Abstract
Tumor vaccines can induce robust immune responses targeting tumor antigens in the clinic, but antitumor effects have been disappointing. One reason for this is ineffective tumor infiltration of the cytotoxic T lymphocytes (CTLs) produced. Oncolytic viruses are capable of selectively replicating within tumor tissue and can induce a strong immune response. We therefore sought to determine whether these therapies could be rationally combined such that modulation of the tumor microenvironment by the viral therapy could help direct beneficial CTLs induced by the vaccine. As such, we examined the effects of expressing chemokines from oncolytic vaccinia virus, including CCL5 (RANTES), whose receptors are expressed on CTLs induced by different vaccines, including type-1-polarized dendritic cells (DC1). vvCCL5, an oncolytic vaccinia virus expressing CCL5, induced chemotaxis of lymphocyte populations in vitro and in vivo, and displayed improved safety in vivo. Interestingly, enhanced therapeutic benefits with vvCCL5 in vivo correlated with increased persistence of the viral agent exclusively within the tumor. When tumor-bearing mice were both vaccinated with DC1 and treated with vvCCL5 a further significant enhancement in tumor response was achieved which correlated with increased levels of tumor infiltrating lymphocytes. This approach therefore represents a novel means of combining biological therapies for cancer treatment.
Introduction
The prognosis of many solid cancers has been shown to be associated with the levels of tumor infiltrating immune cells,1,2,3 suggesting that promoting the entry of tumor-specific type-1 effector T-cells to the sites of primary and metastatic cancers may represent a therapeutic goal. Induction of circulating antitumor cytotoxic T lymphocytes (CTLs) has been demonstrated with a variety of therapeutic cancer vaccine approaches, including peptide, dendritic cell (DC) and viral-based vaccines,4,5,6 however, antitumor effects have been disappointing. This is in part due to a lack of CTL infiltration into the tumor.
Attenuated and tumor-selective strains of vaccinia virus have been shown to be potent oncolytic agents capable of systemic delivery to tumor targets.7,8,9,10,11 Perhaps the most commonly studied oncolytic vaccinia strain contains deletions in the viral thymidine kinase and vaccinia growth factor genes, and is known as vvDD.7,11 Vaccinia virus possesses the ability to both suppress and activate different aspects of cellular and humoral immune responses.12 Antivaccinia immune responses promote the clearance of vvDD and limit the possibility of repeat treatments but can also be advantageous, being able to enhance antitumor effects.10 An attempt to take advantage of the ability of vaccinia to regulate the immune response within and against tumors might be enhanced through the expression of chemokines that are capable of specifically directing key components of an antitumor immune response to the site of infection.
The chemokine CCL5 (RANTES; regulated upon activation, normal T cell expressed and secreted) is of broad clinical importance in a variety of human diseases13 and binds to three receptors; CCR1, CCR3, and CCR514 that are expressed on several types of effector and regulatory T cells. It is produced by both antigen presenting cells and by activated T lymphocytes and is broadly chemoattractive. Guided by our recent observation that type-1-polarized DC (DC1) vaccines are highly effective in inducing functional tumor-targeting CTLs15,16 that display high levels of CCL5-responsiveness, we tested if the effectiveness of oncolytic vaccinia therapy, applied alone or in combination with DC1 cancer vaccines, could be enhanced by the expression of CCL5.
We found that vvCCL5, vvDD constitutively expressing CCL5, enhanced immune infiltration of mouse colorectal tumors in vivo and enhanced therapeutic effects. Interestingly, CCL5 expression also resulted in prolonged persistence of the virus exclusively within the tumor. Further enhancement of antitumor effectiveness was achieved when vvCCL5 was used in conjunction with DC1 vaccination, correlating with increased immune cell infiltration into the tumor and an apparent Th1 skewing of the infiltrating T-cells. This therefore represents a novel means to both enhance oncolytic viral therapy and to attract therapeutic immune cells into the tumor.
Results
vvCCL5 attracts activated T-cells in vitro
We constructed a novel CCL5-expressing recombinant vaccinia virus (vvCCL5) based on the double deleted vaccinia virus (vvDD) backbone. CCL5 was expressed from within the locus of the viral thymidine kinase gene, and was driven by the p7.5 promoter, while DsRed (also expressed from within the TK gene) was driven by the pSE/L promoter, and used as a reporter. Expression and secretion of murine CCL5 was confirmed by enzyme-linked immunosorbent assay following infection of MC38 mouse colon cancer cells (Supplementary Figure S1a). A one-step replication curve of vvCCL5 was compared to vvDD in the MC38 cell line and no significant differences in viral replication were seen (Supplementary Figure S1b).
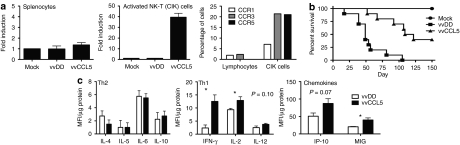
To investigate the ability of vvCCL5 to attract immune cell populations subsequent to infection, chemotaxis assays were performed using supernatant from vvCCL5-infected MC38 cells and either bulk splenocytes or an activated T-cell population, cytokine-induced killer (CIK) cells17 (Figure 1a). It was seen that the activated CIK cells were attracted by vvCCL5-infected MC38 cells with a 40-fold increase relative to supernatant from uninfected or vvDD infected MC38 cells. Bulk splenocytes were used as a control, and did not show significantly increased attraction to vvCCL5 supernatant. The relative levels of CCL5 receptors, CCR1, CCR3, and CCR5 on bulk splenocyte and CIK cell populations were determined (Figure 1a). This confirmed that CCL5 produced from the virus was active and capable of selectively attracting cells expressing their cognate receptor.
Figure 1.
