Abstract
Lentiviral vectors with self-inactivating (SIN) long terminal repeats (LTRs) are promising for safe and sustained transgene expression in dividing as well as quiescent cells. As genome organization and transcription substantially differs between actively dividing and postmitotic cells in vivo, we hypothesized that genomic vector integration preferences might be distinct between these biological states. We performed integration site (IS) analyses on mouse dividing cells (fibroblasts and hematopoietic progenitor cells (HPCs)) transduced ex vivo and postmitotic cells (eye and brain) transduced in vivo. As expected, integration in dividing cells occurred preferably into gene coding regions. In contrast, postmitotic cells showed a close to random frequency of integration into genes and gene spare long interspersed nuclear elements (LINE). Our studies on the potential mechanisms responsible for the detected differences of lentiviral integration suggest that the lowered expression level of Psip1 reduce the integration frequency in vivo into gene coding regions in postmitotic cells. The motif TGGAA might represent one of the factors for preferred lentiviral integration into mouse and rat Satellite DNA. These observations are highly relevant for the correct assessment of preclinical biosafety studies, indicating that lentiviral vectors are well suited for safe and effective clinical gene transfer into postmitotic tissues.
Introduction
Lentiviral self-inactivating (SIN)1 vectors have now been successfully used in their first clinical application. In two patients ex vivo hematopoietic stem cell gene transfer of the ABCD1 transporter transgene has led to the long-term clinical arrest of progressive demyelination in adrenoleukodystrophy.2 Though lentiviral SIN vectors, without doubt, reduce the risk of insertional oncogenesis in gene therapy compared to full long terminal repeat (LTR)-driven γ-retroviral vectors,3,4,5 the preferred integration in gene coding regions remains a concern. In fact, in the recent lentiviral vector–based clinical study for the treatment of β-thalassemia first side effects occurred. Vector integration into the third intron of the HMGA2 proto-oncogene has led to a truncated mRNA and benign clonal dominance of the affected cell clone in one patient.6
Binding of the preintegration complex to particular protein and DNA motifs,7,8,9,10,11 transcriptional activity12,13,14,15 and packaging status of DNA sequences16 have been suggested as some of the mechanisms determining the distribution of retroviral insertions. To date, the causal mechanisms responsible for the preferred integration into particular genomic sites are not fully understood. There are indications that integration site (IS) distribution not only depends on the retroviral subtype, but might also be subject to specific features associated with target cell type, species and structure, e.g., repetitive elements and cellular gene expression. Increased integration of microinjected linear human miniSatellite DNA into repetitive Satellite elements has been described in mouse DNA.17 A relevant correlation between gene expression levels and integration preference in gene coding regions has been so far only established for Psip1 expression in dividing cells.18,19 In addition, a reduction of lentiviral integration frequency in genes of terminally differentiated macrophages significantly correlated with a reduced Psip1 gene expression level, but close to random integration in gene coding regions so far has not been described.18
Many future in vivo clinical applications of lentiviral vectors are intended for postmitotic tissue related to the eye or the central nervous system, often including an in vivo application approach. No systematic study is available for the influence of this vector administration route on IS distribution and related consequences. To address this issue, we have performed a preclinical large scale IS distribution analyses in rodent postmitotic tissues after in vivo administration. We compared the in vivo IS profile in mouse and rat tissues of eye and brain with ex vivo–transduced fibroblasts (SC-1) and primitive hematopoietic progenitor cells (HPCs) that proliferated and divided during transduction.20,21 Our data show a distinctly different, advantageous integration profile in postmitotic cells compared to mitotically active cells for lentiviral vectors, including a close to random IS frequency in gene coding regions.
Results
Close to random in vivo lentiviral integration frequency in gene coding regions in mouse postmitotic cells
To dissect potential differences in IS distribution between dividing and quiescent postmitotic mouse cells, we performed comparative large scale lentiviral SIN vector IS studies by linear amplification–mediated PCR (LAM-PCR) followed by high-throughput sequencing (Sanger- and pyrosequencing) (Experimental strategy is shown in Supplementary Figure S1).20 Dividing cell types were transduced with a green fluorescent protein (GFP) vector either under the control of an internal phosphoglycerate kinase promoter for HPC or a cytomegalovirus promoter for SC-1 cells. The transduction of postmitotic cell types was carried out by direct in vivo injection of a GFP expressing vector into mouse eye cups (subretinally) and mouse brains (stereotactically). For in vivo application, different promoters were used in the vectors: either a spleen focus-forming virus promoter or a cytomegalovirus promoter in the case of retinal cells and a phosphoglycerate kinase promoter for the stereotactical injection into the brain striatum. To assess the lentiviral IS pattern in postmitotic cells of an additional species, a lentiviral SIN vector expressing the therapeutic transgene Mertk under control of the spleen focus-forming virus promoter was subretinally injected into eyes to treat retinitis pigmentosa (Royal College of Surgeons rat model). DNA and RNA isolation for IS analyses were performed from whole eye cups and striatum (brain). To avoid any unwanted methodological biases,22 LAM-PCR analyses on the DNA derived from the different cell types were performed with restriction enzymes Tsp509I and HpyCH4IV (Supplementary Figure S2).
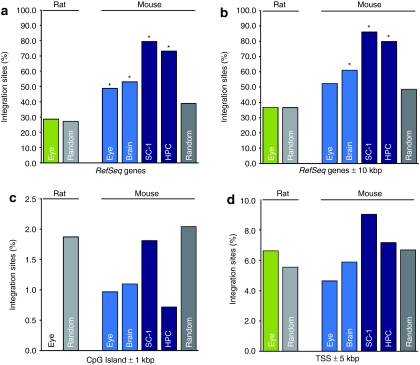
As expected, in mitotically active cells 73.12% and 79.52% of the integrants in dividing HPC and SC-1 cells were found in protein coding RefSeq genes, respectively. This frequency differed significantly (P < 10–17) from an assumed random distribution (Figure 1a and Table 1). In contrast, in vivo–transduced postmitotic murine eye cells only revealed 48.84% of IS located within RefSeq genes, and only 52.94% of IS located in RefSeq genes in case of brain cells. For both, the neuronal and retinal data sets the distribution of IS in RefSeq genes was significantly lower than observed in the mitotically active cells. Dividing cells showed a ~1.5-times higher frequency of IS located inside RefSeq genes (χ2 test, P < 10–11, Figure 1a and Table 1). Interestingly, for postmitotic mouse eyes the genomic IS profile within RefSeq genes and the surrounding 10 kilobase pair (kbp) region revealed a random IS distribution (Peye = 0.1). For brain tissue, the IS distribution in RefSeq genes and the surrounding 10 kbp genomic region was still significantly preferred (P = 5.54 × 10−05, Figure 1a,b and Table 1). The proximity of transcriptional start sites and CpG islands have neither been disfavored nor favored for any of our data sets (Figure 1c,d).
