Abstract
Background. The p38 mitogen-activated protein kinase (p38 MAPK) is an important intracellular signal transduction pathway involved in TGF-β1-induced epithelial–mesenchymal transition (EMT). Sema4C, a member of the semaphorin family, was found to be essential for the activation of p38 MAPK. However, the role of Sema4C in promoting TGF-β1-induced EMT is unclear.
Methods. Renal fibrosis was induced by 5/6 subtotal nephrectomy rat model. In vitro, Sema4C was induced in human proximal tubular epithelial cells (HKC) by treatment with TGF-β1, or was inhibited by siRNA or was over-expressed by Sema4C transfection. The selective p38 MAPK inhibitor, SB203580, was administered to inhibit the p38 pathway. The expression of Sema4C, the markers of EMT, p38 phosphorylation and fibronectin secretion were measured by western blotting, immunohistochemistry, immunocytochemistry or enzyme-linked immunosorbent assay.
Results. The expression of Sema4C increased in HKC cells that were treated with TGF-β1. Knockdown of Sema4C potently inhibited phosphorylation of p38 MAPK and reversed TGF-β1-induced EMT. Over-expression of Sema4C via Sema4C transfection elicited p38 MAPK phosphorylation and promoted EMT. The effects of Sema4C during EMT were blocked by a p38-specific inhibitor. In vivo, the expression of Sema4C increased in the tubular epithelia of 5/6-nephrectomized rats and human fibrotic renal tissue, and similar localization of phosphorylated p38 and Sema4C was demonstrated by immunohistochemistry on serial sections.
Conclusions. Our findings suggest that Sema4C plays an important role in TGF-β1-induced EMT through activation of p38 MAPK in proximal tubular epithelial cells.
Keywords: epithelial to mesenchymal transition, p38 MAPK, Sema4C, TGF-β1
Introduction
The progression of chronic kidney disease leads to widespread tissue fibrosis and irreversible loss of renal function. Epithelial–mesenchymal transition (EMT) of tubular epithelial cells contributes significantly to the onset and pathogenesis of renal fibrosis [1–4]. An extracellular stimulus usually initiates this process, which leads to the loss of junctional contacts, expression of mesenchymal markers, development of cell motility and production of extracellular matrix (ECM) [2,5]. Of the many factors that trigger EMT, transforming growth factor-β1 (TGF-β1) is the most important and well studied [6]. TGF-β1 mediates the EMT process via numerous intracellular signal transduction pathways, including the canonical Smad pathway, mitogen-activated protein kinases (MAPK), PI3K/Akt and small GTPases that control the activity or expression of factors related to EMT [7,8].
In recent years, significant evidence suggests that p38 MAPK pathway is an important intracellular signal transduction pathway involved in TGF-β1-induced EMT in renal tubular epithelial cells [9,10]. The activated p38 MAPK could directly regulate the protein synthesis of α-smooth muscle cell actin (α-SMA) [10], indirectly activate Smad pathway [9], lead to excessive matrix deposition and finally induce the fibrotic process. However, the molecular details of how TGF-β1 could potentially induce p38 MAPK in renal tubular cells have not been elucidated yet.
Sema4C, a member of the semaphorin family, has been shown to be essential for the activation of p38 MAPK [11]. The semaphorins are a large family of secreted or membrane-bound proteins that all have a conserved Sema domain, which is known to regulate tumor progression [12], angiogenesis [13], nervous system development [14] and immune cell interactions [15]. Our previous microarray analysis of metastatic human cervical cancer tissue indicated a significant up-regulation of Sema4C during cancer invasion and metastasis (Table 1; this data had not been published elsewhere), a process that is analogous to that observed during tubular EMT [6,16]. However, it is not known whether Sema4C is involved in EMT. We therefore examined whether TGF-β1-induced EMT is mediated by Sema4C–MAPK pathway. For this purpose, we measured Sema4C in the tubular epithelia of fibrotic renal tissue and in renal tubular cells treated with TGF-β1, examined the effect of Sema4C siRNA on TGF-β1-induced MAPK activation, EMT and fibronectin secretion, and measured the p38 phosphorylation and EMT in Sema4C over-expressed cells.
Table 1.
Genes up-regulated in metastatic human cervical cancer tissue
| Probe set ID | Gene symbol | Description | P-value |
|---|---|---|---|
| 46665_at | Sema4c | Nervous system and cell differentiation development | 0.000219 |
| 217022_s_at | IGHA1 | Immune response, protein–chromophore linkage | 0.000052 |
| 221748_s_at | TNS1 | Intracellular signalling cascade | 0.000147 |
| 229152_at | C4orf7 | Chromosome 4 open reading frame 7 | 0.001953 |
| 234379_at | FLT-4 | Protein–tyrosine kinase activity and receptor activity | 0.01421 |
Materials and methods
Animal model
Male Sprague–Dawley rats (150–200 g) were obtained from the Tongji Laboratory Animal Center (Wuhan, China). All rats were sacrificed 20 weeks after nephrectomy, and serum was collected for determination of creatinine and urea nitrogen. Kidneys were immediately excised; some were fixed with 4% paraformaldehyde and others were frozen in liquid nitrogen for later use. For histological examination, renal tissues were stained with periodic acid–Schiff and Masson's trichrome. All procedures were performed in accordance with our university’s guidelines for animal care.