In vitro chemoattraction of vvCCL5. (a) In vitro chemotaxis of different lymphocyte populations to vvCCL5-infected cells in a transwell assay; (left) bulk unactivated lymphocytes and (centre) cytokine-induced killer (CIK) cells; and expression of CCL5 receptors (CCR1, CCR3, and CCR5) on bulk lymphocytes and CIK cells as determined by flow cytometry (representative of three separate experiments, n = 4). (b) Pathogenicity of vvCCL5. Athymic nu−/nu− mice were treated with 1 × 108 plaque forming unit of the different viruses by intraperitoneal injection and subsequent survival followed (n = 10/group). (c) Immune response to vvDD and vvCCL5. C57/BL6 mice treated intraperitoneally with the different viruses (n = 4/group) were euthanized after 4 days and spleens recovered and processed for luminex assay. Relative normalized levels of different cytokines involved in the Th1 or Th2 response and chemokines known to induce a Th1 response are shown. IFN-γ, interferon-γ.
vvCCL5 displays safety and enhanced therapeutic potential in vivo
We have previously determined the safety of vvDD7 and so looked to compare relative pathogenicity of vvCCL5 and vvDD. Athymic nu−/nu− mice were treated via intraperitoneal injection with 1 × 108 plaque forming unit (PFU) of each virus. Interestingly, vvCCL5 was less pathogenic than vvDD over a period of 90 days (Figure 1b). This was explored further by Luminex assay of cytokine expression in spleens taken from C57/BL6 mice treated with either vvDD or vvCCL5. It was seen (Figure 1c) that although the levels of cytokines typically associated with a Th2 immune response were unaffected by expression of CCL5 from the virus, several cytokines and chemokines associated with a Th1 response (interferon-γ (IFN-γ), interleukin-2 (IL-2), interferon-inducible protein-10) were upregulated to a greater degree in the spleens of mice treated with vvCCL5. Pathogenicity experiments were also run in C57/BL6 immunocompetent mice, with 3 × 108 PFU of each virus delivered via intraperitoneal injection, and in this study all mice survived at least 90 days (data not shown).
Immunocompetent (C57/BL6) mice bearing subcutaneous MC38 tumors were treated with phosphate buffered saline (PBS), vvDD or vvCCL5 (1 × 108 PFU) by intraperitoneal injection. Specific expression of CCL5 within the tumor by vvCCL5 was again confirmed by Luminex assay with fivefold increases in the levels of CCL5 (P < 0.02) (Figure 2a). It was found that a single systemic treatment with vvCCL5 resulted in an effective antitumor response that was significantly better than even vvDD treatment (P < 0.03) (Figure 2b). Biodistribution studies following systemic (intraperitoneal) delivery of vvDD or vvCCL5 determined the levels (PFU/g) of the viruses in different organs at times after delivery. The initial infection patterns were similar between the two viruses (days 2 and 4), while both viruses displayed tumor selective replication and clearance from normal tissues by day 8 (Figure 2c), however, only the vvCCL5 virus showed persistence beyond day 8 within the tumor.
Figure 2.
In vivo analyses of vvCCL5 in tumor-bearing mice. (a) CCL5 levels in tumor samples from MC38 tumor-bearing C57/BL6 mice, treated with intraperitoneal injections of vvCCL5, phosphate buffered saline (PBS) or vvDD (P < 0.02). (b) Antitumor effects of vvCCL5. vvCCL5, vvDD, and PBS delivered via intraperitoneal injection into MC38 tumor-bearing C57/BL6 mice. vvCCL5 showed significant tumor suppression and significantly enhanced survival than vvDD-treated mice (P < 0.03 for vvCCL5 relative to vvDD at all time points after 18 days) (n = 10/group). (c) Biodistribution of vvCCL5 or vvDD after intraperitoneal injections into MC38 tumor-bearing C57/BL6 mice. Mice killed at indicated time points and organs recovered, ground to release viral virions, and titered by plaque assay (n = 3). PFU, plaque forming unit; MFI.
Enhanced therapeutic effect correlated with immune cell infiltration into the tumor
It was unclear whether the enhanced antitumor effects seen with vvCCL5 relative to vvDD are due to attraction of immune effector cells into the tumor, increased oncolytic effects through persistence of the virus or if some other mechanism may be mediating this effect, such as the known ability of CCL5 to attract regulatory T-cells into the tumor.18,19 To analyze this further, we examined subsets of immune cells within the tumor at times after different treatments. It was seen (Figure 3a) that vvCCL5 significantly enhanced the levels of CD4+ lymphocyte and DC (CD11c+) infiltration into the tumor relative to either mock or vvDD infected tumors. Natural killer (NK) cell infiltration was also increased relative to mock, but not vvDD infected tumors, while CD8+ lymphocyte infiltration was not altered (Figure 4c). However, the levels of CD4+FoxP3+ cells showed no increase over control animals at either 3 or 7 days after treatment (Figure 3b).
Figure 3.
Attraction of lymphocytes populations to vvCCL5-infected tumors. (a) Mice (C57/BL6 bearing MC38 tumors) were treated via intraperitoneal injection of phosphate buffered saline (PBS), vvDD or vvCCL5. Tumors were dissociated and single cells stained with anti-CD4 antibody to determine the percentage of CD4+ cells in the tumor or anti-CD11c to look at dendritic cell (DC) infiltration (left panel) or tumor sections stained with antibodies to CD11c+ (DC; red) or CD4+ (green). (b) Immunohistochemistry of MC38 tumor sections following treatment with PBS of vvCCL5 reveals the levels of attraction of CD4+ T-cells (white) and CD4+FoxP3+ cells (black bars) as determined from 10 independent regions from three sections from three individual tumors. Representative sections at day 3 are shown (CD4+ (red) and FoxP3 (green)). (c) Relative attraction of cytokine-induced killer (CIK) (natural killer T) cells to lymphomas infected with different viruses. Mice (friend virus B (FVB)) bearing 6814 bilateral tumors) were treated through intravenous injection with CIK cells expressing luciferase. After 24 hours, the bilateral tumors were injected directly with different viruses, and 48 hours later bioluminescence imaging was used to determine the relative infiltration of each tumor with labeled CIK cells. Left panel; relative attraction of CIK cells to mock infected versus vvCCL5-infected tumors. Right panel; relative attraction of CIK cells to vvDD versus vvCCL5-infected tumors. (P < 0.05). BLI, bioluminescence imaging.
