Abstract
For dominantly inherited disorders development of gene therapies, targeting the primary genetic lesion has been impeded by mutational heterogeneity. An example is rhodopsin-linked autosomal dominant retinitis pigmentosa with over 150 mutations in the rhodopsin gene. Validation of a mutation-independent suppression and replacement gene therapy for this disorder has been undertaken. The therapy provides a means of correcting the genetic defect in a mutation-independent manner thereby circumventing the mutational diversity. Separate adeno-associated virus (AAV) vectors were used to deliver an RNA interference (RNAi)-based rhodopsin suppressor and a codon-modified rhodopsin replacement gene resistant to suppression due to nucleotide alterations at degenerate positions over the RNAi target site. Viruses were subretinally coinjected into P347S mice, a model of dominant rhodopsin-linked retinitis pigmentosa. Benefit in retinal function and structure detected by electroretinography (ERG) and histology, respectively, was observed for at least 5 months. Notably, the photoreceptor cell layer, absent in 5-month-old untreated retinas, contained 3–4 layers of nuclei, whereas photoreceptor ultrastructure, assessed by transmission electron microscopy (TEM) improved significantly. The study provides compelling evidence that codelivered suppression and replacement is beneficial, representing a significant step toward the clinic. Additionally, dual-vector delivery of combined therapeutics represents an exciting approach, which is potentially applicable to other inherited disorders.
Introduction
It is timely to explore gene therapies for autosomal dominantly inherited rhodopsin-linked retinitis pigmentosa (RHO-adRP) given knowledge of the genetic etiology of the disease1 and data suggesting that recombinant adeno-associated virus (AAV)-mediated subretinal gene delivery is well tolerated in the human eye.2,3,4,5,6,7 RHO-adRP leads to the progressive loss of photoreceptors and significant visual dysfunction in 1 in 30,000 people.1,8,9 The rhodopsin (RHO) gene is extremely highly expressed10 and constitutes ~90% of the total protein content of mammalian rod outer segment (OS) disc membranes. Several modes of action of different RHO mutant proteins have been established.11 The disease is immensely heterogeneous with ~150 different RHO mutations identified thus far (www.sph.uth.tmc.edu/RetNet; www.hgmd.cf.ac.uk).
The mutational heterogeneity present in RHO-adRP and many dominant genetic diseases represents a barrier to development of gene-based therapies to correct the primary genetic defect. Therefore, a mutation-independent approach involving two components, RNA interference (RNAi)-based suppression of both mutant and wild-type RHO alleles and provision of a suppression resistant replacement RHO gene,12,13 is investigated here.
In a previous study of RHO suppression and replacement utilizing P23H mice, which simulate human RHO-adRP, a single dual-component AAV vector was evaluated.13 The potential and challenges of the technology were highlighted in the study. Although histological benefit in the retina was obtained, functional improvement was not demonstrated; probably due to the extremely rapid nature of the photoreceptor cell degeneration in the P23H mouse, where photoreceptor cell loss occurs over approximately a 2-week period and in addition, to low levels of expression of the replacement RHO gene. This promoted optimization and separate evaluation of the RNAi suppression and the replacement components of the therapy. Additionally, these studies were performed in P347S mice (another model of RHO-adRP where photoreceptor cell loss is much slower given a much larger window of time for therapeutic intervention). RNAi suppression of the mutant RHO provided benefit in the presence of the endogenous murine rhodopsin (Rho) gene.14 In this study, Rho, resistant to suppression due to natural mismatches at the RNAi target site, effectively functioned as a replacement gene.
Given the high-expression level of endogenous Rho it was apparent that the replacement component of AAV-delivered suppression and replacement required optimization. Therefore, a range of RHO replacement AAV vectors was generated and evaluated in Rho−/− mice.15 Utilization of an optimized rhodopsin promoter, which was far more highly expressed from AAV vectors than earlier constructs, evoked electroretinographic (ERG) responses and resulted in the elaboration of rod OS in Rho−/− mice.15
Although generation of an efficacious dual-component gene therapy (encompassing RNAi-based suppression and codon-modified gene replacement) for dominant disease is challenging, demonstration of the efficacy of each component separately stimulated examination of the potential benefit associated with combining the AAV RHO suppression and AAV RHO replacement therapies. Employing two vectors rather than a single vector enables a further degree of control on relative levels of suppression and replacement. Furthermore, engineering both RNAi-mediated suppression and optimized RHO replacement elements into a single AAV vector results in a transgene size at the limit for optimal production of AAV and therefore may result in compromised infectivity, transgene expression, and viral titers.16 Hence, in this study, we have explored suppression and replacement of RHO utilizing codelivery of the two components of the therapy by two separate AAV vectors and demonstrate the feasibility of this approach in P347S mice. The results obtained provide evidence of significant functional benefit and therefore represent a significant step toward the clinic. The proof of concept obtained in this study supports the view that mutation-independent gene-based therapies may be relevant not only for RHO-adRP but also for other dominant disorders. Additionally, this dual-vector dual-component approach may be applicable to other gene therapies where AAV packaging size is a limiting factor or a flexible/adaptable control of gene expression is desired.
Results
The focus of this study was to determine whether a suppression and replacement gene therapy involving cosubretinal administration of two AAV vectors, one encoding a RHO suppressor (AAV-S) and the other a RHO replacement gene (AAV-R), could provide significant benefit in a mouse model of RHO-adRP, the P347S mouse.
