Abstract
The objective to use gene therapy to provide sustained, therapeutic levels of factor VIII (FVIII) for hemophilia A is compromised by the emergence of inhibitory antibodies that prevent FVIII from performing its essential function as a cofactor for factor IX (FIX). FVIII appears to be more immunogenic than FIX and an immune response is associated more frequently with FVIII than FIX gene therapy strategies. We have evaluated a modified lentiviral delivery strategy that facilitates liver-restricted transgene expression and prevents off-target expression in hematopoietic cells by incorporating microRNA (miRNA) target sequences. In contrast to outcomes using this strategy to deliver FIX, this modified delivery strategy was in and of itself insufficient to prevent an anti-FVIII immune response in treated hemophilia A mice. However, pseudotyping the lentivirus with the GP64 envelope glycoprotein, in conjunction with a liver-restricted promoter and a miRNA-regulated FVIII transgene resulted in sustained, therapeutic levels of FVIII. These modifications to the lentiviral delivery system effectively restricted FVIII transgene expression to the liver. Plasma levels of FVIII could be increased to around 9% that of normal levels when macrophages were depleted prior to treating the hemophilia A mice with the modified lentiviral FVIII delivery system.
Introduction
Severely affected patients with hemophilia A have <1% normal levels of circulating factor VIII (FVIII) and it is these patients who would be benefited the most if their disease could be treated with gene therapy. At present, hemophilia patients receive intravenous infusions of FVIII, either prophylactically to prevent spontaneous bleeding episodes or on demand to stop the prolonged bleeding that is characteristic of the disease. Prophylaxis significantly reduces the risk of joint bleeds and thereby prevents the development of joint arthropathy and the associated chronic disability. Unfortunately, the limited availability and high cost of purified FVIII restricts the use of prophylaxis in developed countries and patients in underdeveloped countries have yet to experience the associated benefits. Although replacement therapy is safe, effective and markedly improves both life expectancy and quality of life, it neither eliminates the risk of bleeding nor completely prevents the chronic joint disease. Furthermore, about 30% of patients who receive replacement therapy eventually mount an immune response against the infused FVIII and the anti-FVIII antibodies (inhibitors) that develop block the procoagulant activity of the FVIII and render the therapy ineffective.1 Currently the development of inhibitors in hemophilia patients is the most significant complication associated with replacement therapy. The risk of developing inhibitors correlates with the type of mutation in the F8 gene, as well as a family history of inhibitor development.2,3 Although, severely affected patients have the highest risk of developing inhibitors, this complication only occurs in a fraction of patients because there are other genetic and nongenetic factors that affect how the infused FVIII interacts with the patient's immune system and influence the risk of antibody development.4
FVIII gene delivery could be the next generation of therapy for hemophilia A if sustained therapeutic levels (>1% of normal) of FVIII could be achieved. This treatment strategy could be more beneficial than FVIII concentrate prophylaxis as it could provide a sustained protective level of clotting factor and would thus minimize the risk of bleeding and prevent chronic joint arthropathy. At first glance, this does not appear to be a difficult task since the normal concentration of FVIII in the plasma is only between 100 and 200 ng/ml. Yet despite significant advances in transgene and vector design, as well as improved transgene delivery strategies, preclinical gene therapy studies have been plagued by low plasma levels of FVIII in treated animals. FVIII, in and of itself, may contribute to this problem since cells do not normally express high levels of FVIII. One reason for this is the requirement for chaperone-mediated folding of FVIII in the endoplasmic reticulum. Furthermore, the endoplasmic reticulum has a complex system that matches protein-folding capacity to protein load, and increases in cellular expression of FVIII activate the unfolded protein response which causes the cell to die by apoptosis.5 A far more serious impediment to long-term expression of FVIII after FVIII gene delivery is the development of inhibitors. FVIII is inherently immunogenic, and by all measures it is more immunogenic than FIX. The incidence of inhibitors is higher in hemophilia A patients (~30%) than in hemophilia B patients (~3%).6 Furthermore, inhibitors are more frequently associated with FVIII than FIX gene delivery. In fact, identical gene delivery strategies that result in long-term expression of FIX in a hemophilia B animal model have not been successful in animal models of hemophilia A because of the anti-FVIII immune response.7,8,9,10,11
Ex vivo cell-based and systemic delivery strategies using a variety of viral vectors have been investigated for FVIII gene therapy and to date, no strategy has emerged as clearly superior. Numerous preclinical studies have only led to two clinical trials of FVIII gene transfer that have yielded limited clinical efficacy.12,13 Interest in lentiviral vector delivery systems has increased over the past several years in part because advances in vector design have enhanced their safety and made them more effective gene delivery systems.14,15 These vectors can transduce a wide array of terminally differentiated cells and, unlike adenoviral or adeno-associated viral vectors, are not compromised by pre-existing immunity. Recently, lentiviral vectors have entered clinical testing in applications of ex vivo hematopoietic cell therapy16,17 and direct central nervous system delivery (http://www.oxfordbiomedica.co.uk/page.asp?pageid=29). The use of lentiviral vectors to deliver the FVIII transgene has shown promising results when delivered systemically to neonatal hemophilia A mice18 or when used in ex vivo strategies.19,20,21 However, systemic delivery of the FVIII transgene via lentiviral vectors into adult hemophilia mice has been complicated by the emergence of neutralizing anti-FVIII antibodies.11,22 This is in contrast to the prolonged, albeit low level of FIX expression observed after systemic delivery of lentiviral vectors containing the FIX transgene into wild-type mice.23 In this latter study, the anti-FIX immune response was overcome by targeting transcription to hepatocytes with the use of a liver-restricted promoter. However, treatment of hemophilia B mice with the same strategy resulted in anti-FIX antibodies.24 Based on speculation that the loss of FIX expression resulted from off-target transgene expression in the antigen presenting cells of mice and, consequently, induction of neutralizing immune response in mice immunologically naive to FIX, an additional layer of post-transcriptional control was added to the FIX trangene cassette that was mediated by target sequences (mir142) that have perfect complimentarily to the hematopoietic-specific microRNA (miRNA), miR-142-3p.25 By this approach, transgene expression is specifically suppressed in hematopoietic lineage cells without being affected in the hepatocytes, and long-term stable FIX expression and tolerance were established in hemophilia B mice.
