To the editor:
Because of the lack of curative treatment options for most advanced cancers, innovative and experimental strategies are being developed. One promising therapeutic approach is the delivery of human sodium iodide symporter (hNIS) with oncolytic viruses.1,2,3,4,5 hNIS provides the dual utility of therapy and imaging of virus distribution with radionuclides such as iodides or technetium. Although therapy is the ultimate goal, imaging of the virus is also critical because all available biodistribution information concerning oncolytic viruses is currently based on animal models, which may not be fully representative for species-specific viruses such as human adenovirus. We and others have published preclinical data on the use of radionuclide transporters as transgenes,2,3,4,5,6,7 and hNIS has thus far been the most popular approach. In general, two approaches have been used to arm oncolytic viruses with human NIS: replication-coupled expression and replication-independent expression. The former is useful for detection of virus replication but may not reveal overall virus biodistribution, and the opposite is true for the latter.
Utilizing replication-independent human NIS expression, Barton et al. recently published an elegant first-in-humans phase I trial.1 Nuclear imaging showed that seven of nine (78%) patients treated intratumorally with the higher viral doses and six of six (100%) treated with the highest dose accumulated technetium-99m (99mTc). These data, together with promising preclinical data on Ad5/3-24-hNIS (ref. 4) and previous clinical experience with different oncolytic adenoviruses,8,9,10,11 led us to treat one patient in a Finnish Medicines Agencyapproved Advanced Therapy Access Program, which allows personalized patient-by-patient therapy under the “hospital exemption.”12 The patient was a 50-year-old woman with chemotherapy-refractory cervical carcinoma metastatic to the lungs, lesser pelvis, pelvic lymph nodes, liver, and bones. She had previously been treated with cisplatin, cisplatin with topotecan, paclitaxel and gemcitabine, and radiotherapy.
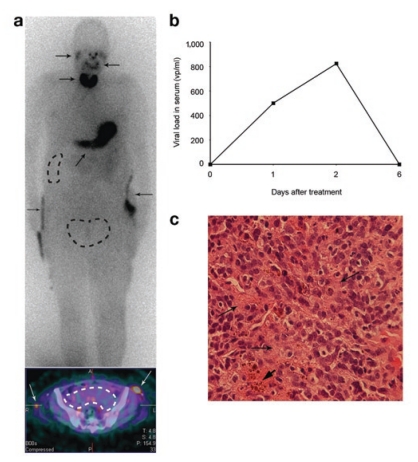
The patient received a total of 3 × 1011 viral particles into the clinically most relevant tumors—as evaluated by positron emission tomography–computed tomography (PET-CT) imaging on the same day—in the pelvis and liver. Twenty hours after treatment with virus, the patient received an injection of iodine-123 (123I), a low-energy γ-emitter with excellent imaging properties that is frequently used for thyroid and brain imaging. A series of scans was performed: a 30-minute dynamic-imaging series at injection time (0 hour), whole-body single-photon emission-computed tomography (SPECT)-CT scans at 4 hours, 8 hours, and 24 hours after 123I injection. These scans did not reveal any accumulation of iodine into the injected regions over baseline readings (Figure 1a). Therefore, the imaging was repeated on the third day (68 hours after virus injection), this time with 99mTcO4 (to exclude iodide-specific issues), resulting in the same negative result. To exclude the possibility of protracted amplification of signal, one more round of 123I imaging was performed on day 6 post virus. Careful examination still did not reveal any signal enhancement other than that resulting from endogenous hNIS expression in the thyroid, stomach, and salivary glands. Urine was visualized due to renal excretion of iodide.
Figure 1.
SPEC-CT imaging of Ad5/3-24-hNIS. (a) A whole-body scan (Siemens Symbia T2, Siemens Medical Solutions, Erlangen, Germany) showing the distribution of 123I 4 hours after 123I injection and 25 hours after intratumoral Ad5/3-24–human sodium iodide symporter (hNIS) injection. Virus was produced by Oncos Therapeutics (Helsinki, Finland). The injection was administered into the tumor mass in the lesser pelvis and into a liver metastasis (dotted lines). The lower panel shows a single-photon emission-computed tomography (SPECT)-computed tomography scan of the pelvic tumor (dotted lines) at the same time point. Only background activity is detected. The images are representative of all the imaging time points, suggesting that with the sensitivity of SPECT, hNIS expression originating from Ad5/3-24-hNIS could not be detected. As positive controls for the imaging, the thyroid, stomach, salivary glands, and pyelostoma catheters can be seen (arrows). (b) Viral load in patient serum as detected by quantitative polymerase chain reaction. Increase on days 1 and 2 suggests virus replication. (c) Hematoxylin–eosin staining of a pretreatment biopsy from the tumor of the lesser pelvis showing 60% poorly differentiated carcinoma,35% fibrotic stroma (arrows), and 5 % necrotic tissue (thick arrowhead).
