Abstract
Wnt signaling plays a crucial role in regulating cell proliferation, differentiation and inducing cardiomyogenesis. Skeletal muscle-derived stem cells (MDSCs) have been shown to be multipotent; however, their potential to aid in the healing of the heart after myocardial infarction appears to be due to the paracrine effects they impart on the host environment. The goal of this study was to investigate whether Wnt11 could promote the differentiation of MDSCs into cardiomyocytes and enhance the repair of infarcted myocardium. MDSCs transduced with a lentivirus encoding for Wnt11 increased mRNA and protein expression of the early cardiac markers NK2 transcription factor related 5 (NKx2.5) and Connexin43 (Cx43) and also led to an increased expression of late-stage cardiac markers including: α, β-myosin heavy chain (MHC) and brain natriuretic protein (BNP) at the mRNA level, and MHC and Troponin I (TnI) at the protein level. We also observed that Wnt11 expression significantly enhanced c-jun N-terminal kinase activity in transduced MDSCs, and that some of the cells beat spontaneously but are not fully differentiated cardiomyocytes. Finally, lentivirus-Wnt11-transduced MDSCs showed greater survival and cardiac differentiation after being transplanted into acutely infarct-injured myocardium. These findings could one day lead to strategies that could be utilized in cardiomyoplasty treatments of myocardial infarction.
Introduction
Wnt family members are secreted, lipid-modified, and highly conserved proteins, and play crucial roles in regulating cell proliferation and differentiation during embryogenesis, adult-tissue homeostasis, and carcinogenesis.1,2,3,4,5 Canonical Wnt signals are transmitted through Frizzled family receptors and LRP5/LRP6 coreceptors to the β-catenin signaling cascade, whereas, noncanonical Wnt signals are transduced through Frizzled family receptors and ROR2/RYK coreceptors to the Dishevelled-dependent (Rho-family GTPases and c-jun NH (2)-terminal kinase) or the Ca (2+)-dependent (NLK and nuclear factor of activated T cells) signaling cascades.2,6
The mammalian genome encodes 19 Wnt protein ligands, 10 Frizzled seven-pass transmembrane receptors, and 2 LRP coreceptors. Among these Wnt ligands, noncanonical Wnt11 was the first found to enhance cardiac tissue formation of early mesoderm and is required for cardiogenesis.7,8 Recent studies also indicate that Wnt 11 is capable of promoting differentiation of embryonic stem cells and human circulating progenitor cells toward a cardiomyogenic cell lineage, activating a myogenic differentiation pathway in bone marrow-derived stem cells, and inducing cardiomyogenesis in unfractionated bone marrow mononuclear cells.9,10,11,12,13
Our research group has isolated populations of murine skeletal muscle-derived stem cells (MDSCs) by using a modified preplate technique,14,15,16 and have shown that MDSCs can undergo differentiation toward muscle, bone, neural, endothelial, and hematopoietic lineages.14,15,16,17 Moreover, when compared to myoblasts, the transplantation of MDSCs into infarcted myocardium results in a robust engraftment with more neoangiogenesis and greater improvements in cardiac function. This superior transplantation capacity appears to be mainly due to the paracrine effects imparted by the implanted cells such as the secretion of vascular endothelial growth factor by the donor cells.18,19 We investigated whether Wnt11 could promote the differentiation of MDSCs into cardiomyocytes and thereby enhance the repair of infarcted myocardium. We found that lentiviral-mediated Wnt11 expression significantly increased the expression of cardiac-specific markers such as NK2 transcription factor related 5 (Nkx2.5), Connexin43 (Cx43), and Troponin I (TnI) and facilitated the cardiomyogenic differentiation of the MDSCs, resulting in some of the differentiated cells beating spontaneously and rhythmically.