Figure 1.
Lentiviral integration site (IS) distribution in rodent RefSeq genes and the surrounding 10 kbp region. (a) The percentage of lentiviral IS located in RefSeq genes and (b) in RefSeq genes including the surrounding 10 kbp region is shown. IS frequency into RefSeq genes is substantially lower in postmitotic cells compared to dividing cells. Including the 10 kbp vicinity of RefSeq genes, distribution of our postmitotic data sets obtained from retinal cells was not statistically different compared to a random distribution (binomial distribution, two-sided test, P < 0.05). Bars denote frequency of IS retrieved from rat eye (green), from mouse eye and brain tissue (light blue) and from mouse dividing cells (dark blue). The percentages of IS in the vicinity of (c) transcriptional start sites (TSS) and (d) CpG islands are displayed. The distribution of IS in TSS and the 5 kbp surrounding genomic region and in CpG Islands and the surrounding 1 kbp genomic region have been analyzed. Statistical testings have been performed by comparing the experimental data to in silico generated IS, no statistical significant differences compared to the random data set have been observed. The frequency of in silico generated IS located in the respective feature are displayed in light gray for the rat genome and in dark gray for the mouse genome. Of note, to date there are 1.56 times more RefSeq genes known from the mouse genome sequencing project compared to the rat genome. *Statistically different integration site distribution compared to in silico generated random data set (binomial distribution, two-sided test, P < 0.05); kbp, kilobase pair(s); SC-1, mouse fibroblasts; HPC, hematopoietic progenitor cells.
Table 1. Frequency of lentiviral integration sites (IS) in RefSeq genes.
Influence of cellular gene expression on lentiviral IS distribution
To test the hypothesis that integration profiles are dependent on the expression status of cellular genes, we compared RNA microarray analyses (Illumina, San Diego, CA) of untransduced SC-1 cells with those of untransduced mouse eyes and brains. The percentage of expressed genes was comparable among mitotically active SC-1 cells (28.58%), postmitotic eye (30.22%), and brain (21.22%) cells, although the proportion of IS found in gene coding regions of SC-1 cells was ~50% higher than in postmitotic cells (Table 1). Therefore, the distinct IS profiles obtained in our dividing and nondividing data sets were not associated with the overall number of expressed genes in the different cell types.
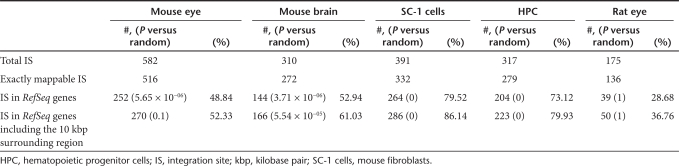
To assess the role of individual gene expression levels of RefSeq genes targeted by our vectors, we studied whether expressed genes were preferred targets for in vivo vector integration in postmitotic cells. Our RNA microarray analysis showed that expressed genes were favored targets for vector integration in dividing cells (Figure 2). In postmitotic tissues, this correlation was less pronounced and did not correspond with medium and high gene expression levels.
Figure 2.
Association between lentiviral integration and gene expression in mouse fibroblasts, mouse eye, and brain tissues. Illumina BeadChip microarray analyses of untransduced dividing and postmitotic mouse cell types revealed a slightly preferred vector integration into (highly) expressed cellular genes for dividing mouse SC-1 fibroblasts (dark blue). The integration preference into expressed genes is less pronounced for mouse postmitotic eyes and brain cells (light blue). The respective Probe IDs with “NM_” accessions on the Illumina MouseRef-8 and Illumina MouseRef-6 Expression BeadChip were grouped and sorted on their expression values (four BINs), (0: likely background expression; 1: low expression; 2: medium expression; 3: medium to high expression). The ratio of integration site within RefSeq genes belonging to one of these four groups to the percentages of overall RefSeq genes which are belonging to these four groups are displayed.
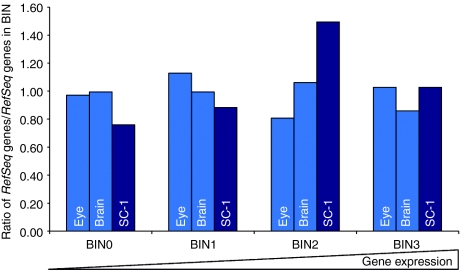
Cellular factors like lens epithelium-derived growth factor (Psip1/Ledgf/p75) are known to bind to HIV integrase and seem to play an important role in integration targeting. A positive correlation of Psip1 gene expression in target cells and integration in gene coding regions was previously suggested for human and mouse cells infected with lentiviral vectors.18,19,23,24 Our microarray analysis revealed that Psip1 expression (Probe ID 1780082) in SC-1 cells was ~1.7 times higher than in brain tissues and 2.5-fold higher compared to the eye. These data were in accordance with the integration frequency we found in RefSeq genes, where the highest proportions of integrants in gene coding regions were noticed in SC-1 cells, followed by brain and eye tissue (Figure 3).
Figure 3.
Relation of expression level of Psip1 and integration site (IS) distribution in RefSeq genes. Expression levels of Psip1 were measured on Illumina MouseRef-8 Expression BeadChip microarray on RNA from untransduced mouse SC-1 fibroblasts, postmitotic eye and brain cells, revealing an increased integration frequency into gene coding regions with increasing Psip1 expression. Diamonds display the average signals of Psip1 in the distinct cell types (left y-axis). The percentages of IS located in RefSeq genes, analyzed by pyrosequencing, are shown in bars (right y-axis). Light blue bars are displaying the results obtained from postmitotic tissues, dark blue bars from dividing cells (SC-1). Standard errors of mean signals are indicated as error bars. ProbeID 1780082 is exemplarily shown. SC-1, mouse fibroblasts.
