Abstract
Endometriosis is a debilitating disease characterized by the growth of ectopic endometrial tissue. It is widely accepted that angiogenesis plays an integral part in the establishment and growth of endometriotic lesions. Recent data from a variety of angiogenesis-dependent diseases suggest a critical role of bone marrow–derived endothelial progenitor cells (EPCs) in neovascularization. In this study we examined the blood levels of EPCs and mature circulating endothelial cells in a mouse model of surgically induced endometriosis. Fluorescence-activated cell sorting analysis revealed elevated levels of EPCs in the blood of mice with endometriosis compared with control subject that underwent a sham operation. EPC concentrations positively correlated with the amount of endometriotic tissue and peaked 1 to 4 days after induction of disease. In a green fluorescent protein bone marrow transplant experiment we found green fluorescent protein–positive endothelial cells incorporated into endometriotic lesions but not eutopic endometrium, as revealed by flow cytometry and immunohistochemistry. Finally, treatment of endometriosis-bearing mice with the angiogenesis inhibitor Lodamin, an oral nontoxic formulation of TNP-470, significantly decreased EPC levels while suppressing lesion growth. Taken together, our data indicate an important role for bone marrow–derived endothelial cells in the pathogenesis of endometriosis and support the potential clinical use of anti-angiogenic therapy as a novel treatment modality for this disease.
Endometriosis, or extra-uterine endometrial-like tissue, affects approximately 10% of women during their reproductive years.1 The disease causes debilitating pain and severely affects quality of life with symptoms mimicking those associated with other chronic pain disorders, such as irritable bowel syndrome and pelvic inflammatory disease. In addition, endometriosis is closely associated with infertility.2 The diagnosis is usually made upon visual inspection of the pelvis during laparoscopy; noninvasive diagnostic tools, such as ultrasound scanning, can reliably detect only severe forms of the disease, such as ovarian endometriotic cysts.3 As a result, women can suffer for 8 to 12 years before receiving a definitive diagnosis and appropriate treatment.4 A biomarker of endometriosis would be invaluable to aid in earlier diagnosis and to monitor therapeutic efficacy.
The treatment options and success rates are limited, partially because of a lack of understanding of the pathogenesis of endometriosis. Treatments include use of hormonal drugs to suppress ovarian function, surgical ablation of endometriotic lesions, and, if necessary, removal of the pelvic organs. In the United States, endometriosis is the third-leading gynecological cause for hospitalization and is associated with significant costs.5, 6 In addition, patients with endometriosis have an increased risk of ovarian cancer and non-Hodgkin's lymphoma,7, 8 which adds to the burden of the disease.
Although the pathogenesis of endometriosis remains uncertain, recent studies by our group and others have demonstrated that the development of a vascular supply is essential for the establishment and growth of endometriotic lesions.9, 10 Thus inhibition of angiogenesis has been suggested as a novel therapeutic approach11 and has shown promise in preclinical studies.12, 13, 14, 15 Neovascularization occurs as a result of local sprouting of endothelial cells, elongation of pre-existing blood vessels, or de novo vasculogenesis.16 Elevated local or systemic levels of angiogenic factors not only result in migration and proliferation of endothelial cells but also recruit endothelial cells from the bone marrow.17, 18 Vascular endothelial growth factor (VEGF), which is highly up-regulated in endometriotic lesions, eutopic endometrium, and peritoneal fluid of patients with endometriosis,19, 20, 21 is a strong stimulus for the recruitment of bone marrow–derived endothelial progenitor cells (EPCs).22 Elevated VEGF serum levels in patients with endometriosis have been reported by some researchers23 but not by others,24 which may be due to the short half-life of VEGF of approximately 3 minutes.25 Considerable debate currently exists about the contribution of EPCs to neovascularization of tumors. Particular interest has focused on whether EPCs incorporate into the growing vascular tree and, if so, to what extent they contribute to neovascularization.26, 27, 28, 29 VEGF inhibition appears to decrease levels of EPCs and increase the detachment of mature circulating endothelial cells (CECs) originating from tumor vasculature.30 Thus it has been suggested that EPCs and CECs can be used as surrogate markers for disease progression and efficacy of anti-angiogenic therapy in tumors.31
Endometriosis and tumor growth both require angiogenesis for expansion of mass.10 Because previous work has suggested the use of EPCs as an indicator of tumor growth and regression, in this study we investigated the role of EPCs as potential biomarkers of endometriosis. We used an established mouse model of surgically induced endometriosis to measure levels of CECs and EPCs. Further, we used a bone marrow transplantation model to quantify the extent of EPC incorporation into the newly forming vasculature of growing endometriotic lesions. Finally, our studies assess the effect of anti-angiogenic therapy on EPC and CEC levels.
Material and Methods
Animal Handling
All procedures were performed in the animal facility at Children's Hospital, Boston, MA. All animal handling and procedures were performed in accordance with federal, local, and institutional guidelines and were approved by the Institutional Animal Care and Use Committee of Children's Hospital. Eight-week-old female 129 × 1/SvJ mice were purchased from Jackson Laboratories (Bar Harbor, MN); 129S6/SvEvTac mice were purchased from Taconic Farms (Hudson, NY), and C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA). C57BL/6J-GFP-mice were a generous gift from Dr. Taturo Udagawa.32 The mice were caged in groups of five to 10 with free access to chow and water; they were acclimated for 3 weeks before any experiment. The animal room was kept constantly at 26°C, 38.5% humidity, with a 12-hour light, 12-hour dark cycle (7:30 AM to 7:30 PM). Mice received no hormonal treatment.
Induction of Endometriosis
Mice were randomly taken from different cages to minimize any potential effects of the stage of the estrous cycle, which was determined by vaginal smears as previously described.12 Consequently, the stages were evenly distributed across the different groups in all experiments (data not shown). All surgical procedures were performed under inhalational anesthesia with isoflurane (Baxter, Deerfield, IL), and mice were observed until they fully recovered. Endometriosis was surgically induced as described previously.33, 34, 35, 36 Briefly, the uterine horns were removed through a midline abdominal incision and opened longitudinally in a Petri dish containing warmed 0.9% saline solution. Four or six biopsy specimens (2 mm in diameter) were obtained with use of a dermal biopsy punch (Miltex, Bethpage, NY), and the lesions were autotransplanted to the peritoneal wall with the endometrial side facing the peritoneum using a braided 7–0 silk suture (Ethicon, Somerville, NJ). The wound was closed with a 5–0 suture (Ethicon). Identical procedures were performed in the “sham group” except that sutures alone were inserted instead of endometrial tissue. Mice in the “control group” did not undergo surgery. For initial experiments (Figure 1), blood was taken 1 week after inoculation with endometriosis. For all of the other experiments, blood was drawn and mice were sacrificed at the indicated time points. At the end of the experiment, lesions were measured at their implantation site as described previously: two perpendicular diameters (D1, D2) of each lesion were measured with a caliper to the nearest tenth of a millimeter.12, 37, 38 Lesion volumes were determined using the formula for a sphere (volume = D1 × D2 × π/4). Lesions were then excised for further processing. In some groups of mice the uteri also were removed for further analysis (described in a later section).
Figure 1.