Results
Transduction with lentivirus-bearing Wnt11 cDNA significantly increased Wnt11 expression in MDSCs
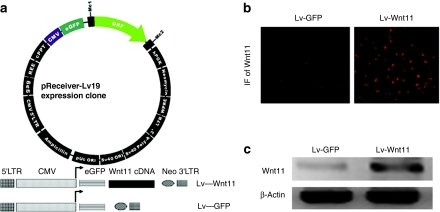
Wnt signaling functions as a regulator of cardiovascular differentiation, morphogenesis, and progenitor self-renewal.1 To explore the role of Wnt11 in cardiac differentiation, we used an feline leukemia virus-based lentiviral vector encoding for a green fluorescent protein (GFP) reporter or Wnt11 gene to transduce the MDSCs (Figure 1a) to promote over expression of these genes. Subsequently, GFP was used to successfully sort the transduced cells. After Wnt11 lentiviral transduction and GFP-sorting, immunofluorescent staining was performed and strong expression of Wnt11 was observed by the MDSCs, compared to the cells transduced with vectors carrying only the GFP reporter gene (Figure 1b). Semiquantitative reverse transcription (RT)-PCR further confirmed that Wnt11 mRNA levels in the MDSCs were increased 2 weeks after lentivirus-Wnt11 (Lv-Wnt11) transduction (Figure 1c).
Figure 1.
Wnt11 expression in sorted mouse muscle-derived stem cells (MDSCs). (a) Schematic illustration of feline leukemia virus (FLV)-based lentiviral vectors used in our experiments. The vectors contain human cytomegalovirus (CMV) immediate-early enhancer/promoter, enhanced green fluorescent protein (eGFP) as an expression reporter and neomycin (Neo) as a selection marker, and Wnt11 gene was inserted into the downstream of eGEP gene. (b) Immunofluorescent (IF) staining of Wnt11 revealed a significant increase in the expression of Wnt11 in MDSCs transduced with Lv-Wnt11 at 2 weeks after infection, whereas low levels of Wn11 expression was also detected in the control cells (magnification: ×200). (c) Semiquantitative RT-PCR analysis showed that Lv-Wnt11 transduction increased the expression of Wnt11 mRNA in MDSCs 2 weeks after infection. Fifty nanogram of RNA extracted from MDSCs by Trizol reagent was used as the template for reverse transcription (RT)-PCR.
Transduction with lentivirus-bearing Wnt11 enables MDSCs to undergo morphological changes
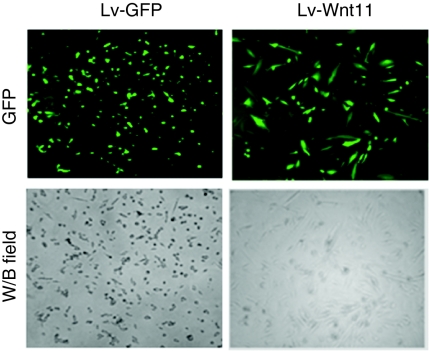
GFP protein was efficiently expressed in GFP-sorted MDSCs after their transduction with control Lv-GFP and Lv-Wnt11 (<90%, Figure 2; upper). 20 days after lentiviral transduction, Wnt11 expression led to morphological changes in the MDSCs, including an increase in cell size and elongation, whereas the control cells remained small in size and round in shape (Figure 2). These morphological changes were also observed in a variety of cellular immunofluorescent staining for cardiac-specific markers as described subsequently. In our experiments, GFP expression in the sorted MDSCs lasted at least 4 months, which indicated that lentiviral transduction could provide long-term expression of reporter and/or target genes (data not shown).
Figure 2.
Morphology of lentivirus-transduced and green fluorescent protein (GFP)-sorted mouse muscle-derived stem cells (MDSCs). High GFP expression (>90%) was shown in the GFP-sorted mouse MDSCs transduced with the Lv-GFP control- or Lv-Wnt11 groups (upper panel). The transduction of lentivirus carrying Wnt11cDNA enables the MDSCs to undergo morphological changes including an increase in size and elongation 20 days after transduction, whereas the control cells remained small and round (magnification: ×200).