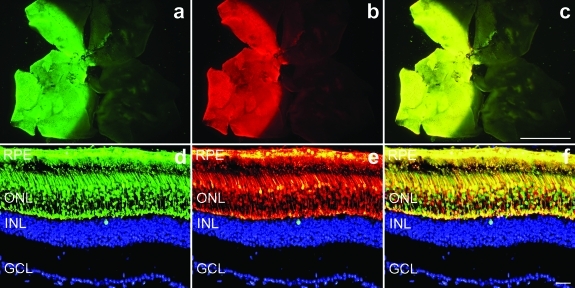
The working hypothesis was that coadministration of two AAVs (AAV-S and AAV-R) would result in coinfection of significant proportions of photoreceptors. In principle, these photoreceptors should receive both suppression and replacement components and therefore should function similarly to wild-type photoreceptors. To test the feasibility of this approach, coadministration of two AAV vectors each encoding a marker gene was undertaken. Both reporter genes were driven from a CMV promoter. A mixture of 1.5 × 109 vector particles (vp) AAV-EGFP and 1.5 × 109 vp AAV-DsRed was subretinally injected into adult wild-type mice and retinas evaluated by histology 2 weeks later (n = 5). Areas of the retinas transduced by the two viruses completely overlapped as determined by microscopic analysis of whole mount retinas (Figure 1a–c). At the cellular level the majority of the transduced cells, coexpressed both markers (yellow) and only a few cells expressed just one marker (red or green; Figure 1d–f). These experiments provide evidence of significant coexpression following coadministration of two AAVs. The results suggest that a similar strategy using two AAV constructs for delivering the suppression and replacement components should also result in significant cotransduction of photoreceptors.
Figure 1.
Cotransduction of adeno-associated viruses (AAVs) in wild-type retinas. Eyes of wild-type mice were subretinally injected with a mixture of 1.5 × 109 vector particles (vp) AAV-EGFP and 1.5 × 109 vp AAV-DsRed. Two weeks postinjection eyes were fixed (n = 5), whole mounted for imaging, then cryosectioned (12 µm) and processed for histology. Nuclei were counterstained with DAPI. (a–c) Representative whole mounts illustrating (a) enhanced green fluorescent protein (EGFP), (b) DsRed, and (c) overlay of EGFP and DsRed signals. Representative sections demonstrate significant coexpression (f) of (d) EGFP and (e) DsRed signals at the cellular level in the outer nuclear layer. In order to obtain a clearer view of the markers the DAPI (blue) signal was edited out from the ONL. Bars = 1 mm (a–c) and 25 µm (d–f). GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; RPE, retinal pigment epithelium.
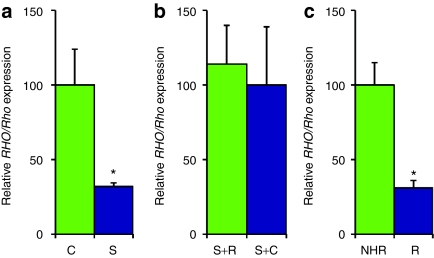
Initially, 6.0 × 108 vp AAV-S or an AAV expressing a nontargeting control RNAi, AAV-C, were subretinally injected into contralateral eyes of P347S mouse pups, which express a human mutant RHO transgene. AAV-S has previously been shown to suppress rhodopsin mRNA by >90% in vivo (also referred to as siBB,13). Two weeks postinjections retinas were harvested, transduced (green) cells collected by fluorescence-activated cell sorting and RNA extracted. Levels of mutant RHO, determined by quantitative real-time reverse transcription PCR (qPCR), using human RHO-specific primers, were suppressed by 68 ± 2.4% (n = 6, P = 0.0187) in cells transduced with AAV-S versus AAV-C, indicating that efficient RHO suppression has occurred in this disease model of RHO-adRP (Figure 2a). Subsequently the resistance to suppression of transcripts expressed from AAV-R was determined in adult wild-type mice. Mixtures of either 6.0 × 108 vp AAV-S and 1.8 × 1010 vp AAV-R or 6.0 × 108 vp AAV-C and 1.8 × 1010 vp AAV-R were subretinally injected into fellow eyes, total RNA extracted 2 weeks postinjection and RNA analyzed. Levels of RHO replacement expressed from AAV-R were determined by qPCR and did not differ significantly between the AAV-S and AAV-R or the AAV-C and AAV-R injected eyes (P = 0.814, n = 7), suggesting that the RHO replacement gene delivered in AAV-R is resistant to AAV-S suppression (Figure 2b). Additionally, subretinal administration of 1.0 × 1010 vp of AAV-R was undertaken in adult wild-type mice (n = 8) and 2 weeks postinjection levels of RHO expression from AAV-R were compared to levels of expression of RHO in normal human rhodopsin (NHR) transgenic mice. The NHR mouse expresses a wild-type human RHO transgene at levels comparable with endogenous levels of mouse Rho expression and displays a wild-type phenotype.13,17 Comparison of RNA from whole retinas of AAV-R administered to wild-type mice and retinas from NHR mice demonstrated that RHO expression from AAV-R was ~31 ± 5% of levels observed in NHR mouse retinas. Notably, since only ~40% of the retina is thought to be transduced by AAV (Figure 2c), this suggests that overall expression levels of RHO from AAV-R may be similar to endogenous murine Rho levels and RHO levels in the NHR transgenic mouse (Figure 2c). Quantitative protein analysis was not undertaken as the human RHO-specific antibody used for immunocytochemistry (see below) is not suitable for western blotting or enzyme-linked immunosorbent assay.
Figure 2.
Rhodopsin mRNA expression levels. (a) Contralateral eyes of adult P347S mice (n = 6) expressing a mutant human RHO transgene were subretinally injected with 6.0 × 108 vp AAV-S or AAV-R. Two weeks postinjection retinas were harvested, transduced (green) cells collected by fluorescence-activated cell sorting (FACS) and RNA extracted. The level of human RHO suppression by AAV-S, determined by qPCR using human-specific RHO primers, was 68 ± 2.4%. (b) Adult wild-type mice (n = 7) were subretinally injected with a mixture of either 6.0 × 108 vp AAV-S and 1.8 × 1010 vp AAV-R or 6.0 × 108 vp AAV-C and 1.8 × 1010 vp AAV-R. Two weeks postinjection total RNA was extracted and levels of replacement RHO RNA determined by qPCR. No significant difference in replacement transcript levels was observed in eyes that received AAV-R and AAV-S versus AAV-R and AAV-C, indicating that no significant suppression of the replacement transcript had occurred (P = 0.814). (c) Adult wild-type mice (n = 8) were subretinally injected with AAV-R. Total RNA was extracted 2 weeks postinjection and levels of RHO replacement expression from AAV-R compared to levels of RHO in normal human rhodopsin (NHR) mouse retinas. Levels of RHO mRNA expression from the AAV-R was 31 ± 5% of levels in NHR mouse retinas.