Given the discrepancy in the observed immune responses between FVIII and FIX after transgene delivery, with the enhanced immunogenicity of FVIII, we wanted to validate this novel strategy in hemophilia A mice. Here we describe modifications that were made to the transgene cassette, as well as to the lentiviral delivery system that led to long-term expression of FVIII in the murine model of hemophilia A.
Results
Targeting FVIII transgene expression to hepatocytes does not result in long-term expression of FVIII
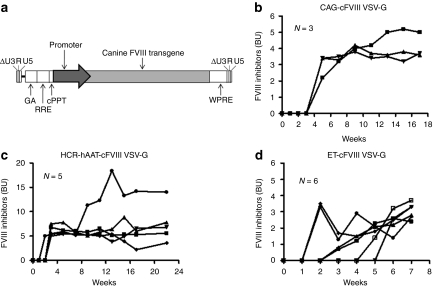
We engineered a lentiviral vector that encodes the B-domain–deleted canine FVIII (cFVIII) transgene under the control of the nonviral, ubiquitous CAG promoter (Figure 1a). Adult, 4–8-week-old, hemophilia A C57BL/6 mice were treated with 1 × 109 IU/mouse via a tail-vein injection and plasma samples were monitored on a weekly basis for FVIII and anti-FVIII inhibitors. Since an objective of this study was to evaluate whether or not modification made to the FVIII transgene could prevent the development of anti-FVIII inhibitors, the appearance of inhibitors in a minimum of 2 treated mice resulted in termination of the evaluation of the specific transgene. Functional FVIII (FVIII:C) could not be detected in any of the three treated mice over the 12-week duration of the study (data not shown) and this lack of expression can be attributed to circulating anti-FVIII inhibitory antibodies that became detectable at 5 weeks post-treatment (Figure 1b). Since our group and others have shown that restricting lentiviral transgene expression to hepatocytes is not associated with inhibitory antibodies,18,23 we substituted the CAG promoter with the hepatocyte-restricted, HCR-hAAT, promoter and infused 1 × 109 IU/mouse via the tail-vein into adult hemophilia A mice. Unlike our previous study that delivered this lentiviral vector to neonatal hemophilia A mice, we saw no evidence of FVIII:C when adult mice were treated with vesicular stomatitis virus glycoprotein (VSV-G) pseudotyped Lenti-HCR-hAAT-cFVIII (data not shown). Once again the lack of FVIII:C resulted from an anti-FVIII immune response (Figure 1c). Previously, we observed that the HCR-hAAT promoter does not completely restrict transgene expression to hepatocytes and in fact some off-target expression was observed in the spleen.26 We wanted to investigate the use of a synthetic promoter expressly designed to improve the specificity, as well as the levels of transgene expression and for that reason we switched the HCR-hAAT promoter with the stronger, liver-specific ET promoter.27 However, although the levels of anti-FVIII inhibitors were marginally lower (Figure 1d), we saw no evidence of FVIII:C in the plasma of the treated mice (data not shown).
Figure 1.
Lentiviral delivery of the canine factor VIII (cFVIII) transgene to hemophilia A mice. (a) Schematic representation of the third-generation, self-inactivating lentiviral vector containing the cFVIII transgene under the control of either a ubiquitous or liver-restricted promoters. The lentivirus was pseudotyped with the vesicular stomatitis virus glycoprotein (VSV-G) envelope glycoprotein. (b-d) Levels of FVIII inhibitors as measured by a Bethesda Assay in hemophilia A mice treated with 1 × 109 IU/mouse of lentiviral vector that contained the cFVIII transgene driven by the (b) ubiquitous CAG or (c) liver-restricted HCR-hAAT or (d) ET promoters. Results are presented as individual Bethesda units (BUs).
Incorporating a miRNA target sequence to prevent expression in antigen presenting cells does not prevent an anti-FVIII immune response
miRNAs are post-transcriptional regulators that silence gene expression by binding to complementary sequence elements found most often in the 3′ untranslated regions of their target mRNAs.28 Incorporating miRNA target sequences specific for a hematopoietic-specific miRNA into the 3′-untranslated region of the FIX transgene cassette was very effective in preventing off-target transgene expression in hematopoietic cells29 and thereby eliminating the anti-FIX immune response.24 We wanted to investigate this strategy in hemophilia A mice and accordingly incorporated four copies of the mir142 target sequence in the 3′-untranslated region of the cFVIII transgene in our three lentiviral constructs (Figure 2a). Individual hemophilia A mice in three different cohorts were treated with intravenous infusions of 1 × 109 IU/mouse of each of the three different lentiviral vectors and plasma FVIII:C levels and anti-FVIII inhibitor titers were monitored on a weekly basis. No FVIII:C was detected in any of the mice and as the results presented in Figure 2b–d show, all treated mice in each of the three cohorts developed anti-FVIII inhibitory antibodies between 4 and 6 weeks after treatment.
Figure 2.