Wnt11 expression in MDSCs leads to the expression of early cardiac markers
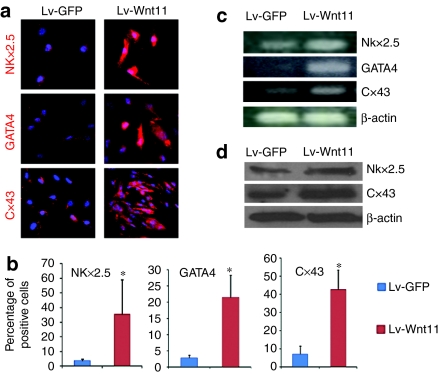
Wnt11 regulates the differentiation capacity of several types of stem/progenitor cells9,10,11,12,13 and also acts as a directional cue to organize the elongation of early muscle fibers.20 We next investigated the effect of increased Wnt11 expression on cardiac differentiation of MDSCs. The transcription factors Nkx2.5 and GATA-binding protein 4 (GATA4) are two markers of cardiac specification, which are expressed in the first heart field and second heart field during cardiac development, consequently we performed immunofluorescent staining for Nkx2.5 and GATA4 to examine the expression difference between Lv-Wnt11-transduced cells and control Lv-GFP transduced cells. We observed that the expression of Wnt11 in sorted MDSCs resulted in increased expression of the early cardiac markers Nkx2.5 and GATA4 which resulted in the nuclear translocation of NKx2.5 in some of the sorted cells (Figure 3a,b, P < 0.05). Furthermore, the staining of the gap junction protein Cx43, a major connexin protein found in the heart which transfers electrical coupling signals during contraction,21 showed an increased expression in sorted Lv-Wnt11 cells (Figure 3a,b, both P < 0.05 versus control). Increases in the expression of Nkx2.5 and Cx43 at the mRNA and protein levels were also confirmed via RT-PCR and western blot, as shown in Figure 3c,d, respectively. We also detected mRNA expression of GATA4 in sorted Lv-Wnt11 cells (Figure 3c). The above results along with the observed morphological changes indicated Wnt11 expression promoted cardiac differentiation.
Figure 3.
Wnt11 promotes the early cardiac differentiation of sorted mouse muscle-derived stem cell (MDSCs). (a) Immunofluorescent (IF) staining for cardiac-specific markers showed that the expression of Wnt11 in sorted MDSCs led to an increase in the expression of the early cardiac marker NK2 transcription factor related 5 (Nkx2.5) and GATA-binding protein 4 (GATA4) and the gap junction protein Connexin43 (Cx43) and promoted cardiac differentiation of the MDSCs as revealed by the morphological changes of cells (magnification: ×400). (b) Percentages of Nkx2.5, GATA4, and Cx43 positive cells in MDSCs 3 weeks following transduction with Lv-Wnt11 or control Lv-GFP lentiviruses (*P < 0.05 versus control). (c,d) The semiquantitative analysis of Nkx2.5 and Cx43 by reverse transcription (RT)-PCR and western blot analysis also showed significantly increased expression of Nkx2.5 and Cx43 in Lv-Wnt11-transduced MDSCs at the level of mRNA and protein when compared to the control Lv-GFP transduced cells (50 ng of RNA was used for RT-PCR and about 10 µg of protein was loaded for western blot analysis). The results are a representative of three independent experiments.