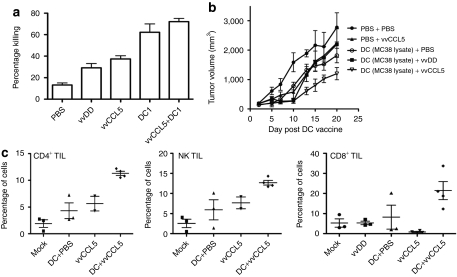
Figure 4.
Combining vvCCL5 and DC1 therapies. (a) Mice bearing MC38 tumors were treated with DC1 vaccine loaded with MC38 lysate (or phosphate buffered saline (PBS)) and 48 hours later with vvCCL5 (or PBS). In vivo CTL assays were performed 7 days after different treatments to assess the levels of antitumor cytotoxic T lymphocyte response produced (n = 3 mice/group). (b) Mice were treated as before, with subsequent tumor volume determined by caliper measurements. vvCCL5 and DC1 vaccine combination results in significantly enhanced tumor responses relative to either therapy alone (P < 0.05, days 13–20) (n = 10/group). (c) Tumors treated as before were dissociated and single cell suspensions stained with anti-CD4 antibody; anti-NK1.1 antibody or anti-CD8 antibody and percentage of positively stained cells determined by flow cytometry (n = 3 or 4). DC, dendritic cell; NK, natural killer; TIL, tumor infiltrating lymphocyte.
We have previously used a population of in vitro cytokine super-activated T-cells (CIK cells) as an immune cell therapy.17 These cells express surface receptors for CCL5 (Figure 1a) and have previously been shown to be capable of trafficking to tumors.20 We used an friend virus B (FVB) transgenic mouse model constitutively expressing luciferase21 as a donor source for expansion of CIK cells expressing luciferase (so avoiding the need to use retroviral labeling of the immune cells ex vivo). However, this meant that we required a FVB tumor model in the recipient mice. We therefore incorporated FVB mice with bilateral lymphoma tumors (6814)22 on the hind flanks. Once tumors had formed, mice were treated with intravenous injection of 1 × 107 CIK cells. After 24 hours these mice were treated with intratumoral injections of PBS (left flank) and vvCCL5 (right flank); or vvDD (left flank) and vvCCL5 (right flank) (1 × 108 PFU/tumor). Our previous studies demonstrated that CIK cells will not enter the tumor until at least 24 hours after application of the therapy.23 For this reason, and to prevent spread of virus between the tumors before CIK cell trafficking has occurred, we waited 24 h between CIK cell and viral treatments. The relative trafficking of the cells to the bilateral tumors was determined by whole animal bioluminescence imaging. In each case, the tumors treated with CCL5 expressing virus displayed significantly enhanced CIK cell trafficking (Figure 3c). It is unclear why CIK signal appears to peak earlier and persist longer in vvCCL5-treated tumors when compared to vvDD-treated controls, but may reflect effects of viral virulence genes in the vvDD-treated tumors.
vvCCL5 enhances the therapeutic effects of cancer vaccines
In order to examine how vvCCL5 might enhance the therapeutic effects of vaccine-based therapies through attraction of effector cells into the tumor, we looked at a DC vaccine approach. Many cancer vaccines have been shown to produce circulating T-cells, but have not produced the expected antitumor responses in cancer patients.24 This may be due to limited tumor infiltration of the antitumor antigen CTLs, and corruption or subversion of these cells once they do reach their tumor targets. We hypothesized that vvCCL5 might overcome these limitations through expression of the chemokine CCL5 (to enhance CTL attraction to the tumor), and through the ability of the immunogenic vaccinia infection to overcome immune suppression within the tumor. We focused on DC1 vaccination, as this approach has been shown to produce high levels of activated Tc1 cells that express CCL5 receptors on their cell surface.25
DC1 were generated and matured and loaded with MC38 cell lysate before being delivered subcutaneously into MC38 tumor-bearing mice. Two days later, the animals were treated systemically with PBS, vvDD or vvCCL5 (N.B.: In this way we expect peak viral gene expression, at 4 days after treatment, to coincide with peak CTL production, about 6 days after vaccination). Initially we examined the level of induction of antitumor CTL immune response through an in vivo assay to determine levels of killing of lymphocytes loaded with MC38 lysate. It was seen that, as we have observed previously,26 viral therapy alone (vvDD or vvCCL5) could induce a CTL response, but this was significantly less potent than the DC1 vaccination. The combination of DC1 and vvCCL5 therapies did not further increase the levels of CTL produced (Figure 4a). We also looked at therapeutic effects of the different therapies alone or in combination; when combined with DC1 vaccination, vvCCL5 treatment produced statistically significant reduction in rates of tumor growth (P < 0.05) (Figure 4b), and increased survival (P < 0.001) relative to vvCCL5 or DC1 alone.