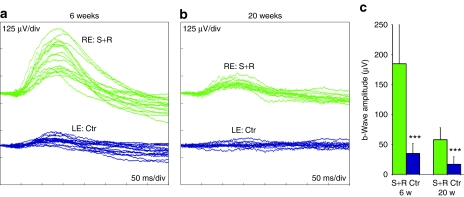
In all subsequent experiments, 5-day-old P347S mice were subretinally injected. In one set of experiments, right eyes were injected with a mixture of 6.0 × 108 vp AAV-S and 1.8 × 1010 vp AAV-R. Fellow left eyes received 6.0 × 108 vp of control AAV-C. As evaluated by 6-week postinjection ERGs, rod-isolated amplitudes in eyes treated with the mixture of AAV-S and AAV-R were found to be 184.5 ± 65.4 µV compared to fellow eyes treated with control AAV-C, whereas the amplitudes were 34.9 ± 16.8 µV (P <0.0001, n = 17; Figure 3a,c). In 20-week postinjection ERGs, rod-isolated responses were 58.1 ± 19.8 µV in treated eyes compared to 16.9 ± 12.6 µV (P < 0.0001, n = 12) in control eyes (Figure 3b,c), the latter being similar to that of uninjected eyes (data not shown).
Figure 3.
Rod-derived electroretinography (ERG) following combined suppression and replacement therapy. The right eyes of P5 P347S mice were subretinally injected with a mixture of 6.0 × 108 vp AAV-S and 1.8 × 1010 vp AAV-R whereas the left eyes were injected with 6.0 × 108 vp AAV-C. Six (n = 17) or twenty weeks (n = 12) postinjection, mice were dark-adapted overnight and rod-isolated ERG responses recorded from both eyes. (a,b) Overlays of the ERG recordings; green and blue lines represent recordings from combined suppression and replacement therapy (S+R) and control (C)-injected eyes, respectively. (c) Mean ERG b-wave amplitudes (µV). Green and blue columns represent values corresponding to S+R and control injected eyes, respectively. Error bars represent SD values and ***P < 0.001.
In order to determine whether the improved retinal responses resulted from the AAV-S and AAV-R suppression and replacement combination therapy or either component singly, effects of AAV-R and AAV-S were assessed separately. To test the suppression component, 6.0 × 108 vp of AAV-S (right eyes) or control AAV-C (left eyes) were subretinally injected into contralateral eyes. Similarly, to test the replacement component, 1.8 × 1010 vp of AAV-R (right eyes) were subretinally injected whereas the fellow left eyes remained uninjected. Rod-isolated ERGs, performed 6 weeks postinjection were not significantly different; 60.5 ± 32.6 µV in AAV-S alone treated eyes compared to 68.1 ± 20.1 µV (n = 12) in control eyes (Supplementary Figure S1a,c) and 63.7 ± 38.6 µV in AAV-R alone treated eyes compared to 46.4 ± 17.9 µV (n = 10) in uninjected eyes (Supplementary Figure S1b,c) indicating that AAV-S alone or AAV-R alone did not provide benefit in P347S mice.
Notably, the benefit observed subsequent to subretinal delivery of the combined suppression and replacement therapy was also observed at the histological level. Six weeks postinjection, outer nuclear layer (ONL) thickness of sections from eyes treated with the mixture of AAV-R and AAV-S was 17.9 ± 3.4 µm compared to 13.3 ± 2.0 µm in sections from control eyes treated with AAV-C (P <0.0001, n = 5). Preservation of retinal structure was also apparent in semithin sections (Supplementary Figure S2a,b). Transmission electron microscopy (TEM) of these retinas (n = 3) demonstrated that only photoreceptor inner segments were present in control retinas whereas photoreceptor inner segments and OS were abundant in AAV-S- and AAV-R-treated retinas (Supplementary Figure S2c,d). Due to the presence of OS, the distance between the ONL and the retinal pigment epithelium (RPE) was greater in the treated eyes (Supplementary Figure S2).
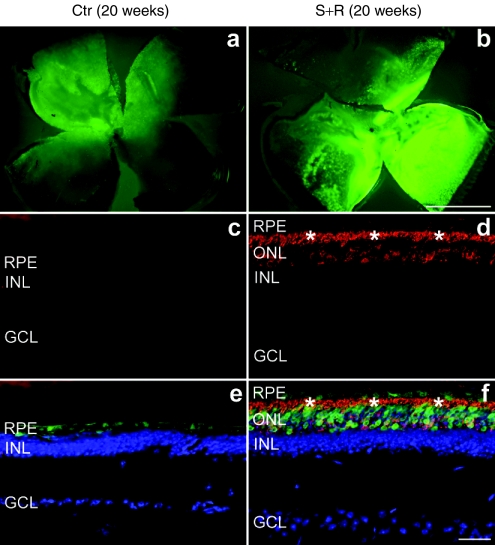
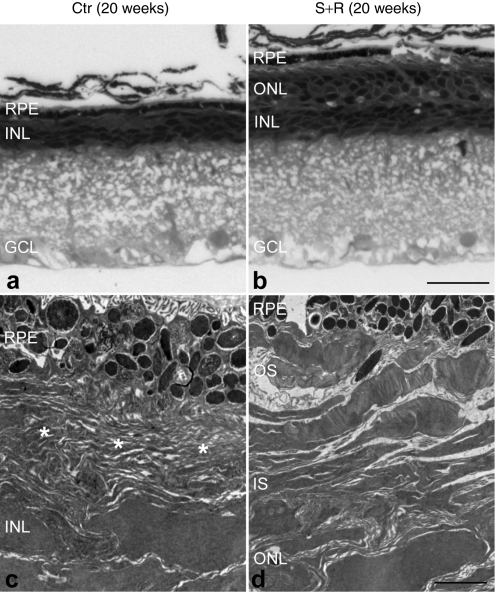
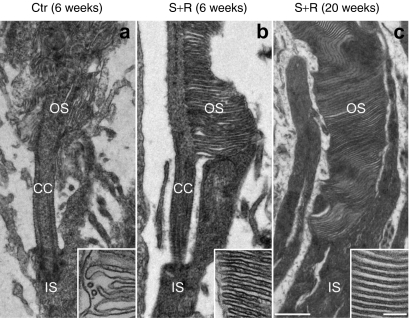
Histological analysis at 20 weeks postinjection demonstrated a striking difference between combined suppression and replacement-treated and control eyes. Whereas vestigial ERG responses were still recorded in some control eyes, the ONL had almost completely disappeared (and therefore ONL thickness was not measurable) and rhodopsin protein was not detectable in the control eyes (Figure 4c,e). In contrast, the ONL in eyes treated with suppression and replacement therapy contained 3–4 layers of photoreceptor nuclei (8.9 ± 1.2 µm thickness; n = 4, Figure 4d,f). Treated retinas were characterized by rhodopsin expression in both the ONL and the photoreceptor segment layer (Figure 4d,f). Note that enhanced green fluorescent protein (EGFP) tracer was only present in the RPE of the control eyes (as no photoreceptor layer remained; Figure 4c) whereas it was present in both RPE and ONL of the suppression- and replacement-treated retinas (Figure 4f). Differences in EGFP intensities between treated and control eyes were apparent in the whole mount retinas (Figure 4a,b) and were independent of the transduction coverage (~40%). Semithin sections demonstrated significant photoreceptor rescue in the treated retinas (Figure 5a,b). At the ultrastructural level, well-preserved OS characterized the treated retina (Figure 5d) whereas only membranous debris was present between the inner nuclear layer and the RPE (Figure 5c) in the control retina. High magnification TEM revealed individual photoreceptor segments, depicted in Figure 6. Short, degenerating OS with inflated and disorganized membrane disks were present in the control retina at 6 weeks postinjection (Figure 6a), whereas no photoreceptors were found at 20 weeks postinjection. In photoreceptor cells treated with AAV-S and AAV-R, the inner segments were attached to well-preserved OS with parallel layers of tightly stacked membrane disks at both 4 and 20 weeks postinjection indicating substantial rescue of the photosensitive OS (Figure 6b,c).