Wnt11 expression in MDSCs leads to the expression of late cardiac markers
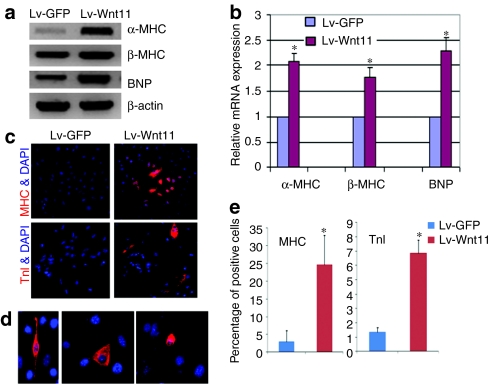
Nkx2.5 and GATA4 are cardiac transcription factors which drive the expression of late cardiac marker genes,22 we further determined expression of several late-stage cardiac markers. Semiquantitative RT-PCR analysis demonstrated that mRNA expression of α, β-myosin heavy chain (MHC) and brain natriuretic protein (BNP) in Lv-Wnt11-transduced MDSCs were significantly increased compared to the control Lv-GFP transduced cells (Figure 4a). We performed real-time quantitative PCR to analyze relative mRNA expression levels of α, β-MHC and BNP, which showed a 2.1-, 1.7-, and 2.3-fold increase in mRNA (all P < 0.01 versus control), respectively, relative to the controls (Figure 4b). Moreover, immunofluorescent staining for cardiac-specific MHC and TnI also revealed that Wnt11 expression resulted in the increased expression of MHC and TnI in Lv-Wnt11-transduced MDSCs compared with Lv-GFP control cells (Figure 4c,e). The cells positive for TnI immuofluorescent staining after Lv-Wnt11 transduction displayed three main types of morphology including spindle, triangular, and round shapes (Figure 4d), and an appropriately seven- and fourfold increase in the number of MHC and TnI positive cells in the Lv-Wnt11-transduced MDSC population was observed relative to the Lv-GFP controls (Figure 4e, both P < 0.01versus control), respectively. Using anti-α-actinin antibodies, which crossreact with both α-skeletal and α-cardiac actinin, we observed positive staining in Lv-Wnt11 and Lv-GFP MDSCs at similar frequencies, suggesting the potential differentiation of the Lv-GFP and Lv-Wnt11 cells toward a skeletal muscle lineage was similar between the groups (Supplementary Figure S1). Under our experimental conditions we could not detect mRNA expression of atrial natriuretic protein (ANP), myosin light chain 2 atrial isoform (MLC2a), or CaV1.2 (L-type calcium channel subunit) or protein expression of MLC2a in the mouse MDSCs. These results suggest that some of the cardiac-specific molecules are either not expressed or expressed at very low levels by the cells during Wnt11-induced differentiation toward a cardiomyocyte lineage. We also found that after one 7-day treatment with other inductive agents such as 5-azacytidine (10 µmol/l), oxytocin (100 nmol/l), and 10 ng/ml of bone morphogenetic protein 4 (BMP4), the expression of MLC2a was not observed in Lv-Wnt11 or Lv-GFP cells. Based on the involvement of BMP signaling in the formation of the heart,23,24,25 we investigated the synergistic effect that BMP4 and Wnt11 had on cardiac differentiation. We observed that a very limited number of Lv-Wnt11 cells and no Lv-GFP cells expressed the MLC2a protein following treatment with 50 ng/ml of BMP4 for 6 days and an additional 8 days of culturing with normal medium (Supplementary Figure S2). This finding suggests that Wnt11 and a given concentration of BMP4 synergistically induces MLC2a expression in Lv-Wnt11 cells. Because human skeletal MDSCs isolated by the preplate technique have already entered the clinical arena for the treatment of stress urinary incontinence26 and cardiac repair we also used Lv-Wnt11 and Lv-GFP to transduce human skeletal muscle-derived MDSCs in order to see whether Wnt11 also had an effect on these cells. Immunofluorescent staining showed that Lv-Wnt11 transduction induced a strong expression of cardiomyocyte-specific TnI in some of the human MDSCs whereas the Lv-GFP-transduced cells were negative for TnI staining (Supplementary Figure S3).
Figure 4.