Tumor infiltrating lymphocyte and cytokine levels in the tumor indicate different types of immune response are produced by different therapies
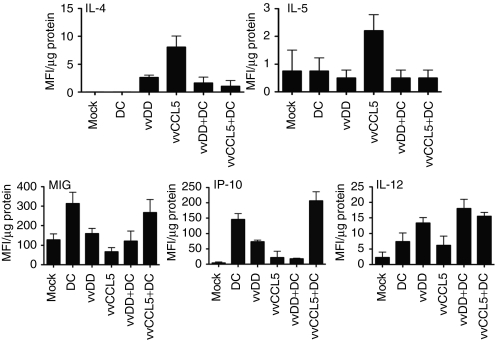
We looked to investigate whether the enhanced therapeutic effects of vvCCL5/DC1 combination therapy correlated with increased levels of tumor infiltrating lymphocytes. Tumor cell suspensions recovered from tumors harvested at 10 days post DC injection (and 8 days after viral delivery) were collected and analyzed by flow cytometry (as this time correlated with when we expected T cells to enter the tumor).15 It was seen (Figure 4c) that as before, vvCCL5 increased the levels of CD4+ T-cells in the tumor, but that these levels were further increased when vvCCL5 was used in conjunction with DC1 vaccination. NK cell infiltration into the tumor was also further enhanced by combining vvCCL5 and DC1 vaccination (Figure 4c). Interestingly, significant increases in the levels of infiltrating CD8+ T cells were only seen when vvCCL5 was used in combination with DC1 vaccination (Figure 4c). In order to analyze in more detail how vvCCL5 was altering the immune environment within the tumor, Luminex assays were run on MC38 tumor samples taken from mice 8 days after different treatments. The results indicated that, in general, an immunostimulatory environment was created within the tumors during viral therapy. However, strikingly, the levels of IL-4 and IL-5 were dramatically increased only in vvCCL5-treated animals (Figure 5), indicating that the CD4+ T-cells attracted into the tumors by this therapy alone are primarily Th2, leading to a humoral immune response. This might also explain the increased persistence of vvCCL5 in the tumor relative to vvDD (Figure 2b), and would explain the reduced levels of CD8+ T-cells in the tumor when vvCCL5 is used as a single agent (Figure 4c). Interestingly, this is the opposite of the effects seen in the spleen, where vvCCL5 induced a more Th1-biased immune response (Figure 1c).
Figure 5.
Cytokine and chemokine profiles in tumors following different treatments. Mice (C57/BL6 bearing MC38 tumors) were treated systemically with the indicated therapies and tumors were analyzed postmortem by Luminex assay for the relative levels of different cytokines. Data for interleukin-4 (IL-4), IL-5, monokine induced by γ-interferon (MIG), interferon-inducible protein-10 (IP-10), and IL-12 levels are shown. MFI, mean fluorescence intensity.
This increase in IL-4 and IL-5 level in the tumor is lost when vvCCL5 is used in conjunction with DC1 vaccination, indicating that the Tc1 nature of the immune response raised by DC1 vaccination may be able to redirect the immune response down this arm, leading to additional increase of CD8+ T-cells in the tumor, and enhanced therapeutic benefit. This hypothesis was further supported by the observation that two chemokines known to specifically attract Tc1 T-cells (monokine induced by γ-interferon and interferon-inducible protein-10) demonstrated increased levels in DC1 and DC1 plus vvCCL5 treatments, but not vvCCL5 single agent treatment (Figure 5). The Th1 cytokine IL-12 was found to display significantly increased levels in the tumor only after combination of vvCCL5 and DC therapy (IFN-γ levels were below the limits of detection in the tumor in this assay, while IL-2 showed no significant differences).
In order to further examine the possibility that the increased persistence and potency of vvCCL5 within the tumor relative to vvDD may be due to the localized production of Th2 cytokines, we examined whether similar effects were seen in IL-4−/− mice (Figure 6), because IL-4 was uniquely elevated in the tumors of vvCCL5-treated mice. It was indeed found that vvCCL5 no longer displayed enhanced persistence (relative to vvDD) in tumors of IL-4−/− mice, indicating that this cytokine was required for viral persistence (Figure 6a). It was also observed that the levels of CD4+ tumor infiltrating lymphocytes were no longer increased in vvCCL5-treated mice over vvDD or mock treated controls when the IL4−/− transgenic was used (Figure 6b). However, it was also noted that the background levels of CD4+ cells in the tumors of IL4−/− mice were greater than for C57/BL6.
Figure 6.
Mice (wild type C57/BL6 and interleukin-4 (IL-4−/−) transgenics) were implanted subcutaneously with MC38 tumors and treated with phosphate buffered saline (PBS), vvDD or vvCCL5 as before. (a) Viral persistence (titers in tumor tissue at day 8 after treatment), and (b) CD4+ cell infiltration were determined. PFU, plaque forming unit.
Discussion
It is typical for front line therapies for cancers to consist of combinations of different therapeutics, because with carefully selected and tested drug combinations it is possible to target multiple cancer pathways, creating additive or occasional synergistic benefits, without increasing toxicity. Similarly, many biological therapies, such as viral or immune cell therapeutics, have been tested in combination with traditional chemo or radiotherapy both in preclinical and clinical studies.4,27,28,29,30,31 However, the experimental nature of these biological platforms has meant that until recently little has been done to investigate how they may be used in combination with each other (other than the use of some immune cell populations as delivery vehicles for viral therapies).23,32
Here we hypothesized that, because oncolytic strains of vaccinia virus are known to be highly immunogenic,33 and many immunotherapies suffer from a limited ability to induce effector cells to infiltrate the tumor and/or to overcome the immunosuppressive environment within the tumor, it is likely that vaccinia infection within the tumor would enhance immune cell or cancer vaccine therapies.
