Abstract
We have recently developed a retargeting system for lentiviral vectors (LVs) that relies on the pseudotyping of LVs with engineered measles virus (MV) glycoproteins (hemagglutinin (H) and fusion protein (F)). Specificity is provided through display of a single-chain antibody (scFv) as targeting domain by fusion to the MV-H protein. As an alternative to scFv, designed ankyrin repeat proteins (DARPins) can be selected to become high-affinity binders to any kind of target molecule. In this study six HER2/neu-specific DARPins exhibiting different affinities and binding to different HER2/neu epitopes were applied as targeting domains. All H-DARPin fusion proteins were efficiently expressed on the cell surface. Upon coexpression with F, syncytia formation was observed in HER2/neu positive cells only and correlated directly with the HER2/neu receptor density. All H-DARPin proteins incorporated into LVs, albeit at different levels. The vectors only transduced HER2/neu-positive cells, while HER2/neu-negative cells remained untransduced. Highest titers were observed with one particular DARPin binding to the membrane distal domain of HER2/neu with medium affinity. When applied in vivo systemically, HER2/neu-targeted LVs showed exclusive gene expression in HER2/neu positive tumor tissue, while vesicular stomatitis virus-glycoprotein (VSV-G) pseudotyped vectors mainly transduced cells in spleen and liver. Thus, DARPins are a promising alternative to scFvs for retargeting of LVs.
Introduction
Virus-derived vectors have become one of the most promising gene delivery systems for human cells. Especially lentiviral vectors (LVs) provide an outstanding therapeutic potential by stable long-term transgene expression in nondividing cells.1,2 The particle surface design of γ-retroviral vectors and LVs has been constantly improved during the last 20 years, starting with the use of murine leukemia virus envelope proteins, those of other γ-retroviruses and finally the glycoprotein (G) of the vesicular stomatitis virus (VSV), which mediates efficient transduction of basically all human cell types.3 More recent developments aim at restricting the vector tropism at the level of cell entry to the target cell population defined by a specific surface antigen. It is expected that this will facilitate in vivo gene transfer strategies and reduce the vector dose required for successful therapy. Promising progress in this direction has recently been obtained by engineering viral envelope proteins that have the receptor attachment and membrane fusion functions separated into two glycoproteins.4,5,6,7
The measles virus (MV) glycoproteins, namely, hemagglutinin (H) and fusion protein (F), mediate cell entry directly at the cell membrane in a pH-independent manner.8 The H-protein attaches the virus to the natural MV receptors CD46 and signaling lymphocyte-activation molecule and forms a complex with F protein to accomplish its fusion helper function. We have recently demonstrated that F and H can be incorporated into LVs, provided their N-terminal cytoplasmic tails are truncated.9 Such MV–LVs were then retargeted to various cell types using a mutant of the H-protein with 4 point mutations that lost use of its natural receptors and fusing a cell type-specific single-chain antibody (scFv) to the C-terminus of the H-protein extracellular domain.9,10
The aim of this study was to evaluate designed ankyrin repeat proteins (DARPins) as an alternative targeting domain to scFvs for MV–LVs. DARPins are based on naturally occurring ankyrin repeat proteins—a ubiquitously expressed protein family that mediates specific protein–protein interactions.11 By a consensus-design approach an ankyrin repeat module comprising 33 amino acid residues was derived in which residues that potentially can interact with the target are randomized. By combining 2–3 of these repeats and flanking them by N- and C-capping repeats (termed N2C or N3C), combinatorial libraries of DARPins have been obtained. These libraries can be selected against almost any kind of ligand using ribosome display (library diversities >1012) or phage display (library diversity >1010).12,13 Specific binders with affinities in the pmol/l range have been obtained at high frequency. These molecules have no cysteines, and show very low tendencies of aggregation. They may thus have great promise in their application as fusion proteins to membrane proteins as the ones investigated here, where other fused targeting proteins, such as some aggregation-prone scFv fragments, would compromise the efficiency of correct assembly and thereby lead to low display levels.
Here, we report that DARPins can be used to re-target LVs to HER2/neu positive cancer cells. Belonging to the epidermal growth factor family HER2/neu is a type-I receptor tyrosine kinase, which is highly expressed on breast, ovarian, colon and pancreatic cancer cells but shows low level or no expression on all normal human tissue.14 By using a panel of six DARPins exhibiting distinct affinities for HER2/neu and using different binding sites, we could show that chimeric H-DARPin proteins are well expressed and incorporated in LVs and able to redirect membrane fusion via HER2/neu. The data suggest that a membrane distal binding site for HER2/neu mediates optimal cell entry of DARPin displaying LVs. By systemic application in a bilateral xenograft mouse model we demonstrate the in vivo targeting potential of these alternative binding molecules.