Cell cultures
HKC cells were cultured in DMEM/F12 (Invitrogen, Inc., Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Gibco, USA). HKC cells were cultured in serum-free medium for 24 h before being used for experiments. To induce renal tubular EMT, cells were then treated with recombinant TGF-β1 (10 ng/mL; B&D Systems Inc., Minneapolis, MN, USA) for 72 h.
SiRNA for Sema4C
For siRNA experiments, RNA primers complementary to human Sema4C were designed and synthesized by the Shanghai Invitrogen Biotechnology Company. HKC cells were transfected with the annealed RNA primer pair by using Lipofectamine 2000 (Invitrogen Inc.), according to the manufacturer's instructions. Five hours after transfection, cells were incubated with 10 ng/mL of TGF-β1 for 72 h to observe the effect of Sema4C silencing.
Production of stable HKC clones over-expressing human Sema4C
The plasmid pcDNA 4.0–Sema4C was transfected into HKC cells with Lipofectamine 2000 according to the manufacturer's instructions. Cells were plated at ~ 70–90% confluence in six-well plates 1 day before transfection. The cells were washed and seeded in growth medium containing zeocin. This selection procedure lasted for 2 weeks, and then Zeocin-resistant clones were harvested and analysed. To block the p38 MAPK pathway, Sema4C-transfected HKC cells were treated with 20 mM SB203580 for 48 h.
Western blotting
Kidney tissues and cells were extracted with lysis buffer containing 1% Triton X-100, 0.5% Nonidet P-40, 20 mM Tris–HCl, 15 mM NaCl, 1 mmol/L EDTA, 1 mmol/L egtazic acid, 1 mM Na3VO4·10H2O, 2 mM NaF, 2 mM Na2P2O4·10H2O, 10 mM β-glycerophosphate disodium salt (pH 8.0) and four protease inhibitors (5 mM phenylmethanesulfonyl fluoride, 5 μg/mL leupeptin, 5 μg/mL pepstatin and 5 μg/mL aprotinin) for 30 min at − 20°C. Then, the extract was centrifuged at 12 000 g for 20 min at 4°C to remove cell debris. Protein concentration was determined by the Bradford protein assay, 80 μg of protein was subjected to SDS–PAGE and proteins were transferred to nitrocellulose membranes. After transfer, the membranes were blocked with 5% non-fat dry milk dissolved in TBS containing 0.1% Tween-20 for 1 h at 37°C and blotted routinely with Sema4C (BD Biosciences, San Jose, CA, USA), E-cadherin (BD Biosciences), vimentin (Abcam, Cambridge, MA, USA), GAPDH (Proteintech Group, Inc.), phosphorylated p38 MAPK and p38 primary antibodies (Cell Signaling Technology, Danvers, MA, USA) at 37°C for 1 h. The bound antibody complexes were visualized by enhanced chemiluminescence (SuperSignal West Femto Kit; Pierce, Rockford, IL, USA), and X-ray films were scanned with a ChemiImager 5500 image analysis system (Alpha Innotech, San Leandro, CA, USA). Quantity One software (Bio-Rad) was used to quantify band density.
Renal biopsy specimens
Renal biopsy specimens were from patients with sclerosing glomerulonephritis or obstructive nephropathy, diagnosed between 2008 and 2010 in the Division of Nephrology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. Informed consent was obtained from each patient when the renal biopsy was performed. The research was in compliance with the Declaration of Helsinki. In all of the renal biopsy specimens, the tubulointerstitial fibrosis was dominant.
Immunohistochemistry and immunocytochemistry
For immunohistochemical analysis, paraffin sections or serial sections were incubated with either primary anti-Sema4C antibody or phosphorylated p38 MAPK antibody at 4°C overnight. The sections were then incubated with biotinylated goat anti-mouse Ig antibody as the secondary antibody, and the antibody reactions were visualized by using diamino benzidine (DAKO, Tokyo, Japan). The serial sections were then analysed to clarify the colocalization of Sema4C and phosphorylated p38 MAPK in these kidneys.