Variable lentiviral integration into repetitive elements
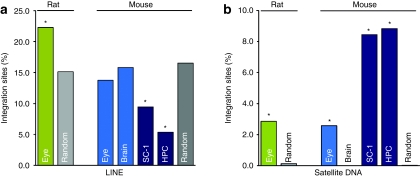
In postmitotic cells, two types of repetitive elements were targeted by the lentiviral vectors in unexpected frequencies (Figure 4 and Supplementary Table S1a). As observed in earlier studies, lentiviral vectors used to transduce dividing cells showed a significantly lower integration frequency in long interspersed nuclear elements (LINE) that comprise gene spare regions (P < 0.0000844). In contrast, integration frequency in LINE found in cells derived from postmitotic mouse eye and brain was not different from a uniform random distribution (Peye = 0.076; Pbrain = 0.81). These data were well in line with our observation of a nearly random integration of lentiviral vectors into gene coding regions after in vivo injection into postmitotic tissue.
Figure 4.
Lentiviral integration site (IS) distribution in rodent repetitive elements. Distinct IS preferences in mouse dividing and postmitotic cells were found for (a) Satellite DNA and (b) LINE. In all data sets, IS distribution in Satellite DNA was significantly overrepresented compared to the random distribution of Satellite DNA in the mouse genome (0.08%). In mouse brain, no IS have been detected in Satellite DNA. IS distribution in LINE corresponded in mouse eye, and in mouse brain tissue to a random distribution (binomial distribution, two-sided test), and was significantly lower for all data sets from dividing cell types compared to in silico generated IS. Two-sided binomial testings for IS obtained from rat eye tissue have been performed on in silico generated IS from the rat genome. A preferred integration in LINE and Satellite DNA has been observed for IS in rat eyes. Green bars denote IS in rat eyes, light blue bars are displaying IS in mouse nondividing cells, and dark blue bars IS in dividing cells. IS in LINE and Satellite DNA generated from an in silico data set are shown in light gray for the rat genome and in dark gray for the mouse genome. HPC, hematopoietic progenitor cells; LINE, long interspersed nuclear element; SC-1, mouse fibroblasts.
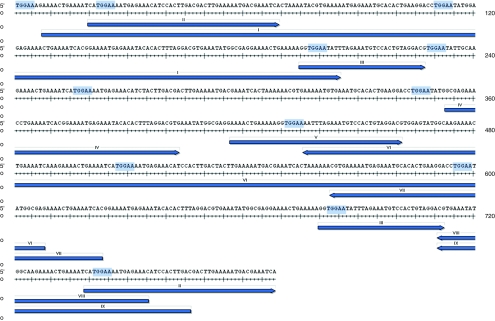
Furthermore, we detected a highly significant increase of integrants located in Satellite DNA in all mouse cell types (P < 10–17), except brain tissues. We identified the sequence motif TGGAA present at the 5′ end and close to the 3′ end of the lentiviral LTR that is also present in the mouse Satellite DNA (Figure 5). To clarify whether IS in Satellite DNA were attracted by the TGGAA motif, we examined the distances between IS and the closest TGGAA motif inside and outside of Satellite DNA. The medium distance between two consecutive TGGAA motifs in Satellite DNA was 293.98 bp, compared to 282.29 bp in the non-Satellite mouse genome (Supplementary Table S1b). In this context it was remarkable that the average distance between IS and TGGAA motifs for IS in mouse eye was ~8 times smaller for IS located in Satellite DNA (17.47 bp) than for IS not located in Satellite DNA (143.11 bp; Supplementary Table S1c), for SC-1 cells and HPC about five times and seven times, respectively, indicating an influence on vector integration by this motif.
Figure 5.
Clustering of lentiviral integration sites (IS) in γ-Satellite. The preferred lentiviral integration into mouse (and rat) Satellite DNA observed in dividing and postmitotic cells, except for mouse brain tissue, is exemplarily shown for a prototypical Satellite sequence to demonstrate how close the insertions are to the motif. In this case, identified IS were aligned to a γ-Satellite DNA (GSAT_MM). The genomic part of linear amplification–mediated PCR amplicons which aligned to this Satellite DNA are shown as blue arrows, TGGAA motif are highlighted in light blue. Numbers above the arrows indicate different IS. IS were obtained by Sanger sequencing.
Similar lentiviral postmitotic IS profiles in rat eyes
To confirm the IS distribution characteristics detected in mouse eye tissue in a second species, we analyzed rat eyecups accordingly. As observed for the mouse eye tissue, we found no obvious preference of lentiviral integration into gene coding regions, only 22.29% of integrants in rat eyes were detected in protein coding RefSeq genes; Figure 1a,b and Table 1). The integration profile in LINE elements and Satellite DNA showed highly similar IS distribution characteristics as observed in mouse eye tissues, where a random integration profile was observed for LINE elements and a favored integration for Satellite DNA, respectively. For rat eye tissue, integration into LINE elements was also close to random, though slightly different to our random data set (P = 0.015, Figure 4a and Supplementary Table S1a). Remarkably, 2.86% of integrants in the rat eye were detected in Satellite DNA (P = 7.98 × 10−06, Figure 4 and Supplementary Table S1a). In rat Satellite DNA, we examined the distribution of the sequence motif TTGAA and TGGGA, a similar motif found in the rat Satellite DNA, which might influence integration into this repetitive element. These data also showed a closer distance between the IS and these motifs in Satellite DNA compared to the non-Satellite rat genome (Supplementary Table S1d).
Discussion
The available genomic distribution analyses of lentiviral SIN vectors in mammalian cells showed uniformly a similar profile of lentiviral vector integration, independent of vector design (internal promoters, transgenes): two-thirds of lentiviral integrations are located in gene coding regions without obvious preferences for transcriptional start sites or other regulatory elements.4,12,13,25,26,27,28,29 These findings are based on in vitro and ex vivo–transduced rodent, nonhuman primate and human cell populations, and were commonly extrapolated to quiescent cell populations. Here, we show that in vivo vector administration of lentiviral vectors into an unperturbed postmitotic state has a hitherto unknown, decisive influence on the distribution of vector insertion. IS were generated using large scale LAM-PCR analyses and high-throughput sequencing technologies.