Results
H-DARPin surface expression levels
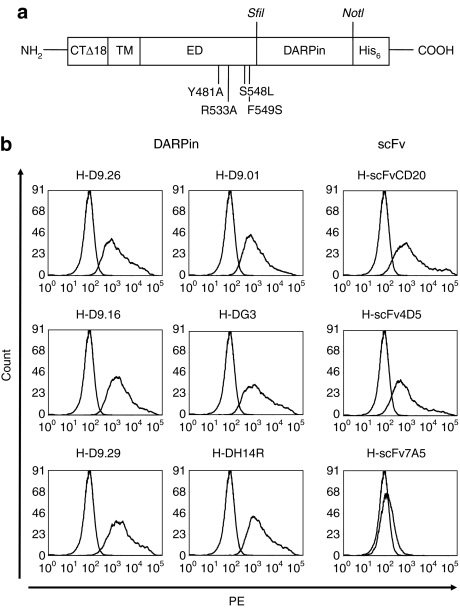
Starting from plasmid pCG-H-α-CD20 encoding an H-protein mutated in its receptor binding sites and fused to a CD20-specific scFv,9 we substituted the scFv coding sequence for that of six HER2/neu-specific DARPins15,16 resulting in constructs pH-D9.29, pH-D9.26, pH-D9.16, pH-D9.01, pH-DH14R, and pH-DG3 (Figure 1a). As controls, we also generated an expression plasmid for an H-protein displaying the HER2/neu-specific scFv 4D5 (pH-scFv4D5)17 and scFv 7A5, which does not allow cell surface expression as H fusion protein.18 As sufficient surface expression of H fusion proteins is a critical prerequisite for the efficient pseudotyping of LVs, we transfected cells with the H-DARPin expression plasmids and determined the cell surface expression levels by fluorescence-activated cell sorter analysis. All six H-DARPin proteins showed high surface expression rates in a similar range (mean fluorescence intensity 1,500–3,200) (Figure 1b). H-DARPin-9.29 exhibited the highest surface expression (mean fluorescence intensity = 3,200), which was about twice that of the H-scFvCD20 protein (mean fluorescence intensity = 1,600) and almost three times higher than that of H-scFv4D5 (MFI = 1,100).
Figure 1.
Organisation of hemagglutinin (H)-designed ankyrin repeat proteins (DARPins) and cell surface expression. (a) Schematic drawing of the modified MV-H used for retargeting, derived from strain Edmonston B (SwissProt P08362).31 Four point mutations in the ectodomain (ED) ablating binding to CD46 and signaling lymphocyte-activation molecule (the natural receptors) are indicated.9 An N-terminal cytoplasmic tail truncation by 18 amino acids (CTΔ18) allows stable incorporation into lentiviral particles.9 The C-terminus of H is fused via a SfiI site to the DARPin, resulting in the sequence EDGTNAAQPADLGKKL where the first underlined sequence is the C-terminus of H and the second is the N-cap of the DARPin. The DARPin terminates in the sequence EILQKLNAAARGSHHHHHH (DARPin C-cap is underlined). The transmembrane domain (TM) and the His-tag are indicated. (b) Expression plasmids for six different H-DARPin and three H-scFv fusion proteins were transfected into HEK-293T cells. Surface expression was analyzed after 48 hours using a His-tag-specific antibody (PE-labeled). H-scFvCD20 and H-scFv7A5 were used as positive and negative controls.
H-DARPin fusion helper function
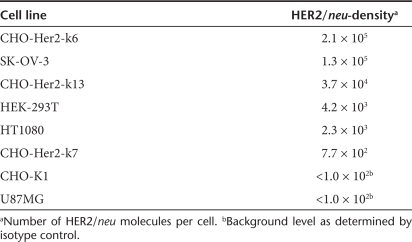
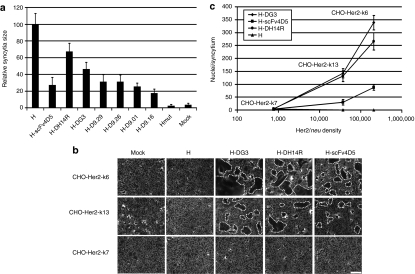
Besides high surface expression levels of the H-DARPin proteins, it is essential that their fusion helper function is maintained for successful retargeting of LVs. To assess the fusion helper function of the H-DARPin proteins, the corresponding expression plasmids were co-transfected with pCG-FΔ30 (encoding a C-terminally truncated F protein,9 Figure 1a) into SK-OV-3 cells, which express high levels of HER2/neu (Table 1). Membrane fusion can then be followed by the formation of syncytia through fusion of adjacent cells. All tested H-DARPin proteins caused syncytia formation. Quantification revealed that the H-DH14R and the H-DG3 proteins exhibited the strongest fusion helper function reaching almost 70% of that of the unmodified H-protein (Figure 2a).
Table 1. HER2/neu surface density of applied cell lines.
Figure 2.
Fusion helper function of hemagglutinin (H)-designed ankyrin repeat proteins (DARPins). (a) SK-OV-3 cells were co-transfected with pCG-FΔ30 and each of the H-DARPin or H-scFv4D5 fusion protein encoding plasmids. pCG-HcΔ18 and pCG-HcΔ18mut (blinded for CD46 and signaling lymphocyte-activation molecule binding) coding for cytoplasmic tail truncated H proteins without fused DARPin were used as controls. Syncytia formation was normalized to that observed for pCG-HcΔ18 transfected cells (i.e., H). The results are expressed as mean values of 20 syncytia analysed for each sample. (b) H-DG3, H-DH14R and H-scFv4D5 mediated syncytia formation on CHO-Her2-k6, CHO-Her2-k13 and CHO-Her2-k7 cells. Untransfected and pCG-HcΔ18 transfected cells served as controls. Syncytia are indicated with dashed lines. Scale bar corresponds to 500 µm. (c) Correlation between syncytia size mediated by H-DG3, H-DH14R or H-scFv4D5 and HER2/neu receptor density. The results are expressed as mean values of 20 syncytia analyzed.