Incorporating a microRNA-target sequence into the lentivector does not prevent an anti-factor VIII (FVIII) immune response. (a) Schematic representation of the canine FVIII (cFVIII)-encoding lentiviral vectors that contain four tandem copies of the mir-142-3p target sequence (mir-142-3pT). The target sequences were inserted directly adjacent to the WPRE. (b–d) Levels of FVIII inhibitors as measured by a Bethesda Assay in hemophilia A mice treated with 1 × 109 IU/mouse of the vesicular stomatitis virus glycoprotein (VSV-G) pseudotyped lentivector that contained the cFVIII transgene driven by the (b) ubiquitous CAG or (c) liver-restricted HCR-hAAT or (d) ET promoters. Results are presented as individual Bethesda units (BUs).
Incorporation of mir142 binding sites and the use of a liver-specific promoter do not restrict transgene expression to the liver
We wanted to investigate whether or not the use of the hepatocyte-restricted ET promoter, with or without the mir142 target sequences was sufficient to restrict transgene expression to the liver. Livers and spleens were isolated from mice treated with Lenti-ET-cFVIII and Lenti-ET-cFVIII-mir142 and lentiviral integration was assessed by quantitative real-time PCR and FVIII transgene expression was quantified by reverse transcription-PCR analysis. Our results indicate that VSV-G-pseudotyped lentiviral vectors transduced cells within the liver and the spleen (Figure 3a) and that the use of the ET promoter alone does not restrict expression to the liver as cFVIII mRNA (320 copies/µg) was detected in the spleen (Figure 3b). It is known that lentivectors transduce hematopoietic lineage cells, such as liver and spleen antigen-presenting cells upon in vivo administration23,30,31,32 and this triggers a type 1 interferon response33 that undoubtedly contributed to the anti-FVIII immune response. However, although incorporating the mir142 target sequences to prevent expression in hematopoietic cells reduced cFVIII expression from 2,521 copies/µg to 1,852 copies/µg in the liver and from 320 copies/µg to 89 copies/µg in the spleen (Figure 3b), it did not restrict expression to the liver and more importantly did not prevent the anti-FVIII immune response.
Figure 3.
Assessment of vesicular stomatitis virus glycoprotein (VSV-G) pseudotyped lentivector integration and canine factor VIII (cFVIII) transgene expression. Adult hemophilia A mice each received 1 × 109 IU of either Lenti-ET-cFVIII or Lenti-ET-cFVIII-mir142 via tail-vein injection. At the termination of the experiment mice were euthanized and livers and spleens were obtained for isolation of DNA and total RNA. (a) DNA was used to assess lentiviral integration by quantitative real-time PCR. The results are presented as mean vector copies/cell plus or minus the standard deviation. (b) cFVIII transgene expression levels were assessed by quantitative reverse transcription-PCR and the results are presented as copies/µg plus or minus the standard deviation.
A GP64-pseudotyped lentivector in conjunction with a liver-restricted promoter and mir142 target sequence results in long-term expression of cFVIII
VSV-G-pseudotyped lentivectors are pantropic and this broad tropism can be problematic for gene therapy. Although incorporating the mir142 target sequence prevents transgene expression in transduced hematopoietic cells, it does not prevent expression in endothelial or stromal cells, both of which are transduced by VSV-G-pseudotyped lentivectors. A number of different envelope glycoproteins have been used with variable success to alter lentiviral tropism or to enhance transduction of hepatocytes.34,35,36,37 Among several envelope pseudotypes reported, the GP64 glycoprotein from baculovirus has been shown to exhibit enhanced tropism for hepatocytes in vivo,34,38 restricts the tropism away from hematopoietic cells,38 and tolerates centrifuge concentration similar to VSV-G.39 Therefore, each of the lentiviral constructs that either contain the ubiquitous CAG, liver-restricted HCR-hAAT or ET promoters and that either have or do not have the mir142 target sequences were pseudotyped with the GP64 glycoprotein and 1 × 109 IU/mouse were delivered to adult hemophilia A mice. FVIII inhibitors developed in mice that received the FVIII transgene regulated by the ubiquitous CAG promoter (Figure 4a) and addition of the mir142 target sequence failed to prevent an anti-FVIII immune response (Figure 5a). Replacing the ubiquitous promoter with either the liver-restricted HCR-hAAT or ET promoters also failed to prevent the anti-FVIII immune response (Figure 4b,c). However, no inhibitory anti-FVIII antibodies were seen in any of the hemophilic mice that received the GP64-pseudotyped lentiviral vectors that contained the FVIII transgene regulated by either the HCR-hAAT or ET promoters, as well as the mir142 target sequence (Figure 5a,b). The results presented in Figure 4a indicate that pseudotyping the lentivectors with GP64 is, in of itself insufficient to prevent the anti-FVIII immune response. Furthermore, restricting transgene expression to hepatocytes with the use of either the HRC-hAAT or the ET promoter, in conjunction with a GP64-pseudotyped virus is also insufficient to prevent the development of FVIII inhibitory antibodies (Figure 4b,c). Levels of FVIII expression in the mice that received the cFVIII regulated by the HCR-hAAT or ET promoter were 2% and 4% of normal FVIII levels.
Figure 4.
Pseudotyping the lentivectors with the GP64 envelope glycoprotein does not prevent the development of factor VIII (FVIII) inhibitors. The lentiviral vectors were pseudotyped with the GP64 envelope glycoprotein and hemophilia A mice were treated with 1 × 109 IU/mouse. The canine FVIII (cFVIII) transgene was regulated by either (a) a ubiquitous CAG promoter, or (b) liver-restricted HCR-hAAT or (c) ET promoters. Levels of FVIII inhibitors were measured by a Bethesda Assay and results are presented as individual Bethesda units (BUs).
Figure 5.
