Abstract
Recently reovirus-based oncotherapy has been successfully implemented for the treatment of prostate cancer. In this report, we show that apart from its primary direct cancer-killing activity, reovirus oncotherapy overrides tumor-associated immune evasion strategies and confers protective antiprostate cancer immunity. Prostate cancer represents an ideal target for immunotherapies. However, currently available immune interventions fail to induce clinically significant antiprostate cancer immune responses, owing to the immunosuppressive microenvironment associated with this disease. We show here that during the process of oncolysis, reovirus acts upon prostate cancer cells and initiates proinflammatory cytokines and major histocompatibility complex (MHC) class I molecule expression. In an immunocompetent transgenic adenocarcinoma of mouse prostate (TRAMP) model, reovirus oncotherapy induces the homing of CD8+ T and NK cells in tumors and the display of tumor-associated antigens (TAAs) on antigen-presenting cells (APCs), and endows dendritic cells (DCs) with a capacity to successfully present TAAs to tumor-specific CD8+ T cells. These newly generated immunological events lead to the development of strong antiprostate cancer T cell responses, which restrict the growth of subsequently, implanted syngeneic tumor in an antigen-specific, but reovirus-independent manner. Such reovirus-initiated antiprostate cancer immunity represents a clinically valuable entity that can promote long-term cancer-free health even after discontinuation of the primary oncotherapy.
Introduction
Prostate cancer, the most common cancer affecting the North American men, caused an estimated 27,360 deaths in 2009 in the United States alone.1 The failure of currently available treatment options to efficiently manage this disease has provoked an intensive search of unconventional therapeutic approaches such as prostate cancer-specific immunotherapies2,3,4 and oncolytic virotherapies.5 Immunotherapies exploit the functions of immune cells (e.g., T cells, dendritic cells [DCs]) or immune mediators (e.g., antibodies, cytokines) and aim at establishing an antitumor immunity. Unfortunately, the prostate cancer-associated immunosuppressive milieu resists the development of clinically meaningful antitumor immune response. Prostate adenocarcinoma is associated with lack of tumor antigen recognition, expression of suppressive cytokines (e.g., interleukin (IL)-10, transforming growth factor-β), presence of inhibitory surface molecules on immune cells (e.g., CTLA-4, programmed death-1) and development of regulatory T cells.4,6 Nonetheless, antiprostate cancer immunotherapies have shown promise in the management of local, advanced, and/or recurrent forms of prostate cancer,2,3,7,8 especially when administered in conjunction with the interventions that mitigate immunological tolerance.9,10
Reovirus, a naturally occurring benign human pathogen, preferentially kills a variety of cancer cells including those of breast, brain, colon, lymphoid, ovarian, spinal cord, bladder as well as prostate origin.11,12,13 Currently, reovirus is undergoing phase III clinical trials in the United Kingdom, the United States, and Belgium.14,15 The susceptibility of tumor cells to reovirus-mediated oncolysis is associated with an activated Ras signaling pathway.11,16,17,18 It is noteworthy that aberrant expression of Ras and associated signaling molecules is observed in >80% of human cancers, including prostate cancer,11,18 making them suitable targets for reovirus oncotherapy.5
The primary mode of action for reovirus oncotherapy is the direct destruction of cancer cells.16,17,18 However, it is believed that antiviral immunological events initiated following administration of oncotherapy also assist in the development of beneficial antitumor immune responses.19,20 In a melanoma mouse model, reovirus oncotherapy invokes antitumor innate as well as adaptive immune components that aid in the tumor regression.21 However, whether reovirus or any other oncolytic virus-based therapy initiates such antitumor immune response during antiprostate cancer oncotherapy is presently unknown. Hence, this study focused on dissecting various oncolytic virus-initiated immunological events that may contribute toward the generation of antiprostate cancer immunity. Our data show that apart from its primary oncolytic activity, reovirus oncotherapy overrides tumor-associated immune evasion mechanisms. Furthermore, reovirus initiates antiprostate cancer T cell immune responses that protect against subsequent tumor challenge in an antigen-dependent manner without requiring a continued presence or an additional administration of reovirus. These anticancer immunotherapeutic activities initiated during reovirus therapy represent a clinically applicable treatment intervention for the efficient management of prostate cancer.
Results
Reovirus targets TRAMP-C1 cells in vitro and in vivo
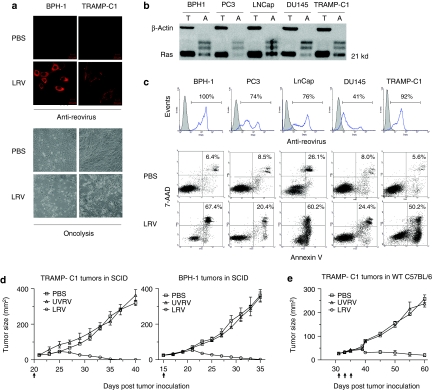
The susceptibility of transgenic adenocarcinoma of mouse prostate-C1 (TRAMP-C1)22 cell line to reovirus infection and oncolysis was tested in vitro and compared with a panel of human prostate cancer cell lines (BPH-1, PC-3, LNCap, and DU145). For this purpose, these cell lines were cultured in the presence or absence of reovirus, then analyzed. As shown in Figure 1a, reovirus infected and killed both TRAMP-C1 and BPH-1 cells with a similar efficiency. Since susceptibility of tumor cells to reovirus-mediated oncolysis is associated with activation of the Ras signaling pathway,16,17,18 we further evaluated the status of both total and activated Ras. Among the cell lines tested, BPH-1, LNCap, and TRAMP-C1 cells contained higher levels of activated Ras protein than PC-3 and DU145 cells (Figure 1b). Although all of the above-mentioned cell lines were susceptible to reovirus infection (Figure 1c, upper panel), higher levels of oncolysis was observed in BPH-1, LNCap, and TRAMP-C1 cells (Figure 1c, lower panel).
Figure 1.