Previous reports and own unpublished data have not revealed any influence of the various vector designs we have used in our studies (i.e., internal promoters and transgenes) on the integration profile of lentiviral vectors. These findings enabled us to combine IS data obtained from cells transduced with vectors varying in their confirmation. We identified a nearly random integration frequency in gene coding regions and in LINE in mouse and rat eyes, as well as in mouse brains. In earlier studies performed on nondividing, terminally differentiated macrophages and growth-arrested lung fibroblasts, these differences have been less pronounced.30,31 The discrepancy in integration profiles observed between earlier studies and our study might, in part, be reasoned by distinct investigated cells types: in our study we focused our research on nondividing cells of neuronal origin, earlier studies investigated the lentiviral integration pattern in macrophages and growth-arrested fibroblasts. Also, the unequal way of vector administration might account for the observed differences. In our study, postmitotic cells were transduced by direct in vivo application of vector, whereas in former studies cells were expanded prior to transduction and further cultured in vitro. We and others already showed that subretinal injection of lentiviral vectors results solely in the transduction of postmitotic cells of the retinal pigment epithelium,32,33 and stereotactical injection of vesicular stomatitis virus G –pseudotyped lentiviral vectors into the striatum was shown to transduce most exclusively postmitotic neurons.34
To uncover potential mechanisms leading to these differences between unperturbed, quiescent cells in vivo and transduced cells in vitro or ex vivo, we performed comparative microarray analysis to clarify whether the lower integration frequency in gene coding regions in postmitotic cells correlated with the cellular gene expression status. However, the distinct integration profiles could not be assigned to the proportion of the overall number of expressed genes in the different cell types, as the frequency of expressed genes was in a comparable range. Furthermore, we investigated the relationship of the expression status of genes prior to transduction which were targeted by our vector in the different analyzed cell types. For eye and brain cells, we noticed a tendency of lentiviral vectors to integrate into nonexpressed genes, whereas in dividing fibroblasts integration took place preferably in moderately to highly expressed genes. Thus, expression of genes may facilitate vector integration in cycling cells, but is not a critical determinant in quiescent cells.
Previous studies have suggested a correlation of the expression level of Psip1 and the frequency of integration in transcription units, postulating the existence of potential tethering mechanisms involved in HIV-1 integration.18 These data also included the integration data set obtained from macrophages mentioned above,30 revealing a lower level of Psip1 expression compared to other cell types which goes in line with a reduced integration frequency in gene coding regions compared to T cells. To uncover the role of Psip1 in integration targeting, earlier studies already investigated the frequencies of IS in transcriptional units in Psip1 knockdown cell lines,19 in embryonic fibroblasts derived from a mouse strain with depleted Psip1 (LEDGF/p75)24 and from gene trap mutants.18 These cell types showed a statistically significant reduced integration frequency inside gene coding regions compared to control cells, however, integration events inside gene coding regions were still statistically significant and enriched compared to matched random controls. Besides of the reduced integration frequencies into genes LEDGF-deficient cell types showed an increased integration frequency near the transcriptional start sites and close to CpG islands in earlier studies. Here, we haven't noticed an enrichment of IS around the transcriptional start sites and CpG island in nondividing cells, but observed reduced, and in this case statistically significant compared to dividing cells, integration events inside gene coding regions in quiescent retinal cell types.
In the next step, we determined the expression level of Psip1 in our different cell types and were able to confirm that a lower frequency of IS in gene coding regions corresponded with lower expression levels of Psip1. Therefore, our data are pointing to a role of Psip1 in integration targeting of lentiviral vectors, however, it still remains difficult to speculate the driving force for the reduced integration frequency in nondividing cells. Cell cycle state, Psip1 expression of the transduced cell types and very likely other yet unknown host and cellular factors might collectively account for this.
Remarkably, the preferred integration into Satellite DNA in the rodent genomes has only been described for microinjected tandem repeated DNA of the human miniSatellite MS3217 and for latent proviral human immunodeficiency virus so far.25 Lewinski et al. have shown preferential lentiviral integration in gene deserts and centromeric heterochromatin in Jurkat cells. Thus, sequence-specific targeting and a breakage prone condensed chromatin structure in Satellite DNA might be responsible for the preferred integration into centromeric mouse Satellite DNA.17 A likely explanation is that insertion is attracted to the vicinity of Satellite sequence due to additional, still unknown factors—the lack of integrants in Satellite DNA in the analyzed brain cells raises this issue—but targeting of the actual insertion locus seems to be facilitated by the presence of the TGGAA motif. Interestingly, this motif ((TGGAA)n) is speculated to be the functional centromeric sequence in humans, due to its localization in centromers, its conservation and high binding affinity to nuclear proteins and indications for the promotion of DNA structures.35,36,37
Our results emphasize the potential of large scale IS mapping studies to obtain deeper insights into the mechanisms underlying viral integration in vivo. Such studies are highly relevant for the correct and validated assessment of vector characteristics in vitro and preclinical biosafety studies. In vivo application of lentiviral vectors into postmitotic tissue disrupts significantly fewer actively transcribed genes than in actively dividing cells, and should be very safe and useful for clinical applications, in particular for the direct in vivo correction of retinal and neuronal disorders.
Materials and Methods
Intraocular and stereotactical brain injections. The procedure of intraocular injections in mice and rats were conducted as approved by the UK Home Office and the Institute of Ophthalmology Research Ethics Committee, in compliance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Stereotactical injections were performed as described in ref. 32. Stereotactical brain injections in mice were performed according to protocols approved by the Animal Care and Use Committee of the San Raffaele Institute (IACUC 225) and communicated to the Ministry of Health and local authorities according to Italian law.
Vector production. Lentiviral vectors L-cSegfpW, L-cCegfp, L-cSmertkW, and LV_hPGK_GFP_wPRE have been described in ref. 3 and ref. 33 and were produced as described previously.
