Melanomas account for ~4% of all dermatological cancers and for 80% of deaths from skin cancers.1 Although many primary melanomas can be cured through surgery, treatment of metastatic melanomas remains challenging.1 Melanoma patients undergoing chemotherapy or even targeted therapy with small-molecule inhibitors aimed at blocking the most frequently mutated oncogene (BRAFV600E) are known to develop drug resistance and tumor recurrence.1,2,3,4 Even though some of the molecular mechanisms underlying acquired drug resistance have recently been described,4,5,6,7 recurrence of initially responsive melanomas can also be due in part to the presence and potential enrichment of tumor subpopulations that are inherently resistant to therapy. Schmidt et al. recently reported that by targeting a small subset (~2%) or subpopulation of tumor cells expressing CD20, a cell surface marker typically associated with B cells,8 long-lasting tumor regression can be achieved in an experimental immunodeficient mouse tumor model,9 whereas targeting of other tumor subpopulations had only minimal effects on tumor regression.
As is the case with other malignancies, melanoma is a highly heterogeneous neoplasia, composed of distinct subpopulations of tumor cells.10,11,12,13 These subpopulations provide the cellular basis for the complex biology of the disease, including phenomena such as self-renewal, differentiation, tumor initiation, progression and maintenance, and therapy resistance. Several phenotypically distinct subpopulations—some with stem cell–like characteristics—have been described in melanoma, including one previously described by us that expresses CD20 (ref. 11). The new study noted above is based on the premise that a small subset of tumor cells is responsible for tumor initiation, maintenance, and disease progression. By successfully targeting and eliminating this small subset of malignant cells, tumor eradication could be more effectively achieved. This finding challenges the current dogma that targeting all tumor cells by biological, chemo-, or targeted therapies is required for a complete cure. The success of the proposed therapy will depend largely on effectively targeting this small tumor subpopulation and also on the assumption that every melanoma patient has this minor CD20+ tumor subpopulation in his or her lesion.
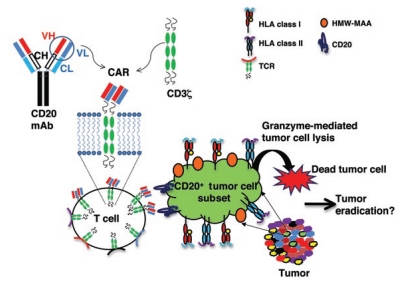
Schmidt et al. report finding a CD20+ melanoma subpopulation in the majority (four of five) of the patients' lesions tested.9 Expression of CD20+ melanoma cells was demonstrated by co-staining melanoma cells with a monoclonal antibody (mAb) against the high-molecular-weight–melanoma-associated antigen (HMW-MAA; a molecule predominantly present on melanomas (ref. 14)) and an antibody against CD20 (ref. 8) via flow cytometry and immunohistology. CD20 expression was further confirmed by reverse transcription–polymerase chain reaction using CD20-specific primers. Polyclonal T cells were engineered to express a chimeric antigen receptor (CAR) composed of a single-chain variable fragment (scFv) derived from an antibody directed against CD20 fused to a CD3z signaling domain of a T-cell receptor (TCR) (Figure 1). The engineered T cells have a redirected specificity to bind to CD20 on melanoma cells in a human leukocyte antigen (HLA)-independent manner and enhanced capacity to lyse CD20+ tumor cells. The advantage of this approach is that CAR-engineered T cells with a single specificity (CD20) can be grown in large numbers in a relatively short period of time and used in adoptive-therapy approaches. The authors further compared the tumor-inhibiting potential of adoptively transferred engineered T cells expressing various CAR specificities, as determined by expression of scFvs that bind to HMW-MAA, melanotransferrin (expressed by all melanomas), or CD19 (a molecule expressed on B cells), in an established immunodeficient mouse tumor model. In the in vivo experiments, they showed that by targeting a small subset of CD20+ tumor cells with engineered T cells with redirected specificity for CD20, complete inhibition of tumor growth in mice could be achieved. Inhibition of tumor growth was long-lasting—no tumor relapse in mice was observed for more than 36 weeks. Engineered T cells with redirected specificities for HMW-MAA, melanotransferrin, or CD19 were either unable or only partially able to inhibit tumor growth. In some mice, inhibition of tumor growth was transient, as lesions reappeared at later time points. Schmidt et al. claim that by solely targeting a small subset of CD20+ cells that are responsible for tumor initiation, maintenance, and progression, tumors can be completely eradicated.
Figure 1.
Polyclonal T cells expressing CD20 svFv-CAR acquire redirected specificity to lyse melanoma tumor cell subset. T cells were engineered to express a chimeric antigen receptor (CAR) comprising a single-chain variable fragment (scFv), derived from a combining region of a monoclonal antibody directed against CD20 and fused to a CD3z signaling domain of a T-cell receptor (TCR). Engineered T cells have a redirected specificity to target and lyse a CD20+ subset of melanoma cells without human leukocyte antigen (HLA) restriction. HMW-MAA, high-molecular-weight–melanoma-associated antigen.
