Abstract
Objective
The objective of this study was to assess the proportion of patients with type 2 diabetes mellitus (T2DM) attaining individual and combined targets of low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), non-HDL-C, and apolipoprotein B (ApoB) after treatment with rosuvastatin (R) + fenofibric acid (FA) compared with corresponding-dose R monotherapy.
Methods
This post hoc analysis evaluated data from the T2DM subset of patients with mixed dyslipidemia (LDL-C ≥130 mg/dL, HDL-C <40/50 mg/dL in men/women, and TG ≥150 mg/dL) from 2 randomized studies. Patients included in the analysis (N = 456) were treated with R (5, 10, or 20 mg), FA 135 mg, or R (5, 10, or 20 mg) + FA 135 mg for 12 weeks. Attainment of LDL-C <100 mg/dL, HDL-C >40/50 mg/dL in men/women, TG <150 mg/dL, non-HDL-C <130 mg/dL, ApoB <90 mg/dL, and the combined targets of these parameters was assessed.
Results
Treatment with R + FA resulted in a significantly higher proportion of patients achieving optimal levels of HDL-C (46.8% vs. 20.8%, P = 0.009 for R 10 mg + FA), TG (60.0% vs. 34.0%, P = 0.02 for R 10 mg + FA; 54.0% vs. 26.4%, P = 0.005 for R 20 mg + FA), non-HDL-C (55.1% vs. 36.4%, P = 0.04 for R 5 mg + FA), ApoB (58.0% vs. 36.4%, P = 0.02 for R 5 mg + FA); and the combined targets of LDL-C, HDL-C, and TG (28.3% vs. 8.3%, P = 0.02 for R 10 mg + FA) and all 5 parameters (26.1% vs. 8.3%, P = 0.03 for R 10 mg + FA) than corresponding-dose R monotherapies.
Conclusions
A significantly greater proportion of T2DM patients achieved individual and combined lipid targets when treated with the combination of R + FA than corresponding-dose R monotherapies.
Key words: Fibrates, Statins, Dyslipidemia
Introduction
Patients with type 2 diabetes mellitus (T2DM) are at increased risk for atherosclerotic cardiovascular disease (CVD) and associated morbidity and mortality [1]. This is likely attributable to a common clustering of CVD risk factors underlying insulin resistance including dyslipidemia, hypertension, hyperglycemia, and a prothrombotic/proinflammatory state [2]. The characteristic dyslipidemic profile seen in patients with T2DM includes elevated triglycerides (TG), low levels of high-density lipoprotein cholesterol (HDL-C), and modestly elevated levels of low-density lipoprotein cholesterol (LDL-C), with an increased number of small dense LDL particles [3–5].
The National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) recommends that patients with DM achieve as a primary target of therapy an LDL-C <100 mg/dL and as a secondary target of therapy a non-HDL-C <130 mg/dL if hypertriglyceridemia (TG ≥200 mg/dL) is present [6]. Additionally, the American Diabetes Association has recommended optimal values for TG of <150 mg/dL and for HDL-C of >40 mg/dL in men and >50 mg/dL in women [7]. A consensus statement on lipoprotein management from the ADA and the American College of Cardiology specified non-HDL-C and apolipoprotein B (ApoB) treatment goals of <130 mg/dL and <90 mg/dL, respectively, in patients with DM [3]. In the presence of DM and at least one additional major CVD risk factor, more aggressive goals apply [3, 8].
Although therapeutic lifestyle changes may constitute initial therapy for patients with T2DM and lipoprotein abnormalities, most patients also will likely require pharmacotherapy to achieve lipid targets [2]. Statin monotherapy, appropriately, is often the initial therapy of choice; however, maximally tolerated doses of statins often fail to achieve desired lipid targets beyond LDL-C, and treatment combining a statin with another lipid-modifying agent may be required [7, 9]. One such therapeutic approach is to combine a statin with fenofibric acid (FA). Fenofibric acid choline salt formulated as enteric-coated mini-tablets in a delayed-release capsule is approved for combined use with a statin to reduce TG and increase HDL-C in patients with mixed dyslipidemia and coronary heart disease (CHD) or a CHD risk equivalent, who are on optimal statin therapy to achieve their LDL-C goal.
Two controlled clinical studies of patients with mixed dyslipidemia evaluated the efficacy and safety of combination therapy with rosuvastatin (R) 5, 10 or 20 mg + FA for 12 weeks compared with individual monotherapies [10, 11]. In both studies, treatment with R (at each dose) + FA was found to be efficacious and generally well tolerated. We present here the results of a post hoc analysis on achievement of individual and combined lipid and lipoprotein targets with R 5, 10, or 20 mg + FA combination therapy compared with corresponding-dose R monotherapies in the subset of patients with T2DM from the aforementioned two studies.
Patients and methods
Patients
This analysis includes patients with T2DM from two phase 3, randomized, controlled studies that compared the efficacy and safety of combination therapy with R 5 mg + FA 135 mg (Study 1; NCT00463606) and R 10 or 20 mg + FA 135 mg (Study 2; NCT00300482) to FA and corresponding-dose R monotherapies in patients with mixed dyslipidemia [10, 11]. The studies randomized patients at 349 sites in North America. The protocol for each study was approved by appropriate ethics committees and review boards, and all patients provided written informed consent.
Men and nonpregnant women ≥18 years of age with fasting lipid results of TG ≥150 mg/dL, HDL-C <40 mg/dL for men or <50 mg/dL for women, and LDL-C ≥130 mg/dL were included in each study. Patients with hemoglobin A1c ≤10.5% in Study 1 and ≤8.5% in Study 2 were included. Additional eligibility criteria have been published [12].
Study design
In Study 1, eligible patients were randomized in a double-blind 1:1:1 ratio to R 5 mg, FA 135 mg, or R 5 mg + FA 135 mg [10]. In Study 2, eligible patients were randomized in a double-blind 2:2:2:2:2:1 ratio to R 10 or 20 mg, FA 135 mg, R 10 or 20 mg + FA 135 mg, or R 40 mg [11]. The R 40 mg group was included in Study 2 as a reference for efficacy and safety assessments and except for comparisons of baseline characteristics was not included in any statistical comparisons. Study design was identical in both studies except for the R dose(s) used. All drugs were self-administered once daily at approximately the same time of day with or without food. In both studies, randomization was stratified by diabetic status and screening TG level (≤250 mg/dL or >250 mg/dL). Diagnosis of T2DM was based on the investigators’ assessment of medical history and/or fasting glucose measurement at screening. Patients and site and sponsor personnel were blinded to lipid parameter results obtained after the baseline visit.