Cell culture. SC-1 cells were cultured in Dulbecco's modified Eagle's medium (GIBCO; Invitrogen, Karlsruhe, Germany) with standard supplements. SC-1 cells were transduced with L-cCegfp at a multiplicity of infection of 900 (qPCR titer, equivalent to 52 enhanced GFP titer) and were kept in culture until day 78 post-transduction. Bone marrow–derived HPCs (lin− cells) were isolated and transduced as described3 previously.
Intraocular injections. Subretinal injections were performed as previously described.33 Female C57Bl/6J mouse eyes (n = 6) were injected with two different lentiviral vectors (L-cSegfpW and L-cCegfp). Mouse eyes were harvested at different points of time after lentiviral injection (14 days to 11 months).
The Royal College of Surgeons' rat model was chosen for lentiviral vector (L-cSmertkW) injections into rat eyes. Vector administration was performed as described in ref. 33. Rat eyes (n = 2) were retrieved 10 weeks after injection.
Brain injections. C57Bl/6J and Cdkn2a−/− mice (n = 4) were stereotactically injected into the brain striatum of the left hemisphere at 2.5 mm from the bregma and 3 mm in depth. Two microliter of concentrated lentiviral vector (LV_hPGK_GFP_wPRE) (titer of 10e10 TU/ml) was injected with a 5 µl Hamilton syringe with a needle of 30 ga. Mice were killed 11 days after injection.
DNA and RNA isolation. Whole mouse or rat eye cups were flash frozen immediately after harvesting and excess extraocular tissue (including muscle, optic nerve, and episclera) was dissected and discarded, dissected tissues were stored at −80 °C. Brain tissues were excised using a stereoscopic dissection microscope under UV illumination, dissected tissues were frozen at −80 °C. DNA was isolated from lentivirally transduced SC-1 cells, mouse eye cups, rat eye cups, and mouse brain using the High Pure PCR Product Purification Kit (Roche, Mannheim, Germany) according to the manufacturer's instructions. Total RNA was isolated from untransduced SC-1 cells, mouse brain and mouse eyes prior to injection of lentiviral vectors using the RNeasy Kit (Qiagen, Hilden, Germany) according to the manufacturer's instruction, RNA was eluted in water. The quality of total RNA was checked by gel analysis using the total RNA Nano chip assay on an Agilent 2100 Bioanalyzer (Agilent Technologies GmbH, Waldbronn, Germany). Only samples with RNA index values >8.5 were selected for expression profiling. RNA concentrations were determined using the NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE). Expression profiling data were analyzed in duplicates for SC-1 cells and uninjected mouse eyes.
3′ LTR IS analysis by LAM-PCR. To retrieve vector-genome junctions, we accomplished LAM-PCR as previously described.21 In brief, 10–100 ng of DNA was preamplified by linear PCR. Biotinylated linear PCR products were enriched via magnetic beads, followed by second strand synthesis, restriction digest, ligation of a linker cassette, and two additional exponential amplifications. All samples were analyzed using two different restriction enzymes (Tsp509I, HpyCH4IV) for LAM-PCR to identify IS in separate approaches. LAM-PCR amplicons were purified for downstream sequencing analyses using the QIAquick PCR Purification Kit (Qiagen) (Sanger sequencing or pyrosequencing).
Fusion primer PCR. To make pyrosequencing more efficient and to increase the number of different samples in a single pyrosequencing run, LAM-PCR products were reamplified using fusion primers as recommended by the manufacturer. Fusion primer PCR was done to add the GS FLX specific amplification and sequencing primers A and B, also needed for immobilization of the PCR products onto DNA capture beads, to both ends of the LAM-PCR amplicons. Primer design was done as suggested by 454 Life Science. In brief, primer B was fused to a linker-specific primer (Fusion Primer B-LK), and primer A was fused to a vector specific LTR primer (fusion primer A-LTR). An individual recognition sequence of 4–6 bases was incorporated into the fusion primer A-LTR to allow the parallel analysis of different samples in a single sequencing run. This configuration required the following fusion primers: 5′-GCCTCCCTCGCGCCATCAG-NNNN(NN)-TGTGTGACTCTGGTAACTAG-3′ (fusion primer A-LTR) and 5′-GCCTTGCCAGCCCGCTCAG-AGTGGCACAGCAGTTAGG-3′ (fusion primer B-LK). 40 ng of each purified LAM-PCR product was amplified using 5–16.6 pmol of each fusion primer in a 50 µl volume. 40–200 ng of purified LAM-PCR products were used as template DNA. Fusion primer PCR conditions were as follows: initial denaturation 2 minutes at 95 °C followed by 10 or 11 cycles of 95 °C for 45 seconds, 60 °C for 45 seconds and 72 °C for 1 minute, followed by a final extension of 5 minutes at 72 °C. 5 µl of each PCR-product was visualized on a 2% agarose gel and DNA concentration was quantified (NanoDrop Technologies).
Pyrosequencing using the Genome Sequencer GS 20 and GS FLX platform (Roche). Sample preparation for pyrosequencing with the GS 20 and GS FLX platform (Roche) was performed using the GS emPCR Kit II as recommended by the manufacturer. LAM-PCR products were not fragmented prior to emulsion PCR. Different numbers of regions (8 and 16) on one PicoTiterPlate device were used. Sequences were reassigned according to their individual barcodes.
Lentiviral IS data analysis. Individual LAM-PCR amplicons were trimmed and aligned to the corresponding host genome assembly (mouse mm9, rat rn4) as described recently.38 The data for the annotation of the IS with genes (RefSeq), repeats (RepeatMasker) and CpG islands were obtained from the UCSC genome browser (http://genome.ucsc.edu/) on November 12th, 2010 for mouse and rat, respectively. IS analysis (exactly mappable IS) data obtained from postmitotic tissues are shown in Supplementary Table S2a–d, IS analysis (exactly mappable IS) data obtained from dividing cells are shown in Supplementary Table S3a–c.
A random IS data set for each restriction enzyme (Tsp509I and HpyCH4IV) was generated. 10,000 random genomic positions were selected as random IS. A random IS was considered as amplifiable if a restriction enzyme cutting site was within a distance of 1000 bp. The remaining sequences were analyzed as described above by omitting the trimming step.