Figure 4.
Immunohistochemical analysis of rhodopsin expression following combined suppression and replacement therapy 20 weeks postinjection. The right eyes of P5 P347S mice were subretinally injected with a mixture of (b,d, and f) 6.0 × 108 vp AAV-S and 1.8 × 1010 vp AAV-R whereas the left eyes were injected with (a,c, and e) 6.0 × 108 vp AAV-C. Note that AAV-S and AAV-C coexpress enhanced green fluorescent protein (EGFP). Eyes were fixed (n = 5), whole mounted for imaging, then cryosectioned (12 µm) and processed for immunocytochemistry using rhodopsin primary and Cy3-conjugated secondary antibodies. Nuclei were counterstained with DAPI. (a,b) Representative whole mounts. (c,d) Representative sections show rhodopsin labeling (red). (e,f) Rhodopsin (red), EGFP (green), and nuclear DAPI (blue) signals overlaid. *Photoreceptor segment layer; ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer; RPE, retinal pigment epithelium. Bars = 1 mm (a,b) and 25 µm (c–f).
Figure 5.
Ultrastructural analysis of combined suppression and replacement-treated retinas 20 weeks postinjection. The right eyes of P5 P347S mice were subretinally injected with a mixture of (b,d) 6.0 × 108 vp AAV-S and 1.8 × 1010 vp AAV-R whereas the left eyes were injected with (a,c) 6.0 × 108 vp AAV-C. Note that AAV-S and AAV-C coexpress enhanced green fluorescent protein (EGFP). Eyes were fixed, whole mounted, and transduced areas identified by EGFP fluorescence and excised. The excised retinal samples were postfixed and processed for transmission electron microscopy (TEM). Semi- and ultrathin sections were analyzed by (a,b) light microscopy or (c,d) TEM. Combined suppression and replacement therapy resulted in preservation of rod photoreceptor outer segments (OS), which extended to the retinal pigment epithelium (RPE; b and d). In contrast, in the control retina, only membranous debris (*) was detected between the RPE and the inner nuclear layer (INL) whereas the outer nuclear layer (ONL) was not present (a,c). IS, inner segment layer, GCL, ganglion cell layer. Bars = 25 µm (a,b) and 2 µm (c,d).
Figure 6.
Photoreceptor morphology rescue following combined suppression and replacement therapy. The right eyes of P5 P347S mice were subretinally injected with a mixture of 6.0 × 108 vp AAV-S and 1.8 × 1010 vp AAV-R (b and c; n = 3 and n = 1, respectively) whereas the left eyes were injected with 6.0 × 108 vp AAV-C (a; n = 4). Note that AAV-S and AAV-C coexpress enhanced green fluorescent protein (EGFP). (a,b) Six and (c) twenty weeks postinjection, eyes were fixed, whole mounted, and transduced areas identified by EGFP fluorescence and excised. The excised retinal samples were processed for transmission electron microscopy (TEM). Combined suppression and replacement therapy resulted in the preservation of rod photoreceptor outer segments (OS) with correctly formed membrane disks (b and c). In contrast in control retinas the rod photoreceptor inner segments (IS) attached to truncated OS with disorganized disks. CC, connecting cilium. Bars = 500 and 100 nm (inserts).
Discussion
The mutational heterogeneity inherent in many autosomal dominantly inherited disorders represents a significant barrier to the development of therapies focused on amending the primary genetic lesion. Suppression and replacement therapies represent a means to circumvent such mutational heterogeneity. In this study, the strategy has been explored for RHO-adRP. The results obtained clearly demonstrate that RNAi-suppression and codon-modified replacement can be used in concert to provide functional benefit in the P347S mouse model of adRP. This should serve to promote the use of the approach for other dominantly inherited conditions such as RDS-linked adRP and COL7A1-linked epidermolysis bullosa amongst others. In this regard, a suppression and replacement gene therapy for SOD-1-linked autosomal dominantly inherited amyotrophic lateral sclerosis has recently been explored in mice.18 RNAi-based suppression and gene replacement of SOD1 following AAV administration of a single vector was achieved as determined by RNA and protein assays. However, the histological and functional effects of AAV-delivered suppression and replacement were not studied.