For immunocytochemical analysis, HKC cells were cultured on sterile glass coverslips in six-well plates. The slides were incubated overnight at 4°C with anti-E-cadherin, anti-vimentin or anti-phosphorylated p38 antibody, followed by incubation with FITC-conjugated secondary antibody at room temperature for 1 h. Finally, slides were counterstained with propidium iodide for E-cadherin, DAPI for vimentin and visualized by confocal laser scanning microscopy.
ELISA assay
Fibronectin (FN) secretion was determined by a competitive ELLSA assay kit (Boster Biological Technology, Wuhan, China) according to the manufacturer's instructions. The OD value was detected by an ELISA Reader in 450-nm wavelength and calculated in the linear part of the curve.
Statistical analyses
All data were analysed by Student’s t-test or a one-way ANOVA using SPSS (version 12.0). Data are expressed as means ± standard errors of the mean (SEM). Significance was assessed at P < 0.05.
Results
Sema4C is involved in renal fibrosis in vivo
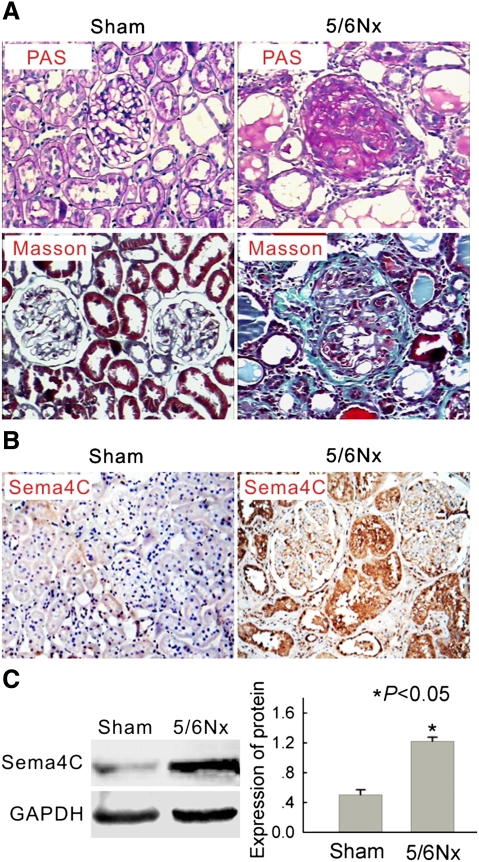
First, we examined the distribution of Sema4C in the fibrotic kidney by use of the 5/6 subtotal nephrectomy rat model. As shown in Table 2, serum urea nitrogen and creatinine were significantly elevated in 5/6-nephrectomized rats compared with sham-operated rats. Light microscopy indicated glomerular sclerosis and interstitial fibrosis in the 5/6-nephrectomized rats, but not in the sham-operated rats (Figure 1A).
Table 2.
Renal function in sham-operated rats and 5/6-nephrectomized rats
| Groups | n | Bun (mmol/L) | Scr (μmol/L) |
|---|---|---|---|
| Sham-operated | 8 | 6.25 ± 1.02 | 27.13 ± 6.13 |
| 5/6-nephrectomized | 8 | 36.79 ± 15.93* | 125.29 ± 58.35* |
*P < 0.01 compared with sham-operated rats.
Bun, serum urea nitrogen; Scr, serum creatinine.
Fig. 1.
Expression of Sema4C is increased in the kidney tissue of 5/6 nephrectomized rats. (A) Representative histology of kidney sections from sham-operated and 5/6-nephrectomized (5/6 NX) rats stained with periodic acid–Schiff (upper) or Masson's trichrome stain (lower). Interstitial fibrosis and glomerular sclerosis are extensive in 5/6 NX rats. The tissue of sham rats was normal. Magnification × 200. (B) Representative immunohistochemical detection of Sema4C in the kidney tissue of sham-operated and 5/6-nephrectomized rats. Sema4C immunostaining is strong in the epithelial tubular cells in 5/6 NX rats and much weaker in sham rats. Magnification × 200. (C) Representative western blot of Sema4C in the kidney tissue of sham-operated and 5/6-nephrectomized rats. The expression of the Sema4C protein was elevated in the kidney tissue of 5/6-nephrectomized rats. The histogram shows the average volume density corrected for the loading control, GAPDH (n = 6). *P < 0.05 compared with sham-operated rats.
Immunohistochemical staining showed that Sema4C was mainly expressed in renal tubular cells of 5/6-nephrectomized rats, with very little staining in the renal tubules of sham-operated rats (Figure 1B). Western blotting also indicated that Sema4C protein expression was significantly elevated in the kidney of 5/6-nephrectomized rats compared with sham-operated rats (Figure 1C). These results suggest that Sema4C is involved in renal fibrosis in this animal model.