Both studies consisted of a 6-week diet/lipid-altering medication washout screening period, a 12-week treatment period, and a 30-day safety evaluation period. Patients stopped any lipid-altering medications and were expected to follow the American Heart Association diet [13]. A week before randomization, fasting (≥12 h) blood lipid profiles were obtained to determine eligibility. Additional fasting blood samples for efficacy analyses were obtained at the randomization visit, two interim visits (weeks 4 and 8 of the treatment period), and the final visit (week 12 or earlier for premature discontinuation). Samples were analyzed at Covance Central Laboratory Services (Indianapolis, IN, USA). Additional study details have been published [10–12].
Statistical analysis
Data collected for patients with a diagnosis of T2DM at randomization from the two studies were included in this analysis; only data from the FA monotherapy group were integrated across the two studies. Of a total of 2197 patients randomized in the two studies, 498 (23%) had T2DM and were treated.
Last observation carried forward method was used to impute values for patients with missing postbaseline values. The number and percentage of patients attaining individual lipid targets of LDL-C <100 mg/dL, HDL-C >40 mg/dL (men) or >50 mg/dL (women), TG <150 mg/dL, non-HDL-C <130 mg/dL, and ApoB <90 mg/dL, and the combined lipid target of two (LDL-C and non-HDL-C), three (LDL-C, HDL-C, and TG), and five (LDL-C, HDL-C, TG, non-HDL-C, and ApoB) parameters at the final visit were compared between each combination-therapy group and the corresponding-dose R monotherapy group using Fisher’s exact test. Patients were not required to have a corresponding baseline value for the summaries of lipid targets. As a sensitivity analysis, a logistic regression analysis of each individual lipid target, in which treatment group and the corresponding baseline value were included as independent variables in the model, was performed.
Statistical comparisons of percent changes in lipid and nonlipid parameters were performed as previously described [11]. For HDL-C, TG, ApoB, and high-sensitivity C-reactive protein (hsCRP), R + FA was compared with corresponding-dose R monotherapy (primary comparison); for LDL-C, the primary comparison was with FA monotherapy. For non-HDL-C, the primary comparisons were between R + FA and FA, followed by a comparison with the corresponding-dose R monotherapy. Data were analyzed using SAS version 8.2 (SAS Institute, Inc., Cary, NC, USA).
Results
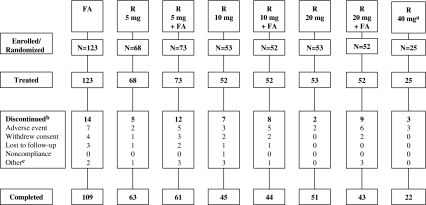
Of 499 patients with T2DM randomized in the two studies, 498 were treated, and 438 completed their respective study (Fig. 1). A total of 456 patients, excluding the R 40 mg arm, had postbaseline efficacy data and were, therefore, included in the analysis evaluating attainment of lipid targets. Baseline demographic and clinical characteristics were similar across all groups (Table 1). Overall (including patients in the R 40 mg group), approximately 70% of patients were <65 years of age; at least 85% of patients in each group weighed ≥70 kg. The incidence of comorbidities was consistent with the increased risk for CVD in this patient population. The most frequently used (>10% overall) DM medications at baseline included metformin (51.4%), glipizide (12.9%), pioglitazone (11.2%), and rosiglitazone (11.4%); no statistically significant differences were observed among the treatment groups in the frequency of the most commonly used diabetic medications at baseline. During the treatment period, metformin was initiated by one or more patients in each treatment group except R 10 mg + FA (3.4% overall). Glipizide, pioglitazone, and rosiglitazone were initiated by 0.8%, 1.6%, and 0.6% of patients, respectively during the treatment period. There were no statistically significant differences between the combination therapy arms and the corresponding rosuvastatin monotherapy arms in the frequency of initiating any of these four diabetic medications during the 12-week treatment period.
Fig. 1.
Patient flowchart. aThe R 40 mg group was not included in predefined statistical comparisons. bPatients may have been counted for more than one reason for discontinuation. cOther reasons for discontinuation included: FA: protocol violation and physician decision; R 5 mg: protocol violation; R 5 mg + FA: error by study coordinator, patient randomized in error, and physician decision; R 10 mg: personal reason, investigator decision, and physician decision; R 10 mg + FA: concomitant illness; and R 20 mg + FA: investigator decision, physician decision, and patient unable to comply with protocol. FA, fenofibric acid 135 mg; R, rosuvastatin (5, 10, or 20 mg)
Table 1.