Probe labeling and Illumina Sentrix BeadChip array hybridization. Biotin-labeled complementary RNA (cRNA) samples for hybridization on one Illumina MouseRef-8 and one Mouse-Sentrix-6 Expression BeadChip arrays (Illumina) were prepared according to Illumina's recommended sample labeling procedure based on the modified Eberwine protocol,39 respectively. In brief, 250 ng of total RNA was used for complementary DNA synthesis, followed by an amplification/labeling step (in vitro transcription) to synthesize biotin-labeled cRNA according to the MessageAmp II aRNA Amplification Kit (Ambion, Austin, TX). Biotin-16-UTP was purchased from Roche Applied Science (Penzberg, Germany). The cRNA was column purified according to TotalPrep RNA Amplification Kit, and eluted in 60 µl of water. Quality of cRNA was controlled using the RNA Nano Chip Assay on an Agilent 2100 Bioanalyzer and spectrophotometrically quantified (NanoDrop; NanoDrop Technologies).
Hybridization was performed at 58 °C, in GEX-HCB buffer (Illumina) at a concentration of 50 ng cRNA/µl, unsealed in a wet chamber for 20 hours. Spike-in controls for low, medium and highly abundant RNAs were added, as well as mismatch control and biotinylation control oligonucleotides. Microarrays were washed twice in E1BC buffer (Illumina) at room temperature for 5 minutes. After blocking for 5 minutes in 4 ml of 1% (w/v) Blocker Casein in phosphate-buffered saline Hammarsten grade (Pierce Biotechnology, Rockford, IL), array signals were developed by a 10 minutes incubation in 2 ml of 1 µg/ml Cy3-streptavidin (Amersham Biosciences, Buckinghamshire, UK) solution and 1% blocking solution. After a final wash in E1BC, the arrays were dried and scanned.
Scanning and data analysis. Microarray scanning was done using a Beadstation array scanner, setting adjusted to a scaling factor of 1 and PMT settings at 430. Data extraction was done for all beads individually, and outliers were removed when MAD excluded 2.5 (median absolute deviation). All remaining data points were used to calculate the mean average signal (and standard deviation) of a given probe.
Data analysis was done by normalization of the signals using the quantile algorithm without background subtraction for Illumina MouseRef-8 Expression BeadChip. Differentially regulated genes were defined by calculating the standard deviation differences of a given probe in a one-by-one comparison of samples or groups.
Gene expression analysis and correlation to IS distribution. To compare the gene expression status of lentivirally targeted genes before transduction, all Probe IDs (restricted to RefSeq genes, NM_Accession) on the two Illumina BeadChips were sorted according to their expression values (E) and P values and grouped into four separate bins. This analysis was performed for all Probe IDs present on Mouse Sentrix-6 and MouseRef-8 Illumina Microarray chips, and for all Probe IDs corresponding to Refseq genes, which have been targeted by the lentiviral vector within the gene coding regions. The relationship of the proportion of IS in the distinct BINs to the proportion of all Probe IDs in the repetitive BIN was calculated. BIN0: mostly likely background expression (P ≥ 0.001); BIN1: no expression (E ≤ 200); BIN2: low expression (E ≤ 1,500); BIN3: medium to high expression (E > 1,500).
IS analysis with respect to Satellite DNA and TGGAA motifs. Repeats from the repeat class Satellite were extracted from the RepeatMasker annotations previously used for IS analysis. These were used to extract the corresponding Satellite DNA sequence from the mouse and rat genome, respectively. The TGGAA motif was localized on the forward and reverse strand of the cellular genome and on the Satellite DNA to access its distribution. IS annotated inside a Satellite by the previous IS analysis were counted. For IS with multiple matches, the IS was considered as located inside a Satellite, if all the matches were located in a Satellite. For all IS the distance to the closest TGGAA motif was calculated.
Statistical analysis. Lentiviral IS distributions in gene coding regions and repetitive elements (LINE, Satellite DNA) in rodent eyes, SC-1 cells and HPCs (lin− cells) were compared to a in silico random IS data set. For each cell type, a two-sided binomial distribution test for significance (α = 0.05) was performed. Differences of lentiviral IS distributions in the distinct cell types were compared by χ2 test.
SUPPLEMENTARY MATERIAL Figure S1. Schematic overview of the experimental strategy. We conducted a comparative lentiviral vector integration site (IS) study of murine (rodent) postmitotic versus dividing cells. Analyzed postmitotic tissues comprised subretinally injected mouse and rat eyes, and stereotactically injected mouse brains in vivo. Dividing mouse hematopoietic progenitor cells (HPC) and SC-1 fibroblasts were transduced ex vivo. To uncover potential differences in IS profiles and to understand underlying causal mechanisms, we accomplished linear amplification-mediated PCR (LAM-PCR) combined with high-throughput Sanger sequencing or 454 pyrosequencing and RNA microarray analysis. RPE, retinal pigment epithelium. Figure S2. Linear amplification–mediated-PCR analyses of different lentivirally transduced rodent cell types. Clonality analysis using the restriction enzyme HpyCH4IV of dividing and postmitotic mouse cells is shown. LAM-PCR was performed repetitively using restriction enzymes Tsp509I (AATT) and HpyCH4IV (ACGT) on 10-100 ng of DNA isolated from dividing hematopoietic progenitor cells (HPC) and fibroblasts (SC-1) (a), from postmitotic mouse eye and brain cells (b) and from rat eye cells (c). LAM-PCR, linear amplification-mediated PCR; SC-1, mouse fibroblasts; M, 100 bp ladder; -C, negative control. Table S1. Integration site distribution in repetitive elements. Table S2. Integration site sequence analysis in postmitotic tissues. Table S3. Integration site sequence analysis in dividing cells.
Acknowledgments
We acknowledge Ina Kutschera (German Cancer Research Center and National Center for Tumor Diseases, Heidelberg, Germany), Nadine Krenzer (German Cancer Research Center and National Center for Tumor Diseases, Heidelberg, Germany), Claudia Prinz (University of Freiburg, Freiburg, Germany), Sonja Schmidt (University of Freiburg, Freiburg, Germany) and Manuela Wissler (University of Freiburg, Freiburg, Germany) for technical assistance. We also thank Alexander Smith (University College London, London, UK) for helpful discussions and Mario Amendola (San Raffaele–Telethon Institute for Gene Therapy (HSR-TIGET), Milan, Italy) for help with the photos of lentiviral injected mouse brain. The authors declare that they have no competing interests. This work was supported by the Deutsche Forschungsgemeinschaft (Grant SPP1230), the German Ministry of Education and Research (Grant TREATID), the European Union (VIth Framework Program, CONSERT and CLINIGENE) and the Helmholtz association. B.K. is supported by the German Ministry of Education and Research (Program NGFN-2, Grant ID 01GR0420). A.J.T. is supported by the Wellcome Trust.