To investigate the influence of the HER2/neu receptor density on cell–cell fusion, we generated a panel of stably transfected CHO clones expressing different HER2/neu surface levels (Table 1). The membrane fusion activity mediated by the H-DG3 and H-DH14R proteins on these cells was compared to that of the H-scFv4D5 protein. All three proteins mediated syncytia formation on cells with high (2 × 105 receptors/cell) and medium (4 × 104 receptors/cell) receptor levels. Low levels (8 × 102 receptors/cell) were not sufficient for syncytia formation to proceed (Figure 2b). Likewise, HER2/neu negative cells did not form syncytia, demonstrating the HER2/neu specificity of the process. Plotting the membrane fusion activities of the proteins against the receptor densities demonstrated a direct correlation between both parameters (Figure 2c). Independent from the receptor levels, the fusion helper function of H-DG3 and H-H14R proteins was about fourfold more efficient than the function of the H-scFv4D5 protein (Figure 2c).
Generation and characterization of HER2/neu-targeted LVs
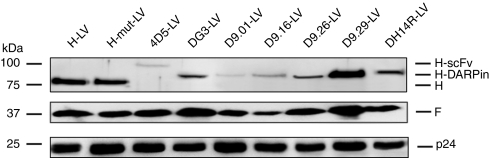
Having demonstrated HER2/neu-specific membrane fusion helper function, the H-DARPin proteins were next used for pseudotyping LVs. The corresponding vector particles (D9.29-LV, D9.26-LV, D9.16-LV, D9.01-LV, DH14R-LV, DG3-LV) were first assayed for the incorporation levels of the H-DARPin proteins. All six H-DARPin proteins were detectable in the particles albeit at different levels. There were only very low levels of the proteins H-D9.01 and H-scFv4D5 incorporated, while H-D9.16 showed moderate incorporation levels (Figure 3, Table 2). High incorporation levels were observed for the proteins H-DG3, H-D9.26 and H-DH14R, and the strongest incorporation for H-DARPin-9.29 (Figure 3, Table 2).
Figure 3.
H-DARPin and F incorporation into HER2/neu targeting vectors. Western Blot analysis of the indicated LVs. Particle amounts loaded onto 10% sodium dodecyl sulfate–polyacrylamide gels were equivalent to 30 ng of p24 for each vector type. Proteins were detected utilizing anti-F, anti-H, or anti-p24 antibodies. Vector particles pseudotyped with unmodified or mutated H-protein (MV–LV, MVmut-LV) were used as controls.
Table 2. Properties of applied designed ankyrin repeat proteins (DARPins) and the corresponding vector particles.
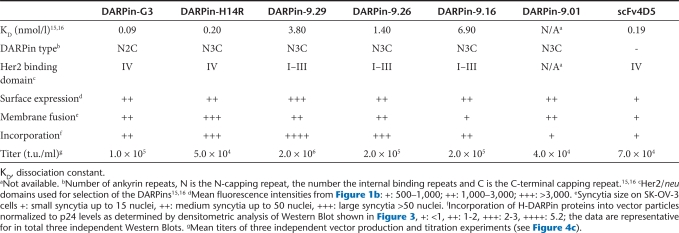
Next, we determined the targeting potential of the DARPin-LV particles by transducing a set of cell lines expressing different HER2/neu levels, i.e., HER2/neu negative CHO-K1, low level HER2/neu expressing HEK-293T and high level HER2/neu expressing CHO-Her2-k6 and SK-OV-3 cells (see Table 1 for receptor densities). None of the DARPin-LVs transduced CHO-K1 cells, while some green fluorescent protein expressing cells were detected on HEK-293T cells (Figure 4a). Many more green fluorescent protein-positive cells were detectable on CHO-Her2-k6 and SK-OV-3 cells, especially upon incubation with D9.29-LV (Figure 4a). Calculation of vector titers revealed that on HEK-293T cells maximal titers of 1 × 104 t.u./ml were reached by vectors D9.29-LV, D9.26-LV, and DG3-LV (Figure 4b). On SK-OV-3 and CHO-Her2-k6 cells, all vectors reached at least tenfold higher titers. Moreover, most DARPin displaying LVs were considerably more efficient in mediating gene transfer via HER2/neu than 4D5-LV, which was 10–30 times less efficient. D9.29-LV was especially effective on SK-OV-3 cells where it showed the highest titers of all vector types tested (Figure 4b).
Figure 4.
Selective transduction of HER2/neu positive cell lines. (a) CHO-K1, CHO-Her2-k6, HEK-293T, or SKOV-3 cells were incubated with vector particle supernatant. Transduced cells were visualized by fluorescence microscopy 48 hours after transduction. Scale bar corresponds to 500 µm. (b) The indicated cell lines were transduced with serial dilutions of each vector stock. Titers (t.u./ml) were calculated upon quantification of green fluorescent protein-positive cells by fluorescence-activated cell sorter analysis. Mean values of three independent transduction experiments are presented.