Wnt11 enhances the expression of late-stage cardiac markers in sorted mouse muscle-derived stem cells (MDSCs). (a) The semiquantitative analysis of α, β-myosin heavy chain (MHC) and brain natriuretic protein (BNP) mRNAs by reverse transcription (RT)-PCR revealed a significant increase in expression in the Lv-Wnt11-transduced MDSCs compared to the control Lv-green fluorescent protein (GFP)-transduced cells. The results are a representative of three independent experiments. (b) Relative mRNA expression of late cardiac marker genes by quantitative real-time PCR analysis using SYBR detect system. mRNA expression of α, β-myosin MHC and BNP was remarkably increased in Lv-Wnt11-transduced MDSCs compared to Lv-GFP transduced MDSCs(*P < 0.01). Data are mean ±SEM of three independent experiments. (c) Immunofluorescent (IF) staining for the cardiac-specific markers showed that the expression of Wnt11 in the sorted MDSCs led to an increase in the expression of late-stage cardiac markers such as MHC and Troponin I (TnI) and promoted cardiac differentiation of the MDSCs (magnification: ×100). (d) Troponin I positive cells displayed three types of cellular shapes including: spindle, triangular, and round (×200). (e) Percentages of TnI and MHC positive cells were quantified (*P < 0.01), respectively.
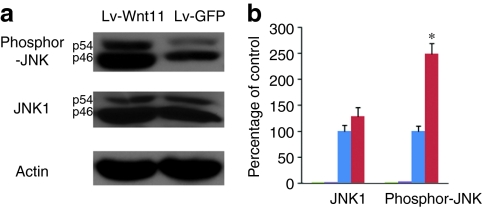
Wnt11 expression results in increased JNK activity in transduced MDSCs
c-Jun-terminal kinase (JNK) signaling has been reported to function in a noncanonical Wnt pathway.2,10,11,27 To detect whether the JNK pathway in MDSCs is activated or enhanced after Wnt11 overexpression, we performed immunoblotting analyses using antibodies against JNK1 and phosphorylated JNK (Thr3/Tyr185), which both recognize 46 and 54 kDa splice variants of JNK1. The results showed that Wnt11 expression leads to a slight increase in JNK1 expression levels, whereas phosphorylated-JNK levels in Lv-Wnt11-transduced cells were significantly higher than in the Lv-GFP-transduced cells (about a 1.5-fold increase, Figure 5a,b), indicating that increased JNK activity is involved in Wnt11-induced cardiomyocytic differentiation of MDSCs.
Figure 5.
Wnt11 expression increases c-Jun-terminal kinase (JNK) activity in muscle-derived stem cells (MDSCs). (a) The expression levels of JNK1 and phosphorylated JNK (Thr183 /Tyr185) in Lv-GFP- or Lv-Wnt11-transduced MDSCs were detected by immunoblotting and increased JNK activity was evidenced by higher levels of phosphorylated JNK after Wnt11 expression. (b) Densitometric analyses of JNK and phosphorylated-JNK signals normalized to actin and expressed as the percentage of the actin control, showing relative JNK activities from three independent experiments. Error bars represent mean ± SEM (*P < 0.01).
Cardiomyocyte-like differentiated cells beat spontaneously, persistently and rhythmically
Considering that some of the critical cardiac proteins were expressed by the Lv-Wnt11-transduced MDSCs, we used a live cell imaging system (Live Cell Imager; Kairos Instruments, Pittsburgh, PA)28 to examine whether the Lv-Wnt11-transduced cells could be shown to have the ability to spontaneously contract. We observed that some of the cardiomyocyte-like differentiated cells in the Lv-Wnt11-transduced cell population beat spontaneously, persistently, and rhythmically (Supplementary Video S1).The fraction of these beating cells was ~2.5% of the total number of cells. The beating cells expressed GFP or were located very near to GFP-expressing cells (data not shown), suggesting that their ability to contract was due to Wnt11's differentiation effect. In contrast, we did not observe any rhythmical beating in the control Lv-GFP-transduced cells. Although our results demonstrate that Wnt11 promotes MDSCs differentiation toward the cardiomyocyte lineage, our electrophysiological analyses suggest that these Wnt11-expressing MDSCs are not fully differentiated cardiomyocytes (data not shown).