Although the work was conducted in an immunodeficient mouse tumor model, it is quite interesting and provocative. Currently there are two views of tumor initiation and progression in melanoma. In a hierarchical cancer stem cell model, melanoma-initiating cells are rare (<0.1%) and a small subset of tumor subpopulation is responsible for tumor initiation, maintenance, and progression.10,11,12 In the second model (stochastic), which is gaining increased attention, every cell has the potential to be a tumor-initiating cell.13,15,16 Complete eradication of tumor cells by targeting a small subset of CD20+ melanoma tumor subpopulation, as shown by Schmidt et al., overwhelmingly supports the hierarchical cancer stem cell model.9 This study potentially offers a new approach to melanoma treatment. However, the approach has many hurdles to clear before it could be tried in a clinical setting. Because of HLA restrictions, each patient's T cells would need to be engineered with CAR for successful elimination of CD20+ tumor subpopulations. This form of personalized therapy has its own limitations. In two previous studies, maintenance of adequate T cell numbers in circulation and minimizing reactivity to normal tissue were issues.17,18 Another challenge is to efficiently engineer all patients' T cells to express CAR. If achieved, will these patients' T cells be as efficient in lysing tumor cells as T cells obtained from normal healthy donors? The small number of data sets presented using patient lymphocytes indicates partial tumor regression in two of five patients' lymphocytes tested. The efficacy of redirected T-cell lysis of tumor cells needs to be confirmed in lymphocyte samples from a larger group of patients. Also, if alternatives such as rituximab or ofatumumab (both reactive against human CD20) are readily available, then why use CAR-engineered T cells? The authors do point out that T cells are more efficient in penetrating tumor tissues as compared with antibodies, and the results confirm that only three injections of T cells are necessary for complete tumor regression. However, only a comparative study using antibodies and engineered T cells will validate this claim. Patients treated with CAR-engineered T cells could also develop resistance similar to that in lymphoma patients in whom CD20 molecules were downmodulated after treatment with rituximab, rendering the antibody treatment ineffective.19
Finally, targeting only a minor subpopulation and leaving behind the bulk of the tumor does not take into account the dynamic nature of tumor cell subsets and the possibility that other minor subpopulations may also have tumor-initiating capabilities.5,20 Moreover, could cells that initially do not express surface markers such as CD20 become CD20+ and acquire stem cell–like properties under the influence of therapy or the tumor microenvironment? Although Schmidt and colleagues propose a novel (and possibly efficient) approach to targeting a minor subset of tumor-initiating cells, a two-pronged approach will most likely be necessary to cure melanoma. This approach should target both the large bulk of highly dynamic and proliferative tumor cells and the phenotypically distinct minor subpopulations. Future studies will be required to validate the strategy proposed by Schmidt and colleagues, its therapeutic impact, and the potential it creates to offer more effective treatments for melanoma patients.
REFERENCES
- Miller AJ., and, Mihm MC., Jr Melanoma. N Engl J Med 2006. 2006;355:51–65. doi: 10.1056/NEJMra052166. [DOI] [PubMed] [Google Scholar]
- Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty KT, Hodi FS., and, Bastian BC. Mutation-driven drug development in melanoma. Curr Opin Oncol. 2010;22:178–183. doi: 10.1097/cco.0b013e32833888ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18:683–695. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch A, Fukunaga-Kalabis M, Schmidt EC, Zabierowski SE, Brafford PA, Vultur A, et al. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell. 2010;141:583–594. doi: 10.1016/j.cell.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468:968–972. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedder TF., and, Engel P. CD20: a regulator of cell-cycle progression of B lymphocytes. Immunol Today. 1994;15:450–454. doi: 10.1016/0167-5699(94)90276-3. [DOI] [PubMed] [Google Scholar]
- Schmidt P, Kopecky C, Hombach A, Zigrino P, Mauch C., and, Abken H. Eradication of melanomas by targeted elimination of a minor subset of tumor cells. Proc Natl Acad Sci USA. 2011;108:2474–2479. doi: 10.1073/pnas.1009069108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328–9337. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- Boiko AD, Razorenova OV, van de Rijn M, Swetter SM, Johnson DL, Ly DP, et al. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature. 2010;466:133–137. doi: 10.1038/nature09161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick JE. Looking ahead in cancer stem cell research. Nat Biotechnol. 2009;27:44–46. doi: 10.1038/nbt0109-44. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay P, Kaveri SV, Byars N, Starkey J, Ferrone S., and, Raychaudhuri S. Human high molecular weight-melanoma associated antigen mimicry by an anti-idiotypic antibody: characterization of the immunogenicity and the immune response to the mouse monoclonal antibody IMel-1. Cancer Res. 1991;51:6045–6051. [PubMed] [Google Scholar]
- Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM., and, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana E, Shackleton M, Foster HR, Fullen DR, Sabel MS, Johnson TM, et al. Phenotypic heterogeneity among tumorigenic melanoma cells from patients that is reversible and not hierarchically organized. Cancer Cell. 2010;18:510–523. doi: 10.1016/j.ccr.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers CH, Sleijfer S, Vulto AG, Kruit WH, Kliffen M, Debets R, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24:e20–22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazirehi AR., and, Bonavida B. Cellular and molecular signal transduction pathways modulated by rituximab (rituxan, anti-CD20 mAb) in non-Hodgkin's lymphoma: implications in chemosensitization and therapeutic intervention. Oncogene. 2005;24:2121–2143. doi: 10.1038/sj.onc.1208349. [DOI] [PubMed] [Google Scholar]
- Shackleton M, Quintana E, Fearon ER., and, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138:822–829. doi: 10.1016/j.cell.2009.08.017. [DOI] [PubMed] [Google Scholar]