Susceptibility of mouse transgenic adenocarcinoma of mouse prostate-C1 (TRAMP-C1) cells to reovirus-mediated oncolysis in vitro and in vivo. (a) BPH-1 and TRAMP-C1 cells were cultured in the presence of live reovirus [LRV; 1–10 multiplicity of infection (MOI)] or phosphate-buffered saline (PBS) (control) for 18–24 hours and then analyzed microscopically. Confocal microscopy (top panel, red: reovirus) and light field contrast (bottom panel) images display reovirus-infected and apoptotic cells, respectively. (b) Total (T) and activated (A) Ras in prostate cancer cells were pulled down using conjugated Raf-domain and detected by anti-Ras antibody. Levels of β-actin served as an internal control. (c) Human and mouse prostate cancer cells were cultured in the presence of PBS or LRV for 24 hours, stained with antireovirus antibody or annexin-V/7-AAD and analyzed in flow cytometry. The numbers indicate the percentages of reovirus-infected (histograms: filled, PBS; open, reovirus) and apoptotic (dot-plots) cells. (d) NOD/SCID mice were subcutaneously (s.c.) implanted with 1 × 106 TRAMP-C1 or 5 × 105 BPH-1 cells and evaluated for tumor growth. When tumors reached a diameter of 25 mm2, mice were intratumorally (i.t.) administered with 5 × 106 plaque-forming units (pfu) of UVRV/LRV or PBS at the time points indicated by arrows, and then monitored for tumor growth. (e) Immunocompetent C57BL/6 mice were implanted with 5 × 106 TRAMP-C1 cells. When tumors reached a diameter of 25 mm2, mice were administered i.t. with 5 × 108 pfu of UVRV/LRV or PBS, at every indicated time point, and monitored for tumor growth. The data is a representative of three independent experiments.
Next, the capacity of reovirus to target TRAMP-C1 tumors in vivo was evaluated in the presence or absence of an immune system. For this, TRAMP-C1 tumor-bearing SCID (Figure 1d) or C57BL/6 (Figure 1e) mice were treated with phosphate-buffered saline (PBS), UV-inactivated reovirus (UVRV) or live reovirus (LRV) and evaluated for the tumor growth. BPH-1-tumor-bearing SCID mice were used as controls. As shown, LRV, but not PBS/UVRV, induced complete regression of both TRAMP-C1 and BPH-1 tumors in SCID mice and attenuated the growth of TRAMP-C1 tumors in C57BL/6 mice. Cumulatively, these results showed that LRV targets TRAMP-C1 mouse cells in vitro as well as in vivo with a comparable efficiency as seen in human prostate cancer cells.
Reovirus initiates proinflammation and lymphoid cell infiltration in prostate cancer microenvironment
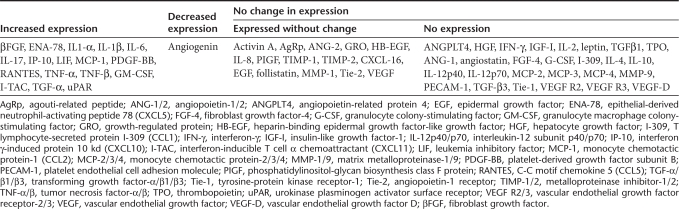
Immunosuppressive cytokines in the tumor microenvironment prevent the priming of antitumor immunity.23 Here, we evaluated whether reovirus acts on prostate tumor cells and initiates the production of cytokines conducive for T cell priming. For this, BPH-1 cells were cultured in the presence or absence of reovirus for 18 hours, and the resultant supernatants were directly evaluated using quantitative cytokine antibody array. As summarized in Table 1 and Supplementary Table S1, following reovirus exposure, BPH-1 cells produced significantly higher levels of proinflammatory cytokines, especially IL-1α, IL-6, RANTES, and granulocyte macrophage colony-stimulating factor, than that of unstimulated cells. Additionally, reovirus stimulated production of βFGF, IL-1β, IL-17, IP-10, MCP-1, TNF-α, I-TAC, and transforming growth factor-α that was undetectable in unstimulated cells. Of note, both stimulated and unstimulated BPH-1 cells produced high levels of follistatin and other soluble factors e.g., ANG-2, IL-8, TIMP-1, TIMP-2, CXCL-16, MMP-1, and VEGF. These cytokines can activate immune cells and direct their traffic into the tumor milieu.
Table 1. Summary of reovirus-induced cytokine protein expression by BPH-1 prostate cancer cells.
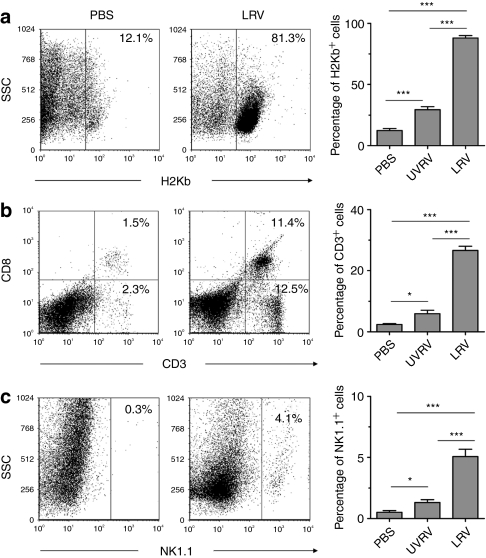
To test whether reovirus augments the tumor infiltration with immune cells, C57BL/6 mice-bearing TRAMP-C1 tumors were injected with PBS/UVRV/LRV (as per Figure 1e) and then analyzed for the presence of tumor-infiltrating lymphoid cells (TILs). Following LRV injection significantly higher percentages of H2Kb+ lymphoid cells (Figure 2a), especially CD3+, CD8+ T (Figure 2b), and NK (Figure 2c) lymphocytes, were observed in TRAMP-C1 tumors than that of PBS control. UVRV injection also induced a small influx of immune cells in the tumors, most probably through activation of toll-like receptors;24 however, the magnitude of such response was significantly lower than that of LRV. Together, data suggests that reovirus oncotherapy initiates higher production of inflammatory cytokines and homing of immune cells into the prostate tumor microenvironemnt.
Figure 2.
Reovirus induces homing of immune cell into prostate tumors. C57BL/6 mice-bearing transgenic adenocarcinoma of mouse prostate-C1 (TRAMP-C1) tumors were administered with therapeutic regimen of phosphate-buffered saline (PBS)/UV-inactivated reovirus (UVRV)/live reovirus (LRV). On day 3 post last injection (p.l.i.), tumor-infiltrating lymphoid cells (TILs) were isolated, stained, and directly analyzed in flow cytometry to determine the frequency of (a) H2Kb+ immune cells, (b) CD3+, CD8+ T cells, and (c) NK cells. Numbers listed on dot-plots indicate the percentages of cells present in a respective quadrant, while bar graphs represent the cumulative data from three independent experiments for respective cell subtype.