Transduction of mixed cell populations and in vivo application of HER2/neu-targeted LVs
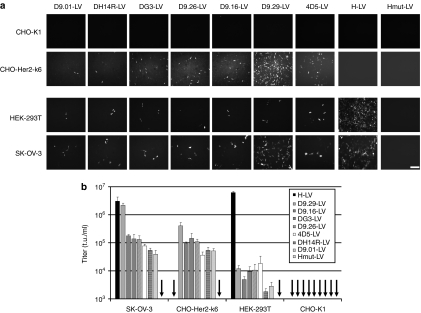
After demonstration of HER2/neu receptor specificity and identification of the most effective H-DARPin protein (i.e., H-DARPin-9.29), we assessed the target cell specificity in co-cultures of CHO-Her2-k6 and CHO-K1 cells. VSV-G–pseudotyped LV (VSV-G-LV) transduced both cell lines at equal efficiency (Figure 5). In contrast, D9.29-LV only transduced CHO-Her2-k6 cells, even when these were clearly underrepresented (Figure 5).
Figure 5.
Target cell specificity of D9.29-LV in mixed cell cultures. CHO-Her2-k6 and CHO-K1 cells were mixed in three different ratios (see percentages in left gates in control panel) and then transduced with D9.29-LV (0.2 µg p24) or VSV-G-LV (0.02 µg p24) at a multiplicity of infection of 2.5, or left untransduced (control). Forty-eight hours after transduction, cells were analyzed by fluorescence-activated cell sorter analysis for green fluorescent protein fluorescence and HER2/neu expression. HER2/neu positive target cells were identified with a PE-labeled HER2/neu-specific antibody.
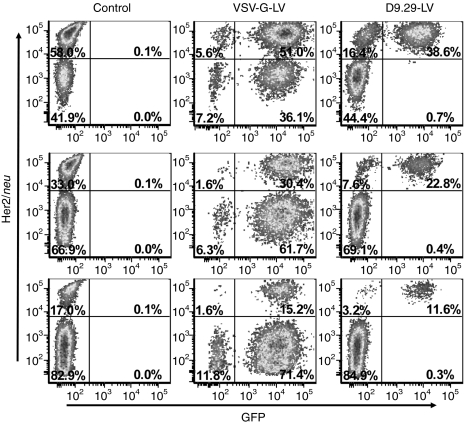
The in vivo targeting potential of D9.29-LV was assessed in a xenograft mouse model carrying a HER2/neu-positive (SK-OV-3 cells) and a HER2/neu-negative tumor (U87MG cells). After both tumors were fully vascularized the luciferase transferring vectors D9.29-LVluc and VSV-G-LVluc were injected systemically through the tail vein and mice were analyzed using charged-coupled device imaging. D9.29-LVluc showed strong luciferase activity in the SK-OV-3 tumor already 3 days after injection while luciferase activity was below the level of detection in the U87MG tumor or elsewhere in the mouse body (data not shown). Also after 2 weeks, luciferase activity could only be detected in the SK-OV-3 tumor, but not in the U87MG tumor or in spleen and liver (Figure 6a). In contrast VSV-G-LVluc injected mice showed the most prominent signals in spleen and liver, whereas both tumors emitted a comparably low signal indistinguishable from other tissues (Figure 6a). Because the intensity of the charged-coupled device imaging can be influenced by variables such as depth of tissue and positioning of the animal during imaging, mice were killed and luciferase activitiy in organ lysates was quantified. D9.29-LVluc injected mice showed the strongest luciferase activity in SK-OV-3 tumor lysates (4.0 × 102 RLU/µg protein), whereas the activity in U87MG tumor lysates was >1000-fold lower and remained at background level (3.0 × 10−1 RLU/µg protein) (Figure 6b). Besides the target tumor, a significant activity just above background was only detected in spleen (0.7 × 101 RLU/µg protein). In contrast, VSV-G-LVluc injected mice revealed by far the most prominent signal in spleen (8.5 × 104 RLU/µg protein) and liver (5.1 × 103 RLU/µg protein), but comparably low luciferase activity in both tumor lysates (2.5 × 102 RLU/µg protein for SKOV and 9.3 × 102 RLU/µg protein for U87MG) (Figure 6b).
Figure 6.
In vivo targeting of HER2/neu positive tumors. (a) Luciferase signals analyzed by in vivo imaging. Six- to 8-week-old CB17 SCID mice were injected with U87MG cells (right flank, yellow arrow) and SK-OV-3 cells (left flank, red arrow). After 11 days, 50 µg p24 of D9.29-LVluc or 5 µg of VSV-G-LVluc were injected into the tail vein. Two weeks postinjection, in vivo imaging data were monitored. Luciferase signal intensity is expressed as photons/second/square centimeter/steradian (p/sec/cm2/sr). (b) Immediately after imaging, organs were isolated and homogenized. Luciferase activities were quantified and normalized to protein content. D9.29-LVluc n = 3; mean ± SD; VSV-G-LVluc n = 4; mean ± SD.