In order to increase the immune-mediated antitumor effects of many oncolytic viruses, cytokines are frequently expressed (such as GM-CSF, tumor necrosis factor-α, or IFN).9,10,34 Although these approaches enhance the overall tumor responses, they may also lead to reduced replication or persistence of the virus and so reduce their oncolytic effects.34 We therefore looked to express chemokines from the virus, such that they would specifically interact with immune cell and/ or vaccine therapies. The chosen chemokine, CCL5 (RANTES) is known to attract a variety of cells that mediate an inflammatory response,14 but was primarily selected on the expectation that it would be capable of preferentially enhancing tumor-targeting of effector T-cells raised during an immune cell or vaccine therapy. Although this chemokine has also been associated with accumulation of regulatory T-cells in tumors,19 we predicted that the highly immunogenic nature of the oncolytic vaccinia infection could prevent extensive proliferation or infiltration of regulatory T-cells.
Initial construction and testing of a strain of the widely used oncolytic vaccinia virus vvDD,7 expressing the murine CCL5 chemokine (vvCCL5) was performed. This was important as vaccinia, like many other large viruses, carries some known chemokine inhibitors.35 In vivo testing of vvCCL5 as a single agent in mouse models verified impressive safety in both immuncompetent and immunodeficient mice, and expression of CCL5 from normal tissues appeared to enhance the clearance of the oncolytic from nontumor tissues. We also demonstrated enhanced antitumor effects (in a colorectal cancer model) for vvCCL5 relative to vvDD. This means that vvCCL5 may also be considered as a single agent therapeutic. Interestingly, vvCCL5 displayed prolonged persistence within the tumor relative to vvDD, despite being more actively cleared from normal tissues, implying that the expression of CCL5 may actually allow for increased replication and oncolytic effect of the virus, rather than enhancing the immunotherapeutic potential. This was initially examined by determining the level and type of immune cells infiltrating into the tumor following different therapies, as CCL5 has been implicated in attraction of T-regs to cancers. No increase in levels of regulatory T-cells were seen in tumors infected with vvCCL5, despite significant increases in the levels of CD4+ T-cells, implying that another mechanism must be necessary for the delayed clearance of this virus. vvCCL5 alone did not increase levels of CD8+ cells in the tumor, whereas an oncolytic adenovirus expressing CCL5 did.36 This may be indicative of the different immune responses induced by the different viruses (with vaccinia encoding many virulence genes that disrupt the Th1 response), or may be due to a less immunosuppressive tumor model being used with the adenovirus.
In a subsequent experiment, the relative infiltration of a luciferase labeled and adoptively transferred immunotherapeutic cell population (CIK cells) into bilateral tumors directly injected with vvDD or vvCCL5 was examined. The preferential infiltration into the vvCCL5-treated tumors demonstrated the possibility of combining vvCCL5 (or related chemokine-expressing viruses) with directly cytolytic and adoptively transferred immune cell therapies. CIK cells have undergone clinical evaluation as single agents,37 and although tumor-trafficking of these cells has been demonstrated, this may be enhanced through combination with vvCCL5. In addition, similar cell types, such as ex vivo derived or expanded tumor-targeting CTLs or chimeric antigen receptor expressing T-cells may also be used in conjunction with vvCCL5. Some NK cell therapies, including lymphokine-activated killer cells that have demonstrated robust cytolytic effects against tumor cells in vitro, but typically display weak tumor-trafficking potential in vivo38 may also benefit from such combination therapies.
Further, we looked at combinations of vvCCL5 with vaccine therapies. The use of vaccine therapies to raise a long-term immune response targeting tumor antigens has the advantage that the cytolytic cells are produced by the host's immune response and after the initial effector phase a long-term memory immune response will be maintained that may lead to immune surveillance and prevention of relapse. However, this approach also suffers from a limited ability to target effector cells into the tumor tissue, and to overcome the immune suppressive nature of large tumors. We therefore examined DC1 vaccination in combination with systemic delivery of vvCCL5. Unlike the use of vvCCL5 as a single agent, the combination with DC1 vaccination led to additional enhanced levels of CD8+ T-cell infiltration, and a significantly enhanced therapeutic response relative to either therapy alone.
Interestingly, when cytokine profiles within the tumor were examined, it was found that vvCCL5 alone increased levels of many cytokines associated with a Th2 type immune response (e.g., IL-4, IL-5), but that this was not the case with vvCCL5 used in conjunction with DC1 vaccination, where instead cytokines and chemokines associated with a Th1 response were seen (IL-12, interferon-inducible protein-10, monokine induced by γ-interferon). This could imply that the CD4+ T-cells attracted to the tumor when vvCCL5 was used alone were primarily Tc2, which in turn might explain the increased persistence of this virus. However, as DC1 vaccination primarily induces Tc1 cells, when vvCCL5 and DC1 are used in combination, the CD4+ cells in the tumor would likely be predifferentiated as Tc1 and this may lead to the additional increase in CD8+ T-cells in the tumor. Further support for this hypothesis was seen with treatment of tumors in IL-4−/− transgenic mice with vvDD or vvCCL5. Here there was no increased persistence of vvCCL5, and no enhanced CD4+ tumor infiltration relative to vvDD.
It therefore appears that, although CCL5 produced by a tumor leads to T-reg infiltration and progression, when CCL5 is expressed from an immunogenic virus within the tumor CD4+ effector T-cell infiltration, a Th2 skewed immune response and increased viral persistence and therapeutic benefit occur. However, the same virus expressing CCL5 in nontumor tissue leads to enhanced safety apparently through increased production of Th1 cytokines. Furthermore, when CCL5 is expressed from the virus and used in conjunction with a Tc1-inducing vaccination, a cytokine profile in the tumor more typically associated with a Th1 immune response is seen coupled with the greatest overall antitumor effects.
Rationally designed combinations of immunotherapy and oncolytic viral therapy may therefore be used to produce improved anticancer effects through targeting of the natural interplay between viruses and the immune response. If harnessed correctly this approach could lead to an array of novel combination cancer therapies.