TGF-β1 increases the expression of Sema4C in HKC cells and Sema4C depletion inhibits TGF-β1-induced EMT
Tubular epithelial cells are the natural targets of TGF-β1 in vivo, which plays a critical role in renal fibrosis in the 5/6 subtotal nephrectomy rat model [17]. Therefore, we examined the expression of Sema4C in proximal epithelial cells (HKC cells) that were treated with TGF-β1 in vitro. The result indicates that Sema4C was significantly elevated in HKC cells after incubation for 72 h with 10 ng/mL TGF-β1 (Figure 2A).
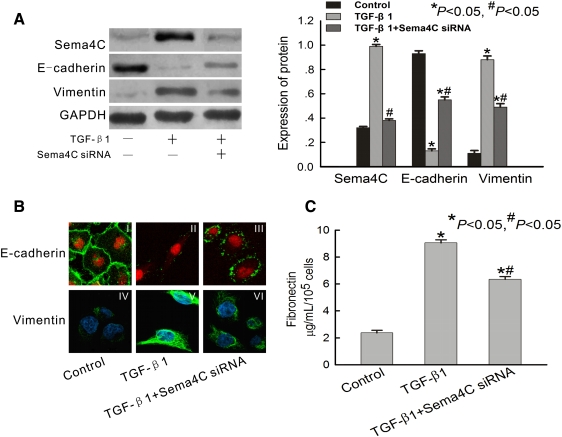
Fig. 2.
Expression of Sema4C is increased in TGF-β1-treated cells, and depletion of Sema4C represses TGF-β1-induced EMT. (A) Representative western blot of Sema4C, E-cadherin and vimentin in control cells, TGF-β1-treated cells and TGF-β1-treated Sema4C-siRNA cells. The histogram shows the average volume density corrected for the loading control, GAPDH (n = 4). *P < 0.05 compared with control cells. #P < 0.05 compared with TGF-β1-treated cells. (B) Representative confocal microscopy of E-cadherin and vimentin in monolayers of control cells, TGF-β1-treated cells and TGF-β1-treated Sema4C-siRNA cells. Cell nuclei were enhanced by staining of cell nuclei with propidium iodide for E-cadherin and DAPI for Vimentin. Magnification × 400. (C) Representative fibronectin secretion in culture media of control cells, TGF-β1-treated cells and TGF-β1-treated Sema4C-siRNA cells (n = 4). *P < 0.05 compared with control cells. #P < 0.05 compared with TGF-β1-treated cells.
We also investigated whether Sema4C could affect TGF-β1-dependent EMT by use of siRNA experiments. As shown in Figure 2A, cells developed EMT after 72 h of incubation with TGF-β1. This was manifested as down-regulation of E-cadherin, an epithelial marker, the decrease of which is a hallmark of EMT. In contrast, the protein vimentin, a mesenchymal marker, was up-regulated in TGF-β1-treated cells as compared with control cells (Figure 2A).
Confocal laser microscopy showed that E-cadherin had a continuous distribution near the perimeter of control cells [Figure 2B (I)], but had a discontinuous distribution near the perimeter of TGF-β1-treated cells [Figure 2B (II)]. Vimentin was present exclusively in the cytosol of TGF-β1-treated cells [Figure 2B (V)], and there was little endogenous expression in control cells [Figure 2B (IV)].
HKC cells transfected with Sema4C-specific siRNA were resistant to TGF-β1-induced EMT. As shown in Figure 2A, Sema4C after silencing almost touched the basal level, which is about 62% lower than that of TGF-β1-treated cells. The EMT resistance manifested as elevated levels of E-cadherin protein (Figure 2A), relocalization of E-cadherin protein to the cell perimeter [Figure 2B (III)] and reduced vimentin expression compared with TGF-β1-treated cells [Figure 2A and 2B (VI)]. All of these results suggest that Sema4C depletion inhibits TGF-β1-induced EMT.
As accumulated interstitial matrix components were a consequence of EMT, fibronectin secretion by HKC cells was measured in culture supernatants. Treatment of HKC cells for 72 h with TGF-β1 resulted in a significant mean 3.8-fold increase (N = 4) in fibronectin protein compared with control cells (Figure 2C). HKC cells transfected with Sema4C-specific siRNA were resistant to TGF-β1-induced fibronectin secretion (Figure 2C). This result was consistent with the finding that Sema4C depletion prevented TGF-β1-induced EMT.
Sema4C is an activator of p38 MAPK in TGF-β1-induced EMT
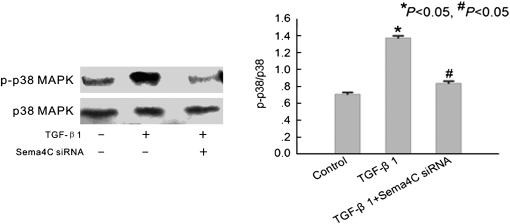
Sema4C has been reported to be a key inducer of p38 MAPK pathway signalling [11]. To investigate whether Sema4C is involved in the TGF-β1-induced EMT through the activation of p38 MAPK, we examined the phosphorylation of p38 MAPK in TGF-β1-treated cells. Western blotting showed that TGF-β1 significantly increased phosphorylation of p38 MAPK in HKC cells after 72 h of treatment (Figure 3). The phosphorylated p38 was about 2-fold higher than that of control, and it could be strikingly inhibited, almost to basal level, by Sema4C-specific siRNA (Figure 3).