Demographics and baseline characteristics
| Characteristic | FA 135 mg (n = 123) | R 5 mg (n = 68) | R 5 mg + FA (n = 73) | R 10 mg (n = 52) | R 10 mg + FA (n = 52) | R 20 mg (n = 53) | R 20 mg + FA (n = 52) |
|---|---|---|---|---|---|---|---|
| Sex, n (%) | |||||||
| Male | 54 (43.9) | 21 (30.9) | 24 (32.9) | 23 (44.2) | 21 (40.4) | 30 (56.6) | 23 (44.2) |
| Race, n (%) | |||||||
| White | 113 (91.9) | 56 (82.4) | 67 (91.8) | 47 (90.4) | 46 (88.5) | 43 (81.1) | 45 (86.5) |
| Black | 9 (7.3) | 12 (17.6) | 2 (2.7) | 4 (7.7) | 6 (11.5) | 8 (15.1) | 5 (9.6) |
| Other | 1 (0.8) | 0 | 4 (5.5) | 1 (1.9) | 0 | 2 (3.8) | 2 (3.8) |
| Age (years) | |||||||
| Mean ± SD | 57.6 ± 10.36 | 58.1 ± 9.57 | 61.0 ± 9.54 | 58.6 ± 9.68 | 59.9 ± 9.76 | 58.2 ± 10.95 | 58.8 ± 10.12 |
| Range | 29–82 | 39–79 | 29–79 | 36–80 | 34–83 | 31–82 | 35–82 |
| Body weight (kg), n (%) | |||||||
| <70 | 15 (12.2) | 8 (11.8) | 8 (11.0) | 4 (7.7) | 7 (13.5) | 2 (3.8) | 1 (1.9) |
| Waist circumference, (cm) | n = 122 | n = 67 | n = 73 | n = 50 | n = 52 | n = 53 | n = 51 |
| Mean ± SD | 107.5 ± 16.50 | 108.1 ± 15.94 | 111.6 ± 18.31 | 112.7 ± 13.48 | 110.4 ± 20.31 | 108.2 ± 13.24 | 111.6 ± 16.06 |
| Comorbidities, n (%)a | |||||||
| CAD | 16 (13.0) | 6 (8.8) | 6 (8.2) | 6 (11.5) | 6 (11.5) | 10 (18.9) | 4 (7.7) |
| Hypertension | 93 (75.6) | 47 (69.1) | 54 (74.0) | 40 (76.9) | 42 (80.8) | 40 (75.5) | 40 (76.9) |
| Metabolic syndromeb | 102 (82.9) | 56 (82.4) | 65 (89.0) | 47 (90.4) | 49 (94.2) | 47 (88.7) | 48 (92.3) |
| Obesity | 39 (31.7) | 30 (44.1) | 27 (37.0) | 21 (40.4) | 18 (34.6) | 22 (41.5) | 18 (34.6) |
| DM medication, n (%)c | |||||||
| Metformin | 64 (52.0) | 38 (55.9) | 38 (52.1) | 22 (42.3) | 28 (53.8) | 26 (49.1) | 26 (50.0) |
| Glipizide | 17 (13.8) | 6 (8.8) | 8 (11.0) | 6 (11.5) | 6 (11.5) | 6 (11.3) | 13 (25.0) |
| Pioglitazone | 15 (12.2) | 7 (10.3) | 7 (9.6) | 6 (11.5) | 7 (13.5) | 5 (9.4) | 6 (11.5) |
| Rosiglitazone | 14 (11.4) | 3 (4.4) | 5 (6.8) | 8 (15.4) | 5 (9.6) | 9 (17.0) | 7 (13.5) |
| Mean lipid values, mg/dL | |||||||
| LDL-C | n = 123 | n = 68 | n = 73 | n = 52 | n = 52 | n = 53 | n = 52 |
| 153.2 | 147.7 | 146.2 | 146.4 | 148.0 | 152.1 | 154.3 | |
| HDL-Cd | n = 120 | n = 68 | n = 73 | n = 50 | n = 50 | n = 51 | n = 51 |
| 39.4 | 41.4 | 40.3 | 36.9 | 38.4 | 37.6 | 38.5 | |
| TG | n = 123 | n = 68 | n = 73 | n = 52 | n = 52 | n = 53 | n = 52 |
| 271.9 | 308.8 | 306.2 | 316.7 | 307.6 | 300.7 | 286.0 | |
| ApoBe | n = 121 | n = 68 | n = 73 | n = 51 | n = 52 | n = 52 | n = 52 |
| 138.6 | 133.6 | 130.8 | 143.9 | 142.7 | 146.2 | 143.8 | |
FA fenofibric acid; R rosuvastatin; CAD coronary artery disease; LDL-C low-density lipoprotein cholesterol; HDL-C high-density lipoprotein cholesterol; TG triglycerides; ApoB apolipoprotein B; DM diabetes mellitus.
aComorbidities were obtained from medical history.
bMetabolic syndrome criteria are as described in the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report [6].
cMost frequently used DM medications (incidence >10% overall) at baseline.
dStatistically significant difference in baseline HDL-C values among treatment groups (P = 0.04).
eStatistically significant difference in baseline ApoB values among treatment groups (P = 0.003).
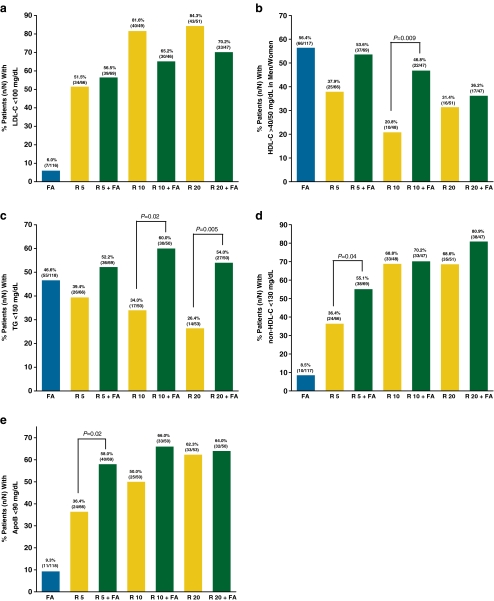
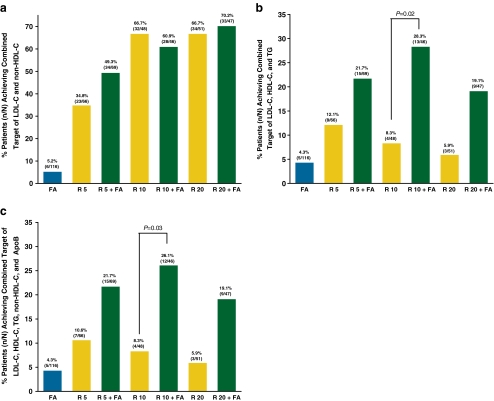
Achievement of individual and combined lipid targets with R + FA combination therapy and R and FA monotherapies is shown in Figs. 2 and 3. No statistically significant differences were observed in the proportion of patients achieving the LDL-C goal of <100 mg/dL between any combination-therapy group and the corresponding-dose R monotherapy group. Treatment with R 10 mg + FA resulted in a significantly higher proportion of patients achieving their individual HDL-C target (46.8% vs. 20.8%, P = 0.009) than R 10 mg. The individual TG target was achieved by a significantly higher proportion of patients treated with R 10 mg + FA (60.0% vs. 34.0%, P = 0.02) and R 20 mg + FA (54.0% vs. 26.4%, P = 0.005) versus the corresponding-dose R monotherapies. The differences for the proportion of patients who achieved the individual non-HDL-C target (55.1% vs. 36.4%, P = 0.04) and the individual ApoB target (58.0% vs. 36.4%, P = 0.02) were significant between R 5 mg + FA and R 5 mg. The combined target of LDL-C and non-HDL-C was achieved by 49.3% of patients treated with R 5 mg + FA versus 34.8% of patients treated with R 5-mg monotherapy (P = 0.12) and by 70.2% of patients treated with R 20 mg + FA versus 66.7% of patients treated with R 20-mg monotherapy (P = 0.83). Treatment with R 10 mg + FA resulted in a significantly higher proportion of patients achieving the combined target of LDL-C, HDL-C, and TG (28.3% vs. 8.3%, P = 0.02) versus R 10 mg. Similarly, combination therapy with R 10 mg + FA resulted in a significantly higher proportion of patients achieving the combined target of all five parameters (26.1% vs. 8.3%, P = 0.03) versus R 10 mg (Fig. 3). The results of sensitivity analyses adjusting for the baseline value were similar. In addition to the statistically significant differences noted above for the individual targets, sensitivity analyses demonstrated statistically significant differences favoring R 5 mg + FA versus R 5 mg for the HDL-C target (odds ratio: 2.2; 95% CI: 1.0, 4.6; P = 0.04), and favoring R 10 mg versus R 10 mg + FA for the LDL-C target (odds ratio: 0.4; 95% CI: 0.1, 1.0; P = 0.05).