Supplementary Material
Schematic overview of the experimental strategy. We conducted a comparative lentiviral vector integration site (IS) study of murine (rodent) postmitotic versus dividing cells. Analyzed postmitotic tissues comprised subretinally injected mouse and rat eyes, and stereotactically injected mouse brains in vivo. Dividing mouse hematopoietic progenitor cells (HPC) and SC-1 fibroblasts were transduced ex vivo. To uncover potential differences in IS profiles and to understand underlying causal mechanisms, we accomplished linear amplification-mediated PCR (LAM-PCR) combined with high-throughput Sanger sequencing or 454 pyrosequencing and RNA microarray analysis. RPE, retinal pigment epithelium.
Linear amplification–mediated-PCR analyses of different lentivirally transduced rodent cell types. Clonality analysis using the restriction enzyme HpyCH4IV of dividing and postmitotic mouse cells is shown. LAM-PCR was performed repetitively using restriction enzymes Tsp509I (AATT) and HpyCH4IV (ACGT) on 10-100 ng of DNA isolated from dividing hematopoietic progenitor cells (HPC) and fibroblasts (SC-1) (a), from postmitotic mouse eye and brain cells (b) and from rat eye cells (c). LAM-PCR, linear amplification-mediated PCR; SC-1, mouse fibroblasts; M, 100 bp ladder; -C, negative control.
Integration site distribution in repetitive elements.
Integration site sequence analysis in postmitotic tissues.
Integration site sequence analysis in dividing cells.
REFERENCES
- Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L.et al. (1998Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery J Virol 729873–9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I.et al. (2009Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy Science 326818–823. [DOI] [PubMed] [Google Scholar]
- Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C.et al. (2006Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration Nat Biotechnol 24687–696. [DOI] [PubMed] [Google Scholar]
- Montini E, Cesana D, Schmidt M, Sanvito F, Bartholomae CC, Ranzani M.et al. (2009The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy J Clin Invest 119964–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlich U, Navarro S, Zychlinski D, Maetzig T, Knoess S, Brugman MH.et al. (2009Insertional transformation of hematopoietic cells by self-inactivating lentiviral and gammaretroviral vectors Mol Ther 171919–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F.et al. (2010Transfusion independence and HMGA2 activation after gene therapy of human ß-thalassaemia Nature 467318–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busschots K, Vercammen J, Emiliani S, Benarous R, Engelborghs Y, Christ F.et al. (2005The interaction of LEDGF/p75 with integrase is lentivirus-specific and promotes DNA binding J Biol Chem 28017841–17847. [DOI] [PubMed] [Google Scholar]
- Cherepanov P. LEDGF/p75 interacts with divergent lentiviral integrases and modulates their enzymatic activity in vitro. Nucleic Acids Res. 2007;35:113–124. doi: 10.1093/nar/gkl885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano M, Vanegas M, Fregoso O, Saenz D, Chung S, Peretz M.et al. (2004LEDGF/p75 determines cellular trafficking of diverse lentiviral but not murine oncoretroviral integrase proteins and is a component of functional lentiviral preintegration complexes J Virol 789524–9537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carteau S, Hoffmann C., and, Bushman F. Chromosome structure and human immunodeficiency virus type 1 cDNA integration: centromeric alphoid repeats are a disfavored target. J Virol. 1998;72:4005–4014. doi: 10.1128/jvi.72.5.4005-4014.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushman FD. Targeting survival: integration site selection by retroviruses and LTR-retrotransposons. Cell. 2003;115:135–138. doi: 10.1016/s0092-8674(03)00760-8. [DOI] [PubMed] [Google Scholar]
- Mitchell RS, Beitzel BF, Schroder AR, Shinn P, Chen H, Berry CC.et al. (2004Retroviral DNA integration: ASLV, HIV, and MLV show distinct target site preferences PLoS Biol 2E234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder AR, Shinn P, Chen H, Berry C, Ecker JR., and, Bushman F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110:521–529. doi: 10.1016/s0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- Mooslehner K, Karls U., and, Harbers K. Retroviral integration sites in transgenic Mov mice frequently map in the vicinity of transcribed DNA regions. J Virol. 1990;64:3056–3058. doi: 10.1128/jvi.64.6.3056-3058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherdin U, Rhodes K., and, Breindl M. Transcriptionally active genome regions are preferred targets for retrovirus integration. J Virol. 1990;64:907–912. doi: 10.1128/jvi.64.2.907-912.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panet A., and, Cedar H. Selective degradation of integrated murine leukemia proviral DNA by deoxyribonucleases. Cell. 1977;11:933–940. doi: 10.1016/0092-8674(77)90304-x. [DOI] [PubMed] [Google Scholar]
- Allen MJ, Jeffreys AJ, Surani MA, Barton S, Norris ML., and, Collick A. Tandemly repeated transgenes of the human minisatellite MS32 (D1S8), with novel mouse gamma satellite integration. Nucleic Acids Res. 1994;22:2976–2981. doi: 10.1093/nar/22.15.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall HM, Ronen K, Berry C, Llano M, Sutherland H, Saenz D.et al. (2007Role of PSIP1/LEDGF/p75 in lentiviral infectivity and integration targeting PLoS ONE 2e1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuffi A, Llano M, Poeschla E, Hoffmann C, Leipzig J, Shinn P.et al. (2005A role for LEDGF/p75 in targeting HIV DNA integration Nat Med 111287–1289. [DOI] [PubMed] [Google Scholar]
- Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA.et al. (2005Genome sequencing in microfabricated high-density picolitre reactors Nature 437376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Schwarzwaelder K, Bartholomae C, Zaoui K, Ball C, Pilz I.et al. (2007High-resolution insertion-site analysis by linear amplification-mediated PCR (LAM-PCR) Nat Methods 41051–1057. [DOI] [PubMed] [Google Scholar]