Fig. 3.
Depletion of Sema4C represses TGF-β1-induced activation of p38 MAPK. Representative western blot of phosphorylated p38 MAPK in control cells, TGF-β1-treated cells and TGF-β1-treated Sema4C-siRNA cells. The histogram shows the average volume density of phosphorylated p38 MAPK corrected for the loading control, total p38 MAPK (n = 4). *P < 0.05 compared with control cells. #P < 0.05 compared with TGF-β1-treated cells.
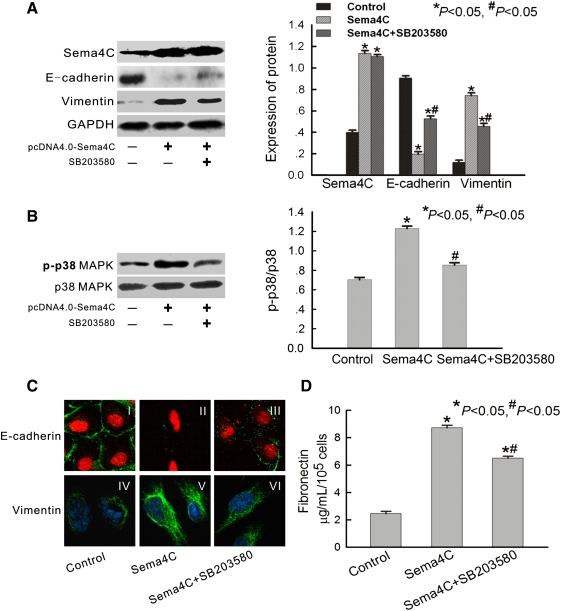
Next, we examined the phosphorylation of p38 and mesenchymal phenotype in Sema4C-transfected HKC cells. The results confirmed high expression of Sema4C and p38 phosphorylation and typical mesenchymal phenotype in Sema4C-transfected cells (Figure 4). As shown in Figure 4B, Sema4C transfection significantly increased the phosphorylation of p38 MAPK. Treatment with SB203580 (a p38 MAPK inhibitor) decreased phosphorylation of p38 MAPK by 31% (Figure 4B). Confocal laser microscopy showed that E-cadherin was linearly localized at cell borders of control cells [Figure 4C (I)], but formed a zipper-like pattern near the perimeter of Sema4C-transfected cells [Figure 4C (II)]. Vimentin was present exclusively in the cytosol of Sema4C-transfected cells [Figure 4C (V)], and there was little endogenous expression in control cells [Figure 4C (IV)]. Treatment with SB203580 inhibited Sema4C-mediated EMT [Figures 4A and 4C (III and VI)].
Fig. 4.
Over-expression of human Sema4C promotes EMT via activation of p38 MAPK. (A) Representative western blots of Sema4C, E-cadherin and vimentin in control cells, Sema4C-transfected cells and SB203580-treated Sema4C-transfected cells. The histogram shows the average volume density corrected for the loading control, GAPDH (n = 4). *P < 0.05 compared with control cells. #P < 0.05 compared with Sema4C-transfected cells. (B) Representative western blot of phosphorylated p38 MAPK in control cells, Sema4C-transfected cells and SB203580-treated Sema4C-transfected cells. The histogram shows the average volume density of phosphorylated p38 MAPK corrected for the loading control, total p38 (n = 4). *P < 0.05 compared with control cells. #P < 0.05 compared with Sema4C-transfected cells. (C) Representative confocal microscopy of E-cadherin and vimentin in monolayers of control cells, Sema4C-transfected cells and SB203580-treated Sema4C-transfected cells. Cell nuclei were enhanced by staining of cell nuclei with propidium iodide for E-cadherin and DAPI for vimentin. Magnification, × 400. (D) Representative fibronectin secretion in culture media of control cells, Sema4C-transfected cells and SB203580-treated Sema4C-transfected cells (n = 4). *P < 0.05 compared with control cells. #P < 0.05 compared with Sema4C-transfected cells.
Fibronectin secretion of HKC cells was also regulated by Sema4C. When HKC cells were transfected with Sema4C, there was a significant increase in fibronectin accumulation in culture supernatants (Figure 4D). However, treatment with SB203580 inhibited the secretion of fibronectin protein compared with Sema4C-transfected cells (Figure 4D). Those results indicate that Sema4C can phosphorylate p38 MAPK in cultured human tubular epithelial cells and induce EMT.