Blood samples were taken on days 0, 1, 2, and 6 and analyzed for adenovirus copy number with quantitative PCR.9 Before treatment, no virus was detected. However, on days 1 and 2, less than 500 virus particles per milliliter (vp/ml) and 825 vp/ml were measured, respectively, suggesting that the highest peak of virus replication occurred exactly when imaging was performed (Figure 1b). On day 6, the value had declined to 0 vp/ml. The patient reported grade 1 lower-limb edema and grade 2 dyspnea and anorexia after the treatment. No changes in laboratory values were observed. The neutralizing-antibody titer measured before treatment was 1:256 and thus only moderately elevated,8,9,10,11 and it rose to 1:16,384 a week after treatment. A pretreatment biopsy contained 60% poorly differentiated carcinoma, 35% fibrotic stroma, and 5% necrotic tissue (Figure 1c). Consequently, neither antibodies nor high stromal content seem likely reasons for low hNIS expression.
Our findings suggest that constructs optimized for oncolysis and featuring replication-coupled transgene expression, such as Ad5/3-24-hNIS, might not be optimal for detection of hNIS expression in humans. By comparing differences in virus design and treatment protocol with Barton et al.,1,5 we believe that important vectorological lessons can be learned. Differences between treatment protocol, dose, and patient type may nevertheless also contribute to the findings. One difference between our construct and Ad5-yCD/mutTKSR39rep-hNIS is the promoter driving hNIS expression. Ad5/3-24-hNIS expresses hNIS from the native E3 promoter, which is activated by virus replication at about 8 hours,4 whereas in Ad5-yCD/mutTKSR39rep-hNIS the transgene is under the ubiquitously active cytomegalovirus (CMV) promoter, which allows for immediate high-level expression of transgene in normal and tumor cells. This might result in a stronger signal when the injected lesion contains both cell types, which may be relevant in that the stromal component of tumors can vary between 5 and 95%. Moreover, the capacity of Ad5-yCD/mutTKSR39rep-hNIS for expressing hNIS in normal tissues was demonstrated in dog prostates.2 Dogs are not permissive for human adenoviruses,13 and the dogs in the study did not have known prostate cancer. Also, the high early activity mediated by CMV, versus slower replication-coupled expression, might be useful in a situation where the cells have limited time to produce hNIS and concentrate radiotracers, before the cell is lysed. In this regard, the high oncolytic potency of Ad5/3-24-hNIS (ref. 4) might work against imaging.
Furthermore, Ad5-yCD/mutTKSR39rep-hNIS is based on dl1520, an adenoviral construct that has been safely used in many clinical trials.14 However, some studies have suggested that the productive replication of dl1520 might be attenuated even in permissive cells.15,16 Moreover, Ad5-yCD/mutTKSR39rep-hNIS represents a complex construct with three transgenes and three CMV promoters that together might further attenuate the replication, allowing for prolonged and enhanced transgene expression. Also, the trial patients received 5-fluorocytosine and valganciclovir prodrug therapy for 3 weeks, which might further hamper viral replication (at least in vitro), thus giving time for CMV-driven hNIS expression. However, it should be noted that the first images were captured before prodrug therapy.16,17 The patients also received radiation therapy, which might influence virus replication and transgene expression.
The administration technique might also contribute to the findings. Our patient received Ad5/3-24-hNIS into a bulky pelvis tumor through three needle tracts and into a liver metastasis through one needle tract, as opposed to subjects in Barton and colleagues' study2 who received the whole dose along one needle tract, resulting in a higher local virus concentration that might have facilitated imaging.
In summary, we believe our experience with Ad5/3-24-hNIS in comparison with the report on Ad5-yCD/mutTKSR39rep-hNIS reveals several important aspects in virus design. First, low oncolytic potency may be useful for expression of transgene products located on the cell surface. Second, slower virus replication (e.g., in combination with replication-attenuating prodrugs) may allow for better imaging of membrane-associated proteins. In addition, high levels of early transgene expression may be preferable over protracted replication-coupled transgene expression. Also, tumor-selective transgene expression may lead to less robust imaging results than expression in all transduced cells. Finally, the sensitivity of SPECT may require relatively high levels of transgene expression. Thus, Ad5-yCD/mutTKSR39rep-hNIS may represent a useful agent for combining oncolytic virotherapy with prodrug-converting enzymes, imaging, and radionuclide therapy, whereas Ad5/3-24-hNIS type viruses are more suitable for optimal oncolysis and expressing transgenes with systemic or paracrine rather than cell membranerestricted effects.9 Therefore, we are currently not planning further treatments with Ad5/3-24-hNIS.
A.H. is founder of and shareholder in Oncos Therapeutics Ltd.