Reovirus enhances the expression of MHC molecules on prostate tumors and facilitates the display of TAA
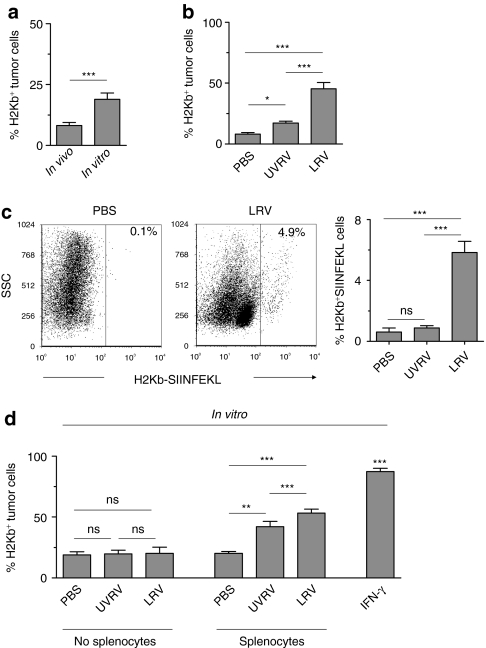
Tumors evade the recognition and attack by immune cells by down modulating the expression of major histocompatibility complex (MHC) molecules that display tumor-associated antigens (TAAs).23,25,26 As shown in Figure 3a, TRAMP-C1 cells express significantly lower levels of MHC class I (H2Kb) when grafted on C57BL/6 mice than when cultured in vitro. Hence, we first investigated whether reovirus alters H2Kb expression on tumors. For this, TRAMP-C1 tumors grafted on C57BL/6 mice were treated with PBS/UVRV/LRV, prepared in single-cell suspension, and then directly evaluated. As shown in Figure 3b, LRV-treated tumors showed significantly higher H2Kb expression than that of PBS/UVRV-treated tumors. This data demonstrates that reovirus oncotherapy enhances the expression of MHC class I molecules on tumors.
Figure 3.
Reovirus enhances the expression of major histocompatibility complex (MHC) class I molecules on prostate tumors and display of tumor-associated antigens (TAA) on antigen-presenting cells (APCs). (a) Transgenic adenocarcinoma of mouse prostate-C1 (TRAMP-C1) tumors from C57BL/6 mice were excised, digested with collagenase, and prepared as single-cell suspension. These TRAMP-C1 tumor cells, in parallel with in-vitro cultured cells, were stained with anti-H2Kb antibodies and analyzed in flow cytometry. (b) Single-cell suspensions prepared from the phosphate-buffered saline (PBS)/UV-inactivated reovirus (UVRV)/live reovirus (LRV)-treated TRAMP-C1 tumors (as per protocol described in Figure 1e) were stained with anti-H2Kb antibodies and analyzed in flow cytometry. Cumulative data from three experiments is shown. (c) TRAMP-C1 tumors expressing a surrogate TAA ovalbumin (ova; TRAMP-C1-ova)27 were grafted on C57BL/6 mice. The resultant tumors were injected with PBS/UV-inactivated reovirus (UVRV)/live reovirus (LRV) (PBS/UVRV/LRV) (as per protocol in Figure 1e). On day 5, TILs were isolated and stained with 25D1.16 antibody.47 Numbers on dot-plot represent the percentages of cells expressing the SIINFEKL–H2Kb complexes. Cumulative data from three independent experiments is shown in a bar graph. (d) TRAMP-C1 cells cultured in the presence or absence of splenocytes were added with PBS/UVRV/UVRV for 24 hours, stained with anti-H2Kb antibodies and analyzed. TRAMP-C1 cells stimulated with 10 units/ml of IFN-γ were used as a positive control. Events were separated based on size scatter properties to evaluate the expression of MHC class I on tumor cells. Data is representative of three independent experiments.
Next, we investigated whether reovirus also facilitates the presentation of prostate TAAs. For this, TRAMP-C1 tumors expressing ovalbumin (ova) as a surrogate TAA (TRAMP-C1-ova)27 were injected with PBS/UVRV/LRV and then processed to obtain TILs. The display of TAA on TIL surface was visualized with 25D1.16 antibody that detects an immunodominant ova peptide (SIINFEKL) complexed with H2Kb. As shown in Figure 3c, LRV-treated TRAMP-C1-ova tumors displayed the presence of significantly greater number of H2Kb-SIINFEKL expressing antigen-presenting cells (APCs) than that of PBS/UVRV-treated tumors, suggesting the exclusive ability of LRV to facilitate the presentation of prostate TAA.
To dissect whether H2Kb expression is caused by reovirus itself or mediated by reovirus-activated TILs, we evaluated H2Kb expression on TRAMP-C1 cells cultured with PBS/UVRV/LRV and incubated in the presence or absence of splenocytes. As shown in Figure 3d, only TRAMP-C1 cells incubated in the presence of both LRV or UVRV and lymphoid cells showed significantly elevated expression of H2Kb compared to the cells incubated with only PBS/LRV/UVRV or in the absence of lymphoid cells. These results suggest that the presence of lymphoid cells is necessary to augment the expression of MHC molecules on tumors. It is believed that, reovirus acts upon TILs to initiate the production of cytokines e.g., interferon-γ (IFN-γ), which in turn can augment the expression of MHC class I on tumor cells. Of note, we have recently shown that reovirus induces the production of IFN-γ from splenocytes.28 However, such IFN-γ secretion is not observed following the stimulation of prostate tumor cells with reovirus (Table 1 and Supplementary Table S1).
Reovirus enhances the presentation of prostate TAA to CD8 T cells
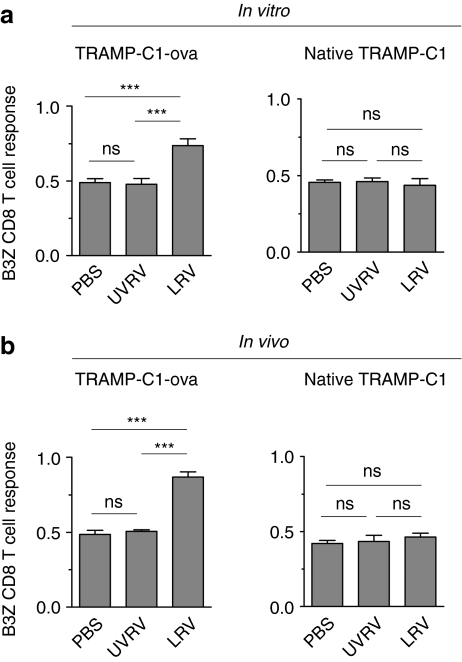
Next, we hypothesized that during oncotherapy, TAA-expressing APCs acquire a functional capacity to activate tumor-specific CD8 T cells. This hypothesis was tested by using the B3Z antigen presentation assay.29 B3Z, a CD8 T cell hybridoma, bears ova-specific T cell receptor and gets activated upon recognizing SIINFEKL–H2Kb complex. To this end, bone marrow-derived DCs (BMDCs) were incubated in the presence of native or ova-expressing TRAMP-C1 cells and PBS/UVRV/LRV, and then evaluated in the B3Z assay. As shown in Figure 4a, BMDCs cultured in the presence of LRV-treated TRAMP-C1-ova initiated significantly higher activation of B3Z cells than those incubated with PBS/UVRV-treated cells. BMDCs cultured in the presence of PBS/UVRV/LRV-treated native TRAMP-C1 cells failed to activate B3Z cells due to the lack of ova expression on the native tumor cells.