A GP64-pseudotyped lentivector in which canine factor VIII (cFVIII) transgene expression is regulated by both a liver-restricted promoter and a microRNA target sequence results in long-term expression of cFVIII. Adult hemophilia A mice were treated with 1 × 109 IU/mouse of the GP64-pseudotyped lentivector that contained cFVIII transgene as well as the mir-142 target sequence. cFVIII expression was regulated by either (a) the ubiquitous CAG promoter, or (b) the liver-restricted HCR-hAAT or (c) ET promoters. Levels of FVIII activity (mU/ml) and FVIII inhibitors (BU) were measured by a chromogenic bioassay and a Bethesda Assay respectively. Results are presented for individual mice.
Restricting cFVIII transgene expression to the liver facilitates long-term expression of cFVIII
An ever-increasing number of studies demonstrate that liver-directed gene transfer can result in long-term expression of the transgene products.40,41,42 In order to investigate if the modifications that were made to the lentiviral vector directed liver-restricted transgene expression, livers and spleens, were isolated from mice treated with the GP64-pseudotyped lentivirus that contained the ET-cFVIII and the ET-cFVIII-mir142 transgenes and lentiviral integration and cFVIII mRNA expression were assessed. The results presented in Figure 6a show that there are no differences in the levels of lentiviral integration in any of the organs examined between the transgene cassette that contained the mir142 target sequences and the cassette that did not. Although expression of cFVIII was substantially higher in the liver, cFVIII mRNA was detected in spleen in mice treated with the GP64-pseudotyped virus in which transgene expression was regulated only by the ET promoter. However, expression of FVIII mRNA was only seen in the liver in the mice treated with the GP64-pseudotyped lentivirus that contained the cFVIII transgene regulated by the liver-restricted ET promoter and the mir142 target sequence. Although these results do not provide conclusive evidence that transgene expression is restricted exclusively to hepatocytes, they do indicate that directing transgene delivery to the liver in immunocompetent hemophilia A mice facilitates long-term expression of FVIII without the development of inhibitory antibodies.
Figure 6.
A GP64-pseudotyped lentiviral vector in conjuction with a liver-restricted promoter and a microRNA target sequence, restricts canine factor VIII (cFVIII) transgene expression to the liver. Adult hemophilia A mice each received 1 × 109 IU of GP64 pseudotyped Lenti-ET-cFVIII or Lenti-ET-cFVIII-mir142 via tail vein injection. Livers and spleens were obtained at the termination of the experiment and DNA and total RNA were isolated. (a) Lentiviral integration was assessed by quantitative real-time PCR and the results are presented as mean vector copies/cell plus or minus the standard deviation. (b) cFVIII transgene expression levels were assessed by quantitative reverse transcription-PCR and the results are presented as copies/µg plus or minus the standard deviation.
Macrophage depletion results in increased levels of plasma FVIII in lentivector-treated hemophilia A mice
Lentiviral vectors are preferentially sequestered by macrophages and this presumably reduces the levels of vector available to transduce hepatocytes.43 Although we observed therapeutic levels of FVIII expression in the treated mice described above, we wanted to determine whether these levels could be increased by depleting macrophages. Mice were treated with clodronate for macrophage depletion and received the GP64-pseudotyped lentiviral vector containing either the ET-cFVIII or the ET-cFVIII-mir142 transgene cassette. The results show that despite the depletion of macrophages, the mir142 target sequence is essential to prevent the formation of inhibitors in the treated mice (Figure 7a) However when the mir142 target sequence is included in the trangene cassette, levels of FVIII expression were substantially higher (Figure 7b) than in mice that did not receive clodronate (refer to Figure 5c). Furthermore, these elevated levels of around 9% persisted over the 62-week duration of the study.
Figure 7.
Depletion of macrophages prior to lentivector administration enhances levels of factor VIII (FVIII) expression. Macrophages were transiently depleted from hemophilia A mice with clodronate prior to infusing 1 × 109 IU of the GP64-pseudotyped lentivectors. The vectors encoded either the canine FVIII (cFVIII) transgene regulated (a) by the ET promoter or (b) by the ET promoter in conjuction with the miRNA-142 target sequences. Levels of FVIII activity were measured by a chromogenic bioassay and results are presented as mU/ml and are shown for individual mice.
Discussion
The use of lentiviral-based vectors in gene therapy strategies for hemophilia has several attractive features. In humans, the prevalence of pre-existing immunity to these viruses is low, although natural immunity to some viral envelope proteins used to pseudotype the vectors has been reported and may affect vector stability upon in vivo delivery;44 the vector can readily accommodate the size of the B-domain–deleted FVIII transgene and can transduce nondividing cells such as hepatocytes where the transgene is integrated into the genome for sustained expression of FVIII. There are also drawbacks of this delivery system, not least of which center on genotoxicity concerns associated with insertional mutagenesis and adverse outcomes resulting from an adaptive immune response against the transgene product. However, there is optimism that these concerns are being addressed by continued improvements in vector engineering. A model system used to study vector genotoxicity reported apparent enhanced safety for the self-inactivating generation of lentiviral vectors45 and encouraging results have emerged from the first clinical trial using lentiviral vectors.17 Furthermore, strategies that impose additional tissue-specific layers of regulation to avoid transgene expression in hematopoietic cells and prevent the adaptive immune response against the transgene product are evolving.24
In general, an immune response against the transgene product is more frequently associated with the delivery of the FVIII than the FIX transgene and therefore, it was not entirely unexpected that a strategy that prevents the rapid clearance of FIX gene-modified cells,24 was unable to prevent the anti-FVIII immune response in our current study. One reason for this may be differences between FVIII and FIX in the requirement to prevent transgene expression in the spleen. When a VSV-G-pseudotyped virus was used and transgene expression was regulated by a liver-restricted promoter in conjunction with the mir142 target sequence, expression was observed in the marginal zone of the spleen.24 Although this off-target transgene expression did not cause an anti-FIX immune response, our data indicates that preventing FVIII transgene expression in the spleen is critical to facilitating long-term expression without the complication of inhibitor development. Consequently, redirecting the tropism of the lentiviral delivery system with the use of the GP64 envelope glycoprotein and presumably preventing transduction of cells within the marginal zone of the spleen prevented the anti-FVIII immune response. Further studies are needed to identify the cell-type within the spleen that is transduced by the VSV-G-pseudotyped lentivirus and the mechanisms by which this cell initiates the anti-FVIII immune response. It is also important to consider that the lack of an anti-FVIII immune response associated with the GP64-pseudotyped lentivirus in combination with the liver-restricted promoter and the mir142 target sequence may in large part be simply due to restricting transgene expression to the liver. Many delivery strategies target the liver for transgene expression, in large part because of its capacity to synthesize proteins and secrete them into the blood stream. However, in addition, there is growing evidence that restricting transgene expression to hepatocytes is associated with the induction of transgene product-specific immune tolerance,40 and it appears that this tolerance is mediated by FoxP3+ T regulatory cells.41,46 Furthermore, there is strong evidence to indicate that FoxP3+ T regulatory cells are important in maintaining long-term immunological tolerance to FVIII after lentiviral delivery.18 Additional studies will be required to determine whether the hemophilia A mice treated in our current study are indeed tolerant to FVIII and, if so, the mechanisms that mediate this tolerance.