Materials and Methods
Cell lines and viruses. MC38 murine colon adenocarcinoma cell line was originally induced with oral dimethylhydrazine in C57BL/6 mice, and has been used extensively by us and others. A total of 6,814 lymphoma cells were provided by Dr Felsher (Stanford University).
All recombinant vaccinia strains were in a Western Reserve background. The vvDD strain, a viral thymidine kinase and vaccinia growth factor double deletion, expressed DsRed. vvCCL5 (a version of vvDD expressing mCCL5) was constructed for this work.
In vitro viral replication assay. MC38 cells infected at multiplicity of infection of 0.1 were harvested at indicated times, subjected to three cycles of freeze-thaw, and the cell lysate homogenized with a FastPrep Cell Disrupter (Model FP120; Qbiogene, Carlsbad, CA) to release virions. Virus was then titered by standard plaque assay on CV-1 cells.
Enzyme-linked immunosorbent assay. MC38 cells were infected with either vvCCL5 or vvDD at an multiplicity of infection = 1.0. Twenty four hours later, supernatants were collected and run on enzyme-linked immunosorbent assay with DuoSet enzyme-linked immunosorbent assay development kit (R&D systems, Minneapolis, MN) according to manufacturer's guidelines.
Immune cell preparation. Activated NKT (CIK) cells were expanded from spleens of Luc+ Tg FVB mice as previously described.17 Briefly, splenocytes were cultured with IFN-γ and anti-CD3e antibody for 24 hours, before being cultured for 10–15 days with IL-2 added. Bulk splenocytes represent whole spleen populations after red blood cell lysis only.
Mouse DC1 cells were prepared as previously described; B16-FTL3L cells were injected subcutaneously into C57BL/6 mice to induce accumulation of DC in the spleen. Ten days later, CD11c+ cells were harvested and sorted with CD11c Micro beads (Miltenyi Biotec, Auburn, CA), cultured with 250 ng/ml LPS, 1,000 u/ml GM-CSF, 1,000 u/ml IL-4, and 1,000 u/ml IFN-γ for 24 hours.39 These DC were loaded with MC38 cell lysate at 37 °C for 1–2 hours and injected subcutaneously.
In vitro transwell chemotaxis assay. Supernatant was added into the lower wells of 3.0 µm pore 96-well Transwell (Corning) plates, while 50,000 immune cells were added into each of the upper wells. These were incubated at 37 °C for 3 hours and the number of migrated immune cells were counted by flow cytometry.
Mouse models. Female athymic nu−/nu− mice, C57BL/6 mice, and FVB mice were obtained from Taconic while 6-weeks-old, IL4−/− C57/BL6 mice were purchased from Jackson. All animal studies were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
In viral pathogenicity studies nu−/nu− or C57BL/6 mice were injected intraperitoneally with PBS or 1 × 108 or 3 × 108 PFU/mouse of either vvDD or vvCCL5. Mice weights were monitored and survival recorded.
For biodistribution, luminex and immunohistochemistry assays, C57BL/6 mice were injected subcutaneously with 200,000 MC38 cells or FVB mice were injected subcutaneously with 500,000 6814 cells into each flank. When the tumors reached 50–100 mm3, animals were treated with intraperitoneal or intravenous injection of PBS, virus or immune cells as indicated. For viral biodistribution, mice were killed and tissues frozen, homogenized and the viral plaque forming units recovered titered; for immune cell biodistribution, cells expressing luciferase were analyzed by bioluminescence imaging on an IVIS200 (Xenogen, part of caliper life sciences, Hopkinton, MA) after injection with luciferin (Caliper, Hopkinton, MA), and anesthesia with 2% isoflurane; for flow cytometry, tumors were harvested and dissociated with enzyme lysis buffer; for immunohistochemistry, tissues were harvested and frozen for subsequent sectioning and staining; for Luminex array assays, tissues were collected, weighed, and grounded in PBS buffer; bicinchoninic acid protein assays were performed and the samples normalized. For tumor response assays, tumor sizes were measured every other day by caliper measurement.
In some experiments an in vivo CTL assay was run. Briefly, splenocytes from a naive mouse were split and half labeled with high doses of carboxy-fluorescein diacetate succinimidyl ester and exposed to MC38 lysate, while the other half labeled with low doses of carboxy-fluorescein diacetate succinimidyl ester. These populations were mixed 1:1 and injected intravenously into mice to be tested. After 16 hours mice were killed and splenocytes run on a flow cytometer to determine the relative amounts of high and low carboxy-fluorescein diacetate succinimidyl ester-labeled cells recovered. The relative killing of the MC38 lysate exposed cells was used to quantify the CTL response against these tumor cells.
Ex vivo assays. Antibodies used in flow cytometry included labeled anti-CD4, anti-CD8, anti-NK1.1, and anti-CD11c (BD Pharmingen, San Diego, CA), and Mouse Regulatory T cell staining Kit (eBioscience, San Diego, CA); samples were analyzed on a Beckman Coulter XL32 EXPO or a DAKO Cyan flow cytometer.
Immunohistochemistry and immunofluorescence sections were incubated with anti-CD4 or CD8 followed by appropriate secondary antibodies (eBioscience). Epifluorescence images were taken using an Olympus Provis light microscope (Olympus America, Center Valley, PA) and MetaMorph (Molecular Devices, Sunny Vale, CA) was used for image analysis.
Luminex array assays were performed with Milipore Mouse 32-Plex in the University of Pittsburgh Cancer Institute facility.
Statistical methods. Unpaired and paired Student's t-tests were used to determine statistical significance (defined as P < 0.05).