Figure 4.
Reovirus enhances the presentation of prostate tumor-associated antigen (TAA) to tumor-specific CD8 T cells. (a) 1 × 105 bone marrow-derived dendritic cells (BMDCs) cultured in the presence of 1 × 105 native TRAMP-C1 or TRAMP-C1-ova tumor cells were incubated with phosphate-buffered saline (PBS) or 1 multiplicity of infection (MOI)/cell of UV-inactivated reovirus (UVRV) or live reovirus (LRV) for 24 hours. Then, 5 × 104 B3Z cells were added to these cultures for an additional 18 hours and then evaluated for the breakdown of β-galactosidase substrate to define CD8 T cell activation. (b) Native or ova-expressing TRAMP-C1 tumor-bearing C57BL/6 mice were treated with PBS/UV-inactivated reovirus (UVRV)/live reovirus (LRV) (PBS/UVRV/LRV) (as per Figure 1e). On day 5 p.l.i., tumor-infiltrating dendritic cells were isolated and assessed in the B3Z antigen presentation assay (see Materials and Methods section). Data is representative of four independent experiments.
Such a capacity of reovirus was further analyzed in the tumor-infiltrating DCs collected from PBS/UVRV/LRV-treated mice. As shown in Figure 4b, only DCs isolated from LRV-treated TRAMP-C1-ova tumors initiated activation of ova-specific CD8 T cells. Taken together, our results suggest that LRV exclusively endows either in vitro propagated BMDCs or tumor-infiltrating DCs with a capacity to successfully present TAA to tumor-specific CD8 T cells.
Reovirus induces antiprostate cancer T cell immune response
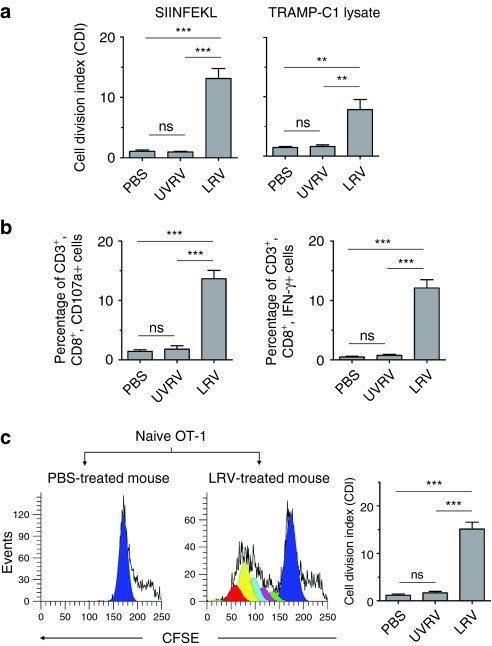
Next, we proceeded to determine whether reovirus oncotherapy initiates antiprostate cancer T cell responses, using proliferation and IFN-γ/CD107a production assays. First, TILs from PBS/UVRV/LRV-treated mice were stimulated in vitro and then analyzed in the proliferation and IFN-γ/CD107a assay. As shown in Figure 5a, CD3+ T cells from LRV-treated native or ova-expressing tumors underwent significant proliferation following stimulation with the peptide SIINFEKL or TRAMP tumor lysate, respectively, whereas respective T cells obtained from PBS/UVRV-injected mice did not show such reactivity. Additionally, TILs collected from LRV-treated mice contained significantly higher number of intrinsic IFN-γ as well as CD107a-producing CD3+, CD8+ T cells than those from UVRV/PBS-treated animals (Figure 5b).
Figure 5.
Reovirus oncotherapy initiates antiprostate T cell immunity. Native or ova-expressing transgenic adenocarcinoma of mouse prostate-C1 (TRAMP-C1) tumor-bearing C57BL/6 mice were injected with phosphate-buffered saline (PBS)/UV-inactivated reovirus (UVRV)/live reovirus (LRV) (PBS/UVRV/LRV) (as per Figure 1e). On day 7 post last injection (p.l.i), tumor-infiltrating lymphoid cells (TILs) were isolated. (a) TILs were labeled with CFSE from native or ova-expressing tumors were stimulated with SIINFEKL or TRAMP-C1 tumor lysate, respectively, and then analyzed in proliferation assay. (b) TILs from PBS/UVRV/LRV-treated TRAMP-C1-ova tumors were stimulated with SIINFEKL for 6 hours and then evaluated in interferon-γ (IFN-γ)/CD107a assay. (c) B6.PL-Thy1a/CYJ mice (thy1.1+; congenic to C57BL/6) were injected with TRAMP-C1-ova tumor cells, allowed to develop tumors and then injected with PBS/UV-inactivated reovirus (UVRV)/live reovirus (LRV) (PBS/UVRV/LRV) (as per Figure 1e). After 5 days p.l.i., these thy1.1+ mice were injected (i.v.) with naive, CFSE-labeled ova-specific transgenic T cells isolated from the spleens of OT-1 mice (thy1.2+). After 7 days, the lymph nodes of recipient thy1.1 mice were harvested, stained with anti-thy1.2 and CD3 antibodies, and assessed in CFSE assay. Histograms display characteristic halving of CFSE fluorescence in proliferating CD3+, thy1.2+ OT-1 donor cells as analyzed with ModFit LT. Sequential daughter cell generations present in proliferating donor cells are represented by various shades/colors in histograms. Bar graph represents a cumulative data from three independent experiments.