Discussion
Here, we present proof of principle for applying DARPins as targeting domain of LV vectors. While adenovirus has recently been retargeted with DARPins19 to our knowledge, this is the first description and in vivo demonstration of this type of application for DARPins for lentiviral gene transfer vector. Six different HER2/neu-specific DARPins, previously selected from DARPin libraries by ribosome or phage display,15,16 were fused to the H-protein ectodomain at its C-terminus. A critical parameter for efficient LV particle incorporation of H proteins fused to targeting domains is their efficient cell surface expression. It was remarkable that all six H-DARPin proteins tested were readily expressed at the surface of HEK-293T cells. We did not observe any difference in surface expression between DARPins consisting of two or of three ankyrin repeats between the capping repeats. In each case, the surface expression levels were higher than those of H proteins fused to an scFv fragment, the HER2/neu-specific 4D5 or the CD20-specific scFv,20 previously used to establish this targeting system.9 Accordingly, all the H-DARPin fusion proteins were detected in vector particles, most of them at higher levels than H-scFv proteins. Moreover, while six out of six tested H-DARPin proteins were expressed at the cell surface and incorporated into vector particles, this was the case for only 12 of in total 25 H-scFv proteins tested so far (Schneider and Buchholz, unpublished results). When directly compared to a specific-scFv (4D5),17 which recognized the same domain (IV) of HER/neu with a similar high affinity as DARPins G3 and H14R, between ten and 30-fold higher gene transfer efficiencies were observed for the DARPin displaying vectors (Table 2). Thus, DARPins seem to be better suited as targeting domain in context of the MV H-protein than scFv fragments. Even though in the current comparison only one scFv fragment was included, 4D5 scFv is known to be stable and well expressed,21 and thus one with already above-average biophysical properties. DARPins have no disulfide bonds and are very resistant to aggregation, and thus probably more compatible with the folding and assembly of integral membrane proteins.
Most retrovirus receptors are multiple transmembrane spanning integral membrane proteins with rather small extracellular domains thus mediating binding in close proximity to the cell membrane.22 For MV, it was shown that the distance between the viral binding site and the cell membrane correlates reciprocally with membrane fusion efficiency.23 However, in such a receptor protein, the virus binding site is well accessible. If a membrane protein with a large, multi-domain extracellular region is used as receptor for the virus, it is essential that the epitope is accessible for the targeting domain within the context of the viral surface protein, in this case the MV H-protein. Unlike other members of the epidermal growth factor -receptor family, HER2/neu dimerises in absence of ligand contact.24 This results in an exposed dimerisation domain, but covers partially the membrane proximal domain IV,25 which therefore may be difficult to access by LVs binding to this domain. This may explain why DARPins binding to the membrane distal domains I–III of HER2/neu mediated more efficient gene transfer than those binding to the membrane proximal domain IV. It appears that affinity plays a smaller role, as gene transfer efficiency did not correlate with affinity (Table 2). One explanation for this finding would be multivalent binding, which would increase the functional affinity of the viral particle.
To assess the influence of the HER2/neu density on membrane fusion and gene transfer efficiency we analyzed a panel of cell lines expressing HER2/neu in a range of three orders of magnitude. We found that HER2/neu densities below 1,000 molecules per cell were insufficient to allow efficient membrane fusion and particle entry. With increasing densities, both syncytia formation and particle entry increased. Optimal gene transfer efficiency was observed above a density of 105 molecules per cell. Such an expression level of HER2/neu is found in certain types of cancer cells, such as e.g., the ovarian cancer cell line SK-OV-3. Thus, targeting specificity of these vectors critically depends on receptor density, which will facilitate the discrimination between cancer cells and noncancer cells.
Recently, a panel of recombinant MVs displaying HER2/neu-specific scFvs that ranged in their affinities from 10−6 to 10−11 mol/l have been described.26 An affinity threshold of 1.6 × 10−8 mol/l for the displayed scFv was identified at which cell–cell fusion and cell entry of the virus proceeded efficiently. The affinities of the DARPins we used ranged from 7 × 10−8 mol/l to 10−10 mol/l, and thus were all above this threshold level. Our data are thus in line with those of Hasegawa et al. and extend them by demonstrating that, at least for MV–LV vector particles, above the threshold level, the location of the targeting domain binding site on HER2/neu is more important than affinity.
A surprising outcome of our study is the high tumor target specificity of D9.29-LV when applied by tail vein injection. It is well established that VSV-G-LV mainly accumulates in spleen and liver.6,27 Our results are well in line with this revealing at least 100-fold more luciferase activity in spleen than in tumor tissue for VSV-G-LV. Previous attempts to detarget LVs from spleen and liver by surface engineering had limited success. Morizono et al. targeted lung tumor cells applying LVs pseudotyped with Sindbis virus envelope proteins bound to a tumor cell surface antigen-specific monoclonal antibody. They achieved a threefold higher luciferase activity in target compared to nontarget tissue.5 D9.29-LV in contrast results in at least 40-fold more luciferase activity in the target tumor than in off-target tissue. It is important to mention that off target tissue was not only of murine origin, which is devoid of MV receptors, but also included a human cell–based tumor expressing the MV receptor CD46 at high density. Thus, targeted MV–LV vectors are not only highly specific in vitro9,10 or after local in vivo application10 but also when applied systemically. The possibility to apply DARPins for LV targeting further broadens the applicability of MV–LV vectors. DARPins can be generated with specificity for essentially any surface molecule, and many such DARPins are already available.16,28
Materials and Methods
Cell culture. HEK-293T (ATCC CRL-11268), SK-OV-3 (ATCC HTB-77), HT1080 (ATCC CCL-121), and CHO-K1 (ATCC CCL-61) cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and 1% glutamine at 37 °C in a humidified atmosphere containing 5% CO2. CHO–Her2 clones expressing different densities of HER2/neu were established by stable transfection of CHO-K1 cells with pEX-Z2866-MO2 (GeneCopoeia, Germantown, MD), followed by G418 (Invitrogen, Karsruhe, Germany) selection and single cell cloning (1.2 mg/ml for CHO-Her2-k6 and CHO-Her2-k7; 0.5 mg/ml for CHO-Her2-k13).