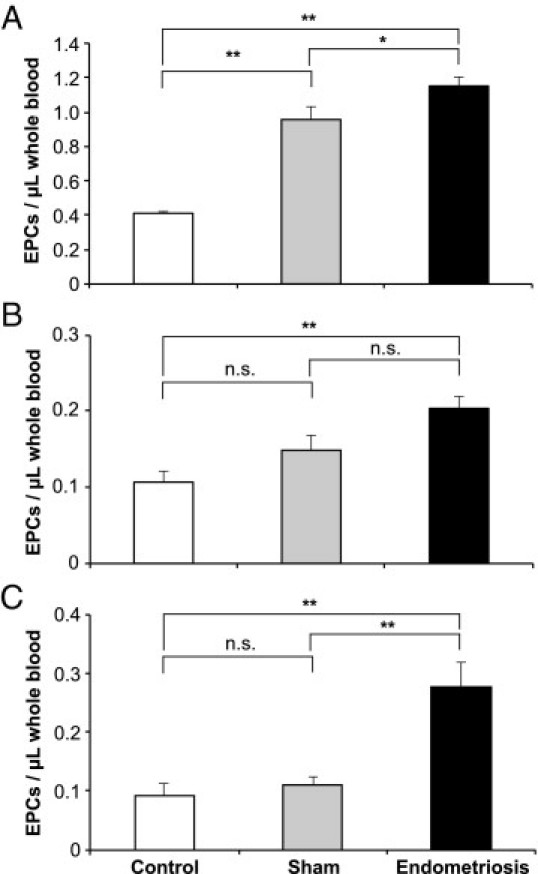
Blood EPC numbers in mice. Female mice received endometriotic (black columns) or sham lesions (gray columns) during abdominal surgery or were not operated upon (control subjects, white columns). Whole blood was taken after 1 week and stained for EPC markers (flow cytometry). Female C57BL/6 mice received four lesions (A) or six lesions (B) (*P < 0.05; **P < 0.001, posthoc Bonferroni test). Female 129SvJ mice received six lesions (C). All experiments were performed with n = 10 per group. Values shown are mean ± SEM (*P < 0.05; **P = 0.007, posthoc Bonferroni test).
Lodamin Treatment
129S6/SvEvTac mice were divided into three groups (“endometriosis,” “sham,” and “control”; n = 20/group). Half of the mice received 15 mg/kg Lodamin, an oral formulation of the angiogenesis inhibitor TNP-470, or vehicle daily by oral gavage for 14 days.39 Mice were monitored daily for changes in weight. Daily vaginal smears were taken, and uterine horns were removed at the end of the experiment to determine the potential effect of Lodamin on estrous cycling. In the bone marrow transplantation experiments, C57BL/6J mice with transplanted green fluorescent protein (GFP) bone marrow received endometriotic tissue from wild-type C57BL/6J mice.
Bone Marrow Transplantation
Bone marrow transplantation was performed as described previously.40 In brief, bone marrow was harvested from C57BL/6J-GFP mice. The donor mice were euthanized using carbon dioxide, and their femurs were dissected and then flushed with 1 mL sterile 0.9% saline solution to collect bone marrow. Anesthetized recipient C57BL/6 mice (Charles River Laboratories) were lethally irradiated with 950 Gy. The harvested GFP bone marrow was then injected into the retro-orbital plexus of into the irradiated recipients in 100 μL 0.9% saline solution. The bone marrow was allowed to engraft for 6 weeks. Engraftment was verified by GFP-positive blood samples before endometriosis induction.
Flow Cytometry Analysis of Circulating Endothelial Cells
Circulating EPCs and CECs were measured in the peripheral blood of all of the mice. After induction of anesthesia using isoflurane, 500 μL to 1000 μL of blood was collected by retro-orbital puncture and anticoagulated with use of sodium citrate. Blood was kept on ice until processing, and 150 μL of blood was used to quantify circulating endothelial cells using four-color flow cytometry as previously described.30, 41, 42 Red blood cells were lysed using FACSLyse Solution (BD Biosciences, San Jose, CA), according to the manufacturer's directions. For the fluorescence-activated cell sorting analysis of GFP-positive cells in tissue, eutopic endometrium and endometriotic lesions were taken from mice that had undergone a bone marrow transplantation and wild-type mice. Tissue was incubated in Dispase 2 (Roche, Nutley, NJ) for 30 minutes at 37°C per the manufacturer's instructions to dissociate tissue into single-cell suspensions, re-suspended in Dulbecco's modified Eagle's medium with 10% fetal bovine serum to neutralize enzymes, and washed in PBS with 1% bovine serum albumin before staining with antibodies and performing fluorescence-activated cell sorting analysis.
The following directly conjugated antibodies were used to detect EPCs and CECs in peripheral mouse blood: anti-mouse CD45-PerCP, CD31-APC (platelet/endothelial cell adhesion molecule-1), Flk-1-PE (mouse VEGFR-2; all BD Biosciences, San Jose, CA), and CD133-fluorescein isothiocyanate (Prominin-1; eBioscience, San Diego, CA). Tissue was stained with the same antibodies except the CD133 fluorescein isothiocyanate antibody. Flow cytometry was performed using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) and acquired data were analyzed with FLOWJO flow cytometry analysis software (Treestar, Ashland, OR), with analysis gates designed to remove residual platelets and cellular debris. Fluorescence minus one control subjects were used for gating. Between 500,000 and 1000,000 events were counted in each sample. SV40-transformed murine endothelial (MS-1) cells43 were spiked into blood samples as a positive control for compensation and gating purposes.30
Circulating endothelial cells in mouse peripheral blood were assessed using flow cytometry and then classified into mature (CECs: CD45−, VEGFR-2+, CD31+, CD133−) and bone marrow–derived progenitor endothelial cells (EPCs: CD45−, VEGFR-2+, CD31+, CD133+) as previously described.30, 41, 42, 44
Immunohistochemistry
Immunohistochemical staining was carried out on 8-μm frozen sections. For CD31-Cy3 staining, sections were dried for 2 to 3 hours at room temperature and circled with an Immedge Pen (Vector Laboratories, Burlingame, CA). Sections were immersed in 0.3% Triton X-100 PBS (T-PBS), washed twice with PBS, and then blocked for 1 hour with 5% goat serum (Jackson ImmunoResearch, West Grove, PA) in PBS + 0.3% Triton X-100 + 0.01% bovine serum albumin + 0.01% Thimerosal. The same solution without goat serum was used to dilute antibodies. Incubation with primary antibody (rat anti-mouse CD31, 1:500, BD Biosciences) was carried out overnight. Slides were then washed with T-PBS and incubated with secondary antibody (Cy3-goat anti-rat 1:400, Jackson ImmunoResearch) for 4 to 5 hours. Slides were then washed with T-PBS and fixed with 4% paraformaldehyde for 15 minutes. The final washes with PBS were done before mounting with Vectashield Mounting Medium with DAPI (Vector Laboratories) according to the manufacturer's instructions.
Uterine horns and ovaries were removed from mice in all groups after Lodamin treatment. Tissues were snap frozen in liquid nitrogen and mounted in OCT embedding medium (Thermo Fisher Scientific, Waltham, MA). Sections that were 5- to 7-μm thick were cut on a cryostat machine and stained with H&E.
Statistics
All statistical comparisons were done using SigmaStat software (Aspire Software International, Leesburg, VA) as previously described.40 The number of mature CECs and EPCs detected in each mouse was expressed as absolute number of cells per microliter of blood analyzed. Mean CEC and EPC values were then calculated and compared between the different groups. Means and Student's t-test for parametric data or median and rank-sum test for nonparametric data were applied to compare groups. EPCs in whole blood were compared using one-way analysis of variance (analysis of variance) with posthoc testing based on the Bonferroni procedure to minimize type I errors (false-positive results) due to multiple group comparisons.45 Lesion size was compared with use of repeated-measures analysis of variance.