We then evaluated whether the oncotherapy-modulated immunological microenvironment can support the priming of externally transferred naive, tumor-specific T cells in vivo. To this end, CFSE-labeled ova-specific transgenic T cells (OT-1; Thy1.2+)30 were adoptively transferred into the PBS/UVRV/LRV-treated, TRAMP-C1-ova bearing B6.PL-Thy1a/CYJ mice (thy1.1+) and monitored for cell division in CFSE assay. As shown in Figure 5c, CD3+, thy1.2+ T cells from LRV-treated mice displayed characteristic halving of CFSE fluorescence, whereas such a reduction of fluorescence was absent in T cells obtained from PBS-treated animals. As summarized in bar graph (Figure 5c), OT-1 cells transferred into LRV-treated mice became activated in vivo and underwent cell division. Together, our results suggest that reovirus oncotherapy primes the intrinsic prostate tumor-specific T cell responses identifying not only surrogate but also native tumor antigens.
Oncotherapy-initiated antiprostate T cell responses protect against subsequent tumor challenge in an antigen-specific manner
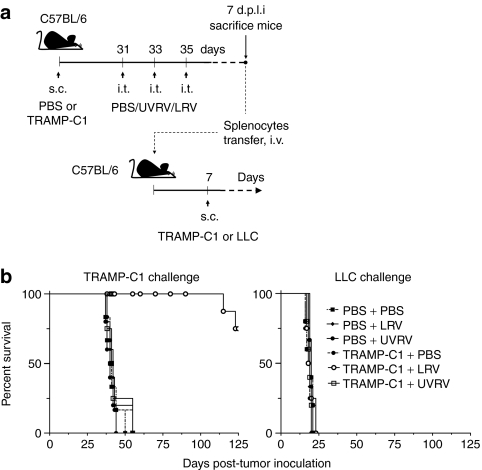
Antitumor immune responses bear clinical significance only if they can restrict tumor growth and/or relapse. Hence, we evaluated the capacity of reovirus oncotherapy-induced antitumor T cell responses to protect against subsequent tumor challenge. For this purpose, splenocytes from PBS/UVRV/LRV-treated mice were adoptively injected into cancer-naive mice and analyzed for their capacity to restrict the growth of freshly implanted TRAMP-C1 or Lewis lung carcinoma cells (Figure 6a). As shown in Figure 6b (left graph), only lymphocytes from LRV-treated, TRAMP-C1 tumor-bearing mice increased the survival in TRAMP-C1 challenged mice than did the lymphocytes from PBS/UVRV-treated mice. These results demonstrate that (i) reovirus oncotherapy-initiated antitumor T cell responses can restrict the growth of freshly implanted tumor cells and (ii) the induction of the protective antitumor immunity is exclusively associated with oncotherapy comprising of LRV. This data also shows that, once appropriately activated, antitumor responses act in a reovirus-independent manner as the protection against tumor challenge is achieved without administrating secondary reovirus injection. Finally, splenocytes collected from all above-mentioned study groups, including LRV-treated TRAMP-C1 tumor mice, failed to affect survival in the mice challenged with Lewis lung carcinoma cells and demonstrated that the oncotherapy-initiated antitumor immune responses can target only syngenic, antigenically homogeneous tumors (Figure 6b, right graph). Taken together, the data from this experiment conclusively show that reovirus-initiated antiprostate cancer immunity confers protection against subsequent tumor challenge in an antigen-dependent but reovirus-independent manner.
Figure 6.
Oncotherapy-initiated antitumor immunity protects against subsequent tumor challenge. (a) C57BL/6 mice were subcutaneously (s.c.) injected with phosphate-buffered saline (PBS) or transgenic adenocarcinoma of mouse prostate-C1 (TRAMP-C1) cells, and then administered with therapeutic regimen of PBS, UV-inactivated reovirus (UVRV), or live reovirus (LRV) at indicated time points. Seven days p.l.i., splenocytes were harvested and adoptively transferred into naive C57BL/6 mice. (b) After additional 7 days, recipient mice were further challenged with either 5 × 106 TRAMP-C1 or 5 × 105 Lewis lung carcinoma (LLC) tumor cells and monitored for tumor growth to determine survival.
Discussion
For the first time, our study conclusively demonstrates that the oncotherapy-initiated immunological events override prostate cancer-associated immune evasion mechanisms and prime “clinically meaningful” antitumor immunity. Thus far, the premise of antitumor immunotherapy has been received with some skepticism in view of the low immunogenicity, self-reactivity, and/or unavailability of tumor antigens. However, the unique anatomical and developmental features of the prostate make it an ideal candidate for immunotherapy. The relatively slow growth of prostate cancer would allow the body sufficient time to generate antiprostate immune response.3 Evidence demonstrating the presence of TILs and prostate antigen-specific antibodies suggests that prostate cancer is more immunogenic than was previously thought.31,32 Furthermore, advances in proteomics and genomics have identified several tissue-specific proteins that can be utilized for the precise development of prostate-specific immunotherapies.33,34,35 Most importantly, the nonessential nature of the prostate gland makes prostate-specific immunotherapies a safer option than in other cancers, in the event these therapies also target normal tissue instead of just cancerous cells.36
The tumor-associated immune suppressive microenvironment aids in the persistence of prostate cancer. For example, tumor cells and the surrounding stroma secrete proteins like IL-10 and transforming growth factor-β and contribute in local and systemic immunosuppression. In our study, reovirus acts upon prostate cancer cells and augments the production of inflammatory cytokines. We have recently shown that reovirus also initiates similar responses from DCs and other lymphoid cells.28 Such production of inflammatory cytokines from the tumor and the residing immune cells can create a chemoattracting gradient to direct the traffic of immune cells to a tumor site where reovirus preferentially replicates. This notion agrees with our observation that higher numbers of T and NK cells are found in prostate tumors following an intratumoral LRV injection. It is important to note that the patients with higher frequency of TILs in their tumor specimens also live on an average 10 years longer than those with no TILs,37 suggesting a possible clinical benefit of reovirus-initiated immune cell infiltration.
Tumor-specific T cells identify their targets by recognizing TAAs presented through MHC molecules. However, tumors evolve strategies to avoid TAA presentation. Metastatic human prostate cancer cells underexpress MHC and associated molecules, e.g., β2 microglobulin and antigen peptide transporter 2, involved in antigen presentation.25,26 In our study TRAMP-C1 cells expressed less MHC class I molecules when grafted on immunocompetent animals as compared to when cultured in vitro. This data suggests a possible antigen presentation evasion strategy by which prostate tumors can avoid recognition by CD8+ T cells. Importantly, reovirus oncotherapy overturns such inhibition. However, reovirus alone is unable to induce such an increase in MHC expression in vitro. Our study shows that reovirus acts upon lymphoid cells to produce inflammatory cytokines, which then stimulate the increase in MHC expression on tumor cells. Additionally, reovirus increases the TAA display and presentation. Taken together it can be surmised that reovirus oncotherapy overrides the prostate cancer-associated antigen presentation anomalies.