A GP64-pseudotyped FIV-based lentiviral vector has been used to deliver the human FVIII transgene to hemophilia A mice and long-term expression of FVIII was observed.34 It is interesting to note that the FIV-based delivery system only required the liver-restricted promoter and not the mir142 target sequence to prevent the development of anti-FVIII inhibitors. HIV-based lentiviral vectors, whether pseudotyped with VSV-G or GP64, induce a strong but transient type I interferon response and it is presumably this early innate response that contributes to the anti-FVIII immune response. Given that the type I interferon response occurs regardless of which envelope glycoprotein is used, the response is likely a function of other vector components.33 Unfortunately, no similar studies have been carried out to assess the host innate immune response directed against FIV-based lentiviral vectors and therefore the reasons for the different outcomes remain unclear. However, it is unlikely that it can be attributed to differences in immunogenicity between human and cFVIII transgene products as we have used the HIV-based lentiviral vector to deliver both human and cFVIII transgenes to hemophilia A mice and the levels of FVIII inhibitors were higher in the mice treated with the human transgene (data not shown). Furthermore, given that both the FIV- and HIV-based vectors were pseudotyped with GP64, it is unlikely that differences in vector tropism account for the different outcomes, since the tropism is largely governed by the interactions between the envelope glycoprotein and its host cell receptor.
We have treated hemophilia A mice as neonates with a VSV-G-pseudotyped lentiviral vector containing the cFVIII transgene that is only under the regulation of a liver-restricted promoter. As adults, these mice were tolerant to FVIII and the FVIII tolerance was mediated by FoxP3+ T regulatory cells (Tregs).18 We also observed much higher levels of FVIII expression in these mice. There are two aspects of treating mice as neonates that account for these differences when compared to that seen in mice treated as adults. Most importantly, in contrast to mice that are treated as adults, neonatal therapy involves vector administration at a time when the immune system is immature and when central tolerance to neoantigens is readily established. Second, in mice that are treated as neonates with lentiviral vectors, the FVIII transgene integrates into the host's genome and as the neonate grows, expansion of the transduced, FVIII-expressing hepatocytes occurs and accounts for the higher levels of FVIII expression observed in the mature mice. When adult mice are treated with lentiviral vectors lower levels of FVIII are observed because of a lack of hepatocyte expansion. In both of these studies, as well as in normal dogs, levels of FVIII mRNA appear to be quite low relative to the amount of FVIII protein detected in the plasma. Presumably this discrepancy is a reflection of the observed stability of the cFVIII mRNA. In adult mice, macrophages, and in particular Kuppfer cells, rapidly sequester the administered virus and reduce the levels of vector available for transduction of hepatocytes. As a proof of concept, we have demonstrated that depletion of macrophages, prior to administering vector results in increased levels of plasma FVIII. This prior removal of macrophages has no effect on the overall anti-FVIII immune response (Figure 7a). This is in large part because lentiviral vectors induce type I interferon production only from plasmacytoid but not myeloid dendritic cells or macrophages and it is the production of the type I interferon that facilitates the initiation of the anti-FVIII adaptive immune response.33
In conclusion, the results of this study emphasize the distinct nature of specific transgene products with regards to their immunogenic potential. In this instance, the inherently immunogenic nature of FVIII required a combination strategy to prevent transgene expression in the spleen and target long-term expression in the liver. In contrast, previous studies have indicated that this level of restriction will not be necessary for other transgenes, for which the likelihood of an adverse immune response is far less likely.