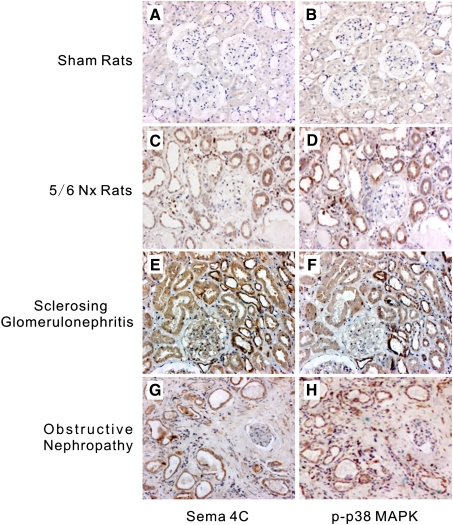
To further confirm the relationship between Sema4C and activation of p38 MAPK on tubules in vivo, we evaluated sequential kidney sections from 5/6-nephrectomized rats and patients with renal fibrosis. Sema4C expression in tubules was highly correlated with p-p38 MAPK expression in these kidneys (Figure 5). In fibrotic kidney from either rats or human beings, along with an increase in Sema4C, phosphorylated p38 MAPK was over-expressed in the renal epithelia, and the distribution patterns of phosphorylated p38 MAPK and Sema4C were highly congruent in the renal tubules (Figure 5C–H). In contrast, tubules in normal rats barely expressed either Sema4C or p-p38 MAPK (Figure 5A–B). These results suggest that Sema4C is involved in TGF-β1-induced renal fibrosis via the p38 MAPK pathway.
Fig. 5.
Immunohistochemistry of Sema4C and phosphorylated p38 MAPK on serial sections of rats or patients with renal fibrosis. Sema4C and p38 MAPK were co-expressed in tubules from rats or patients with tubulointerstitial fibrosis. These were representative photomicrographs from kidneys of sham rats immunostained with Sema4C (A) or p-p38 MAPK (B), 5/6-nephrectomized rats immunostained with Sema4C (C) or p-p38 MAPK (D), patients with sclerosing glomerulonephritis immunostained with Sema4C (E) or p-p38 MAPK (F) and patients with obstructive nephropathy immunostained with Sema4C (G) or p-p38 MAPK (H). Magnification, × 200.
Taken together, our results indicate that Sema4C directly activates p38 kinase in TGF-β1-treated cells, and that Sema4C-MAPK signalling pathway is important in TGF-β1-induced EMT.
Discussion
The epithelial–mesenchymal transition (EMT) of tubular epithelial cells is a critical process of renal tubulointerstitial fibrosis [2,18]. TGF-β1 signalling pathway plays a central role in regulating tubular EMT [5,18]. TGF-β1 transduces cellular signals via heterotetrameric complexes of type II (TβR-II) and type I (TβR-I) receptors [19]. The binding of TGF-β1 to TβR-II leads to phosphorylation of TβR-I and then activates receptor-activated (R) Smads (Smad2 and Smad3) and triggers EMT [20]. However, the activation of EMT is thought to occur through the signals arising not only from Smad2/3 but also those activated in response to p38 MAPK [21–23]. Recent studies have established a direct link between the TGF-β receptors and p38 MAPK [10,21,22]. TGF-β1 enables src to phosphorylate TβR-II on Tyr284. Phosphorylated TβR-II functions as an Src homology 2 (SH2) domain-binding site for growth factor receptor-bound protein 2 (Grb2), leading to substantial phosphorylation of p38 MAPK [21,22]. This, in turn, triggers intracellular signalling pathways related to EMT. Despite recent advances in understanding this pathway, the molecular mechanisms that enable TGF-β1 to activate p38 MAPK remain to be fully elucidated.
In the current study, Sema4C, a transmembrane protein and member of the semaphorin family, was reported to be essential for the activation of p38 MAPK [11]. The cytoplasmic domain of Sema4C contains a proline-rich region that may interact with Src homology 3 (SH3) domains and mediate intracellular signalling via interaction with a SH3 domain-containing protein. The adapter protein Grb2 is composed almost exclusively of SH3 domains [24]. Thus, it is possible that Sema4C may recruit Grb2 to the cellular membrane, promote association of TβR-II and Grb2, and ultimately facilitate the activation of p38 MAPK. Therefore, we postulated that Sema4C promote p38 MAPK signalling in the TGF-β1-induced renal tubular EMT.