Results
EPC Levels Are Elevated in a Mouse Model of Endometriosis
To examine the levels of EPCs in a mouse model of endometriosis, we initially measured EPC counts by flow cytometry in C57BL/6 mice with four syngeneic endometriotic lesions. As we have previously described,30 EPCs were identified by their expression of three cell surface markers including CD31 (endothelial marker), Flk-1 (VEGFR-2, endothelial marker), and CD133 (Prominin-1, stem cell marker) and the absence of CD45 (hematopoietic cell marker) (see Supplemental Figure S1 at http://ajp.amjpathol.org). To distinguish between CECs and EPCs, further characterization was done by gating on CD133 and CD31 expression. Similar to previous reports, in a typical experiment with C57BL/6 mice we found 0.2 to 0.5 EPC per microliter of blood and five to 10 CECs per microliter of blood.30, 40 In the presence of four and six lesions, a highly statistically significant overall difference between the three groups was observed 1 week after endometriosis surgery (four lesions: F = 51.48, P < 0.0001, Figure 1A; six lesions: F = 8.41, P = 0.001, Figure 1B). However, the difference between the endometriosis and sham groups were only marginally significant or not significant.
Because various inbred murine genetic backgrounds have been demonstrated to have variable angiogenic responses,31, 46 we chose to examine one of the most angiogenic strains, 129/SvJ mice. Thus we implanted six lesions per mouse into 129/SvJ mice, which have been shown to have baseline higher circulating EPC levels.46 Our studies demonstrated a significant overall mean difference between groups (Figure 1C) (F = 7.39, P = 0.003). Statistical analysis further indicated significantly higher circulating blood EPC levels in the endometriosis group compared with control subjects (P = 0.007) or compared with mice that underwent a sham operation (P = 0.007) (Figure 1C). No significant difference was detected between sham treated mice versus control subjects (P = 0.99).
CEC and EPC Levels Decrease Over Time
To determine whether the numbers of EPCs or CECs fluctuated over time after the implantation of endometriotic lesions, mice were terminally bled on various days after disease initiation. We found a highly significant change in lesion area over time (F = 15.78, P < 0.001), with posthoc analysis revealing a significant reduction in the first 10 days (P = 0.04) and then a dramatic increase from day 10 to day 28 (P < 0.001). We next examined the changes in mean EPCs per microliter of whole blood between sham and endometriosis groups at specific time points. Our results indicated overall significantly greater circulating EPC levels in the endometriosis group (F = 10.54, P = 0.002) (Figure 2B) with significant differences at 1 day (P = 0.008), 4 days (P = 0.03), and 10 days (P = 0.04) but no differences at 7 days (P = 0.48), 14 days (P = 0.30), or 28 days (P = 0.47).
Figure 2.
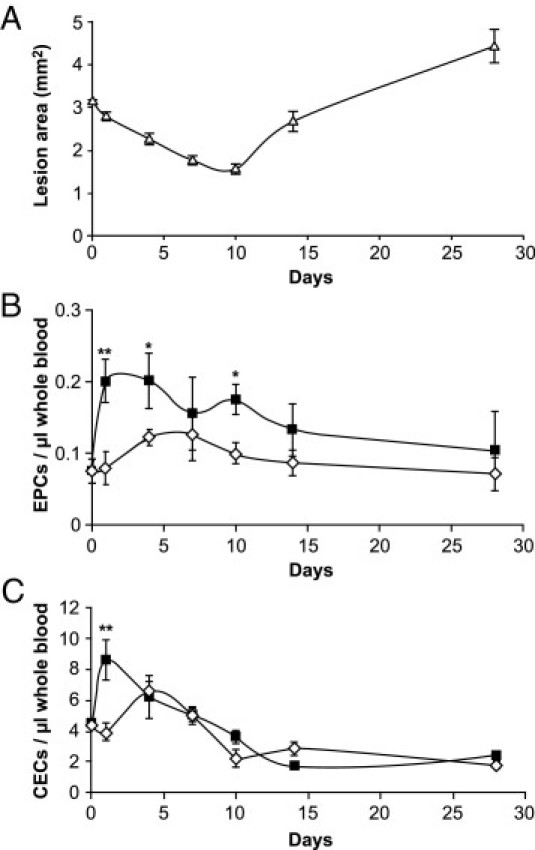
Blood levels of endothelial cells over time. Whole blood was collected from mice with six endometriotic lesions after different time points (n = 5 per time point and group). A: Following blood collection, endometriotic lesions were measured with a caliper using the formula of an ellipse as previously described.12, 34, 37, 38, 77 Values shown are mean ± SEM. B: EPCs stained negative for CD45 and positive for CD31, VEGRR-2, and CD133 (*P ≤ 0.04; **P = 0.008; posthoc Bonferroni test). C: CECs stained negative for CD45 and CD133 and positive for CD31 and VEGFR-2 [endometriosis group (black squares); sham operated group (white diamonds)] (**P < 0.001; posthoc Bonferroni test).
We then investigated CEC levels in mice that had endometriosis versus mice that underwent a sham operation over time. Overall, significantly higher CEC levels were found in the endometriosis group (F = 4.21, P = 0.04) (Figure 2C), which was confirmed by posthoc analysis for day 1 (P < 0.001), but not thereafter.
Bone Marrow-Derived Cells Incorporate into the Vasculature of Endometriotic Implants
Endometriotic lesions were implanted into irradiated wild-type mice transplanted with GFP-expressing bone marrow. Endothelial cells in the circulation, in the endometriotic lesions, and in the uterine tissue were quantified at various time points after surgery. Uterine tissue and endometriotic lesions were isolated and dissociated into single cell suspensions, and endothelial cells were identified using flow cytometry by the expression of two different cell surface markers (CD31+ and VEGFR2+) and exclusion of a hematopoietic cell surface antigen (CD45−) (Figure 3A). We detected a highly significant increase over time in the numbers of endothelial cells incorporated into the endometriotic lesions at 21 days (F = 31.97, P < 0.001) but no change in uterine tissue (F = 1.73, P = 0.21).
Figure 3.
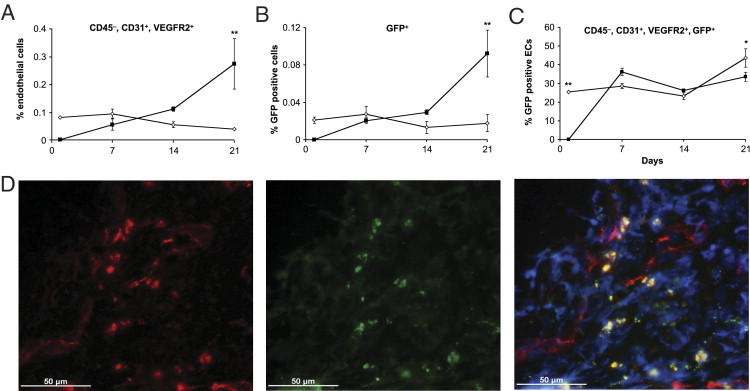
Endothelial cells in endometriotic lesions. After irradiating with lethal doses, mice received GFP+/+ bone marrow from donor mice. Six weeks later, endometriotic lesions from wild-type animals were implanted into these mice. Lesions and uterine tissue were removed at different time points from different mice (n = 5 per group and time point), digested, and single cell suspensions were stained and measured using flow cytometry [endometriotic lesions (black squares); uterine tissue (white diamonds)]. A: Percentage of CD45−, CD31+, and VEGFR-2+ endothelial cells of all cells were measured. B: Percentage of GFP+ cells of all cells measured. C: Percentage of CD45−, CD31+, VEGFR-2+, and GFP+ endothelial cells. Values shown are mean ± SEM (*P < 0.003; **P < 0.001; posthoc Bonferroni test). D: Immunohistochemistry of wild-type endometriotic lesions in irradiated mice with transplanted GFP+ bone marrow. GFP fluorescence (middle panel) and after staining for CD31 (Cy-3; left panel). Fluorescent confocal microscopy showed some co-localization of GFP+ and CD31+ cells (right panel; bar = 50 μmol/L).