Materials and Methods
Lentiviral vector construction. The B-domain–deleted cFVIII transgene was chosen for use in these studies as the long-term objective is to evaluate the efficacy of the lentiviral delivery system in the canine model of hemophilia A. The lentiviral backbone plasmid, plenti-cytomegalovirus (CMV)-cFVIII, containing the cFVIII transgene and has previously been described.19 The plasmid constructs pRRLsinCAG.hF8pptpre, pRRLsin.ppt.Enh1mTTR.eGFP.Wpre and pBlue.mir-142as.T1 were used to isolate the CAG and ET promoters and the mir142 target sequences, respectively. The CAG promoter contains the CMV immediate early enhancer and the chichen β-actin promoter47 and the ET promoter consists of a synthetic hepatocyte-specific enhancer fused to the murine transthyretin promoter.24 To prevent transgene expression in hematopoietic cells,29 four copies of the micro-RNA142-3pT (mir142) target sequence were excised from pBlue.mir-142as.T1 with SalI and XhoI, blunt ends were created with DNA polymerase I Klenow fragment and the 112 basepair fragment was ligated into the SmaI digested plenti-CMV-cFVIII and HCR-hAAT-cFVIII backbones to generate plenti-CMV-cFVIII-mir142 and plenti-HCR-hAAT-cFVIII-mir142 respectively. The HCR-hAAT promoter consists of the human apolipoprotein locus control region (HCR) and the human α1-anti-trypsin (hAAT) promoter. The four copies of the mir142 target sequences are situated in the transgene 3′-untranslated region downstream to the WPRE. To engineer plenti-CAG-cFVIII and plenti-CAG-cFVIII-mir142, the ClaI/XbaI fragment containing the CAG promoter was removed from pRRLsinCAG.hF8pptpre and ligated into the similarly digested plenti-CMV-cFVIII and plenti-CMV-cFVIII-mir142 plasmids. The ET promoter was amplified from pRRLsin.ppt.Enh1mTTR.eGFP.Wpre using 5′ and 3′ primers that contained NarI and a NheI cleavage sites respectively. After NarI and NheI digestion, the purified fragment was ligated into ClaI/XbaI digested plenti-CMV-cFVIII and the plenti-CMV-cFVIII-mir142 plasmids to generate plenti-ET-cFVIII and plenti-ET-cFVIII-mir142, respectively. The integrity of all new constructs was confirmed by sequence analysis.
Viral vector production and vector titering. Lentiviral vectors were pseudotyped with either VSV-G or GP64. GP64 is the envelope glycoprotein from baculovirus Autographa californica. The plasmid used to generate GP64-pseudotyped vector has already been described.34 Lentiviral vectors were produced by co-transfecting 293T cells with the two packaging plasmids (GAG/Pol and REV) along with the vector and envelope (VSV-G or GP64) plasmids and the functional titer for each viral preparation was determined as previously outlined.19 To avoid overestimating the viral titer that results from residual plasmid contamination, all viral preparations were treated with DNase prior to determining the functional titer. On average viral titers ranged between 5 × 108 and 2 × 109 IU/ml. An endotoxin assay was carried out on each of the lentiviral preparations using the QCL-1000 test kit (Biowhittaker, Walkersville, MD) according to the manufacturer's recommendations and in general endotoxin contamination levels were found to be <7 EU/ml.
Animal procedures. The hemophilia A mice used in this study are C57BL/6 strain in which the FVIII gene has been disrupted by insertion of the neomycin gene into Exon 16.48 Unanesthetized 6- to 8-week-old mice received a total lentivector dose of 1 × 109 IU/mouse, delivered via two tail vein injections over 2 consecutive days. Peripheral vein blood samples were collected from the saphenous vein. All animal procedures were reviewed and approved by the Queen's University Animal Care Committee.
Assays for FVIII activity and neutralizing anti-FVIII antibodies. Blood samples were collected on a weekly basis and a chromogenic bioassay (DiaPharma, West Chester, OH) was used to measure plasma cFVIII functional activity as previously described.19 Normal pooled canine plasma was used to establish the FVIII standard curve and the sensitivity of this assay is 10 mU/ml. Anti-FVIII inhibitory antibodies were measured by the Bethesday assay as previously described.19
Quantification of vector copy number and cFVIII mRNA expression. At the end of each study, mice were euthanized by CO2 inhalation and organs were collected. DNA was extracted and the lentiviral integrated copy number was determined as previously described.25 The β-actin gene was used to normalize for cell numbers. To assess cFVIII transgene expression, RNA was isolated from the harvested organs using TRIzol (Invitrogen, Carlsbad, CA) and treated with RNase-free DNase. In vivo cFVIII mRNA was measured by quantitative reverse transcription-PCR as previously described.19
Macrophage depletion. Clodronate, a gift from Roche Diagnostics GmbH (Mannheim, Germany) was used to transiently deplete macrophages from mice. Mice received 200 µl per 20 g body weight of the standard suspension (0.25g/ml) of clodronate liposomes diluted in phosphate buffered saline via intravenous injection. Two infusions of clodronate were administered, the first was 24 hours pre and the second was 24 hours postviral infusion.
Acknowledgments
This research was funded by an operating grant from the Canadian Institutes of Health Research (Ottawa, ON; MOP-10912) and the Bayer/Canadian Blood Services Partnership Fund. At the time of the study H.M. held an Industry-Partnered Fellowship with the Canadian Institutes of Health Research. D.L. holds a Canada Research Chair in Molecular Hemostasis.