The findings of our study support the hypothesis that Sema4C plays an important role in mediating renal tubular EMT through p38 MAPK signalling. Our in vivo experiments indicated that Sema4C increased in the tubular epithelial cells of fibrotic kidneys, and in vitro experiments indicated that TGF-β1 treatment induced over-expression of Sema4C in human tubular epithelial cells (HKC) accompanying characteristic changes of EMT. Loss of E-cadherin (a cell adhesion molecule present in the membranes of most epithelial cells) occurred, and this protein developed a discontinuous distribution along the cell perimeters. Vimentin, a cytoskeletal protein in many mesenchymal cells, was also induced. Fibronectin secretion, a consequence of EMT, was significantly increased in HKC cell culture supernatants.
Over-expression of Sema4C, performed with a Sema4C-transfected cell culture system, also remarkably accelerated the differentiation of epithelial HKC into mesenchymal cells. In addition, Sema4C siRNA knockdown in TGF-β1-treated HKC cells maintained E-cadherin, blocked vimentin expression and inhibited fibronectin secretion, suggesting a delay of the EMT process. Taken together, these results suggest that Sema4C contributes to TGF-β1-induced EMT.
Haitao Wu et al. [11] have previously demonstrated that p38 MAPK is a key element for Sema4C signalling, and Sema4C is an activator for p38 MAPK. In this study, we confirmed that p38 MAPK requires Sema4C for the regulation of EMT. Sema4C initiates p38 MAPK phosphorylation in Sema4C-transfected cells, and SB203580 (a p38 MAPK inhibitor) suppresses the activation of p38 MAPK and EMT. Knockdown of Sema4C dramatically impairs the phosphorylation of p38 MAPK during TGF-β1 treatment (Figure 3). Those results indicated that Sema4C mediated TGF-β1-induced EMT through the activation of p38 MAPK. Furthermore, we demonstrated in vivo that the distribution pattern of phosphorylated p38 MAPK is highly congruent with that of Sema4C in tubules of fibrotic kidney (Figure 5). As tubular epithelial cells are the natural targets of TGF-β1 in vivo [17], this result further supported that TGF-β1 exerts its fibrogenic effect through Sema4C-mediated activation of p38 MAPK.
Our study provides the first evidence for this hypothesis and shows that the TGF-β1 stimulation of tubular EMT is intimately linked to the Sema4C and the associated phosphorylation of p38 MAPK. From these findings, we propose to identify the formation and distribution of Sema4C–Grb2 complex and indicate its necessity for the activation of p38 MAPK during TGF-β1 treatment in future studies. Future studies are also needed to determine whether therapeutic targeting of Sema4C may function in alleviating the development of the TGF-β1-induced EMT. Furthermore, the knockdown of Sema4C (Figure 2A–B) or inhibition of p38 MAPK (Figure 4A, C) did not substantially preserve EMT, suggesting that other activated pathways are involved. The crosstalk between Sema4C/p38 MAPK and other intracellular signal transduction pathways and the therapeutic strategy targeting multiple kinases may need to be studied in the future.
In summary, the results presented here demonstrate that TGF-β1-induced activation of p38 MAPK and EMT is regulated by Sema4C in renal proximal tubular epithelial cells. Depletion of Sema4C inhibited activation of p38 MAPK and blocked TGF-β1-induced EMT. Our results indicate the importance of Sema4C-MAPK signalling pathway in the development and progression of renal fibrosis, and thus suggest that this is a potential therapeutic target for the treatment of renal fibrosis.
Acknowledgments
This work was supported by the National Natural Sciences Foundation of China (30800525 and 30971372), Doctoral Fund for Youth Scholars of Ministry of Education of China (200804871042) and under the auspices of the New Century Excellent Talents Grant by the Ministry of Education of China (NCET004-0712). We greatly thank Prof. Ming Fan (Chinese Academy of Medical Sciences) for the generous gift of the pcDNA 4.0–Sema4C plasmid. We also thank Prof. Changlin Mei (The Kidney Center of PLA) for the generous gift of HKC cells.
Conflict of interest statement. None declared.