The percentage of GFP-positive bone marrow–derived cells was significantly elevated in endometriotic lesions after 21 days (F = 30.62, P < 0.001) but remained constant in uterine tissue (F = 0.88, P = 0.48) (Figure 3B). To determine whether GFP bone marrow–derived cells were endothelial cells, we analyzed GFP+ cells that were also VEGFR2 and CD31 positive but negative for CD45 (Figure 3C). None of these cells could be detected in the endometriotic lesions on the day after the transplantation. Two-way analysis of variance confirmed a highly significant increase over time in the percentage of GFP-positive endothelial cells in endometriotic tissue (F = 33.06, P < 0.001) and in uterine tissue (F = 21.69, P = 0.003). Furthermore, incorporation of GFP-positive cells into the vasculature of endometriotic lesions was confirmed by immunofluorescence with an antibody to CD31, an endothelial cell specific marker that co-localized with GFP expression (Figure 3D). These studies confirmed the presence of endothelial progenitor cells incorporated into endometriotic lesions.
Lodamin Inhibits the Up-Regulation of EPCs and CECs
Anti-angiogenic therapy has been shown to suppress endometriotic growth in various mouse models.15, 37, 38 Lodamin is an oral nontoxic formulation of TNP-470, one of the most potent angiogenesis inhibitors known to date.39 Cohorts of wild-type mice were either designated as control (untouched) or had sham surgery or endometriotic lesions implanted. Mice were then treated daily with either 15 mg/kg Lodamin or vehicle alone by oral gavage. Weight loss among the different cohorts was not statistically significant, indicating the lack of toxicity with daily Lodamin treatment for 1 week (data not shown). After 1 week of treatment, blood was drawn to measure both CEC and EPC concentrations. Statistical analysis indicated significantly lower CECs per microliter of whole blood for treatment compared with vehicle in the endometriosis group (P = 0.02) but no significant difference between vehicle and treatment for control (P = 0.09) or sham (P = 0.21) groups (Figure 4A).
Figure 4.
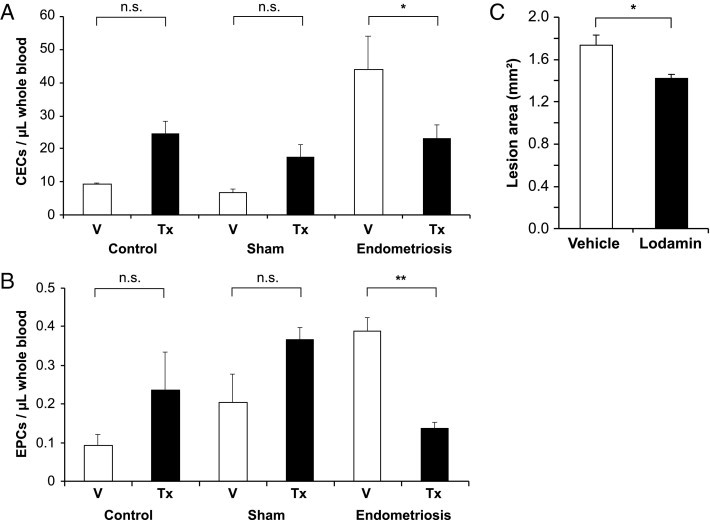
Effect of antiangiogenic treatment on EPCs and CECs. Female 129 SvJ mice underwent endometriosis or sham surgery or no surgery (control subjects) (n = 10 per group). Half of the mice of each group received 15 mg/kg Lodamin (Tx; black columns), an oral, nontoxic form of the angiogenesis inhibitor TNP-470, over 7 days. Control subjects received vehicle (V; white columns). Whole blood was taken at the end of the experiment, stained for (A) CEC markers (CD45−, CD31+, VEGFR-2+, CD133−) (*P = 0.02; two-way analysis of variance) and (B) EPC markers (CD45−, CD31+, VEGFR-2+, CD133+) (**P < 0.001; two-way analysis of variance) and measured using flow cytometry. C: Endometriosis-like lesions were measured with a caliper using the formula of an ellipse. Mean cross-sectional area of endometriosis-like lesions (n = 6 lesions) of mice treated with vehicle or Lodamin (*P = 0.022; repeated-measures analysis of variance). Values shown are mean ± SEM.
When we looked at EPCs, overall highly significant differences were observed (F = 6.72, P = 0.006), with significantly lower EPCs per microliter after treatment of the endometriosis (P < 0.001), but no significant differences in the control group (P = 0.11) or the sham group (P = 0.07) (Figure 4B). This finding could indicate that anti-angiogenic therapy with Lodamin suppresses the mobilization of both circulating endothelial cells and endothelial progenitor cells in the presence of an angiogenesis-dependent disease.
When the lesion size was measured after 1 week of Lodamin treatment, results indicated a significantly smaller lesion area in the Lodamin group compared with vehicle (1.74 mm2 versus 1.41 mm2, F = 7.99, P = 0.022) (Figure 4C).
Discussion
In the present study we show that circulating endothelial progenitor cells are elevated in an established mouse model of endometriosis and provide evidence that these cells incorporate into the growing vasculature of the endometriotic lesions. EPC up-regulation appears to be highest during early lesion establishment, coinciding with increased vascular growth as previously demonstrated.38 However, treatment with the potent angiogenesis inhibitor Lodamin resulted in a significant inhibition of this surge of circulating EPCs and subsequent suppression of lesion growth. These findings imply a potential role for EPCs in the angiogenesis-dependent growth of endometriotic lesions and may indicate the use of EPCs as a biomarker of disease progression and surrogate marker of treatment efficacy.
Endometriosis is similar to tumors in that it requires blood vessel growth for disease establishment and progression. Although the exact mechanisms are unknown, angiogenesis has been proposed to occur in endometriosis through classic mechanisms such as proliferation of endothelial and supporting cells, intussusception, and elongation of existing blood vessels.16, 47 In our current study, we demonstrate for the first time another possible mechanism, the incorporation of bone marrow–derived circulating endothelial progenitor cells into the growing vascular tree. Blood EPC levels rose within 2 days of “disease” induction and peaked during the first 10 days, in a manner that was inversely correlated with the size of the lesions.
Considerable debate exists about the role of EPCs, in particular regarding the extent and significance of their incorporation into tumor vasculature.28, 44 In our study, we found that up to 37% of all endothelial cells in the lesions were bone marrow–derived 1 week after transplantation. No significant increase of general bone marrow–derived cells in the uterus was observed during this period. However, the percentage of bone marrow–derived endothelial cells did increase over time, which may be a result of changes in the estrous cycle in these animals. Human studies have shown higher blood EPC levels during the follicular48 and the luteal phase,49 but in this model, we did not see estrous cycle–related differences in blood EPC levels (data not shown). Eggermont et al demonstrated in a xenotransplant model that human lesions contained murine CD31-positive cells as early as day 5 after implantation, peaking around day 15.50 Because host endothelial cells invaded the interface between the peritoneal stroma and the lesion, they suggested that the source of the endothelial cells most likely was in the proliferating surrounding blood vessels. Our data suggest that bone marrow–derived endothelial cells may contribute to this process of blood vessel growth, especially during early lesion establishment.