REFERENCES
- Wight J., and, Paisley S. The epidemiology of inhibitors in haemophilia A: a systematic review. Haemophilia. 2003;9:418–435. doi: 10.1046/j.1365-2516.2003.00780.x. [DOI] [PubMed] [Google Scholar]
- Astermark J, Oldenburg J, Escobar M, White GC., 2nd, and, Berntorp E. The Malmö International Brother Study (MIBS). Genetic defects and inhibitor development in siblings with severe hemophilia A. Haematologica. 2005;90:924–931. [PubMed] [Google Scholar]
- Boekhorst J, Lari GR, D'Oiron R, Costa JM, Nováková IR, Ala FA.et al. (2008Factor VIII genotype and inhibitor development in patients with haemophilia A: highest risk in patients with splice site mutations Haemophilia 14729–735. [DOI] [PubMed] [Google Scholar]
- Astermark J, Altisent C, Batorova A, Diniz MJ, Gringeri A, Holme PA.et al. (2010Non-genetic risk factors and the development of inhibitors in haemophilia: a comprehensive review and consensus report Haemophilia 16747–766. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW.et al. (2008Antioxidants reduce endoplasmic reticulum stress and improve protein secretion Proc Natl Acad Sci USA 10518525–18530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimichele D. The North American Immune Tolerance Registry: contributions to the thirty-year experience with immune tolerance therapy. Haemophilia. 2009;15:320–328. doi: 10.1111/j.1365-2516.2008.01880.x. [DOI] [PubMed] [Google Scholar]
- Ye P, Thompson AR, Sarkar R, Shen Z, Lillicrap DP, Kaufman RJ.et al. (2004Naked DNA transfer of Factor VIII induced transgene-specific, species-independent immune response in hemophilia A mice Mol Ther 10117–126. [DOI] [PubMed] [Google Scholar]
- Ye X, Loeb KR, Stafford DW, Thompson AR., and, Miao CH. Complete and sustained phenotypic correction of hemophilia B in mice following hepatic gene transfer of a high-expressing human factor IX plasmid. J Thromb Haemost. 2003;1:103–111. doi: 10.1046/j.1538-7836.2003.00024.x. [DOI] [PubMed] [Google Scholar]
- Xu L, Mei M, Ma X., and, Ponder KP. High expression reduces an antibody response after neonatal gene therapy with B domain-deleted human factor VIII in mice. J Thromb Haemost. 2007;5:1805–1812. doi: 10.1111/j.1538-7836.2007.02629.x. [DOI] [PubMed] [Google Scholar]
- Xu L, Mei M, Haskins ME, Nichols TC, O'donnell P, Cullen K.et al. (2007Immune response after neonatal transfer of a human factor IX-expressing retroviral vector in dogs, cats, and mice Thromb Res 120269–280. [DOI] [PubMed] [Google Scholar]
- Park F, Ohashi K., and, Kay MA. Therapeutic levels of human factor VIII and IX using HIV-1-based lentiviral vectors in mouse liver. Blood. 2000;96:1173–1176. [PubMed] [Google Scholar]
- Powell JS, Ragni MV, White GC., 2nd, , Lusher JM, Hillman-Wiseman C, Moon TE.et al. (2003Phase 1 trial of FVIII gene transfer for severe hemophilia A using a retroviral construct administered by peripheral intravenous infusion Blood 1022038–2045. [DOI] [PubMed] [Google Scholar]
- Roth DA, Tawa NE, Jr, O'Brien JM, Treco DA., and, Selden RF. Nonviral transfer of the gene encoding coagulation factor VIII in patients with severe hemophilia A. N Engl J Med. 2001;344:1735–1742. doi: 10.1056/NEJM200106073442301. [DOI] [PubMed] [Google Scholar]
- Vigna E., and, Naldini L. Lentiviral vectors: excellent tools for experimental gene transfer and promising candidates for gene therapy. J Gene Med. 2000;2:308–316. doi: 10.1002/1521-2254(200009/10)2:5<308::AID-JGM131>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Mátrai J, Chuah MK., and, VandenDriessche T. Recent advances in lentiviral vector development and applications. Mol Ther. 2010;18:477–490. doi: 10.1038/mt.2009.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine BL, Humeau LM, Boyer J, MacGregor RR, Rebello T, Lu X.et al. (2006Gene transfer in humans using a conditionally replicating lentiviral vector Proc Natl Acad Sci USA 10317372–17377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I.et al. (2009Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy Science 326818–823. [DOI] [PubMed] [Google Scholar]
- Matsui H, Shibata M, Brown B, Labelle A, Hegadorn C, Andrews C.et al. (2009A murine model for induction of long-term immunologic tolerance to factor VIII does not require persistent detectable levels of plasma factor VIII and involves contributions from Foxp3+ T regulatory cells Blood 114677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H, Shibata M, Brown B, Labelle A, Hegadorn C, Andrews C.et al. (2007Ex vivo gene therapy for hemophilia A that enhances safe delivery and sustained in vivo factor VIII expression from lentivirally engineered endothelial progenitors Stem Cells 252660–2669. [DOI] [PubMed] [Google Scholar]
- Van Damme A, Thorrez L, Ma L, Vandenburgh H, Eyckmans J, Dell'Accio F.et al. (2006Efficient lentiviral transduction and improved engraftment of human bone marrow mesenchymal cells Stem Cells 24896–907. [DOI] [PubMed] [Google Scholar]
- Tiede A, Eder M, von Depka M, Battmer K, Luther S, Kiem HP.et al. (2003Recombinant factor VIII expression in hematopoietic cells following lentiviral transduction Gene Ther 101917–1925. [DOI] [PubMed] [Google Scholar]
- Kootstra NA, Matsumura R., and, Verma IM. Efficient production of human FVIII in hemophilic mice using lentiviral vectors. Mol Ther. 2003;7 5 Pt 1:623–631. doi: 10.1016/s1525-0016(03)00073-x. [DOI] [PubMed] [Google Scholar]
- Follenzi A, Battaglia M, Lombardo A, Annoni A, Roncarolo MG., and, Naldini L. Targeting lentiviral vector expression to hepatocytes limits transgene-specific immune response and establishes long-term expression of human antihemophilic factor IX in mice. Blood. 2004;103:3700–3709. doi: 10.1182/blood-2003-09-3217. [DOI] [PubMed] [Google Scholar]