References
- 1.Maria P, Rastaldi FF, Giardino L, et al. Epithelial–mesenchymal transition of tubular epithelial cells in human renal biopsies. Kidney Int. 2002;62:137–146. doi: 10.1046/j.1523-1755.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15:1–12. doi: 10.1097/01.asn.0000106015.29070.e7. [DOI] [PubMed] [Google Scholar]
- 3.MP R. Epithelial–mesenchymal transition and its implications for the development of renal tubulointerstitial fibrosis. J Nephrol. 2006;19:407–412. [PubMed] [Google Scholar]
- 4.Surmeet Bedi AV, Arjang D. Epithelial-to-mesenchymal transition and chronic allograft tubulointerstitial fibrosis. Transplant Rev (Orlando) 2008;22:1–5. doi: 10.1016/j.trre.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lan HY. Tubular epithelial–myofibroblast transdifferentiation mechanisms in proximal tubule cells. Curr Opin Nephrol Hypertens. 2003;12:25–29. doi: 10.1097/00041552-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad H, Bani-Hania MTC, Daniel R, et al. Cytokines in epithelial–mesenchymal transition: a new insight into obstructive nephropathy. J Urol. 2008;180:461–468. doi: 10.1016/j.juro.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Moustakas AHC. Non-Smad TGF-beta signals. J Cell Sci. 2005;118:3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- 8.Zhang YE. Non-Smad pathways in TGF-signaling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong Young Rhyu YY, Hunjoo H, et al. Role of reactive oxygen species in TGF-1-induced mitogen-activated protein kinase activation and epithelial–mesenchymal transition in renal tubular epithelial cells. J Am Soc Nephrol. 2005;16:667–675. doi: 10.1681/ASN.2004050425. [DOI] [PubMed] [Google Scholar]
- 10.Attila Sebe S-KL, Attila F, et al. Transforming growth factor-β-induced alpha-smooth muscle cell actin expression in renal proximal tubular cells is regulated by p38β mitogen-activated protein kinase, extracellular signal-regulated protein kinase1,2 and the Smad signaling during epithelial–myofibroblast transdifferentiation. Nephrol Dial Transplant. 2008;23:1537–1545. doi: 10.1093/ndt/gfm789. [DOI] [PubMed] [Google Scholar]
- 11.Wu H, Wang X, Liu S, et al. Sema4C participates in myogenic differentiation in vivo and in vitro through the p38 MAPK pathway. Eur J Cell Biol. 2007;86:331–344. doi: 10.1016/j.ejcb.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Kruger RP, Aurandt J, Guan K-L. Semaphorins command cells to move. Nat Rev Mol Cell Biol. 2005;6:789–800. doi: 10.1038/nrm1740. [DOI] [PubMed] [Google Scholar]
- 13.Basile JR, Barac A, Zhu T, et al. Class IV semaphorins promote angiogenesis by stimulating Rho-initiated pathways through plexin-B. Cancer Res. 2004;64:5212–5224. doi: 10.1158/0008-5472.CAN-04-0126. [DOI] [PubMed] [Google Scholar]
- 14.Pasterkamp RJ, Peschon JJ, Spriggs MK, et al. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature. 2003;424:398–405. doi: 10.1038/nature01790. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki K, Kumanogoh A, Kikutani H. Semaphorins and their receptors in immune cell interactions. Nat Immunol. 2008;9:17–23. doi: 10.1038/ni1553. [DOI] [PubMed] [Google Scholar]
- 16.JP TJS. Complex networks orchestrate epithelial–mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 17.Hou CC, Wang W, Huang XR, et al. Ultrasound-microbubble-mediated gene transfer of inducible Smad7 blocks transforming growth factor-β signaling and fibrosis in rat remnant kidney. Am J Pathol. 2005;166:761–771. doi: 10.1016/s0002-9440(10)62297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Böttinger EP. TGF-beta in renal injury and disease. Semin Nephrol. 2007;27:309–320. doi: 10.1016/j.semnephrol.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Yigong Shi JM. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 20.Ulrich Valcourt MK, Hideki N, Carl-Henrik H, et al. TGF-β and the Smad signaling pathway support transcriptomic reprogramming during epithelial–mesenchymal cell transition. Mol Biol Cell. 2005;16:1987–2002. doi: 10.1091/mbc.E04-08-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galliher AJ, Schiemann WP. Src phosphorylates Tyr284 in TGF-β type II receptor and regulates TGF-β stimulation of p38 MAPK during breast cancer cell proliferation and invasion. Cancer Res. 2007;67:3752–3758. doi: 10.1158/0008-5472.CAN-06-3851. [DOI] [PubMed] [Google Scholar]
- 22.Galliher-Beckley AJ, Schiemann WP. Grb2 binding to Tyr284 in TβR-II is essential for mammary tumor growth and metastasis stimulated by TGF-β. Carcinogenesis. 2008;29:244–251. doi: 10.1093/carcin/bgm245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bakin AV, Rinehart C, Tomlinson AK, et al. p38 mitogen-activated protein kinase is required for TGFβ-mediated fibroblastic transdifferentiation and cell migration. J Cell Sci. 2002;115:3193–3206. doi: 10.1242/jcs.115.15.3193. [DOI] [PubMed] [Google Scholar]
- 24.Lewitzky M, Kardinal C, Gehring NH, et al. The C-terminal SH3 domain of the adapter protein Grb2 binds with high affinity to sequences in Gab1 and SLP-76 which lack the SH3-typical P-x-x-P core motif. Oncogene. 2001;20:1052–1062. doi: 10.1038/sj.onc.1204202. [DOI] [PubMed] [Google Scholar]