Endometriosis is a chronic, progressive disease that frequently presents with multiple sites of extra-uterine tissue in the peritoneal cavity. Pro-angiogenic factors such as VEGF, basic fibroblast growth factor, and IL-8 are elevated in endometriotic tissue, peritoneal fluid, or serum of patients with endometriosis.21, 51, 52 VEGF is a well-studied mitogen for endothelial cells and strongly increases vascular permeability.53 It has been previously demonstrated that VEGF plays a significant role in the mobilization of bone marrow– derived progenitor cells for postnatal neovascularization.22
Assuming that VEGF is one of the main stimuli for the recruitment of EPCs in this model, our current data support our previous findings that showed high VEGF levels in the transplanted lesions during the first week after transplantation. This rise and subsequent fall in EPC concentration and VEGF expression could be explained by a hypoxic environment after the lesions lose their blood supply followed by a normoxic one once the lesions acquire new blood vessels. In fact, we have previously demonstrated a concomitant rise in levels of hypoxia inducible factor-1 α and its downstream target, VEGF, in these lesions during the first few days after transplantation.37 Concentrations of both molecules decline thereafter when neovascularization results in the delivery of oxygen and nutrients.38, 50 The small, delayed rise in blood EPC levels in the mice that underwent a sham operation is possibly due to wound healing in these animals. Other factors have been shown to stimulate EPC recruitments such as stromal cell-derived factor-1, placental growth factor, and matrix metalloprotease-9.54, 55, 56 Interestingly, they all have been associated with endometriosis.57, 58, 59, 60
Blood levels of mature endothelial cells also rose in both mice that underwent a sham operation and in mice with endometriosis, although this effect was delayed in the sham group. We speculate that wound healing, mainly of the abdominal wall and uterine stump, resulted in the shedding of these cells from the local tissue in all animals. The fact that this phenomenon occurred earlier in the endometriosis group supports this theory, because transplantation increases the tissue burden undergoing regeneration. CEC concentrations in both groups were similar after a few days, suggesting that most of the endothelial cells originating from the transplanted lesions had gone.
Anti-angiogenic therapy may have a role as a novel therapeutic approach in patients with endometriosis.9 However, potential teratogenicity and inhibitory effects on the reproductive system demonstrated by some angiogenesis inhibitors are posing a significant hurdle to the clinical testing of angiogenesis inhibitors in patients with endometriosis.47, 61, 62, 63 We previously demonstrated that the endogenous angiogenesis inhibitor endostatin and its fragment mP-1 had no negative effects on the reproductive cycle and offspring of mice, while endometriotic growth was suppressed.12, 34 TNP-470, a synthetic fumagillin analog, is a very powerful angiogenesis inhibitor that has been shown to inhibit endometriotic growth15, 64, 65 but that caused neurotoxicity in clinical trials.66, 67 Lodamin is an oral, nontoxic form of the fumagillin derivative TNP-470, which inhibits tumor growth and angiogenesis in mice.39 Possible TNP-470 mechanisms on endothelial cells include affecting the cell cycle through p53 activation by binding to methionine aminopeptidase (MetAP-2), preventing Rac1 activation and induction of p21 (CIP/WAF).68, 69, 70
In our current study we found that Lodamin suppressed the levels of EPCs in the blood of mice that had endometriosis while inhibiting growth of endometriotic lesions. We have seen a similar effect with caplostatin (unpublished data), an injectable, nontoxic formulation of TNP-470 that inhibited endometriotic growth and angiogenesis.38 TNP-470 has been shown to inhibit VEGF-induced endothelial cell growth, migration, and vascular hyperpermeability.71, 72, 73 In vitro data suggest that VEGF expression by tumor cells is inhibited by TNP-470.74 Because VEGF is considered to play a central role in bone marrow recruitment, it is conceivable that TNP-470 treatment inhibits VEGF expression in the endometriotic lesions, resulting in a reduced stimulus for EPC mobilization.75 We have previously demonstrated that VEGF is highly up-regulated in endometriotic tissue through hypoxia inducible factor-1 α.37 Therefore if the TNP-470 derivative Lodamin inhibits this pathway it could explain the effect on EPC levels in the treated animals. In fact, Lodamin inhibits VEGF-induced angiogenesis in a mouse corneal micropocket assay.39 Treatment with Lodamin resulted in the arrest of the estrous cycle at metestrus (data not shown). Therefore it is conceivable that the potential effect of the drug on estrogen levels also may affect EPC (and CEC levels). This question is currently under investigation.
In summary, we demonstrate that bone marrow–derived endothelial cells contribute to neovascularization in a mouse model of endometriosis. These findings highlight the importance of angiogenesis in the establishment and growth of endometriotic lesions, and they also support the hypothesis that endothelial progenitor cells are involved in the pathogenesis of the disease.76 From a clinical perspective it will be highly interesting to investigate EPC and CEC levels in women who have the disease and the effect of therapy on these levels. If circulating endothelial progenitor and mature endothelial cells are elevated in patients, they may be promising and much needed biomarkers for both diagnosis and therapeutic efficacy.
Acknowledgments
We thank Kristin Johnson for her excellent work with graphics, Dr. Taturo Udagawa for fruitful discussions and the gift of the GFP mice, and Muna El-Kasti and Janet Carver for their technical help.
Footnotes
Supported by the Endometriosis Millennium Fund from the Royal College of Obstetrics and Gynaecology, United Kingdom; an MRC New Investigator Award (G0601458 to C.M.B.); the Oxford Partnership Comprehensive Biomedical Research Centre with funding from the Department of Health's NIHR Biomedical Research Centres scheme (C.M.B.); and a grant from the Sydney Swensrud Foundation (R.J.D.). C.M.B. was supported by a Postdoctoral Research Exchange Fellowship from the Max Kade Foundation. P.B. was supported by an Alberta Heritage Foundation for Medical Research Clinical Fellowship. S.R. was supported by the Garrett B. Smith Foundation.
Supplemental material for this article can be found on http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2010.12.037.
Supplementary data
Typical set-up for flow cytometry to detect murine EPCs. A: Initial gate used to exclude red cell and platelet debris. B: Selection of CD45-PerCp negative and VEGFR2-PE-positive cells, which encompasses both mature CECs and CEPs. Upper panel showing whole blood (control) from mice and lower panel showing whole blood mixed with the murine endothelial cell line MS-1 cells (control + MS-1). C: CECs are further characterized using CD31-APC and progenitor marker CD133-FITC. D: Isotype controls for CD45-PerCP, VEGFR2-PE, CD31-APC, and CD133-FITC.