REFERENCES
- Barton KN, Stricker H, Brown SL, Elshaikh M, Aref I, Lu M, et al. Phase I study of noninvasive imaging of adenovirus-mediated gene expression in the human prostate. Mol Ther. 2008;16:1761–1769. doi: 10.1038/mt.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton KN, Tyson D, Stricker H, Lew YS, Heisey G, Koul S, et al. GENIS: gene expression of sodium iodide symporter for noninvasive imaging of gene therapy vectors and quantification of gene expression in vivo. Mol Ther. 2003;8:508–518. doi: 10.1016/s1525-0016(03)00153-9. [DOI] [PubMed] [Google Scholar]
- Dingli D, Diaz RM, Bergert ER, O'Connor MK, Morris JC., and, Russell SJ. Genetically targeted radiotherapy for multiple myeloma. Blood. 2003;102:489–496. doi: 10.1182/blood-2002-11-3390. [DOI] [PubMed] [Google Scholar]
- Hakkarainen T, Rajecki M, Sarparanta M, Tenhunen M, Airaksinen AJ, Desmond RA, et al. Targeted radiotherapy for prostate cancer with an oncolytic adenovirus coding for human sodium iodide symporter. Clin Cancer Res. 2009;15:5396–5403. doi: 10.1158/1078-0432.CCR-08-2571. [DOI] [PubMed] [Google Scholar]
- Barton KN, Freytag SO, Nurushev T, Yoo S, Lu M, Yin FF, et al. A model for optimizing adenoviral delivery in human cancer gene therapy trials. Hum Gene Ther. 2007;18:562–572. doi: 10.1089/hum.2007.004. [DOI] [PubMed] [Google Scholar]
- Peerlinck I, Merron A, Baril P, Conchon S, Martin-Duque P, Hindorf C, et al. Targeted radionuclide therapy using a Wnt-targeted replicating adenovirus encoding the Na/I symporter. Clin Cancer Res. 2009;15:6595–6601. doi: 10.1158/1078-0432.CCR-09-0262. [DOI] [PubMed] [Google Scholar]
- Merron A, Baril P, Martin-Duque P, de la Vieja A, Tran L, Briat A, et al. Assessment of the Na/I symporter as a reporter gene to visualize oncolytic adenovirus propagation in peritoneal tumours. Eur J Nucl Med Mol Imaging. 2010;37:1377–1385. doi: 10.1007/s00259-009-1379-3. [DOI] [PubMed] [Google Scholar]
- Cerullo V, Pesonen S, Diaconu I, Escutenaire S, Arstila PT, Ugolini M, et al. Oncolytic adenovirus coding for granulocyte macrophage colony-stimulating factor induces antitumoral immunity in cancer patients. Cancer Res. 2010;70:4297–4309. doi: 10.1158/0008-5472.CAN-09-3567. [DOI] [PubMed] [Google Scholar]
- Koski A, Kangasniemi L, Escutenaire S, Pesonen S, Cerullo V, Diaconu I, et al. Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF. Mol Ther. 2010;18:1874–1884. doi: 10.1038/mt.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nokisalmi P, Pesonen S, Escutenaire S, Särkioja M, Raki M, Cerullo V, et al. Oncolytic adenovirus ICOVIR-7 in patients with advanced and refractory solid tumors. Clin Cancer Res. 2010;16:3035–3043. doi: 10.1158/1078-0432.CCR-09-3167. [DOI] [PubMed] [Google Scholar]
- Pesonen S, Nokisalmi P, Escutenaire S, Särkioja M, Raki M, Cerullo V, et al. Prolonged systemic circulation of chimeric oncolytic adenovirus Ad5/3-Cox2L-D24 in patients with metastatic and refractory solid tumors. Gene Ther. 2010;17:892–904. doi: 10.1038/gt.2010.17. [DOI] [PubMed] [Google Scholar]
- European Commission, Directorate General for Health and Therapeutics Advanced Therapies < http://ec.europa.eu/ health/human-use/advanced-therapies/index_en.htm >
- Hemminki A, Kanerva A, Kremer EJ, Bauerschmitz GJ, Smith BF, Liu B, et al. A canine conditionally replicating adenovirus for evaluating oncolytic virotherapy in a syngeneic animal model. Mol Ther. 2003;7:163–173. doi: 10.1016/s1525-0016(02)00049-7. [DOI] [PubMed] [Google Scholar]
- Kirn D. Oncolytic virotherapy for cancer with the adenovirus dl1520 (Onyx-015): results of phase I and II trials. Expert Opin Biol Ther. 2001;1:525–538. doi: 10.1517/14712598.1.3.525. [DOI] [PubMed] [Google Scholar]
- Dix BR, Edwards SJ., and, Braithwaite AW. Does the antitumor adenovirus ONYX-015/dl1520 selectively target cells defective in the p53 pathway. J Virol. 2001;75:5443–5447. doi: 10.1128/JVI.75.12.5443-5447.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freytag SO, Rogulski KR, Paielli DL, Gilbert JD., and, Kim JH. A novel three-pronged approach to kill cancer cells selectively: concomitant viral, double suicide gene, and radiotherapy. Hum Gene Ther. 1998;9:1323–1333. doi: 10.1089/hum.1998.9.9-1323. [DOI] [PubMed] [Google Scholar]
- Dias JD, Liikanen I, Guse K, Foloppe J, Sloniecka M, Diaconu I, et al. Targeted chemotherapy for head and neck cancer with a chimeric oncolytic adenovirus coding for bifunctional suicide protein FCU1. Clin Cancer Res. 2010;16:2540–2549. doi: 10.1158/1078-0432.CCR-09-2974. [DOI] [PubMed] [Google Scholar]