Contrary to previously published report38 UVRV also initiated tumor infiltration by immune cells, albeit of a significantly lesser magnitude than that of LRV. We have previously shown that UVRV can initiate the production of proinflammatory cytokines and maturation of DCs,28 most likely through the activation of toll-like receptor pathway.39 UVRV could initiate similar immune response in vivo and direct the influx of immune cells into the tumor milieu. This type of immune response is exploited by the toll-like receptor-based adjuvants40 and observed in the cultured prostate cancer cells.24 Of note, the experiments done in previous report38 had used heat, instead of UV, to inactivate reovirus, which can potentially disrupt the tertiary molecular structures of pathogen-associated molecular patterns that may lead to the failure of toll-like receptor stimulation. Nonetheless, UVRV fails to induce TAA presentation and subsequent activation of antitumor immunity. Our view is that since UVRV is unable to lyse tumor cells, it fails to expose TAAs for the processing by APCs required for the activation of tumor-specific T cells.
Prostate-cancer infiltrating CD8+ lymphocytes display impaired functionalities.41,42 In this study, our data suggest that reovirus therapy appropriately activates proliferative and IFN-γ/CD107a response from CD4+ and CD8+ T cells, respectively. These antitumor immune responses protect against subsequent tumor challenge in a reovirus-independent manner as the growth of tumors after challenge is solely inhibited by adoptively transferred immune cells. Such antiprostate immune responses bear a potential to maintain active surveillance against cancer relapse at local as well as distant metastatic sites even after discontinuation of oncolytic virus therapy.
Materials and Methods
Reovirus and cell lines. Reovirus (serotype 3, Dearing strain) was grown and purified as previously described.12 BPH-1, LNCap, DU145, PC-3, Lewis lung carcinoma, and TRAMP-C1 cell lines were originally purchased from ATCC, Manassas, VA. TRAMP-C1-ova27 and B3Z29 cells were kindly provided by Dr Weinberg, Providence Portland Medical Center, Portland, OR and Dr N. Shastri, University of California, Berkeley, CA, respectively.
Antibodies and reagents. Antibodies and reagents purchased from respective vendors: eBioscience (San Diego, CA): PerCp-Nk1.1, Alexa647-anti-H2Kb, Alexa 488-anti-CD11c, PE-anti-mouse MHC class I molecule Kb bound to the peptide SIINFEKL (25-D1.16), unconjugated anti-mouse CD16/32, unconjugated anti-mouse CD49d; Invitrogen (Carlsbad, CA): PE-anti-CD3, Alexa 488-IFN-γ, APC-anti-CD107a, unconjugated anti-mouse CD28, 5- (and -6)-carboxyfluorescein diacetate (CFSE); PerCp anti-CD90.2; BD Biosciences (Mississauga, ON): PerCp-anti-CD8. GenScript (Piscataway, NJ): SIINFEKL (ova257B264) and KAVYNFATM (LCMV gp33–41) peptides. Ras activation assay kit (Millipore, Billerica, MA) was used according to manufacturer's instructions to detect Ras protein.
Animals and in vivo experimental manipulations. The experimental procedures were governed by the ethics committee at Dalhousie University, Halifax, Nova Scotia, Canada. Eight- to ten-weeks old wild-type male C57BL/6 mice (thy1.2+) were obtained from Charles River Laboratory (Montreal, Quebec, Canada), whereas B6.PL-Thy1a/CYJ (thy1.1+; congenic to thy1.2+ C57BL/6 mice) and OT-1 mice were purchased from the Jackson Laboratories (Bar Harbor, ME). Transgenic OT-1 mice bear T cell receptor Vα2β5 specific for the ova257B264 peptide (SIINFEKL).30
Isolation of lymphocytes, enrichment of DCs, and generation of BMDCs. Ficoll–Paque Plus (GE Healthcare, Uppsala, Sweden) density gradient was used to isolate the lymphocytes from spleens, lymph nodes, or tumors as previously described.43,44 These lymphoid cells were incubated with MACS beads (Invitrogen) containing anti-CD3, anti-CD14, anti-B220 antibodies, and then passed through magnetic column to obtain enriched DCs by negative selection.
Bone marrow cells were collected from femur and tibia bones and cultured for 6–8 days in RPMI 1640 medium supplemented with 10% fetal calf serum, 2 mmol/l glutamine, 0.1 mmol/l nonessential amino acids, 50 U/ml penicillin/streptomycin, 0.1 mmol/l 2-ME, and 20 ng/ml granulocyte macrophage colony-stimulating factor to obtain BMDCs.
Cytokine production by prostate cancer cells. BPH-1 cells were exposed to 1 multiplicity of infection of reovirus or PBS. After 18–24 hours, culture supernatants were directly evaluated for the presence of 60 cytokines using Antibody-based Quantitative Cytokine Arrays (Raybiotech, Norcross, GA). The change in cytokine level was considered significant when the difference between the means of cytokine concentrations from stimulated and unstimulated samples was ±2.1 fold.
B3Z antigen presentation assay. 1 × 105 BMDCs cocultured with 1 × 105 reovirus-treated tumor cells or enriched DCs obtained from tumor were added with 1 × 105 B3Z CD8 T cells for 18–24 hours. Then, cultures were supplemented with 0.15 mmol/l of chlorophenol red-β--galactopyranoside and incubated for additional 4 hours. The breakdown of chlorophenol red-β--galactopyranoside was visualized by reading absorbance at 570 nm and defined as a CD8 T cell response.29
Analysis of antitumor T cell response in vitro and in vivo. Lymphocytes from PBS/UVRV/LRV-treated tumor-bearing mice were labeled with 1 µmol/l of CFSE dye44 and then stimulated with BMDCs pulsed with SIINFEKL, tumor lysate45 and control LCMVgp33–41 peptide (5 µg/ml) or left unstimulated. After 5 days, cells were harvested, stained with anti-CD3 antibodies and analyzed in flow cytometry to define cell division index.44 The intracellular production of IFN-γ and degranulation of CD107a (lysosomal-associated membrane protein-1; LAMP-1) in CD3+, CD8+ T cells following stimulation with 5 µg/ml of SIINFEKL or control peptide was detected as described.46
To visualize the priming of antiprostate T cell response in vivo, splenocytes from naive OT-1 mice were collected, labeled with CFSE and then transferred into PBS/UVRV/LRV-treated, TRAMP-C1-ova bearing B6.PL-Thy1a/CYJ mice. After 5 days, lymphocytes from lymph nodes were stained with anti-thy1.2 and anti-CD3 antibodies and analyzed in flow cytometry.