- Gabriel R, Eckenberg R, Paruzynski A, Bartholomae CC, Nowrouzi A, Arens A.et al. (2009Comprehensive genomic access to vector integration in clinical gene therapy Nat Med 151431–1436. [DOI] [PubMed] [Google Scholar]
- Engelman A., and, Cherepanov P. The lentiviral integrase binding protein LEDGF/p75 and HIV-1 replication. PLoS Pathog. 2008;4:e1000046. doi: 10.1371/journal.ppat.1000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shun MC, Raghavendra NK, Vandegraaff N, Daigle JE, Hughes S, Kellam P.et al. (2007LEDGF/p75 functions downstream from preintegration complex formation to effect gene-specific HIV-1 integration Genes Dev 211767–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinski MK, Bisgrove D, Shinn P, Chen H, Hoffmann C, Hannenhalli S.et al. (2005Genome-wide analysis of chromosomal features repressing human immunodeficiency virus transcription J Virol 796610–6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felice B, Cattoglio C, Cittaro D, Testa A, Miccio A, Ferrari G.et al. (2009Transcription factor binding sites are genetic determinants of retroviral integration in the human genome PLoS ONE 4e4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GP, Levine BL, Binder GK, Berry CC, Malani N, McGarrity G.et al. (2009Analysis of lentiviral vector integration in HIV+ study subjects receiving autologous infusions of gene modified CD4+ T cells Mol Ther 17844–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs S, Guenechea G, Gonzalez-Murillo A, Zsuzsanna Nagy K, Luz Lozano M, del Val C.et al. (2006Lentiviral vector integration sites in human NOD/SCID repopulating cells J Gene Med 81197–1207. [DOI] [PubMed] [Google Scholar]
- Beard BC, Dickerson D, Beebe K, Gooch C, Fletcher J, Okbinoglu T.et al. (2007Comparison of HIV-derived lentiviral and MLV-based gammaretroviral vector integration sites in primate repopulating cells Mol Ther 151356–1365. [DOI] [PubMed] [Google Scholar]
- Barr SD, Ciuffi A, Leipzig J, Shinn P, Ecker JR., and, Bushman FD. HIV integration site selection: targeting in macrophages and the effects of different routes of viral entry. Mol Ther. 2006;14:218–225. doi: 10.1016/j.ymthe.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Ciuffi A, Mitchell RS, Hoffmann C, Leipzig J, Shinn P, Ecker JR.et al. (2006Integration site selection by HIV-based vectors in dividing and growth-arrested IMR-90 lung fibroblasts Mol Ther 13366–373. [DOI] [PubMed] [Google Scholar]
- Bainbridge JW, Stephens C, Parsley K, Demaison C, Halfyard A, Thrasher AJ.et al. (2001In vivo gene transfer to the mouse eye using an HIV-based lentiviral vector; efficient long-term transduction of corneal endothelium and retinal pigment epithelium Gene Ther 81665–1668. [DOI] [PubMed] [Google Scholar]
- Yáñez-Muñoz RJ, Balaggan KS, MacNeil A, Howe SJ, Schmidt M, Smith AJ.et al. (2006Effective gene therapy with nonintegrating lentiviral vectors Nat Med 12348–353. [DOI] [PubMed] [Google Scholar]
- Blömer U, Naldini L, Kafri T, Trono D, Verma IM., and, Gage FH. Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. J Virol. 1997;71:6641–6649. doi: 10.1128/jvi.71.9.6641-6649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Chou SH., and, Reid BR. A single G-to-C change causes human centromere TGGAA repeats to fold back into hairpins. Proc Natl Acad Sci USA. 1996;93:12159–12164. doi: 10.1073/pnas.93.22.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Chou SH., and, Reid BR. The structure of a novel DNA duplex formed by human centromere d(TGGAA) repeats with possible implications for chromosome attachment during mitosis. J Mol Biol. 1995;254:623–637. doi: 10.1006/jmbi.1995.0643. [DOI] [PubMed] [Google Scholar]
- Chou SH, Chin KH., and, Wang AH. Unusual DNA duplex and hairpin motifs. Nucleic Acids Res. 2003;31:2461–2474. doi: 10.1093/nar/gkg367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paruzynski A, Arens A, Gabriel R, Bartholomae CC, Scholz S, Wang W.et al. (2010Genome-wide high-throughput integrome analyses by nrLAM-PCR and next-generation sequencing Nat Protoc 51379–1395. [DOI] [PubMed] [Google Scholar]
- Eberwine J, Yeh H, Miyashiro K, Cao Y, Nair S, Finnell R.et al. (1992Analysis of gene expression in single live neurons Proc Natl Acad Sci USA 893010–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schematic overview of the experimental strategy. We conducted a comparative lentiviral vector integration site (IS) study of murine (rodent) postmitotic versus dividing cells. Analyzed postmitotic tissues comprised subretinally injected mouse and rat eyes, and stereotactically injected mouse brains in vivo. Dividing mouse hematopoietic progenitor cells (HPC) and SC-1 fibroblasts were transduced ex vivo. To uncover potential differences in IS profiles and to understand underlying causal mechanisms, we accomplished linear amplification-mediated PCR (LAM-PCR) combined with high-throughput Sanger sequencing or 454 pyrosequencing and RNA microarray analysis. RPE, retinal pigment epithelium.
Linear amplification–mediated-PCR analyses of different lentivirally transduced rodent cell types. Clonality analysis using the restriction enzyme HpyCH4IV of dividing and postmitotic mouse cells is shown. LAM-PCR was performed repetitively using restriction enzymes Tsp509I (AATT) and HpyCH4IV (ACGT) on 10-100 ng of DNA isolated from dividing hematopoietic progenitor cells (HPC) and fibroblasts (SC-1) (a), from postmitotic mouse eye and brain cells (b) and from rat eye cells (c). LAM-PCR, linear amplification-mediated PCR; SC-1, mouse fibroblasts; M, 100 bp ladder; -C, negative control.
Integration site distribution in repetitive elements.
Integration site sequence analysis in postmitotic tissues.
Integration site sequence analysis in dividing cells.