishing; 2006. pp. 307–337. [Google Scholar]
- Bandura A, Cervone D. Self-evaluative and self-efficacy mechanisms governing the motivational effects of goal systems. J. Pers. Soc. Psychol. 1983;45:1017–1028. [Google Scholar]
- Bandura A, Locke EA. Negative self-efficacy and goal effects revisited. J. Appl. Psychol. 2003;88:87–99. doi: 10.1037/0021-9010.88.1.87. [DOI] [PubMed] [Google Scholar]
- Bandura A, Wood R. Effect of perceived controllability and performance standards on self-regulation of complex decision making. J. Pers. Soc. Psychol. 1989;56:805–814. doi: 10.1037//0022-3514.56.5.805. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Berry JM, West RL. Cognitive self-efficacy in relation to personal mastery and goal setting across the life span. Int. J. Behav. Dev. 1993;16:351–379. [Google Scholar]
- Bouffard-Bouchard T. Influence of self-efficacy on performance in a cognitive task. J. Soc. Psychol. 1990;130:353–363. [Google Scholar]
- Cervone D, Peake PK. Anchoring, efficacy, and action: The influence of judgmental heuristics on self-efficacy judgments and behavior. J. Pers. Soc. Psychol. 1986;50:492–501. [Google Scholar]
- Chiu PH, Deldin PJ. Neural evidence for enhanced error detection in major depressive disorder. Am. J. Psychiatry. 2007;164:608–616. doi: 10.1176/ajp.2007.164.4.608. [DOI] [PubMed] [Google Scholar]
- Coles MGH, Scheffers MK, Holroyd CB. Why is there an ERN/Ne on correct trials? Response representations, stimulus-related components, and the theory of error-processing. Biol. Psychol. 2001;56:173–189. doi: 10.1016/s0301-0511(01)00076-x. [DOI] [PubMed] [Google Scholar]
- Compumedics Neuroscan. Offline analysis of acquired data (SCAN 4.3 – Vol. II, EDIT 4.3). [Software Manual] El Paso, TX: Author; 2003. [Google Scholar]
- Compton RJ, Robinson MD, Ode S, Quandt LC, Fineman SL, Carp J. Error-monitoring ability predicts daily stress regulation. Psychol. Sci. 2008;19:702–708. doi: 10.1111/j.1467-9280.2008.02145.x. [DOI] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, Dywan J, Pailing PE. Error-negativity and positivity as they relate to other ERP indices of attentional control and stimulus processing. Biol. Psychol. 2001;56:191–206. doi: 10.1016/s0301-0511(01)00080-1. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonresearch task. Percept. Psychophys. 1974;16:143–149. [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of errors in choice reaction tasks on the ERP under focused and divided attention. In: Brunia CHM, Gaillard AWK, Kok A, editors. Psychophysiological brain research. vol. 1. Tilberg, the Netherlands: Tilberg University Press; 1990. pp. 192–195. [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components: II. Error processing in choice reaction tasks. Electroencephal. Clin. Neurophysiol. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: A tutorial. Biol. Psychol. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. Changes of error-related ERPs with age. Exp. Brain Res. 2001;138:258–262. doi: 10.1007/s002210100712. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RAP, Stein EA. Dissociable executive functions in the dynamic control of behavior: Inhibition, error detection, and correction. Neuroimage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychol. Sci. 1993;4:385–390. [Google Scholar]
- Gehring WJ, Himle J, Nisenson LG. Action-monitoring dysfunction in obsessive-compulsive disorder. Psychol. Sci. 2000;11:1–6. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. Anxiety and error-related brain activity. Biol. Psychol. 2003;64:77–90. doi: 10.1016/s0301-0511(03)00103-0. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. Error-related psychophysiology and negative affect. Brain Cogn. 2004;56:189–197. doi: 10.1016/j.bandc.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Yeung N, Simons RF. On the ERN and the significance of errors. Psychophysiology. 2005;42:151–160. doi: 10.1111/j.1469-8986.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Hirsh JB, Inzlicht M. Error-related negativity predicts academic performance. Psychophysiology. 2010;47:192–196. doi: 10.1111/j.1469-8986.2009.00877.x. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, Pizzagalli DA. Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Arch. Gen. Psychiatry. 2008;65:179–188. doi: 10.1001/archgenpsychiatry.2007.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychol. Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Judge TA, Erez A, Bono JE, Thoresen CJ. Are measures of self-esteem, neuroticism, locus of control, and generalized self-efficacy indicators of a common core construct? J. Pers. Soc. Psychol. 2002;83:693–710. doi: 10.1037//0022-3514.83.3.693. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, III, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Sowon H, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, Colcombe A. Ageing, fitness, and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- Lachman ME, Jelalian E. Self-efficacy and attributions for intellectual performance in young and elderly adults. J. Gerontol. 1984;39:577–582. doi: 10.1093/geronj/39.5.577. [DOI] [PubMed] [Google Scholar]