Plasmids. The DARPin-coding sequences15,16 have been PCR amplified as SfiI/NotI fragments using the primers D1-for (5′-GCTTGGCCCAGCCGGCCGACCTGGGTAAGAAACTAC-3′) and D1-rev (5′-TTTTCCTTTTGCGGCCGCATTAAGCTTTTGCAGGATTTC-3′) for DARPins G3 and 9.26 and D2-for (5′-GCTTGGCCCAGCCGGCCGACCTGGGTAAGAAACTGC-3′) and D1-rev for all other DARPins. PCR products were ligated in-frame via SfiI/NotI into the plasmid pCG-H-α-CD20.9 The coding sequences for the scFv fragments 4D517 and 7A518 were subcloned from plasmids pIG6_4D5++ and pTC53-7A5neo, respectively, into pCG-H-α-CD20.
Vector particle production. Vector particles were generated as described previously.9 Briefly, HEK-293T cells were transfected using calcium phosphate transfection. For transfection, 3.0 µg of pCG-FcΔ30, 1.0 µg of the H-protein variants encoding plasmids, 10.72 µg of the HIV-1 packaging plasmid pCMVΔR8.9,29 and 11.27 µg of the green fluorescent protein transfer vector plasmid pSEW30 were used for transfection of cells in T75 flasks. Vector particles pseudotyped with the VSV-G protein were produced by co-transfection of 4.55 µg of the plasmid pMD.G2 along with 8.45 µg of pCMVΔR8.9 and 13.00 µg of transfer plasmid. For vector particles transferring the luciferase gene, the luc coding sequence was cloned from the pGL4.50 vector system (Promega, Mannheim, Germany) into the pSEW transfer vector and the resulting transfer vector was used for vector production. Twenty-four hours after transfection, the cell supernatant was filtered (0.45 µm filter) and concentrated and purified by centrifugation at 25,000g and 4 °C over a 20% sucrose cushion for at least 3 hours. The pellet was resuspended in 200 µl fetal calf serum-free medium.
Transduction of cell lines. For transduction, 5 × 104 cells were seeded into a single well of a 12-well plate. Forty-eight hours later, vector particles were serially diluted in 1:10 steps and a total of 500 µl of the dilutions were added per well, incubated for 7 hours, and then replaced with 1 ml of fresh medium. After 48 hours, titers were calculated by determining the number of green fluorescent protein-positive cells under the fluorescence microscope. Alternatively, titers were determined by fluorescence-activated cell sorter analysis based on the indicated percentage of green fluorescent cells.
Flow cytometry. Flow cytometry was performed on a Becton Dickinson LSRII flow cytometry system (Becton Dickinson, Heidelberg, Germany) as described preciously.9 Briefly, for surface expression experiments, 1 × 106 HEK-293T cells were transfected with H-DARPin expression plasmids (p-H-DARPin) using Lipofectamin 2000 transfection reagent (Invitrogen) according to manufacturers' instructions. After 48 hours, cells were detached and separated. Cells were washed and incubated with a 1:20 dilution in phosphate-buffered saline (PBS) of the mouse antihuman His-PE antibody (Miltenyi Biotec, Bergisch Gladbach, Germany) to detect the His-tagged H-DARPin protein. The data were analyzed using the BD FACSDiva software version 6.0 (Becton Dickinson).
HER2/neu expression levels were determined as described previously26 using a phycoerythrin-conjugated anti-HER2/neu antibody (Becton Dickinson). HER2/neu receptor density was determined using the QuantiBrite phycoerythrin quantitation kit (Becton Dickinson), according to the manufacturers' instructions.
Quantification of syncytia size. To demonstrate functional surface expression of H-DARPin fusion proteins, the level of cell-to-cell fusion was quantified by counting the number of nuclei in each syncytium after co-transfection of 0.5 µg H-DARPin and 0.5 µg pCG-FcΔ309 into 5 × 105 HER2/neu-expressing cells using Lipofectamin 2000 transfection reagent (Invitrogen). Twelve hours after transfection, cells were washed with PBS and fixed with 10% formaldehyde in PBS for 12 hours at 4 °C. Subsequently, cells were stained using 1 ml of 0.1% methylrosanilium and 18% ethanol in PBS.
Quantification of p24. The quantity of p24 gag in purified vector suspensions was determined using the Innotest HIV antigen mAb (Innogenetics, Gent, Belgium) according to the manufacturers' instructions.