References
- 1.Cramer D.W., Missmer S.A. The epidemiology of endometriosis. Ann NY Acad Sci. 2002;955:11–22. doi: 10.1111/j.1749-6632.2002.tb02761.x. discussion 34–16, 396–406. [DOI] [PubMed] [Google Scholar]
- 2.Bulun S.E. Endometriosis. N Engl J Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy S., Bergqvist A., Chapron C., D'Hooghe T., Dunselman G., Greb R., Hummelshoj L., Prentice A., Saridogan E. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20:2698–2704. doi: 10.1093/humrep/dei135. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy S., Hadfield R., Mardon H., Barlow D. Age of onset of pain symptoms in non-twin sisters concordant for endometriosis. Hum Reprod. 1996;11:403–405. doi: 10.1093/humrep/11.2.403. [DOI] [PubMed] [Google Scholar]
- 5.Simoens S., Hummelshoj L., D'Hooghe T. Endometriosis: cost estimates and methodological perspective. Hum Reprod Update. 2007;13:395–404. doi: 10.1093/humupd/dmm010. [DOI] [PubMed] [Google Scholar]
- 6.Zhao S.Z., Wong J.M., Davis M.B., Gersh G.E., Johnson K.E. The cost of inpatient endometriosis treatment: an analysis based on the Healthcare Cost and Utilization Project Nationwide Inpatient Sample. Am J Manag Care. 1998;4:1127–1134. [PubMed] [Google Scholar]
- 7.Brinton L.A., Gridley G., Persson I., Baron J., Bergqvist A. Cancer risk after a hospital discharge diagnosis of endometriosis. Am J Obstet Gynecol. 1997;176:572–579. doi: 10.1016/s0002-9378(97)70550-7. [DOI] [PubMed] [Google Scholar]
- 8.Varma R., Rollason T., Gupta J.K., Maher E.R. Endometriosis and the neoplastic process. Reproduction. 2004;127:293–304. doi: 10.1530/rep.1.00020. [DOI] [PubMed] [Google Scholar]
- 9.Becker C.M., D'Amato R.J. Angiogenesis and antiangiogenic therapy in endometriosis. Microvasc Res. 2007;74:121–130. doi: 10.1016/j.mvr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Taylor R.N., Lebovic D.I., Mueller M.D. Angiogenic factors in endometriosis. Ann NY Acad Sci. 2002;955:89–100. doi: 10.1111/j.1749-6632.2002.tb02769.x. discussion 118, 396–406. [DOI] [PubMed] [Google Scholar]
- 11.Taylor R.N., Mueller M.D. Anti-angiogenic treatment of endometriosis: biochemical aspects. Gynecol Obstet Invest. 2004;57:54–56. [PubMed] [Google Scholar]
- 12.Becker C.M., Sampson D.A., Rupnick M.A., Rohan R.M., Efstathiou J.A., Short S.M., Taylor G.A., Folkman J., D'Amato R.J. Endostatin inhibits the growth of endometriotic lesions but does not affect fertility. Fertil Steril. 2005;84(Suppl 2):1144–1155. doi: 10.1016/j.fertnstert.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 13.Dabrosin C., Gyorffy S., Margetts P., Ross C., Gauldie J. Therapeutic effect of angiostatin gene transfer in a murine model of endometriosis. Am J Pathol. 2002;161:909–918. doi: 10.1016/S0002-9440(10)64251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laschke M.W., Elitzsch A., Vollmar B., Vajkoczy P., Menger M.D. Combined inhibition of vascular endothelial growth factor (VEGF), fibroblast growth factor and platelet-derived growth factor, but not inhibition of VEGF alone, effectively suppresses angiogenesis and vessel maturation in endometriotic lesions. Hum Reprod. 2006;21:262–268. doi: 10.1093/humrep/dei308. [DOI] [PubMed] [Google Scholar]
- 15.Nap A.W., Griffioen A.W., Dunselman G.A., Bouma-Ter Steege J.C., Thijssen V.L., Evers J.L., Groothuis P.G. Antiangiogenesis therapy for endometriosis. J Clin Endocrinol Metab. 2004;89:1089–1095. doi: 10.1210/jc.2003-031406. [DOI] [PubMed] [Google Scholar]
- 16.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 17.Asahara T., Murohara T., Sullivan A., Silver M., van der Zee R., Li T., Witzenbichler B., Schatteman G., Isner J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 18.Spring H., Schuler T., Arnold B., Hammerling G.J., Ganss R. Chemokines direct endothelial progenitors into tumor neovessels. Proc Natl Acad Sci USA. 2005;102:18111–18116. doi: 10.1073/pnas.0507158102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donnez J., Smoes P., Gillerot S., Casanas-Roux F., Nisolle M. Vascular endothelial growth factor (VEGF) in endometriosis. Hum Reprod. 1998;13:1686–1690. doi: 10.1093/humrep/13.6.1686. [DOI] [PubMed] [Google Scholar]
- 20.McLaren J., Prentice A., Charnock-Jones D.S., Smith S.K. Vascular endothelial growth factor (VEGF) concentrations are elevated in peritoneal fluid of women with endometriosis. Hum Reprod. 1996;11:220–223. doi: 10.1093/oxfordjournals.humrep.a019023. [DOI] [PubMed] [Google Scholar]
- 21.Shifren J.L., Tseng J.F., Zaloudek C.J., Ryan I.P., Meng Y.G., Ferrara N., Jaffe R.B., Taylor R.N. Ovarian steroid regulation of vascular endothelial growth factor in the human endometrium: implications for angiogenesis during the menstrual cycle and in the pathogenesis of endometriosis. J Clin Endocrinol Metab. 1996;81:3112–3118. doi: 10.1210/jcem.81.8.8768883. [DOI] [PubMed] [Google Scholar]
- 22.Asahara T., Takahashi T., Masuda H., Kalka C., Chen D., Iwaguro H., Inai Y., Silver M., Isner J.M. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xavier P., Belo L., Beires J., Rebelo I., Martinez-de-Oliveira J., Lunet N., Barros H. Serum levels of VEGF and TNF-alpha and their association with C-reactive protein in patients with endometriosis. Arch Gynecol Obstet. 2006;273:227–231. doi: 10.1007/s00404-005-0080-4. [DOI] [PubMed] [Google Scholar]
- 24.Gagne D., Page M., Robitaille G., Hugo P., Gosselin D. Levels of vascular endothelial growth factor (VEGF) in serum of patients with endometriosis. Hum Reprod. 2003;18:1674–1680. doi: 10.1093/humrep/deg326. [DOI] [PubMed] [Google Scholar]
- 25.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 26.Gao D., Nolan D.J., Mellick A.S., Bambino K., McDonnell K., Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 27.Kerbel R.S., Benezra R., Lyden D.C., Hattori K., Heissig B., Nolan D.J., Mittal V., Shaked Y., Dias S., Bertolini F., Rafii S. Endothelial progenitor cells are cellular hubs essential for neoangiogenesis of certain aggressive adenocarcinomas and metastatic transition but not adenomas. Proc Natl Acad Sci USA. 2008;105:E54. doi: 10.1073/pnas.0804876105. author reply E55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyden D., Hattori K., Dias S., Costa C., Blaikie P., Butros L., Chadburn A., Heissig B., Marks W., Witte L., Wu Y., Hicklin D., Zhu Z., Hackett N.R., Crystal R.G., Moore M.A., Hajjar K.A., Manova K., Benezra R., Rafii S. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 29.Purhonen S., Palm J., Rossi D., Kaskenpaa N., Rajantie I., Yla-Herttuala S., Alitalo K., Weissman I.L., Salven P. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci USA. 2008;105:6620–6625. doi: 10.1073/pnas.0710516105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beaudry P., Force J., Naumov G.N., Wang A., Baker C.H., Ryan A., Soker S., Johnson B.E., Folkman J., Heymach J.V. Differential effects of vascular endothelial growth factor receptor-2 inhibitor ZD6474 on circulating endothelial progenitors and mature circulating endothelial cells: implications for use as a surrogate marker of antiangiogenic activity. Clin Cancer Res. 2005;11:3514–3522. doi: 10.1158/1078-0432.CCR-04-2271. [DOI] [PubMed] [Google Scholar]