Fig. 2.
a–e. Proportion of T2DM patients achieving individual target of (a) LDL-C <100 mg/dL; (b) HDL-C >40/50 mg/dL in men/women; (c) TG <150 mg/dL; (d) non-HDL-C <130 mg/dL; and (e) ApoB <90 mg/dL at final visit. P-values were obtained using Fisher’s exact test to test for a difference between each rosuvastatin (R) + fenofibric acid (FA) combination-therapy group and the corresponding-dose R monotherapy group. ApoB, apolipoprotein B; FA, fenofibric acid 135 mg; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; R, rosuvastatin (5, 10, or 20 mg); T2DM, type 2 diabetes mellitus; TG, triglycerides
Fig. 3.
a–c. Proportion of T2DM patients achieving combined targets of (a) LDL-C and non-HDL-C; (b) LDL-C, HDL-C, and TG; and (c) LDL-C, HDL-C, TG, non-HDL-C, and ApoB. Targets were defined as LDL-C <100 mg/dL, HDL-C >40/50 mg/dL in men/women, TG <150 mg/dL, non-HDL-C <130 mg/dL, and ApoB <90 mg/dL at final visit. ApoB, apolipoprotein B; FA, fenofibric acid 135 mg; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; R, rosuvastatin (5, 10, or 20 mg); T2DM, type 2 diabetes mellitus; TG, triglycerides
R + FA combination therapy resulted in statistically significant greater mean percent increases in HDL-C (25.5% vs. 17.0%, P = 0.02 for R 5 mg + FA and 19.6% vs. 5.7%, P = 0.002 for R 10 mg + FA) compared with R 5- or 10-mg monotherapies, respectively (Table 2). All three doses of R + FA resulted in statistically significant greater mean percent decreases in TG (39.9% vs. 23.6%, P < 0.001 for R 5 mg + FA; 44.8% vs. 28.6%, P = 0.002 for R 10 mg + FA; and 42.6% vs. 26.9%, P = 0.002 for R 20 mg + FA) than corresponding dose of R. Compared with R 5 mg, reductions in non-HDL-C (41.1% vs. 33.5%, P = 0.004) and ApoB (34.9% vs. 28.1%, P = 0.008) were significant with R 5 mg + FA, but not with the higher doses of R + FA versus corresponding-dose R monotherapies.
Table 2.
Percent change from baseline to final visit in efficacy parameters
| Variable | FA 135 mg | R 5 mg | R 5 mg + FA | R 10 mg | R 10 mg + FA | R 20 mg | R 20 mg + FA |
|---|---|---|---|---|---|---|---|
| HDL-C | n = 114 | n = 66 | n = 69 | n = 46 | n = 45 | n = 49 | n = 46 |
| Baseline mean, mg/dL | 39.3 | 41.1 | 40.3 | 36.8 | 38.4 | 37.7 | 38.6 |
| Final mean, mg/dL | 46.5 | 46.3 | 49.2 | 39.3 | 46.1 | 41.1 | 44.5 |
| Mean change, % (SE) | 19.0 (2.03) | 17.0 (2.68) | 25.5 (2.61) | 5.7 (3.20) | 19.6 (3.24) | 11.0 (3.10) | 16.4 (3.19) |
| P-valuea | 0.02c | 0.002c | 0.22c | ||||
| TG | n = 118 | n = 66 | n = 69 | n = 50 | n = 50 | n = 53 | n = 50 |
| Baseline mean, mg/dL | 272.8 | 311.2 | 306.7 | 320.8 | 304.0 | 300.4 | 285.4 |
| Final mean, mg/dL | 164.6 | 207.0 | 157.5 | 218.5 | 169.1 | 195.2 | 152.0 |
| Mean change, % (SE) | −34.5 (2.41) | −23.6 (3.20) | −39.9 (3.13) | −28.6 (3.68) | −44.8 (3.68) | −26.9 (3.57) | −42.6 (3.68) |
| P-valuea | <0.001c | 0.002c | 0.002c | ||||
| LDL-C | n = 116 | n = 66 | n = 69 | n = 49 | n = 46 | n = 51 | n = 47 |
| Baseline mean, mg/dL | 154.4 | 147.9 | 146.6 | 147.2 | 143.9 | 151.9 | 152.9 |
| Final mean, mg/dL | 142.1 | 102.4 | 95.7 | 83.9 | 91.3 | 81.6 | 91.9 |
| Mean change, % (SE) | −5.2 (1.67) | −28.1 (2.22) | −32.8 (2.17) | −43.3 (2.57) | −36.8 (2.67) | −44.3 (2.52) | −37.0 (2.63) |
| P-valuea | <0.001d | <0.001d | <0.001d | ||||
| Non-HDL-C | n = 114 | n = 66 | n = 69 | n = 46 | n = 45 | n = 49 | n = 46 |
| Baseline mean, mg/dL | 218.2 | 217.8 | 218.3 | 217.4 | 213.9 | 222.5 | 216.3 |
| Final mean, mg/dL | 178.3 | 144.6 | 124.9 | 121.8 | 120.7 | 118.5 | 117.0 |
| Mean change, % (SE) | −17.5 (1.41) | −33.5 (1.86) | −41.1 (1.82) | −44.6 (2.22) | −43.6 (2.26) | −45.8 (2.16) | −44.9 (2.23) |
| P-valuea | <0.001d | <0.001d | <0.001d | ||||
| 0.004c | 0.77c | 0.77c | |||||
| ApoB | n = 116 | n = 66 | n = 69 | n = 49 | n = 50 | n = 52 | n = 50 |