Immunoblotting. Concentrated vector particles were denatured by incubation for 10 minutes at 95 °C in 2× urea sample buffer (5% sodium dodecyl sulfate, 8 mol/l urea, 200 mmol/l Tris–HCl, 0.1 mmol/l EDTA, 0.03% bromphenol blue, 2.5% dithiothreitol, pH 8.0), separated by electrophoresis on 10% sodium dodecyl sulfate–polyacrylamide electrophoresis gels, and electrotransferred onto nitrocellulose membranes (Amersham Biosciences, Freiburg, Germany). The membranes were incubated with rabbit anti-F (F 431, 1:2,000) serum, rabbit anti-H (H 606, 1:1,000) serum, or mouse anti-p24 (clone 38/8.7.47, 1:1,000; Gentaur, Aachen, Germany) monoclonal antibody, to detect F, H, and p24 gag, respectively. Subsequently, the corresponding secondary antibodies conjugated to horseradish peroxidase (1:2,000; DakoCytomation, Hamburg, Germany) were used. Signals were detected using ECL Plus Western Blotting Detection System (GE Healthcare, München, Germany). The F and H specific sera had been raised by immunization of rabbits with peptides CTVTREDGTNRR (H606) or QVGSRRYPDAVYLHRC (F431), respectively (Eurogentec, Köln, Germany).
In vivo analysis of HER2/neu-specific vectors. Experimental mouse work was carried out in compliance with the regulations of the German animal protection law. CB17 SCID female mice (6- to 8-week-old) were obtained from Harlan Laboratories (Eystrup, Germany) and maintained under pathogen-free conditions. Tumors were established by subcutaneous injections of 2 × 106 U87MG cells or 5 × 106 SK-OV-3 cells, respectively, in 100 µl of PBS into the right or left flank of mice. Systemic vector application was performed 2 weeks later by injection of vector particles (50 µg p24/mouse for D9.29-LVluc, 5 µg p24/mouse for VSV-G-LVluc) into the tail vein in a volume of 100 µl diluted in PBS. After 2 weeks, mice were intraperitoneally injected with 150 mg D-Luciferin/kg body weight (Caliper Life Sciences, Mainz, Germany) and anesthetized. Imaging data were obtained 10 minutes after substrate injection using a noninvasive cooled charged-coupled device (IVIS Spectrum; Caliper Life Sciences). Data were analyzed using the Living Image Software (Caliper Life Sciences). Mice were killed after charged-coupled device imaging and organ excision was performed immediately. Organs were homogenized (FastPrep System; MP Biomedicals, Illkirch, France) and cell lysates were analyzed for luciferase signals using the Dual-Luciferase Reporter Assay System (Promega).
Acknowledgments
This work was supported by grants of the Priority Programme “Mechanisms of gene vector entry and persistence” (SPP 1230) of the Deutsche Forschungsgemeinschaft to C.J.B. and K.C. and of the 7th European Community programme project “PERSIST” to C.J.B. S.K., K.C., and C.J.B. are listed as inventors in an international Patent Cooperation Treaty European patent application (PCT/EP2007/008384) assigned to the Paul Ehrlich Institut, which includes as claims the generation of targeted lentiviral vectors.
REFERENCES
- Cockrell AS., and, Kafri T. Gene delivery by lentivirus vectors. Mol Biotechnol. 2007;36:184–204. doi: 10.1007/s12033-007-0010-8. [DOI] [PubMed] [Google Scholar]
- Naldini L. Medicine. A comeback for gene therapy. Science. 2009;326:805–806. doi: 10.1126/science.1181937. [DOI] [PubMed] [Google Scholar]
- Frecha C, Szécsi J, Cosset FL., and, Verhoeyen E. Strategies for targeting lentiviral vectors. Curr Gene Ther. 2008;8:449–460. doi: 10.2174/156652308786848003. [DOI] [PubMed] [Google Scholar]
- Buchholz CJ, Mühlebach MD., and, Cichutek K. Lentiviral vectors with measles virus glycoproteins - dream team for gene transfer. Trends Biotechnol. 2009;27:259–265. doi: 10.1016/j.tibtech.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Morizono K, Xie Y, Ringpis GE, Johnson M, Nassanian H, Lee B, et al. Lentiviral vector retargeting to P-glycoprotein on metastatic melanoma through intravenous injection. Nat Med. 2005;11:346–352. doi: 10.1038/nm1192. [DOI] [PubMed] [Google Scholar]
- Morizono K, Xie Y, Helguera G, Daniels TR, Lane TF, Penichet ML, et al. A versatile targeting system with lentiviral vectors bearing the biotin-adaptor peptide. J Gene Med. 2009;11:655–663. doi: 10.1002/jgm.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizono K, Pariente N, Xie Y., and, Chen IS. Redirecting lentiviral vectors by insertion of integrin-tageting peptides into envelope proteins. J Gene Med. 2009;11:549–558. doi: 10.1002/jgm.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagi Y, Takeda M., and, Ohno S. Measles virus: cellular receptors, tropism and pathogenesis. J Gen Virol. 2006;87 Pt 10:2767–2779. doi: 10.1099/vir.0.82221-0. [DOI] [PubMed] [Google Scholar]
- Funke S, Maisner A, Mühlebach MD, Koehl U, Grez M, Cattaneo R, et al. Targeted cell entry of lentiviral vectors. Mol Ther. 2008;16:1427–1436. doi: 10.1038/mt.2008.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anliker B, Abel T, Kneissl S, Hlavaty J, Caputi A, Brynza J, et al. Specific gene transfer to neurons, endothelial cells and hematopoetic progenitors with lentiviral vectors. Nat Methods. 2010;7:929–935. doi: 10.1038/nmeth.1514. [DOI] [PubMed] [Google Scholar]