SUPPLEMENTARY MATERIAL Figure S1. Characterization of vvCCL5. (A). In vitro expression of murine CCL5 from vvCCL5-infected MC38 cells in vitro as determined by ELISA (p < 0.0007 relative to vvDD); (B) Viral growth curve of vvCCL5 and vvDD in MC38 cancer cells. Cells infected at MOI of 0.1 and viral titers determined by plaque assays at subsequent times (p > 0.05).
Acknowledgments
The authors would like to thank Dean Felsher (Stanford University) for the 6814 cells; Simon Watkins' group for staining of tumor sections; Rachel Sikorski for help with culturing of cytokine-induced killer cells. Ravi Muthuswamy for help with chemotaxis assay; Lisa Bailey for isolation of natural killer cells; and Dr Anna Lokshin and Yi-Fan Chen for statistical analysis of Luminex data. This work was supported by the NCI (PO1CA132714 to P.K); and the Alliance of Cancer Gene Therapy to S.H.T.
Supplementary Material
Characterization of vvCCL5. (A). In vitro expression of murine CCL5 from vvCCL5-infected MC38 cells in vitro as determined by ELISA (p < 0.0007 relative to vvDD); (B) Viral growth curve of vvCCL5 and vvDD in MC38 cancer cells. Cells infected at MOI of 0.1 and viral titers determined by plaque assays at subsequent times (p > 0.05).
REFERENCES
- Kloor M. Lymphocyte infiltration and prognosis in colorectal cancer. Lancet Oncol. 2009;10:840–841. doi: 10.1016/S1470-2045(09)70245-1. [DOI] [PubMed] [Google Scholar]
- Atreya I., and, Neurath MF. Immune cells in colorectal cancer: prognostic relevance and therapeutic strategies. Expert Rev Anticancer Ther. 2008;8:561–572. doi: 10.1586/14737140.8.4.561. [DOI] [PubMed] [Google Scholar]
- Laghi L, Bianchi P, Miranda E, Balladore E, Pacetti V, Grizzi F.et al. (2009CD3+ cells at the invasive margin of deeply invading (pT3-T4) colorectal cancer and risk of post-surgical metastasis: a longitudinal study Lancet Oncol 10877–884. [DOI] [PubMed] [Google Scholar]
- Harrop R, Drury N, Shingler W, Chikoti P, Redchenko I, Carroll MW.et al. (2008Vaccination of colorectal cancer patients with TroVax given alongside chemotherapy (5-fluorouracil, leukovorin and irinotecan) is safe and induces potent immune responses Cancer Immunol Immunother 57977–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh B, Ko A, Venook A, Margolin K, Zeh H, Lotze M.et al. (2007Vaccination of metastatic colorectal cancer patients with matured dendritic cells loaded with multiple major histocompatibility complex class I peptides J Immunother 30762–772. [DOI] [PubMed] [Google Scholar]
- Alves PM, Viatte S, Fagerberg T, Michielin O, Bricard G, Bouzourene H.et al. (2007Immunogenicity of the carcinoembryonic antigen derived peptide 694 in HLA-A2 healthy donors and colorectal carcinoma patients Cancer Immunol Immunother 561795–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCart JA, Ward JM, Lee J, Hu Y, Alexander HR, Libutti SK.et al. (2001Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes Cancer Res 618751–8757. [PubMed] [Google Scholar]
- Guo ZS, Naik A, O'Malley ME, Popovic P, Demarco R, Hu Y.et al. (2005The enhanced tumor selectivity of an oncolytic vaccinia lacking the host range and antiapoptosis genes SPI-1 and SPI-2 Cancer Res 659991–9998. [DOI] [PubMed] [Google Scholar]
- Kim JH, Oh JY, Park BH, Lee DE, Kim JS, Park HE.et al. (2006Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF Mol Ther 14361–370. [DOI] [PubMed] [Google Scholar]
- Kirn DH, Wang Y, Le Boeuf F, Bell J., and, Thorne SH. Targeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 2007;4:e353. doi: 10.1371/journal.pmed.0040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne SH, Hwang TH, O'Gorman WE, Bartlett DL, Sei S, Kanji F.et al. (2007Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963 J Clin Invest 1173350–3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BH, Hwang T, Liu TC, Sze DY, Kim JS, Kwon HC.et al. (2008Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial Lancet Oncol 9533–542. [DOI] [PubMed] [Google Scholar]
- Levy JA. The unexpected pleiotropic activities of RANTES. J Immunol. 2009;182:3945–3946. doi: 10.4049/jimmunol.0990015. [DOI] [PubMed] [Google Scholar]
- Mueller A., and, Strange PG. The chemokine receptor, CCR5. Int J Biochem Cell Biol. 2004;36:35–38. doi: 10.1016/s1357-2725(03)00172-9. [DOI] [PubMed] [Google Scholar]
- Mailliard RB, Wankowicz-Kalinska A, Cai Q, Wesa A, Hilkens CM, Kapsenberg ML.et al. (2004alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity Cancer Res 645934–5937. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Foon KA, Mailliard RB, Muthuswamy R., and, Kalinski P. Type 1-polarized dendritic cells loaded with autologous tumor are a potent immunogen against chronic lymphocytic leukemia. J Leukoc Biol. 2008;84:319–325. doi: 10.1189/jlb.1107737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu PH., and, Negrin RS. A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J Immunol. 1994;153:1687–1696. [PubMed] [Google Scholar]