Recent clinical trials employing AAV-mediated subretinal delivery of an RPE65 replacement gene for Leber congenital amaurosis have suggested that AAV is well tolerated in the human eye and have promoted the use of AAV for retinal gene delivery.2,3,4,5,6,7 In addition, an inverse relationship between age of treatment and level of benefit was observed.5 Given the encouraging result from the Leber congenital amaurosis clinical trials, AAV (2/5) was adopted in the present study as the virus of choice to administer suppression and replacement therapies to P347S mice.
Although the clinical applications of AAV make it an attractive choice for gene delivery to the retina, transgene size constraints associated with AAV promoted the exploration of viral mixtures consisting of two separate AAV suppression and replacement vectors. Using reporter viruses (AAV-EGFP and AAV-DsRed) initially, it was established that a substantial photoreceptor coinfection with the two vector after a single subretinal injection of vector mixtures (Figure 1a–f). This encouraged the subsequent assessment of therapeutic coadministration of AAV-S and AAV-R in P347S mice. Of note, the dual vector strategy also allows a further level of control on the relative RHO suppression and replacement levels. To limit the probability of RHO suppression in the absence of RHO replacement, a greater amount of the AAV-R vector was employed. The dose of AAV-R chosen was the maximum possible given the AAV-R viral titer and one that had been previously shown to provide structural and functional benefit in the Rho−/− mouse.15 The dose of AAV-S used was the minimum required to achieve high levels of suppression.14 With regard to transgene size, there have been reports of transgenes of ~9 kb in AAV2/5 vectors.19 However, potential effects on titer and transgene expression remain unresolved.16 Three groups have since independently attempted to repeat packaging of large cassettes20,21,22 but their studies to date indicate that the packaging capacity of AAV is usually limited to about 5 kb. Hence, a dual vector approach for suppression and replacement was adopted in the present study.
Subretinal injections were undertaken at P5 in P347S mice to enable early administration of the gene therapy while minimizing surgical trauma that had previously been observed in younger animals, for example, in P0 and P1 injected eyes (data not shown). Significant and consistent evidence of therapeutic benefit was obtained using both electrophysical and histological readouts. ERG comparisons between treated and control eyes at 6 weeks and 5 months demonstrated significantly improved responses in AAV-S and AAV-R dual-treated eyes (Figure 3a–c). The beneficial effects observed using ERG were also observed using histological analyses employing both light and electron microscopy. Retention of ONL (3–4 rows) was observed in treated P347S eyes 5 months subsequent to a single subretinal injection of AAV-S and AAV-R, whereas in contrast, there were no remaining rows of photoreceptor nuclei in the ONL of control eyes (Figure 4). Likewise, improved photoreceptor OS ultrastucture was detected by TEM in treated versus control P347S mouse eyes (Figures 5 and 6).
Although the AAV-S and AAV-R combination therapy was found to provide functional benefit in P347S mouse eyes when compared to control eyes, it was important to establish if either of these components alone provided the beneficial effect. Subretinal injection of AAV-S alone or AAV-R alone (using the same vector doses as employed in the AAV-S and AAV-R combination therapy) did not provide significant therapeutic benefit in P347S mice when treated and control eyes were compared (Supplementary Figure S1a–c). Note that the relatively low dose of AAV-S used resulted in a modest but not significant reduction in ERG in the AAV-S treated eyes compared to eyes treated with AAV-C. In contrast, ERG responses in AAV-R, alone treated eyes were slightly enhanced when compared to control eyes but this difference did not reach statistical significance. Indeed, it was the combination of AAV-mediated suppression in conjunction with AAV-mediated replacement that was required to obtain benefit in this mouse model of RHO-adRP. It is worth noting that while the use of two separate AAV vectors for gene delivery is of direct relevance for suppression and replacement therapies where two components are essential, employing a dual-vector approach may also be of value where delivery of two synergistic therapies could potentially augment benefit. One example may be the delivery of a gene replacement therapy together with a neurotrophic factor for some autosomal recessively inherited disorders23 amongst other combinations. Undoubtedly, the use of dual-vectors will be explored more extensively in the future in an attempt to optimize gene-based therapeutics for many disorders. The results from the present study validate the principle of a two-vector approach.
Suppression and replacement provides a means of correcting the primary genetic defect and will be particularly relevant where the mutant protein drives the disease process. Alternative therapeutic approaches focused on modulating secondary effects associated with the disease are also being contemplated for adRP; many of these are focused on modulating secondary effects associated with the disease. Some of these strategies have been evaluated in rodent models of adRP and beneficial effects of the therapy observed. Such approaches include provision of neurotrophic factors and/or antiapoptotic factors, modulation of oxidative stress, cell replacement strategies, and reduction of protein aggregates.24,25,26,27,28 Typically these approaches are being evaluated not solely for retinal degenerations but a broad range of neurodegenerative conditions. Indeed at times multivalent therapies involving a combination of strategies may be required to protect photoreceptors from degeneration precipitated by the presence of dominant mutations. The modes of action of different dominant RHO mutations can vary significantly and for some RHO mutations remain undetermined. These mechanisms may involve, for example, incorrect rhodopsin transport to the OSs, rhodopsin misfolding or may affect endocytosis or protein stability.11 In principle, RHO suppression and replacement is relevant to RHO-linked adRP patients irrespective of the mode of action of a particular RHO mutation.
The potential power of viral vector-mediated RNAi as a therapeutic tool has been reviewed.29 In the present study, a strategy to correct the primary genetic defect in RHO-linked adRP was evaluated using two AAV viruses to deliver a dual-component therapy. RNAi-mediated suppression of RHO in conjunction with provision of an RNAi-resistant RHO replacement gene engineered using codon redundancy was found to provide functional benefit in the P347S mouse. This represents the first demonstration for any dominant condition that an AAV-delivered dual-component therapy (involving gene suppression and replacement) targeted at amending the primary defect can provide functional benefit. Employing RNAi in concert with replacement genes exploiting codon redundancy provides a means to overcome the mutational diversity associated with many autosomal dominant conditions. The results obtained provide the impetus to progress this therapeutic approach for RHO-adRP toward clinical trial.