| Baseline mean, mg/dL | 138.8 | 133.7 | 131.5 | 144.7 | 141.2 | 146.1 | 142.9 |
| Final mean, mg/dL | 117.9 | 97.7 | 86.6 | 90.3 | 88.6 | 88.7 | 87.8 |
| Mean change, % (SE) | −14.8 (1.37) | −28.1 (1.82) | −34.9 (1.79) | −36.3 (2.11) | −36.2 (2.09) | −37.2 (2.05) | −36.2 (2.08) |
| P-valuea | 0.008c | 0.97c | 0.75c | ||||
| hsCRP | n = 117 | n = 66 | n = 69 | n = 49 | n = 50 | n = 52 | n = 50 |
| Baseline median, mg/L | 2.88 | 3.50 | 3.43 | 3.90 | 3.94 | 3.24 | 3.96 |
| Final median, mg/L | 2.62 | 2.82 | 2.37 | 2.42 | 2.71 | 2.14 | 2.55 |
| Median change, % | −7.2 | −11.5 | −26.8 | −31.1 | −28.0 | −34.2 | −40.7 |
| P-valueb | 0.20c | 0.52c | 0.99c |
FA fenofibric acid; R rosuvastatin; HDL-C high-density lipoprotein cholesterol; LDL-C low-density lipoprotein cholesterol; TG triglycerides; ApoB apolipoprotein B; hsCRP high-sensitivity C-reactive protein. Only patients with both baseline and at least one postbaseline value were included in this analysis.
a P-value is from an analysis of covariance with the corresponding baseline value as the covariate and with effects for treatment group and screening TG level (≤250 mg/dL, >250 mg/dL).
b P-value is from the van Elteren’s test with screening TG level as stratum.
c P-value for difference between R + FA group and corresponding-dose R monotherapy group.
d P-value for difference between R + FA group and FA monotherapy group.
The safety profile of combination therapy with R + FA was consistent with the profiles of the individual monotherapies, and there were no unexpected adverse effects. Adverse events (AEs) were the most frequent reason cited for discontinuation in all groups. The incidence of discontinuations attributed to AEs was higher with combination therapy than with FA monotherapy or corresponding dose R monotherapy (Fig. 1). The incidence of myalgia was similar across treatment groups, rhabdomyolysis was not reported, and elevations in creatine phosphokinase were observed infrequently in this cohort (Table 3). Hepatic-related AEs or laboratory abnormalities were rare. Increased creatinine levels were observed primarily with FA monotherapy and combination therapy (R 10 or 20 mg + FA; Table 3). Adverse events consistent with hyperglycemia or increased hemoglobin A1c were reported infrequently across all treatment groups.
Table 3.
Adverse events and laboratory variables related to muscle, hepatic, and renal function
| Adverse events and laboratory variables | FA 135 mg (n = 123) | R 5 mg (n = 68) | R 5 mg + FA (n = 73) | R 10 mg (n = 52) | R 10 mg + FA (n = 52) | R 20 mg (n = 53) | R 20 mg + FA (n = 52) |
|---|---|---|---|---|---|---|---|
| Muscle-related | |||||||
| Myalgia, n (%)a | 3 (2.4) | 2 (2.9) | 1 (1.4) | 3 (5.8) | 1 (1.9) | 1 (1.9) | 2 (3.8) |
| CK increased, n (%)a | 4 (3.3) | 0 | 2 (2.7) | 1 (1.9) | 0 | 2 (3.8) | 0 |
| CK >5× ULN, n/N (%) | 0/119 | 0/68 | 0/71 | 0/50 | 1/51 (2.0) | 0/53 | 0/51 |
| CK >10× ULN, n/N (%) | 0/119 | 0/68 | 0/71 | 0/50 | 1/51 (2.0) | 0/53 | 0/51 |
| Hepatic-related | |||||||
| ALT >5× ULN, n/N (%) | 0/119 | 0/68 | 1/71 (1.4) | 0/50 | 0/50 | 0/53 | 0/51 |
| Renal-related | |||||||
| Creatinine increased, n (%)a | 3 (2.4) | 0 | 0 | 0 | 1 (1.9) | 0 | 3 (5.8) |
| Creatinine ≥50% increase from baseline and >ULN, n/N (%) | 12/119 (10.1) | 0/68 | 1/71 (1.4)b | 2/50 (4.0) | 2/50 (4.0) | 0/53 | 2/51 (3.9) |
| Creatinine ≥100% increase from baseline, n/N (%) | 1/119 (0.8) | 0/68 | 0/71 | 0/50 | 0/50 | 0/53 | 0/51 |
| Creatinine >2 mg/dL, n/N (%) | 8/119 (6.7) | 0/68 | 0/71b | 1/50 (2.0) | 2/50 (4.0) | 0/53 | 1/51 (2.0) |
FA fenofibric acid; R rosuvastatin; CK creatine phosphokinase; ULN upper limit of normal; ALT alanine aminotransferase; MedDRA Medical Dictionary for Regulatory Activities.
aMedDRA Version 11.1 preferred term.
b P < 0.05 for comparison between R + FA group and FA monotherapy group using Fisher’s exact text.