Antitumor immunity-mediated protection against tumor challenge. Splenocytes from PBS/UVRV/LRV-treated TRAMP-C1 tumor-bearing and nontumor-bearing C57BL/6 mice were transferred into naive C57BL/6 mice as per protocol shown in Figure 6a. After 7 days, recipient mice were challenged with TRAMP-C1 or Lewis lung carcinoma cells and monitored for the development of tumors. Tumor dimensions were calculated by measuring longest diameter of the tumor X smallest diameter of the tumor and expressed as mm2. Animals were sacrificed when tumors reached 250 mm2 to define survival.
Flow cytometry and statistical analysis. Flow cytometry data was acquired with BD FACSCalibur cytometer and analyzed with BD CellQuest Pro, FCS Express V3 and/or ModFit LT softwares. The statistical analysis was performed using two-tailed, Student t-test or Kaplan–Meier survival analysis. P values of <0.05 were considered statistically significant and represented as: *P < 0.05, **P < 0.005, ***P < 0.0001.
SUPPLEMENTARY MATERIAL Table S1. Quantitation of proinflammatory cytokines and chemokines produced by BPH-1 cells in the presence or absence of reovirus.
Acknowledgments
This work was supported by an operating grant from the Canadian Cancer Society Research Institute (CCSRI) through the Terry Fox Foundation, and Canadian Institutes for Health Research (CIHR) to P.W.K.L. S.A.G. and D.P. receive postdoctoral and doctoral fellowships, respectively, from the Cancer Research Training Program (CRTP).
Supplementary Material
Quantitation of proinflammatory cytokines and chemokines produced by BPH-1 cells in the presence or absence of reovirus.
REFERENCES
- Jemal A, Siegel R, Ward E, Hao Y, Xu J., and, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- Fong L., and, Small EJ. Immunotherapy for prostate cancer. Semin Oncol. 2003;30:649–658. doi: 10.1016/s0093-7754(03)00350-6. [DOI] [PubMed] [Google Scholar]
- Drake CG. Immunotherapy for prostate cancer: walk, don't run. J Clin Oncol. 2009;27:4035–4037. doi: 10.1200/JCO.2009.22.2299. [DOI] [PubMed] [Google Scholar]
- Drake CG. Prostate cancer as a model for tumour immunotherapy. Nat Rev Immunol. 2010;10:580–593. doi: 10.1038/nri2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirukkumaran CM, Nodwell MJ, Hirasawa K, Shi ZQ, Diaz R, Luider J, et al. Oncolytic viral therapy for prostate cancer: efficacy of reovirus as a biological therapeutic. Cancer Res. 2010;70:2435–2444. doi: 10.1158/0008-5472.CAN-09-2408. [DOI] [PubMed] [Google Scholar]
- Vieweg JW. Prostate cancer: targeting complexity. Curr Opin Urol. 2008;18:261–262. doi: 10.1097/MOU.0b013e3282f9b413. [DOI] [PubMed] [Google Scholar]
- McNeel DG, Dunphy EJ, Davies JG, Frye TP, Johnson LE, Staab MJ, et al. Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase in patients with stage D0 prostate cancer. J Clin Oncol. 2009;27:4047–4054. doi: 10.1200/JCO.2008.19.9968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling A, Füssel S, Wehner R, Bachmann M, Wirth MP, Rieber EP, et al. Advances in specific immunotherapy for prostate cancer. Eur Urol. 2008;53:694–708. doi: 10.1016/j.eururo.2007.11.043. [DOI] [PubMed] [Google Scholar]
- Drake CG., and, Antonarakis ES. Update: immunological strategies for prostate cancer. Curr Urol Rep. 2010;11:202–207. doi: 10.1007/s11934-010-0106-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonarakis ES., and, Drake CG. Current status of immunological therapies for prostate cancer. Curr Opin Urol. 2010;20:241–246. doi: 10.1097/MOU.0b013e3283381793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcato P, Shmulevitz M., and, Lee PW. Connecting reovirus oncolysis and Ras signaling. Cell Cycle. 2005;4:556–559. doi: 10.4161/cc.4.4.1600. [DOI] [PubMed] [Google Scholar]
- Coffey MC, Strong JE, Forsyth PA., and, Lee PW. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282:1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- Kelly K, Nawrocki S, Mita A, Coffey M, Giles FJ., and, Mita M. Reovirus-based therapy for cancer. Expert Opin Biol Ther. 2009;9:817–830. doi: 10.1517/14712590903002039. [DOI] [PubMed] [Google Scholar]
- Lal R, Harris D, Postel-Vinay S., and, de Bono J. Reovirus: Rationale and clinical trial update. Curr Opin Mol Ther. 2009;11:532–539. [PubMed] [Google Scholar]
- Harrington KJ, Vile RG, Melcher A, Chester J., and, Pandha HS. Clinical trials with oncolytic reovirus: moving beyond phase I into combinations with standard therapeutics. Cytokine Growth Factor Rev. 2010;21:91–98. doi: 10.1016/j.cytogfr.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcato P, Shmulevitz M, Pan D, Stoltz D., and, Lee PW. Ras transformation mediates reovirus oncolysis by enhancing virus uncoating, particle infectivity, and apoptosis-dependent release. Mol Ther. 2007;15:1522–1530. doi: 10.1038/sj.mt.6300179. [DOI] [PubMed] [Google Scholar]
- Shmulevitz M, Marcato P., and, Lee PW. Activated Ras signaling significantly enhances reovirus replication and spread. Cancer Gene Ther. 2010;17:69–70. doi: 10.1038/cgt.2009.46. [DOI] [PubMed] [Google Scholar]
- Shmulevitz M, Marcato P., and, Lee PW. Unshackling the links between reovirus oncolysis, Ras signaling, translational control and cancer. Oncogene. 2005;24:7720–7728. doi: 10.1038/sj.onc.1209041. [DOI] [PubMed] [Google Scholar]
- Prestwich RJ, Ilett EJ, Errington F, Diaz RM, Steele LP, Kottke T, et al. Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication. Clin Cancer Res. 2009;15:4374–4381. doi: 10.1158/1078-0432.CCR-09-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwich RJ, Harrington KJ, Vile RG., and, Melcher AA. Immunotherapeutic potential of oncolytic virotherapy. Lancet Oncol. 2008;9:610–612. doi: 10.1016/S1470-2045(08)70163-3. [DOI] [PubMed] [Google Scholar]