Materials and Methods
Vector construction and AAV production. RHO-targeting RNAi (shBB; target position nucleotide 254–274, accession no. NM_000539.2 and nontargeting control RNAi (shNT; 5-TTCTCCCAACGAGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTT-3) were cloned into pAAV-MCS (Stratagene, La Jolla, CA) as described.14 shNT was guaranteed by the manufacturer (Qiagen, Venlo, The Netherlands) not to target any human or murine RNA sequences. shNT has previously been compared to other nontargeting control shRNAs and was shown to be equivalent (data not shown). A CMV promoter (CMV-P) including the CMV enhancer (CMVE; accession no. EF550208) and the SV40 polyadenylation were used in both shRNA constructs to drive expression of an EGFP reporter gene (accession no. U57608; Clontech, Mountain View, CA), creating pAAV-S (shBB-EGFP) and pAAV-C (shNT-EGFP). Replacement human RHO complementary DNA sequence was constructed by modifying the wild-type human RHO sequence (accession no. NM_000539.2) at nucleotide 254–274 as follows 5′-ATAAATTTTTTGACCCTGTAT-3′ (altered bases underlined13). Replacement RHO (pAAV-R) was driven by a hybrid murine Rho promoter originally described as pAAV-BB24,15 which contains a 1.7-kb mouse rhodopsin promoter (Rho-P) together with two conserved Rho-P elements (element E, accession no. NT_005612, nucleotide 35742513–35742578 and element B, accession no. AC142099.3, nucleotide 24955-24880), a 0.4 kb fragment of the endogenous 3′-UTR, and a minimal polyadenylation signal.30 The EGFP (accession no. U57608; Clontech) and DsRed-Express2 genes (DsRed; accession no. FJ226077; Clontech) driven by a CMV promoter were also cloned into pAAV-MCS.
Constructs were packaged into helper-free recombinant AAV2/5 viruses (AAV) as described,13 to generate AAV-shBB-EGFP (AAV-S), AAV-shNT-EGFP (AAV-C), AAV-BB24 (AAV-R), AAV-EGFP, and AAV-DsRed. Briefly, the expression cassettes were transfected into human embryonic kidney-293 cells [ATCC (accession no. CRL-1573]) with pRep2/Cap531 and pHelper (Stratagene). Fifty 150-mm plates of confluent cells were transfected using polyethylenimine. Forty-eight hours after transfection, crude viral lysates were cleared and purified by cesium-gradient centrifugation. AAV-containing fractions were dialyzed against phosphate-buffered saline. Genomic titers (vp/ml) were determined by qPCR.32
Animals and subretinal injections. Mutant transgenic RHO-Pro347Ser+/− Rho+/− (P347S14,33,34) and wild-type mice were used in this study. All animals were on a 129 S2/SvHsd (Harlan, Loughborough, UK) background. Mice were maintained under specific pathogen-free housing conditions. Subretinal injections were carried out in strict compliance with the European Communities Regulations 2002 and 2005 (Cruelty to Animals Act) and the Association for Research in Vision and Ophthalmology statement for the use of animals as described.13 Briefly, adult mice were anesthetized by intraperitoneal injection of medetomidine and ketamine (10 and 750 mg/10 g body weight, respectively). Pupils were dilated with 1% cyclopentolate and 2.5% phenylephrine, and, using topical anesthesia (Amethocaine), a small puncture was made in the sclera. A 34-gauge blunt ended microneedle attached to a 10-µl Hamilton syringe was inserted through the puncture, and AAV was administered to the subretinal space. Following subretinal injection, an anesthetic reversing agent (100 mg/10 g body weight; Atipamezole Hydrochloride) was delivered by intraperitoneal injection. Body temperature was maintained using a homeothermic heating device. Five-day-old mice were prepared for subretinal injection as described.35 Adult and 5-day-old mice were injected with 3 and 0.6 µl AAV, respectively. Typically between five and seven adult mice were used per group in order to obtain significance. However, between 10 and 17, 5-day old mice per group were used, due to greater injection variability associated with small eye sizes. All animal studies have been approved by the authors' institutional review board.
RNA extraction and qPCR analysis. Adult P347S mice (n = 6) were subretinally injected with 6.0 × 108 vp AAV-S whereas fellow eyes received 6.0 × 108 vp AAV-C. Retinas were harvested 2 weeks postinjection, transduced (green) cells collected by fluorescence-activated cell sorting and RNA extracted from these cells.14 Levels of mutant RHO expression were determined by qPCR using human RHO-specific primers previously described.13 Adult wild-type mice were subretinally injected with mixtures of 6.0 × 108 vp AAV-S and 1.8 × 1010 vp AAV-R (n = 7) or 6.0 × 108 vp AAV-C and 1.8 × 1010 vp AAV-R (n = 7). Two weeks postinjection eyes were harvested, RNA extracted from whole retinas and qPCRs, using human RHO-specific primers, performed to determine levels of replacement RHO expression as described.13 Adult wild-type mice were subretinally injected with 1.0 × 1010 vp of AAV-R (n = 8). Two weeks postinjection retinas were collected, total RNA extracted and levels of replacement RHO expression compared to levels of RHO mRNA in NHR mouse retinas by qPCR using human RHO-specific primers.13
ERG. ERG procedures have been described in detail.13,14 Briefly, intraperitoneal administration of ketamine and xylazine (16 and 1.6 mg/10 g body weight, respectively) were used for anesthesia. Pupils were dilated with 1% cyclopentolate and 2.5% phenylephrine and eyes were maintained in a proptosed position throughout the examination. Reference and ground electrodes were positioned subcutaneously, ~1 mm from the temporal canthus and anterior to the tail, respectively. The ERG responses were recorded simultaneously from both eyes using goldwire electrodes (Roland Consult, Brandenburg, Germany); these were positioned to touch the central cornea of each eye. Corneal hydration and electrical contact were maintained by the application of Vidisic (Dr Mann Pharma, Berlin, Germany) to the cornea. Standardized flashes of light were presented to the mouse in a Ganzfeld bowl. Responses were analyzed using a RetiScan RetiPort electrophysiology unit (Roland Consulting). The protocol used was based on that approved by the International Clinical Standards Committee for human ERG. Rod-isolated responses were recorded using a dim white flash (−25 dB maximal intensity where maximal flash intensity was 3 candelas/m2/s) presented in the dark-adapted state. The a-waves were measured from the baseline to the trough and b-waves from the baseline (standard convention). Following 10 minutes light adaptation (30 candelas/m2) cone responses were recorded to the standard flash presented at 0.5 and 10 Hz flicker against the rod suppressing background.