Discussion
This post hoc analysis was performed on pooled data for the subset of patients with T2DM from two phase 3 studies that evaluated the lipid-altering efficacy and safety of R + FA combination therapy in patients with mixed dyslipidemia. The primary objective of this analysis was to evaluate the achievement of individual and combined lipid targets. In this analysis, a significantly higher proportion of patients achieved individual targets for HDL-C with R 10 mg + FA, TG with R 10 or 20 mg + FA, non-HDL-C with R 5 mg + FA, and ApoB with R 5 mg + FA, compared with corresponding-dose R monotherapies. The proportion of patients who achieved the combined target of two lipid parameters (LDL-C and non-HDL-C) was greater with the combinations of R 5 mg + FA and R 20 mg + FA than with the corresponding-dose R monotherapies; these differences were not statistically significant. The proportion of patients who achieved the combined target of three lipid parameters (LDL-C, HDL-C, and TG) and five lipid parameters (LDL-C, HDL-C, TG, non-HDL-C, and ApoB) was 3-fold greater and statistically significant with R 10 mg + FA versus R 10 mg; R 10 mg + FA had the highest incidence of patients attaining the combined target of three and five parameters compared with R 10 mg (28.3% vs. 8.3% for three parameters and 26.1% vs. 8.3% for five parameters). It is noted that a dose-related increase in the proportion of patients achieving the combined target of three and five parameters was not observed with the combination of R 20 mg + FA, compared with R 10 mg + FA. The reason for this is unclear, but may be related to the severity of hypertriglyceridemia in the patients of this study. Nonetheless, the R 20 mg + FA combination may be required for patients in clinical practice who require a higher statin dose in order to reach their individual LDL-C target, in addition to fibrate therapy to treat HDL-C and TG abnormalities. In a separate study of the fixed-dose combination of rosuvastatin and fenofibric acid in patients with elevated LDL-C but more modestly elevated TG, a numerically greater proportion of patients achieved the combined target of five parameters with rosuvastatin/fenofibric acid 20 mg/135 mg versus 10 mg/135 mg (50.9% vs. 45.1%) [14].
Because T2DM is designated as a CHD risk equivalent in the NCEP ATP III guidelines, the primary treatment goal in patients with T2DM is LDL-C <100 mg/dL. As noted earlier, guidelines suggest treating other lipid components in addition to LDL-C to reduce the overall risk of CVD. Patients with T2DM frequently present with mixed dyslipidemia, which is characterized by abnormalities in LDL particle size, low levels of HDL-C, and elevated levels of TG. In this patient population, ApoB and LDL particle concentration more accurately define residual CVD risk and are stronger predictors of cardiovascular outcome than LDL-C [15]. In both Study 1 and Study 2, FA, a fibrate that effectively raises HDL-C and lowers TG, in combination with R, improved multiple lipid parameters in patients with mixed dyslipidemia [10, 11].
The achievement of LDL-C goals has been evaluated in clinical trials of statin monotherapy and combination therapy [16–23]. In studies in which R was used, given its efficacy, approximately 80% or more of patients treated with R monotherapy or combination therapy achieved LDL-C goals [16, 18, 19, 21–23]. In CORALL, a study that evaluated LDL-C goal attainment in patients with T2DM, 82% and 84% of patients treated with R 10 and 20 mg, respectively, achieved the LDL-C goal of <100 mg/dL at 24 weeks of treatment [23]. Although there were differences in study design and duration of treatment, the goal attainment results of the CORALL study are similar to the proportion of patients who achieved LDL-C goals with R 10 and 20 mg monotherapy in the present analysis. The proportions of T2DM patients who achieved the LDL-C goal of <100 mg/dL in the present studies with R + FA combination therapy were slightly lower than the proportions who achieved this goal with corresponding-dose R monotherapies. This is not surprising, because it has been shown that treatment with fibrates results in either a small increase or no change in the measured LDL-C content in patients with high TG and low HDL-C levels, likely because of accelerated fibrate-induced catabolism of TG-rich very-low-density lipoprotein (VLDL) particles [24–26]. Treatment with fibrates also results in a shift in LDL particle size from a small and dense to a larger phenotype [27–29].
Attainment of the LDL-C goal is just one component of the recommended lipid profile for patients with T2DM. In order to reduce CVD risk, these patients must also increase HDL-C levels and decrease TG levels [30–33]. In this analysis of the two controlled studies, patients with T2DM showed significantly greater mean percent changes in LDL-C, HDL-C, TG, non-HDL-C, and ApoB with R 5 mg + FA; LDL-C, HDL-C, and TG with R 10 mg + FA; and LDL-C and TG with R 20 mg + FA, compared with prespecified monotherapies.
The results of this analysis are supported by the findings reported in a study comparing the efficacy of R 5 or 10 mg + fenofibrate combination therapy with that of each monotherapy component in patients with T2DM who had high levels of TG and total cholesterol at baseline [34]. In that study, decreases in TG levels of 34% and 30% and changes in LDL-C of +1% and −47% were achieved after 24 weeks of treatment with fenofibrate monotherapy and R monotherapy, respectively. Administration of R 10 mg + fenofibrate resulted in a 47% decrease in TG, which was significantly greater (P = 0.001) than that seen with R monotherapy.
The ACCORD (Action to Control Cardiovascular Risk in Diabetes) Lipid component of the overall ACCORD trial was designed to test whether CVD event reduction with the combination of a statin and a fibrate would exceed what is achieved with statin monotherapy in patients with T2DM. It should be noted that although the combination of simvastatin and fenofibrate in the ACCORD Lipid study did not significantly reduce the rate of nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death compared with simvastatin alone, a prespecified analysis demonstrated in patients with both high TG (≥204 mg/dL) and low HDL-C (≤34 mg/dL) a 31% relative risk reduction in cardiovascular events with combination therapy compared with the monotherapy group [35]. Furthermore, the ACCORD Eye study, which evaluated the progression of diabetic retinopathy in a subgroup of patients from the ACCORD study found that treatment with simvastatin and fenofibrate resulted in a significant 40% reduction in the progression of diabetic retinopathy versus simvastatin monotherapy [36].
Combination therapy with R + FA was generally well tolerated in the subgroup of patients with T2DM. The safety profile of R + FA combination therapy was consistent with that of each monotherapy. Although, the risk for increased muscle-related AEs is a safety concern when combining fibrates with statins, the incidence of myalgia was lower with R 5 or 10 mg + FA, compared with the corresponding-dose R monotherapy, and there were no reports of rhabdomyolysis in this analysis. Similarly, the ACCORD Lipid study found no increased risk for muscle-related AEs with simvastatin and fenofibrate combination treatment for up to 4.5 years compared with simvastatin alone [35]. Elevations in liver enzymes were infrequent occurrences in the present analysis and abnormal creatinine values were observed predominantly with FA monotherapy and combination therapy.