- Brown BD, Cantore A, Annoni A, Sergi LS, Lombardo A, Della Valle P.et al. (2007A microRNA-regulated lentiviral vector mediates stable correction of hemophilia B mice Blood 1104144–4152. [DOI] [PubMed] [Google Scholar]
- Chen CZ, Li L, Lodish HF., and, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- Brown BD, Shi CX, Rawle FE, Tinlin S, McKinven A, Hough C.et al. (2004Factors influencing therapeutic efficacy and the host immune response to helper-dependent adenoviral gene therapy in hemophilia A mice J Thromb Haemost 2111–118. [DOI] [PubMed] [Google Scholar]
- Vigna E, Amendola M, Benedicenti F, Simmons AD, Follenzi A., and, Naldini L. Efficient Tet-dependent expression of human factor IX in vivo by a new self-regulating lentiviral vector. Mol Ther. 2005;11:763–775. doi: 10.1016/j.ymthe.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BD, Venneri MA, Zingale A, Sergi Sergi L., and, Naldini L. Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer. Nat Med. 2006;12:585–591. doi: 10.1038/nm1398. [DOI] [PubMed] [Google Scholar]
- VandenDriessche T, Thorrez L, Naldini L, Follenzi A, Moons L, Berneman Z.et al. (2002Lentiviral vectors containing the human immunodeficiency virus type-1 central polypurine tract can efficiently transduce nondividing hepatocytes and antigen-presenting cells in vivo Blood 100813–822. [DOI] [PubMed] [Google Scholar]
- Pan D, Gunther R, Duan W, Wendell S, Kaemmerer W, Kafri T.et al. (2002Biodistribution and toxicity studies of VSVG-pseudotyped lentiviral vector after intravenous administration in mice with the observation of in vivo transduction of bone marrow Mol Ther 619–29. [DOI] [PubMed] [Google Scholar]
- Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage FH.et al. (1996In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector Science 272263–267. [DOI] [PubMed] [Google Scholar]
- Brown BD, Sitia G, Annoni A, Hauben E, Sergi LS, Zingale A.et al. (2007In vivo administration of lentiviral vectors triggers a type I interferon response that restricts hepatocyte gene transfer and promotes vector clearance Blood 1092797–2805. [DOI] [PubMed] [Google Scholar]
- Kang Y, Xie L, Tran DT, Stein CS, Hickey M, Davidson BL.et al. (2005Persistent expression of factor VIII in vivo following nonprimate lentiviral gene transfer Blood 1061552–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie TC, Kobinger GP, Kootstra NA, Radu A, Sena-Esteves M, Bouchard S.et al. (2002Efficient transduction of liver and muscle after in utero injection of lentiviral vectors with different pseudotypes Mol Ther 6349–358. [DOI] [PubMed] [Google Scholar]
- Kang Y, Stein CS, Heth JA, Sinn PL, Penisten AK, Staber PD.et al. (2002In vivo gene transfer using a nonprimate lentiviral vector pseudotyped with Ross River Virus glycoproteins J Virol 769378–9388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park F. Correction of bleeding diathesis without liver toxicity using arenaviral-pseudotyped HIV-1-based vectors in hemophilia A mice. Hum Gene Ther. 2003;14:1489–1494. doi: 10.1089/104303403769211691. [DOI] [PubMed] [Google Scholar]
- Schauber CA, Tuerk MJ, Pacheco CD, Escarpe PA., and, Veres G. Lentiviral vectors pseudotyped with baculovirus gp64 efficiently transduce mouse cells in vivo and show tropism restriction against hematopoietic cell types in vitro. Gene Ther. 2004;11:266–275. doi: 10.1038/sj.gt.3302170. [DOI] [PubMed] [Google Scholar]
- Kumar M, Bradow BP., and, Zimmerberg J. Large-scale production of pseudotyped lentiviral vectors using baculovirus GP64. Hum Gene Ther. 2003;14:67–77. doi: 10.1089/10430340360464723. [DOI] [PubMed] [Google Scholar]
- LoDuca PA, Hoffman BE., and, Herzog RW. Hepatic gene transfer as a means of tolerance induction to transgene products. Curr Gene Ther. 2009;9:104–114. doi: 10.2174/156652309787909490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annoni A, Brown BD, Cantore A, Sergi LS, Naldini L., and, Roncarolo MG. In vivo delivery of a microRNA-regulated transgene induces antigen-specific regulatory T cells and promotes immunologic tolerance. Blood. 2009;114:5152–5161. doi: 10.1182/blood-2009-04-214569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CS, Kang Y, Sauter SL, Townsend K, Staber P, Derksen TA.et al. (2001In vivo treatment of hemophilia A and mucopolysaccharidosis type VII using nonprimate lentiviral vectors Mol Ther 3850–856. [DOI] [PubMed] [Google Scholar]
- van Til NP, Markusic DM, van der Rijt R, Kunne C, Hiralall JK, Vreeling H.et al. (2005Kupffer cells and not liver sinusoidal endothelial cells prevent lentiviral transduction of hepatocytes Mol Ther 1126–34. [DOI] [PubMed] [Google Scholar]
- Schauber-Plewa C, Simmons A, Tuerk MJ, Pacheco CD., and, Veres G. Complement regulatory proteins are incorporated into lentiviral vectors and protect particles against complement inactivation. Gene Ther. 2005;12:238–245. doi: 10.1038/sj.gt.3302399. [DOI] [PubMed] [Google Scholar]
- Montini E, Cesana D, Schmidt M, Sanvito F, Bartholomae CC, Ranzani M.et al. (2009The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy J Clin Invest 119964–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao O, Dobrzynski E, Wang L, Nayak S, Mingle B, Terhorst C.et al. (2007Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer Blood 1101132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, Yamamura K., and, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Bi L, Lawler AM, Antonarakis SE, High KA, Gearhart JD., and, Kazazian HH., Jr Targeted disruption of the mouse factor VIII gene produces a model of haemophilia A. Nat Genet. 1995;10:119–121. doi: 10.1038/ng0595-119. [DOI] [PubMed] [Google Scholar]