Microscopy and statistical analysis. Rhodopsin immunocytochemistry and fluorescence microscopy were performed as described.13 For immunocytochemistry, the primary rhodopsin antibody was used in 1:100 dilution (courtesy of R. Molday). ONL thickness was measured in AAV transduced parts of the retina. Measurements were made at three points per section and three sections per retina. The sections were ~100 mm apart and within 300 mm of the optic nerve head. Measurements were made using the ruler tool in Photoshop (Adobe Systems Europe, Glasgow, UK). Procedures for TEM were previously described.15,36 Briefly, for tissue preparation eyes were enucleated, fixed in 4% paraformaldehyde in phosphate-buffered solution, and whole mounted. Using the EGFP tracer, EGFP+ areas from the central part of the retinas were excised and fixed in 2.5% glutaraldehyde in 0.1 mol/l cacodylate buffer (pH 7.3) for 2 hours at room temperature. Specimens were washed and fixed in buffered 2% osmium tetroxide, dehydrated, and embedded in araldite. Semi- and ultrathin sections were cut and ultrastructural analyses were performed using a Tecnai 12 BioTwin transmission electron microscope (FEI, Eindhoven, NL).
Statistical analysis. Means and SD values of ERG, mRNA, and histology data sets were calculated. Student's t-tests were used to determine statistical significance between corresponding data sets. Paired t-tests were applied to ERG data. In addition, Wilcoxon-signed rank tests were undertaken on mRNA and histology data sets and paired sign tests on ERG data sets to establish that statistical significance was maintained using nonparametric statistical models. Differences of P < 0.05 were considered statistically significant.
SUPPLEMENTARY MATERIAL Figure S1. Rod-derived ERG following individual delivery of either suppression or replacement AAVs. Figure S2. Ultrastructural analysis of combined suppression and replacement treatment 6 weeks postinjection.
Acknowledgments
We thank Prof Wolfgang Baehr (University of Utah, Salt Lake City, UT) for the original RHO cDNA construct, Prof Tiensen Li for the P347S mouse (Harvard Medical School, Boston, MA), Elisabeth Sehn (Johannes Gutenberg University, Mainz, Germany) for skillful technical assistance, Prof Robert S. Molday (University of British Colombia, Vancouver, CA) for the rhodopsin primary antibody and the staff of the Bioresources Unit, Trinity College Dublin. The research was supported by grant awards from Science Foundation Ireland, Fighting Blindness Ireland, Foundation Fighting Blindness-National Neurovision Research Institute (USA), Enterprise Ireland, I.R.C.S.E.T. “The embark initiative,” EviGenoRet (LSHG-CT-2005–512036), Deutsche Forschungsgemeinschaft (GRK1044), FAUN-Stiftung. G.J.F. and P.F.K. are directors of Genable Technologies; N.C. A.P. M.O'R. and S.M.-W. are consultants for Genable Technologies. These authors have no conflict of interest.
Supplementary Material
Rod-derived ERG following individual delivery of either suppression or replacement AAVs.
Ultrastructural analysis of combined suppression and replacement treatment 6 weeks postinjection.
REFERENCES
- Farrar GJ, Palfi A., and, O'Reilly M. Gene therapeutic approaches for dominant retinopathies. Curr Gene Ther. 2010;10:381–388. doi: 10.2174/156652310793180661. [DOI] [PubMed] [Google Scholar]
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19:979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr, Mingozzi F, Bennicelli J, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F, et al. Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374:1597–1605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL, et al. Gene therapy for Leber's congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther. 2010;18:643–650. doi: 10.1038/mt.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenekoop RK. Successful RPE65 gene replacement and improved visual function in humans. Ophthalmic Genet. 2008;29:89–91. doi: 10.1080/13816810802216480. [DOI] [PubMed] [Google Scholar]
- Farrar GJ, Kenna PF., and, Humphries P. On the genetics of retinitis pigmentosa and on mutation-independent approaches to therapeutic intervention. EMBO J. 2002;21:857–864. doi: 10.1093/emboj/21.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AF, Chakarova CF, Abd El-Aziz MM., and, Bhattacharya SS.2010Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait Nat Rev Genetepub ahead of print). [DOI] [PubMed]
- Palczewski K, Hofmann KP., and, Baehr W. Rhodopsin–advances and perspectives. Vision Res. 2006;46:4425–4426. doi: 10.1016/j.visres.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Mendes HF, van der Spuy J, Chapple JP., and, Cheetham ME. Mechanisms of cell death in rhodopsin retinitis pigmentosa: implications for therapy. Trends Mol Med. 2005;11:177–185. doi: 10.1016/j.molmed.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Millington-Ward S, O'Neill B, Tuohy G, Al-Jandal N, Kiang AS, Kenna PF, et al. Strategems in vitro for gene therapies directed to dominant mutations. Hum Mol Genet. 1997;6:1415–1426. doi: 10.1093/hmg/6.9.1415. [DOI] [PubMed] [Google Scholar]
- O'Reilly M, Palfi A, Chadderton N, Millington-Ward S, Ader M, Cronin T, et al. RNA interference-mediated suppression and replacement of human rhodopsin in vivo. Am J Hum Genet. 2007;81:127–135. doi: 10.1086/519025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadderton N, Millington-Ward S, Palfi A, O'Reilly M, Tuohy G, Humphries MM, et al. Improved retinal function in a mouse model of dominant retinitis pigmentosa following AAV-delivered gene therapy. Mol Ther. 2009;17:593–599. doi: 10.1038/mt.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palfi A, Millington-Ward S, Chadderton N, O'Reilly M, Goldmann T, Humphries MM, et al. Adeno-associated virus-mediated rhodopsin replacement provides therapeutic benefit in mice with a targeted disruption of the rhodopsin gene. Hum Gene Ther. 2010;21:311–323. doi: 10.1089/hum.2009.119. [DOI] [PubMed] [Google Scholar]