- Errington F, Steele L, Prestwich R, Harrington KJ, Pandha HS, Vidal L, et al. Reovirus activates human dendritic cells to promote innate antitumor immunity. J Immunol. 2008;180:6018–6026. doi: 10.4049/jimmunol.180.9.6018. [DOI] [PubMed] [Google Scholar]
- Foster BA, Gingrich JR, Kwon ED, Madias C., and, Greenberg NM. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997;57:3325–3330. [PubMed] [Google Scholar]
- Rabinovich GA, Gabrilovich D., and, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli R, Starace D, Busà R, Angelini DF, Paone A, De Cesaris P, et al. TLR stimulation of prostate tumor cells induces chemokine-mediated recruitment of specific immune cell types. J Immunol. 2010;184:6658–6669. doi: 10.4049/jimmunol.0902401. [DOI] [PubMed] [Google Scholar]
- Younger AR, Amria S, Jeffrey WA, Mahdy AE, Goldstein OG, Norris JS, et al. HLA class II antigen presentation by prostate cancer cells. Prostate Cancer Prostatic Dis. 2008;11:334–341. doi: 10.1038/sj.pcan.4501021. [DOI] [PubMed] [Google Scholar]
- Sanda MG, Restifo NP, Walsh JC, Kawakami Y, Nelson WG, Pardoll DM, et al. Molecular characterization of defective antigen processing in human prostate cancer. J Natl Cancer Inst. 1995;87:280–285. doi: 10.1093/jnci/87.4.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond WL, Gough MJ, Charbonneau B, Ratliff TL., and, Weinberg AD. Defects in the acquisition of CD8 T cell effector function after priming with tumor or soluble antigen can be overcome by the addition of an OX40 agonist. J Immunol. 2007;179:7244–7253. doi: 10.4049/jimmunol.179.11.7244. [DOI] [PubMed] [Google Scholar]
- Gujar SA, Marcato P, Pan D., and, Lee PW. Reovirus virotherapy overrides tumor antigen presentation evasion and promotes protective antitumor immunity. Mol Cancer Ther. 2010;9:2924–2933. doi: 10.1158/1535-7163.MCT-10-0590. [DOI] [PubMed] [Google Scholar]
- Sanderson S., and, Shastri N. LacZ inducible, antigen/MHC-specific T cell hybrids. Int Immunol. 1994;6:369–376. doi: 10.1093/intimm/6.3.369. [DOI] [PubMed] [Google Scholar]
- Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ., and, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Wang X, Yu J, Sreekumar A, Varambally S, Shen R, Giacherio D, et al. Autoantibody signatures in prostate cancer. N Engl J Med. 2005;353:1224–1235. doi: 10.1056/NEJMoa051931. [DOI] [PubMed] [Google Scholar]
- McNeel DG., and, Malkovsky M. Immune-based therapies for prostate cancer. Immunol Lett. 2005;96:3–9. doi: 10.1016/j.imlet.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Taylor BS, Varambally S., and, Chinnaiyan AM. Differential proteomic alterations between localised and metastatic prostate cancer. Br J Cancer. 2006;95:425–430. doi: 10.1038/sj.bjc.6603274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes DR, Barrette TR, Rubin MA, Ghosh D., and, Chinnaiyan AM. Meta-analysis of microarrays: interstudy validation of gene expression profiles reveals pathway dysregulation in prostate cancer. Cancer Res. 2002;62:4427–4433. [PubMed] [Google Scholar]
- Vieweg J. Immunotherapy for advanced prostate cancer. Rev Urol. 2007;9 Suppl 1:S29–S38. [PMC free article] [PubMed] [Google Scholar]
- Harzstark AL., and, Small EJ. Immunotherapeutics in development for prostate cancer. Oncologist. 2009;14:391–398. doi: 10.1634/theoncologist.2008-0240. [DOI] [PubMed] [Google Scholar]
- Vesalainen S, Lipponen P, Talja M., and, Syrjänen K. Histological grade, perineural infiltration, tumour-infiltrating lymphocytes and apoptosis as determinants of long-term prognosis in prostatic adenocarcinoma. Eur J Cancer. 1994;30A:1797–1803. doi: 10.1016/0959-8049(94)e0159-2. [DOI] [PubMed] [Google Scholar]
- Diaz RM, Galivo F, Kottke T, Wongthida P, Qiao J, Thompson J, et al. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007;67:2840–2848. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- Finberg RW, Wang JP., and, Kurt-Jones EA. Toll like receptors and viruses. Rev Med Virol. 2007;17:35–43. doi: 10.1002/rmv.525. [DOI] [PubMed] [Google Scholar]
- Steinhagen F, Kinjo T, Bode C., and, Klinman DM.2010TLR-based immune adjuvants Vaccineepub ahead of print). [DOI] [PMC free article] [PubMed]
- Sfanos KS, Bruno TC, Meeker AK, De Marzo AM, Isaacs WB., and, Drake CG. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1+ Prostate. 2009;69:1694–1703. doi: 10.1002/pros.21020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfanos KS, Bruno TC, Maris CH, Xu L, Thoburn CJ, DeMarzo AM, et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujar SA., and, Michalak TI. Primary occult hepadnavirus infection induces virus-specific T-cell and aberrant cytokine responses in the absence of antiviral antibody reactivity in the Woodchuck model of hepatitis B virus infection. J Virol. 2009;83:3861–3876. doi: 10.1128/JVI.02521-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujar SA., and, Michalak TI. Flow cytometric quantification of T cell proliferation and division kinetics in woodchuck model of hepatitis B. Immunol Invest. 2005;34:215–236. [PubMed] [Google Scholar]
- Ladekarl M, Agger R, Fleischer CC, Hokland M, Hulgaard EF, Kirkin A, et al. Detection of circulating tumor lysate-reactive CD4+ T cells in melanoma patients. Cancer Immunol Immunother. 2004;53:560–566. doi: 10.1007/s00262-004-0502-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- Porgador A, Yewdell JW, Deng Y, Bennink JR., and, Germain RN. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity. 1997;6:715–726. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quantitation of proinflammatory cytokines and chemokines produced by BPH-1 cells in the presence or absence of reovirus.