- Sedgwick SG., and, Smerdon SJ. The ankyrin repeat: a diversity of interactions on a common structural framework. Trends Biochem Sci. 1999;24:311–316. doi: 10.1016/s0968-0004(99)01426-7. [DOI] [PubMed] [Google Scholar]
- Kohl A, Binz HK, Forrer P, Stumpp MT, Plückthun A., and, Grütter MG. Designed to be stable: crystal structure of a consensus ankyrin repeat protein. Proc Natl Acad Sci USA. 2003;100:1700–1705. doi: 10.1073/pnas.0337680100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binz HK, Amstutz P, Kohl A, Stumpp MT, Briand C, Forrer P, et al. High-affinity binders selected from designed ankyrin repeat protein libraries. Nat Biotechnol. 2004;22:575–582. doi: 10.1038/nbt962. [DOI] [PubMed] [Google Scholar]
- Yarden Y. Biology of HER2 and its importance in breast cancer. Oncology. 2001;61 Suppl 2:1–13. doi: 10.1159/000055396. [DOI] [PubMed] [Google Scholar]
- Zahnd C, Wyler E, Schwenk JM, Steiner D, Lawrence MC, McKern NM, et al. A designed ankyrin repeat protein evolved to picomolar affinity to Her2. J Mol Biol. 2007;369:1015–1028. doi: 10.1016/j.jmb.2007.03.028. [DOI] [PubMed] [Google Scholar]
- Steiner D, Forrer P., and, Plückthun A. Efficient selection of DARPins with sub nanomolar affinities using SRP phage display. J Mol Biol. 2008;382:1211–1227. doi: 10.1016/j.jmb.2008.07.085. [DOI] [PubMed] [Google Scholar]
- Ramm K, Gehrig P., and, Plückthun A. Removal of the conserved disulfide bridges from the scFv fragment of an antibody: effects on folding kinetics and aggregation. J Mol Biol. 1999;290:535–546. doi: 10.1006/jmbi.1999.2854. [DOI] [PubMed] [Google Scholar]
- Engelstädter M, Bobkova M, Baier M, Stitz J, Holtkamp N, Chu TH, et al. Targeting human T cells by retroviral vectors displaying antibody domains selected from a phage display library. Hum Gene Ther. 2000;11:293–303. doi: 10.1089/10430340050016030. [DOI] [PubMed] [Google Scholar]
- Dreier B, Mikheeva G, Belousova N, Parizek P, Boczek E, Jelesarov I, et al. 2010Her2-specific multivalent adapters confer designed tropism to adenovirus for gene targeting J Mol Biolepub ahead of print). [DOI] [PMC free article] [PubMed]
- Bucheit AD, Kumar S, Grote DM, Lin Y, von Messling V, Cattaneo RB, et al. An oncolytic measles virus engineered to enter cells through the CD20 antigen. Mol Ther. 2003;7:62–72. doi: 10.1016/s1525-0016(02)00033-3. [DOI] [PubMed] [Google Scholar]
- Jäger M, Gehrig P., and, Plückthun A. The scFv fragment of the antibody hu4D5-8: evidence for early premature domain interaction in refolding. J Mol Biol. 2001;305:1111–1129. doi: 10.1006/jmbi.2000.4342. [DOI] [PubMed] [Google Scholar]
- Overbaugh J, Miller AD., and, Eiden MV. Receptors and entry cofactors for retroviruses include single and multiple transmembrane-spanning proteins as well as newly described glycophosphatidylinositol-anchored and secreted proteins. Microbiol Mol Biol Rev. 2001;65:371–89, table of contents. doi: 10.1128/MMBR.65.3.371-389.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz CJ, Schneider U, Devaux P, Gerlier D., and, Cattaneo R. Cell entry by measles virus: long hybrid receptors uncouple binding from membrane fusion. J Virol. 1996;70:3716–3723. doi: 10.1128/jvi.70.6.3716-3723.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26:6469–6487. doi: 10.1038/sj.onc.1210477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselga J., and, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9:463–475. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- Hasegawa K, Hu C, Nakamura T, Marks JD, Russell SJ., and, Peng KW. Affinity thresholds for membrane fusion triggering by viral glycoproteins. J Virol. 2007;81:13149–13157. doi: 10.1128/JVI.01415-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang KX, Kim C, Chow E, Chen IS, Jia W., and, Rennie PS. Targeting trastuzumab-resistant breast cancer cells with a lentivirus engineered to bind antibodies that recognize HER-2. Breast Cancer Res Treat. 2011;125:89–97. doi: 10.1007/s10549-010-0828-9. [DOI] [PubMed] [Google Scholar]
- Stumpp MT., and, Amstutz P. DARPins: a true alternative to antibodies. Curr Opin Drug Discov Devel. 2007;10:153–159. [PubMed] [Google Scholar]
- Zufferey R, Nagy D, Mandel RJ, Naldini L., and, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]
- Demaison C, Parsley K, Brouns G, Scherr M, Battmer K, Kinnon C, et al. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency [correction of imunodeficiency] virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum Gene Ther. 2002;13:803–813. doi: 10.1089/10430340252898984. [DOI] [PubMed] [Google Scholar]
- Moll M, Klenk HD., and, Maisner A. Importance of the cytoplasmic tails of the measles virus glycoproteins for fusogenic activity and the generation of recombinant measles viruses. J Virol. 2002;76:7174–7186. doi: 10.1128/JVI.76.14.7174-7186.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]