- Hirsch ML, Agbandje-McKenna M., and, Samulski RJ. Little vector, big gene transduction: fragmented genome reassembly of adeno-associated virus. Mol Ther. 2010;18:6–8. doi: 10.1038/mt.2009.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson JE, Gordon JW, Pawlyk BS, Roof D, Hayes A, Molday RS, et al. Transgenic mice with a rhodopsin mutation (Pro23His): a mouse model of autosomal dominant retinitis pigmentosa. Neuron. 1992;9:815–830. doi: 10.1016/0896-6273(92)90236-7. [DOI] [PubMed] [Google Scholar]
- Kubodera T, Yamada H, Anzai M, Ohira S, Yokota S, Hirai Y, et al. 2010In vivo application of an RNAi strategy for the selective suppression of a mutant allele Hum Gene Therepub ahead of print). [DOI] [PubMed]
- Allocca M, Doria M, Petrillo M, Colella P, Garcia-Hoyos M, Gibbs D, et al. Serotype-dependent packaging of large genes in adeno-associated viral vectors results in effective gene delivery in mice. J Clin Invest. 2008;118:1955–1964. doi: 10.1172/JCI34316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B, Nakai H., and, Xiao W. Characterization of genome integrity for oversized recombinant AAV vector. Mol Ther. 2010;18:87–92. doi: 10.1038/mt.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Yue Y., and, Duan D. Evidence for the failure of adeno-associated virus serotype 5 to package a viral genome >or = 8.2 kb. Mol Ther. 2010;18:75–79. doi: 10.1038/mt.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Yang H., and, Colosi P. Effect of genome size on AAV vector packaging. Mol Ther. 2010;18:80–86. doi: 10.1038/mt.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch PK, MacLaren RE, Durán Y, Balaggan KS, MacNeil A, Schlichtenbrede FC, et al. In contrast to AAV-mediated Cntf expression, AAV-mediated Gdnf expression enhances gene replacement therapy in rodent models of retinal degeneration. Mol Ther. 2006;14:700–709. doi: 10.1016/j.ymthe.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Gorbatyuk MS, Knox T, LaVail MM, Gorbatyuk OS, Noorwez SM, Hauswirth WW, et al. Restoration of visual function in P23H rhodopsin transgenic rats by gene delivery of BiP/Grp78. Proc Natl Acad Sci USA. 2010;107:5961–5966. doi: 10.1073/pnas.0911991107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam LC, Kiang AS, Campbell M, Keaney J, Farrar GJ, Humphries MM, et al. Prevention of autosomal dominant retinitis pigmentosa by systemic drug therapy targeting heat shock protein 90 (Hsp90) Hum Mol Genet. 2010;19:4421–4436. doi: 10.1093/hmg/ddq369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory-Evans K, Chang F, Hodges MD., and, Gregory-Evans CY. Ex vivo gene therapy using intravitreal injection of GDNF-secreting mouse embryonic stem cells in a rat model of retinal degeneration. Mol Vis. 2009;15:962–973. [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Mohand-Said S, Danan A, Simonutti M, Fontaine V, Clerin E, et al. Functional cone rescue by RdCVF protein in a dominant model of retinitis pigmentosa. Mol Ther. 2009;17:787–795. doi: 10.1038/mt.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samardzija M, Wenzel A, Thiersch M, Frigg R, Remé C., and, Grimm C. Caspase-1 ablation protects photoreceptors in a model of autosomal dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2006;47:5181–5190. doi: 10.1167/iovs.06-0556. [DOI] [PubMed] [Google Scholar]
- Couto LB., and, High KA. Viral vector-mediated RNA interference. Curr Opin Pharmacol. 2010;10:534–542. doi: 10.1016/j.coph.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Levitt N, Briggs D, Gil A., and, Proudfoot NJ. Definition of an efficient synthetic poly(A) site. Genes Dev. 1989;3:1019–1025. doi: 10.1101/gad.3.7.1019. [DOI] [PubMed] [Google Scholar]
- Hildinger M, Auricchio A, Gao G, Wang L, Chirmule N., and, Wilson JM. Hybrid vectors based on adeno-associated virus serotypes 2 and 5 for muscle-directed gene transfer. J Virol. 2001;75:6199–6203. doi: 10.1128/JVI.75.13.6199-6203.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr UP, Wulf MA, Stahn S, Steidl U, Haas R., and, Kronenwett R. Fast and reliable titration of recombinant adeno-associated virus type-2 using quantitative real-time PCR. J Virol Methods. 2002;106:81–88. doi: 10.1016/s0166-0934(02)00138-6. [DOI] [PubMed] [Google Scholar]
- Li T, Snyder WK, Olsson JE., and, Dryja TP. Transgenic mice carrying the dominant rhodopsin mutation P347S: evidence for defective vectorial transport of rhodopsin to the outer segments. Proc Natl Acad Sci USA. 1996;93:14176–14181. doi: 10.1073/pnas.93.24.14176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MM, Rancourt D, Farrar GJ, Kenna P, Hazel M, Bush RA, et al. Retinopathy induced in mice by targeted disruption of the rhodopsin gene. Nat Genet. 1997;15:216–219. doi: 10.1038/ng0297-216. [DOI] [PubMed] [Google Scholar]
- Matsuda T., and, Cepko CL. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc Natl Acad Sci USA. 2004;101:16–22. doi: 10.1073/pnas.2235688100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfrum U. Cytoskeletal elements in arthropod sensilla and mammalian photoreceptors. Biol Cell. 1992;76:373–381. doi: 10.1016/0248-4900(92)90441-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Rod-derived ERG following individual delivery of either suppression or replacement AAVs.
Ultrastructural analysis of combined suppression and replacement treatment 6 weeks postinjection.