- Larson GE, Saccuzzo DP, Brown J. Motivation: Cause or confound in information processing/intelligence correlations? Acta Psychol. 1994;85:25–37. doi: 10.1016/0001-6918(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Luu P, Collins P, Tucker DM. Mood, personality, and self-monitoring: Negative affect and emotionality in relation to frontal lobe mechanisms of error monitoring. J. Exp. Psychol. Gen. 2000;129:43–60. doi: 10.1037//0096-3445.129.1.43. [DOI] [PubMed] [Google Scholar]
- Mathewson KJ, Dywan J, Segalowitz SJ. Brain bases of error-related ERPs as influenced by age and task. Biol. Psychol. 2005;70:88–104. doi: 10.1016/j.biopsycho.2004.12.005. [DOI] [PubMed] [Google Scholar]
- McAuley E, Morris KS, Doerksen SE. Measures of cognitive self-efficacy. Exercise Psychology Laboratory, Department of Kinesiology and Community Health, University of Illinois at Urbana-Champaign; 2005. Unpublished manuscript. [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GPH, Kok A. Error-related brain potentials are differentially related to awareness of response errors: Evidence from an antisaccade task. Psychophysiology. 2001;38:752–760. [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Talsma D, Coles MGH, Holroyd CB, Kok A, van der Molen MW. A computational account of altered error processing in older age: Dopamine and the error-related negativity. Cogn. Affect Behav. Neurosci. 2002;2:19–36. doi: 10.3758/cabn.2.1.19. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olvet D, Hajcak G. The error-related negativity (ERN) and psychopathology: Toward an endophenotype. Clin. Psychol. Rev. 2008;28:1343–1354. doi: 10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet D, Hajcak G. The stability of error-related brain activity with increasing trials. Psychophysiology. 2009;46:957–961. doi: 10.1111/j.1469-8986.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Pailing PE, Segalowitz SJ. The error-related negativity as a state and trait measure: Motivation, personality, and ERPs in response to errors. Psychophysiology. 2004a;41:84–95. doi: 10.1111/1469-8986.00124. [DOI] [PubMed] [Google Scholar]
- Pailing PE, Segalowitz SJ. The effects of uncertainty in error monitoring on associated ERPs. Brain Cogn. 2004b;56:215–233. doi: 10.1016/j.bandc.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, Jr, Miller GA, Ritter W, Ruchkin DS, Rugg MD, Taylor MJ. Guidelines for using human event-related-potentials to study cognition: Recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- Pontifex MB, Scudder MR, Brown ML, O’Leary KC, Wu C-T, Themanson JR, Hillman CH. On the number of trials necessary for stabilization of error-related brain activity across the life span. Psychophysiology. 2010;47:767–773. doi: 10.1111/j.1469-8986.2010.00974.x. [DOI] [PubMed] [Google Scholar]
- Rabbitt PMA. Errors and error correction in choice-response tasks. J. Exp. Psychol. 1966;71:264–272. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Davis HP. Organization of cognitive abilities and neuropsychological variables across the lifespan. Dev. Rev. 2006;26:31–54. [Google Scholar]
- Sobel ME. Asymptotic intervals for indirect effects in structural equation models. In: Leinhart S, editor. Sociological methodology 1982. San Francisco: Jossey-Bass; 1982. pp. 290–312. [Google Scholar]
- Stajkovic AD, Luthans F. Self-efficacy and work-related performance: A meta-analysis. Psychol. Bull. 1998;124:240–261. [Google Scholar]
- Themanson JR, Hillman CH. Cardiorespiratory fitness and acute aerobic exercise effects on neuroelectric and behavioral measures of action monitoring. Neuroscience. 2006;141:757–767. doi: 10.1016/j.neuroscience.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Themanson JR, Hillman CH, Curtin JJ. Age and physical activity influences on neuroelectric indices of action monitoring during task switching. Neurobiol. Aging. 2006;27:1335–1345. doi: 10.1016/j.neurobiolaging.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Themanson JR, Hillman CH, McAuley E, Buck SM, Doerksen SE, Morris KS, Pontifex MB. Self-efficacy effects on neuroelectric and behavioral indices of action monitoring in older adults. Neurobiol. Aging. 2008;29:1111–1122. doi: 10.1016/j.neurobiolaging.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tops M, Boksem MAS, Wester AE, Lorist MM, Meijman TF. Task engagement and the relationships between the error-related negativity, agreeableness, behavioral shame proneness and cortisol. Psychoneuroendocrinology. 2006;31:847–858. doi: 10.1016/j.psyneuen.2006.04.001. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulated cortex. J. Cogn. Neurosci. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol. Bull. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol. Rev. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
- Zimmerman BJ, Kitsantas A. Developmental phases in self-regulation: Shifting from process goals to outcome goals. J. Educ. Psychol. 1997;89:29–36. [Google Scholar]