The major limitations for application of these results to clinical practice are attributable to constraints of study design. Combination therapy with R + FA was initiated in the studies that provided data for this analysis, whereas in clinical practice statin monotherapy is often initiated first with FA added to the regimen if treatment goals are not met or if further titration of the statin is contraindicated. Although the duration of treatment, which was limited to 12 weeks in these studies, was sufficient to demonstrate efficacy, it probably is not an accurate reflection of full treatment effect in clinical practice. The results of a long-term clinical trial in which patients with mixed dyslipidemia, who completed Study 2, received R 20 mg + FA for 52 weeks confirmed additional benefit with a longer duration of combination treatment [37].
Despite these limitations, this analysis clearly demonstrated the effectiveness and safety of R + FA combination therapy in attaining individual and combined lipid targets in patients with T2DM, compared with R monotherapy.
Acknowledgments
We thank all investigators, study coordinators, and patients who participated in the study. Hsiaoming Sun, Min Tian, and Aditya Lele, of Abbott provided assistance with statistical analyses; Noreen Travers, and Lura Morris, of Abbott provided assistance with clinical study management. Geeta Thakker and Darryl Sleep provided medical writing assistance on behalf of Abbott. Rachelle Weiss, of Ledell Group, provided medical writing support for this paper and was compensated by Abbott for this support.
Robert S. Rosenson is a consultant for Abbott; serves on the speaker’s bureau for AstraZeneca; serves on the scientific advisory board for Residual Risk Reduction Initiative and has received honorarium from Residual Risk Reduction Initiative. Dawn Carlson, Maureen Kelly, Carolyn Setze, James Stolzenbach, and Laura Williams are employees of Abbott. Boaz Hirshberg is an employee of AstraZeneca. Abbott and AstraZeneca sponsored the studies.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Clinical Trial Registration Number: www.clinicaltrials.gov. NCT00463606, NCT00300482
References
- 1.Steiner G. Treating lipid abnormalities in patients with type 2 diabetes mellitus. Am J Cardiol. 2001;88(suppl):37N–40N. doi: 10.1016/S0002-9149(01)02151-8. [DOI] [PubMed] [Google Scholar]
- 2.Kendall DM. The dyslipidemia of diabetes mellitus: giving triglycerides and high-density lipoprotein cholesterol a higher priority? Endocrinol Metab Clin N Am. 2005;34:27–48. doi: 10.1016/j.ecl.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Brunzell JD, Davidson M, Furburg CD, Goldberg RB, Howard BV, Stein JH, et al. Lipoprotein management in patients with cardiometabolic risk. Consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care. 2008;31:811–822. doi: 10.2337/dc08-9018. [DOI] [PubMed] [Google Scholar]
- 4.Rosenson RS, Otvos JD, Hsia J. Effects of rosuvastatin and atorvastatin on LDL and HDL particle concentrations in patients with metabolic syndrome. Diabetes Care. 2009;32:1087–1091. doi: 10.2337/dc08-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garvey WT, Kwon S, Zheng D, Shaughnessy S, Wallace P, Hutto A, et al. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes. 2003;52:453–462. doi: 10.2337/diabetes.52.2.453. [DOI] [PubMed] [Google Scholar]
- 6.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed]
- 7.American Diabetes Association 2009 Executive summary: Standards of medical care in diabetes–2009. Diabetes Care. 2009;32(Suppl 1):S6–S12. [DOI] [PMC free article] [PubMed]
- 8.Grundy SM, Cleeman JI, Merz CNB, Brewer HB, Jr, Clark LT, Hunninghake DB, et al. Coordinating Committee of the National Cholesterol Education Program. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 9.Fruchart JC, Sacks FM, Hermans MP, Assmann G, Brown WV, Ceska R, et al. The residual risk reduction initiative: a call to action to reduce residual vascular risk in dyslipidaemic patient. Diab Vasc Dis Res. 2008;5:319–335. doi: 10.3132/dvdr.2008.046. [DOI] [PubMed] [Google Scholar]
- 10.Roth EM, Rosenson RS, Carlson DM, Fukumoto SM, Setze CM, Blasetto JW, et al. Efficacy and safety of rosuvastatin 5 mg in combination with fenofibric acid 135 mg in patients with mixed dyslipidemia—A phase 3 study. Cardiovasc Drugs Ther. 2010 doi: 10.1007/s10557-010-6266-4. [DOI] [PubMed] [Google Scholar]
- 11.Jones PH, Davidson MH, Kashyap ML, Kelly MT, Buttler SM, Setze CM, et al. Efficacy and safety of ABT-335 (fenofibric acid) in combination with rosuvastatin in patients with mixed dyslipidemia: a phase 3 study. Atherosclerosis. 2009;204:208–215. doi: 10.1016/j.atherosclerosis.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 12.Jones PH, Bays HE, Davidson MH, Kelly MT, Buttler SM, Setze CM, et al. Evaluation of a new formulation of fenofibric acid, ABT-335, co-administered with statins: study design and rationale of a phase III clinical programme. Clin Drug Investig. 2008;28:625–634. doi: 10.2165/00044011-200828100-00003. [DOI] [PubMed] [Google Scholar]
- 13.American Heart Association. An eating plan for healthy Americans—our American Heart Association diet. Dallas (TX): AHA, 2004. AHA Publication No. 50-1481A.