- 31.Shaked Y., Bertolini F., Man S., Rogers M.S., Cervi D., Foutz T., Rawn K., Voskas D., Dumont D.J., Ben-David Y., Lawler J., Henkin J., Huber J., Hicklin D.J., D'Amato R.J., Kerbel R.S. Genetic heterogeneity of the vasculogenic phenotype parallels angiogenesis; implications for cellular surrogate marker analysis of antiangiogenesis. Cancer Cell. 2005;7:101–111. doi: 10.1016/j.ccr.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 32.Udagawa T., Puder M., Wood M., Schaefer B.C., D'Amato R.J. Analysis of tumor-associated stromal cells using SCID GFP transgenic mice: contribution of local and bone marrow-derived host cells. FASEB J. 2006;20:95–102. doi: 10.1096/fj.04-3669com. [DOI] [PubMed] [Google Scholar]
- 33.Becker C.M., Sampson D.A., Rupnick M.A., Rohan R.M., Efstathiou J.A., Short S.M., Taylor G.A., Folkman J., D'Amato R.J. Endostatin inhibits the growth of endometriotic lesions but does not affect fertility. Fertil Steril. 2005;84(Suppl 2):1144–1155. doi: 10.1016/j.fertnstert.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 34.Becker C.M., Sampson D.A., Short S.M., Javaherian K., Folkman J., D'Amato R.J. Short synthetic endostatin peptides inhibit endothelial migration in vitro and endometriosis in a mouse model. Fertil Steril. 2006;85:71–77. doi: 10.1016/j.fertnstert.2005.07.1290. [DOI] [PubMed] [Google Scholar]
- 35.Cummings A.M., Metcalf J.L. Induction of endometriosis in mice: a new model sensitive to estrogen. Reprod Toxicol. 1995;9:233–238. doi: 10.1016/0890-6238(95)00004-t. [DOI] [PubMed] [Google Scholar]
- 36.Vernon M.W., Wilson E.A. Studies on the surgical induction of endometriosis in the rat. Fertil Steril. 1985;44:684–694. [PubMed] [Google Scholar]
- 37.Becker C.M., Rohwer N., Funakoshi T., Cramer T., Bernhardt W., Birsner A., Folkman J., D'Amato R.J. 2-Methoxyestradiol inhibits hypoxia-inducible factor-1{alpha} and suppresses growth of lesions in a mouse model of endometriosis. Am J Pathol. 2008;172:534–544. doi: 10.2353/ajpath.2008.061244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Becker C.M., Wright R.D., Satchi-Fainaro R., Funakoshi T., Folkman J., Kung A.L., D'Amato R.J. A novel noninvasive model of endometriosis for monitoring the efficacy of antiangiogenic therapy. Am J Pathol. 2006;168:2074–2084. doi: 10.2353/ajpath.2006.051133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benny O., Fainaru O., Adini A., Cassiola F., Bazinet L., Adini I., Pravda E., Nahmias Y., Koirala S., Corfas G., D'Amato R.J., Folkman J. An orally delivered small-molecule formulation with antiangiogenic and anticancer activity. Nature Biotechnol. 2008;26:799–807. doi: 10.1038/nbt1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beaudry P., Hida Y., Udagawa T., Alwayn I.P., Greene A.K., Arsenault D., Folkman J., Heymach J.V., Ryeom S., Puder M. Endothelial progenitor cells contribute to accelerated liver regeneration. J Pediatr Surg. 2007;42:1190–1198. doi: 10.1016/j.jpedsurg.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 41.Bertolini F., Paul S., Mancuso P., Monestiroli S., Gobbi A., Shaked Y., Kerbel R.S. Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res. 2003;63:4342–4346. [PubMed] [Google Scholar]
- 42.Schuch G., Heymach J.V., Nomi M., Machluf M., Force J., Atala A., Eder J.P., Jr, Folkman J., Soker S. Endostatin inhibits the vascular endothelial growth factor-induced mobilization of endothelial progenitor cells. Cancer Res. 2003;63:8345–8350. [PubMed] [Google Scholar]
- 43.Arbiser J.L., Moses M.A., Fernandez C.A., Ghiso N., Cao Y., Klauber N., Frank D., Brownlee M., Flynn E., Parangi S., Byers H.R., Folkman J. Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc Natl Acad Sci USA. 1997;94:861–866. doi: 10.1073/pnas.94.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bertolini F., Shaked Y., Mancuso P., Kerbel R.S. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer. 2006;6:835–845. doi: 10.1038/nrc1971. [DOI] [PubMed] [Google Scholar]
- 45.Cabral H.J. Multiple comparisons procedures. Circulation. 2008;117:698–701. doi: 10.1161/CIRCULATIONAHA.107.700971. [DOI] [PubMed] [Google Scholar]
- 46.Rohan R.M., Fernandez A., Udagawa T., Yuan J., D'Amato R.J. Genetic heterogeneity of angiogenesis in mice. FASEB J. 2000;14:871–876. doi: 10.1096/fasebj.14.7.871. [DOI] [PubMed] [Google Scholar]
- 47.Taylor R.N., Yu J., Torres P.B., Schickedanz A.C., Park J.K., Mueller M.D., Sidell N. Mechanistic and therapeutic implications of angiogenesis in endometriosis. Reprod Sci. 2009;16:140–146. doi: 10.1177/1933719108324893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robb A.O., Mills N.L., Smith I.B., Short A., Tura-Ceide O., Barclay G.R., Blomberg A., Critchley H.O., Newby D.E., Denison F.C. Influence of menstrual cycle on circulating endothelial progenitor cells. Hum Reprod. 2009;24:619–625. doi: 10.1093/humrep/den411. [DOI] [PubMed] [Google Scholar]
- 49.Matsubara K., Abe E., Matsubara Y., Kameda K., Ito M. Circulating endothelial progenitor cells during normal pregnancy and pre-eclampsia. Am J Reprod Immunol. 2006;56:79–85. doi: 10.1111/j.1600-0897.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- 50.Eggermont J., Donnez J., Casanas-Roux F., Scholtes H., Van Langendonckt A. Time course of pelvic endometriotic lesion revascularization in a nude mouse model. Fertil Steril. 2005;84:492–499. doi: 10.1016/j.fertnstert.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 51.Bourlev V., Larsson A., Olovsson M. Elevated levels of fibroblast growth factor-2 in serum from women with endometriosis. Am J Obstet Gynecol. 2006;194:755–759. doi: 10.1016/j.ajog.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 52.Ryan I.P., Tseng J.F., Schriock E.D., Khorram O., Landers D.V., Taylor R.N. Interleukin-8 concentrations are elevated in peritoneal fluid of women with endometriosis. Fertil Steril. 1995;63:929–932. [PubMed] [Google Scholar]
- 53.Benjamin L.E. The controls of microvascular survival. Cancer Metastasis Rev. 2000;19:75–81. doi: 10.1023/a:1026552415576. [DOI] [PubMed] [Google Scholar]