- Yurchenko E, Tritt M, Hay V, Shevach EM, Belkaid Y., and, Piccirillo CA. CCR5-dependent homing of naturally occurring CD4+ regulatory T cells to sites of Leishmania major infection favors pathogen persistence. J Exp Med. 2006;203:2451–2460. doi: 10.1084/jem.20060956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan MC, Goedegebuure PS, Belt BA, Flaherty B, Sankpal N, Gillanders WE.et al. (2009Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer J Immunol 1821746–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger M, Cao YA, Verneris MR, Bachmann MH, Contag CH., and, Negrin RS. Revealing lymphoma growth and the efficacy of immune cell therapies using in vivo bioluminescence imaging. Blood. 2003;101:640–648. doi: 10.1182/blood-2002-06-1751. [DOI] [PubMed] [Google Scholar]
- Cao YA, Bachmann MH, Beilhack A, Yang Y, Tanaka M, Swijnenburg RJ.et al. (2005Molecular imaging using labeled donor tissues reveals patterns of engraftment, rejection, and survival in transplantation Transplantation 80134–139. [DOI] [PubMed] [Google Scholar]
- Karlsson A, Giuriato S, Tang F, Fung-Weier J, Levan G., and, Felsher DW. Genomically complex lymphomas undergo sustained tumor regression upon MYC inactivation unless they acquire novel chromosomal translocations. Blood. 2003;101:2797–2803. doi: 10.1182/blood-2002-10-3091. [DOI] [PubMed] [Google Scholar]
- Thorne SH, Negrin RS., and, Contag CH. Synergistic antitumor effects of immune cell-viral biotherapy. Science. 2006;311:1780–1784. doi: 10.1126/science.1121411. [DOI] [PubMed] [Google Scholar]
- Dudley ME., and, Rosenberg SA. Adoptive cell transfer therapy. Semin Oncol. 2007;34:524–531. doi: 10.1053/j.seminoncol.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watchmaker PB, Berk E, Muthuswamy R, Mailliard RB, Urban JA, Kirkwood JM.et al. (2010Independent regulation of chemokine responsiveness and cytolytic function versus CD8+ T cell expansion by dendritic cells J Immunol 184591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne SH, Liang W, Sampath P, Schmidt T, Sikorski R, Beilhack A.et al. (2010Targeting localized immune suppression within the tumor through repeat cycles of immune cell-oncolytic virus combination therapy Mol Ther 181698–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Gao L, Yeagy B., and, Reid T. Virus combinations and chemotherapy for the treatment of human cancers. Curr Opin Mol Ther. 2008;10:371–379. [PubMed] [Google Scholar]
- Galanis E, Okuno SH, Nascimento AG, Lewis BD, Lee RA, Oliveira AM.et al. (2005Phase I-II trial of ONYX-015 in combination with MAP chemotherapy in patients with advanced sarcomas Gene Ther 12437–445. [DOI] [PubMed] [Google Scholar]
- Post DE, Fulci G, Chiocca EA., and, Van Meir EG. Replicative oncolytic herpes simplex viruses in combination cancer therapies. Curr Gene Ther. 2004;4:41–51. doi: 10.2174/1566523044577988. [DOI] [PubMed] [Google Scholar]
- Yu DC, Chen Y, Dilley J, Li Y, Embry M, Zhang H.et al. (2001Antitumor synergy of CV787, a prostate cancer-specific adenovirus, and paclitaxel and docetaxel Cancer Res 61517–525. [PubMed] [Google Scholar]
- Twigger K, Vidal L, White CL, De Bono JS, Bhide S, Coffey M.et al. (2008Enhanced in vitro and in vivo cytotoxicity of combined reovirus and radiotherapy Clin Cancer Res 14912–923. [DOI] [PubMed] [Google Scholar]
- Cole C, Qiao J, Kottke T, Diaz RM, Ahmed A, Sanchez-Perez L.et al. (2005Tumor-targeted, systemic delivery of therapeutic viral vectors using hitchhiking on antigen-specific T cells Nat Med 111073–1081. [DOI] [PubMed] [Google Scholar]
- Kirn DH., and, Thorne SH. Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer. 2009;9:64–71. doi: 10.1038/nrc2545. [DOI] [PubMed] [Google Scholar]
- Banaszynski LA, Sellmyer MA, Contag CH, Wandless TJ., and, Thorne SH. Chemical control of protein stability and function in living mice. Nat Med. 2008;14:1123–1127. doi: 10.1038/nm.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahar MW, Kenyon JC, Putz MM, Abrescia NG, Pease JE, Wise EL.et al. (2008Structure and function of A41, a vaccinia virus chemokine binding protein PLoS Pathog 4e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapteva N, Aldrich M, Weksberg D, Rollins L, Goltsova T, Chen SY.et al. (2009Targeting the intratumoral dendritic cells by the oncolytic adenoviral vaccine expressing RANTES elicits potent antitumor immunity J Immunother 32145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemhuis T, Wells S, Scheffold C, Edinger M., and, Negrin RS. A phase I trial of autologous cytokine-induced killer cells for the treatment of relapsed Hodgkin disease and non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2005;11:181–187. doi: 10.1016/j.bbmt.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Melder RJ, Whiteside TL, Herberman RB., and, Jain RK. Preferential localization of human adherent lymphokine-activated killer cells in tumor microcirculation. J Natl Cancer Inst. 1991;83:433–437. doi: 10.1093/jnci/83.6.433. [DOI] [PubMed] [Google Scholar]
- Giermasz AS, Urban JA, Nakamura Y, Watchmaker P, Cumberland RL, Gooding W.et al. (2009Type-1 polarized dendritic cells primed for high IL-12 production show enhanced activity as cancer vaccines Cancer Immunol Immunother 581329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characterization of vvCCL5. (A). In vitro expression of murine CCL5 from vvCCL5-infected MC38 cells in vitro as determined by ELISA (p < 0.0007 relative to vvDD); (B) Viral growth curve of vvCCL5 and vvDD in MC38 cancer cells. Cells infected at MOI of 0.1 and viral titers determined by plaque assays at subsequent times (p > 0.05).