- 14.Roth EM, McKenney JM, Kelly MT, Setze CM, Carlson DM, Gold A, et al. Efficacy and safety of rosuvastatin and fenofibric acid combination therapy versus simvastatin monotherapy in patients with hypercholesterolemia and hypertriglyceridemia: a randomized, double-blind study. Am J Cardiovasc Drugs. 2010;10:175–186. doi: 10.2165/11533430-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Rosenson RS, Davidson MH, Pourfarzib R. Underappreciated opportunities for low-density lipoprotein management in patients with cardiometabolic residual risk. Atherosclerosis. 2010 doi: 10.1016/j.atherosclerosis.2010.03.038. [DOI] [PubMed] [Google Scholar]
- 16.Berne C, Siewert-Delle A. URANUS study investigators. Comparison of rosuvastatin and atorvastatin for lipid lowering in patients with type 2 diabetes mellitus: results from the URANUS study. Cardiovasc Diabetol. 2005;4:7. doi: 10.1186/1475-2840-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ballantyne CM, Abate N, Yuan Z, King TR, Palmisano J. Dose-comparison study of the combination of ezetimibe and simvastatin (Vytorin) versus atorvastatin in patients with hypercholesterolemia: the Vytorin versus atorvastatin (VYVA) study. Am Heart J. 2004;149:464–473. doi: 10.1016/j.ahj.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 18.Davidson MH, Ballantyne CM, Kerzner B, Melani L, Sager PT, Lipka L, Ezetimibe Study Group et al. Efficacy and safety of ezetimibe coadministered with statins: randomised, placebo-controlled, blinded experience in 2382 patients with primary hypercholesterolemia. Int J Clin Pract. 2005;58:746–755. doi: 10.1111/j.1368-5031.2004.00289.x. [DOI] [PubMed] [Google Scholar]
- 19.Jones PH, Davidson MH, Stein EA, Bays HE, McKenney JM, Miller E, STELLAR Study Group et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* trial) Am J Cardiol. 2003;92:152–160. doi: 10.1016/S0002-9149(03)00530-7. [DOI] [PubMed] [Google Scholar]
- 20.McKenney J, Ballantyne CM, Feldman TA, Brady WE, Shah A, Davies MJ, et al. LDL-C goal attainment with ezetimibe plus simvastatin coadministration vs atorvastatin or simvastatin monotherapy in patients at high risk of CHD. MedGenMed. 2005;7:3. [PMC free article] [PubMed] [Google Scholar]
- 21.McKenney JM, Jones PH, Angeli Adamczyk M, Cain VA, Bryzinski BS, STELLAR Study Group et al. Comparison of the efficacy of rosuvastatin versus atorvastatin, simvastatin, and pravastatin in achieving lipid goals: results from the STELLAR trial. Curr Med Res Opin. 2003;19:689–698. doi: 10.1185/030079903125002405. [DOI] [PubMed] [Google Scholar]
- 22.Stalenhoef AFH, Ballantye CM, Sarti C, Murin J, Tonstad S, Rose H, et al. A COmparative study with rosuvastatin in subjects with METabolic Syndrome: results of the COMETS study. Eur Heart J. 2005;26:2664–2672. doi: 10.1093/eurheartj/ehi482. [DOI] [PubMed] [Google Scholar]
- 23.Wolffenbuttel BHR, Franken AAM, Vincent HH, Dutch CORALL Study Group Cholesterol-lowering effects of rosuvastatin compared with atorvastatin in patients with type 2 diabetes—CORALL study. J Intern Med. 2005;257:531–539. doi: 10.1111/j.1365-2796.2005.01499.x. [DOI] [PubMed] [Google Scholar]
- 24.Lemieux I, Laperrière L, Dzavik V, Tremblay G, Bourgeois J, Després JP. A 16-week fenofibrate treatment increases LDL particle size in type IIA dyslipidemic patients. Atherosclerosis. 2002;162:363–371. doi: 10.1016/S0021-9150(01)00711-0. [DOI] [PubMed] [Google Scholar]
- 25.Elisaf M. Effects of fibrates on serum metabolic parameters. Curr Med Res Opin. 2002;18:269–276. doi: 10.1185/030079902125000516. [DOI] [PubMed] [Google Scholar]
- 26.Rosenson RS, Wolff DA, Huskin AL, Helenowski IB, Rademaker AW. Fenofibrate therapy ameliorates fasting and postprandial lipoproteinemia, oxidative stress, and the inflammatory response in subjects with hypertriglyceridemia and the metabolic syndrome. Diabetes Care. 2007;30:1945–1951. doi: 10.2337/dc07-0015. [DOI] [PubMed] [Google Scholar]
- 27.Campos H, Genest JJ, Jr, Blijlevens E, McNamara JR, Jenner JL, Ordovas JM, et al. Low density lipoprotein particle size and coronary artery disease. Arterioscler Thromb. 1992;12:187–195. doi: 10.1161/01.atv.12.2.187. [DOI] [PubMed] [Google Scholar]
- 28.Campos H, Blijlevens E, McNamara JR, Ordovas JM, Posner BM, Wilson PW, et al. LDL particle size distribution. Results from the Framingham Offspring Study. Arterioscler Thromb. 1992;12:1410–1419. doi: 10.1161/01.atv.12.12.1410. [DOI] [PubMed] [Google Scholar]
- 29.Griffin BA, Freeman DJ, Tait GW, Thomson J, Caslake MJ, Packard CJ, et al. Role of plasma triglyceride in the regulation of plasma low density lipoprotein (LDL) subfractions: relative contribution of small, dense LDL to coronary heart disease risk. Atherosclerosis. 1994;106:241–253. doi: 10.1016/0021-9150(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 30.Assmann G, Schulte H. Relation of high-density lipoprotein cholesterol and triglycerides to incidence of atherosclerotic coronary artery disease (the PROCAM experience). Prospective Cardiovascular Munster study. Am J Cardiol. 1992;70:733–737. doi: 10.1016/0002-9149(92)90550-I. [DOI] [PubMed] [Google Scholar]
- 31.Assmann G, Schulte H, Cullen P, Seedorf U. Assessing risk of myocardial infarction and stroke: new data from the Prospective Cardiovascular Munster (PROCAM) study. Eur J Clin Invest. 2007;37:925–932. doi: 10.1111/j.1365-2362.2007.01888.x. [DOI] [PubMed] [Google Scholar]
- 32.Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk. 1996;3:213–219. doi: 10.1097/00043798-199604000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, et al. Triglycerides and the risk of coronary heart disease: 10, 158 incident cases among 262, 565 participants in 29 Western prospective studies. Circulation. 2007;115:450–458. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 34.Durrington PN, Tuomilehto J, Hamann A, Kallend D, Smith K. Rosuvastatin and fenofibrate alone and in combination in type 2 diabetes patients with combined hyperlipidaemia. Diabetes Res Clin Pract. 2004;64:137–151. doi: 10.1016/j.diabres.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 35.The ACCORD Study Group Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.The ACCORD Study Group and ACCORD Eye Study Group Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;263:233–244. doi: 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bays HE, Jones PH, Mohiuddin SM, Kelly MT, Sun H, Setze CM, et al. Long-term safety and efficacy of fenofibric acid in combination with statin therapy for the treatment of patients with mixed dyslipidemia. J Clin Lipidol. 2008;2:426–435. doi: 10.1016/j.jacl.2008.10.001. [DOI] [PubMed] [Google Scholar]