- 54.Hattori K., Heissig B., Wu Y., Dias S., Tejada R., Ferris B., Hicklin D.J., Zhu Z., Bohlen P., Witte L., Hendrikx J., Hackett N.R., Crystal R.G., Moore M.A., Werb Z., Lyden D., Rafii S. Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1(+) stem cells from bone-marrow microenvironment. Nat Med. 2002;8:841–849. doi: 10.1038/nm740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heissig B., Hattori K., Dias S., Friedrich M., Ferris B., Hackett N.R., Crystal R.G., Besmer P., Lyden D., Moore M.A., Werb Z., Rafii S. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamaguchi J., Kusano K.F., Masuo O., Kawamoto A., Silver M., Murasawa S., Bosch-Marce M., Masuda H., Losordo D.W., Isner J.M., Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 57.Becker C.M., Louis G., Exarhopoulos A., Mechsner S., Ebert A.D., Zurakowski D., Moses M.A. Matrix metalloproteinases are elevated in the urine of endometriosis patients. Fertil Steril. 2010;94:2343–2346. doi: 10.1016/j.fertnstert.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 58.Chung H.W., Lee J.Y., Moon H.S., Hur S.E., Park M.H., Wen Y., Polan M.L. Matrix metalloproteinase-2, membranous type 1 matrix metalloproteinase, and tissue inhibitor of metalloproteinase-2 expression in ectopic and eutopic endometrium. Fertil Steril. 2002;78:787–795. doi: 10.1016/s0015-0282(02)03322-8. [DOI] [PubMed] [Google Scholar]
- 59.Furuya M., Suyama T., Usui H., Kasuya Y., Nishiyama M., Tanaka N., Ishiwata I., Nagai Y., Shozu M., Kimura S. Up-regulation of CXC chemokines and their receptors: implications for proinflammatory microenvironments of ovarian carcinomas and endometriosis. Hum Pathol. 2007;38:1676–1687. doi: 10.1016/j.humpath.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 60.Suzumori N., Sugiura-Ogasawara M., Katano K., Suzumori K. Women with endometriosis have increased levels of placental growth factor in the peritoneal fluid compared with women with cystadenomas. Hum Reprod. 2003;18:2595–2598. doi: 10.1093/humrep/deg491. [DOI] [PubMed] [Google Scholar]
- 61.D'Amato R.J., Loughnan M.S., Flynn E., Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci USA. 1994;91:4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klauber N., Rohan R.M., Flynn E., D'Amato R.J. Critical components of the female reproductive pathway are suppressed by the angiogenesis inhibitor AGM-1470. Nat Med. 1997;3:443–446. doi: 10.1038/nm0497-443. [DOI] [PubMed] [Google Scholar]
- 63.Van Langendonckt A., Donnez J., Defrere S., Dunselman G.A., Groothuis P.G. Antiangiogenic and vascular-disrupting agents in endometriosis: pitfalls and promises. Mol Hum Reprod. 2008;14:259–268. doi: 10.1093/molehr/gan019. [DOI] [PubMed] [Google Scholar]
- 64.Ingber D., Fujita T., Kishimoto S., Sudo K., Kanamaru T., Brem H., Folkman J. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature. 1990;348:555–557. doi: 10.1038/348555a0. [DOI] [PubMed] [Google Scholar]
- 65.Nap A.W., Dunselman G.A., Griffioen A.W., Mayo K.H., Evers J.L., Groothuis P.G. Angiostatic agents prevent the development of endometriosis-like lesions in the chicken chorioallantoic membrane. Fertil Steril. 2005;83:793–795. doi: 10.1016/j.fertnstert.2004.06.080. [DOI] [PubMed] [Google Scholar]
- 66.Bhargava P., Marshall J.L., Rizvi N., Dahut W., Yoe J., Figuera M., Phipps K., Ong V.S., Kato A., Hawkins M.J. A Phase I and pharmacokinetic study of TNP-470 administered weekly to patients with advanced cancer. Clin Cancer Res. 1999;5:1989–1995. [PubMed] [Google Scholar]
- 67.Herbst R.S., Madden T.L., Tran H.T., Blumenschein G.R., Jr., Meyers C.A., Seabrooke L.F., Khuri F.R., Puduvalli V.K., Allgood V., Fritsche H.A., Jr., Hinton L., Newman R.A., Crane E.A., Fossella F.V., Dordal M., Goodin T., Hong W.K. Safety and pharmacokinetic effects of TNP-470, an angiogenesis inhibitor, combined with paclitaxel in patients with solid tumors: evidence for activity in non-small-cell lung cancer. J Clin Oncol. 2002;20:4440–4447. doi: 10.1200/JCO.2002.04.006. [DOI] [PubMed] [Google Scholar]
- 68.Nahari D., Satchi-Fainaro R., Chen M., Mitchell I., Task L.B., Liu Z., Kihneman J., Carroll A.B., Terada L.S., Nwariaku F.E. Tumor cytotoxicity and endothelial Rac inhibition induced by TNP-470 in anaplastic thyroid cancer. Mol Cancer Ther. 2007;6:1329–1337. doi: 10.1158/1535-7163.MCT-06-0554. [DOI] [PubMed] [Google Scholar]
- 69.Sin N., Meng L., Wang M.Q., Wen J.J., Bornmann W.G., Crews C.M. The anti-angiogenic agent fumagillin covalently binds and inhibits the methionine aminopeptidase, MetAP-2. Proc Natl Acad Sci USA. 1997;94:6099–6103. doi: 10.1073/pnas.94.12.6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Y., Griffith E.C., Sage J., Jacks T., Liu J.O. Cell cycle inhibition by the anti-angiogenic agent TNP-470 is mediated by p53 and p21WAF1/CIP1. Proc Natl Acad Sci USA. 2000;97:6427–6432. doi: 10.1073/pnas.97.12.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kusaka M., Sudo K., Matsutani E., Kozai Y., Marui S., Fujita T., Ingber D., Folkman J. Cytostatic inhibition of endothelial cell growth by the angiogenesis inhibitor TNP-470 (AGM-1470) Br J Cancer. 1994;69:212–216. doi: 10.1038/bjc.1994.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Satchi-Fainaro R., Mamluk R., Wang L., Short S.M., Nagy J.A., Feng D., Dvorak A.M., Dvorak H.F., Puder M., Mukhopadhyay D., Folkman J. Inhibition of vessel permeability by TNP-470 and its polymer conjugate, caplostatin. Cancer Cell. 2005;7:251–261. doi: 10.1016/j.ccr.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 73.Yoshida A., Anand-Apte B., Zetter B.R. Differential endothelial migration and proliferation to basic fibroblast growth factor and vascular endothelial growth factor. Growth Factors. 1996;13:57–64. doi: 10.3109/08977199609034566. [DOI] [PubMed] [Google Scholar]
- 74.Kaya M., Wada T., Nagoya S., Kawaguchi S., Yamashita T., Yamamoto N., Yoshimoto M., Okada F., Ishii S. TNP-470 Suppresses the tumorigenicity of HT1080 fibrosarcoma tumor through the inhibition of VEGF secretion from the tumor cells. Sarcoma. 2001;5:197–202. doi: 10.1080/13577140120099182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khakoo A.Y., Finkel T. Endothelial progenitor cells. Annu Rev Med. 2005;56:79–101. doi: 10.1146/annurev.med.56.090203.104149. [DOI] [PubMed] [Google Scholar]
- 76.Gargett C.E. Uterine stem cells: what is the evidence? Hum Reprod Update. 2007;13:87–101. doi: 10.1093/humupd/dml045. [DOI] [PubMed] [Google Scholar]
- 77.Efstathiou J.A., Sampson D.A., Levine Z., Rohan R.M., Zurakowski D., Folkman J., D'Amato R.J., Rupnick M.A. Nonsteroidal antiinflammatory drugs differentially suppress endometriosis in a murine model. Fertil Steril. 2005;83:171–181. doi: 10.1016/j.fertnstert.2004.06.058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Typical set-up for flow cytometry to detect murine EPCs. A: Initial gate used to exclude red cell and platelet debris. B: Selection of CD45-PerCp negative and VEGFR2-PE-positive cells, which encompasses both mature CECs and CEPs. Upper panel showing whole blood (control) from mice and lower panel showing whole blood mixed with the murine endothelial cell line MS-1 cells (control + MS-1). C: CECs are further characterized using CD31-APC and progenitor marker CD133-FITC. D: Isotype controls for CD45-PerCP, VEGFR2-PE, CD31-APC, and CD133-FITC.