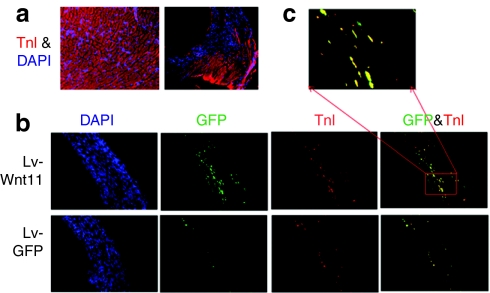
Wnt11 enhances the survival and cardiac differentiation capacity of the MDSCs transplanted into acutely ischemic myocardium NOD-SCID mice
To investigate whether Wnt11 expression augments cardiomyogenic differentiation of the MDSCs in vivo, Lv-Wnt11-transduced cells, as well as controls Lv-GFP cells, were intramyocardially administrated into ischemic myocardium of NOD-SCID mice. After 3 weeks the mice were sacrificed, the hearts sectioned and double immunofluorescent stained for GFP and cardiac TnI were performed. At the 3-week time point, none of the injected donor cells (GFP+ cells) had engrafted into the remote and border areas of the infarct, and that the cardiomyocytes in these areas were alive and stained positively for TnI (Figure 6a). We found no significant difference in the infarct area and thickness of the ischemic myocardium between the Lv-Wnt11-transduced cell group and the Lv-GFP transduced cell group; however, GFP staining revealed that there were many more cells that survived in the Lv-Wnt11-transduced cell group relative to the Lv-GFP control group (appropriately an 11-fold increase) (Figure 6b; left). Moreover, as shown by double immunofluorescent staining for GFP and cardiac TnI (Figure 6b,c), the transplanted Lv-Wnt11-transduced cells found in the infarct area showed a fourfold increase in cardiomyocytic differentiation compared to the Lv-GFP transduced cell group. These results indicate that Wnt11 promoted the survival and cardiac differentiation of the transduced MDSCs after their transplantation into ischemic myocardium.
Figure 6.
Wnt11 enhances the survival and cardiac differentiation of mouse muscle-derived stem cells (MDSCs) in vivo. (a) Troponin I (TnI) staining (red) of frozen sections from remote area (left) and infarct border area (right) of myocardium injected with Lv-Wnt11 or Lv-green fluorescent protein (GFP) transduced MDSCs, 3 weeks after acute myocardial infarction and cell transplantation (magnification: ×100). Nuclei were counterstained with 4′, 6-diamidino-2-phenylindole (DAPI) (blue). (b) Representative double immunofluorescent staining for GFP (green) and TnI (red) inside the infarct area (magnification: ×100). More cells inside infracted hearts injected with Lv-Wnt11-transduced cells survived than the hearts injected with Lv-GFP transduced cells (as shown by GFP staining). Markedly increased cardiomyocytic differentiation occurred in the Lv-Wnt11-transduced cell group as revealed by GFP and TnI colocalization. (c) A greater magnification of the colocalization of GFP and TnI from a frozen section of a heart injected with Lv-Wnt11transduced cells (magnification: ×400), indicating in vivo cardiomyocyte differentiation.
Discussion
In the present study, we investigated the role that Wnt11 played in the cardiomyocytic differentiation of mouse MDSCs. After transduction with an Lv-Wnt11 lentiviral vector, the mouse MDSCs expressed cardiac genes, including Nkx2.5, GATA4, Cx43, α-MHC, β-MHC, BNP and cardiac proteins Nkx2.5, GATA4, Cx43, MHC, and TnI, at higher levels. Moreover, cardiomyocyte-specific TnI positive cells displayed three main types of morphology including spindle, round, and triangular-shaped cells. Furthermore, some of these transduced cells were shown to spontaneously, persistently, and rhythmically beat. We also found that Lv-Wnt11-transduced cells showed significantly enhanced survival and a cardiac differentiation capacity after transplantation into acute ischemic myocardium relative to Lv-GFP cells. Our results indicate that the overexpression of Wnt11 can promote the differentiation of MDSCs toward a cardiomyocyte lineage.
Due to the fact that skeletal MDSCs are easily accessed from a donor through a simple muscle biopsy and the fact that the cells can be rapidly expanded prior to autologous transplantation they represent an excellent cell source for cellular therapeutic approaches to treat myocardial infarction.18,19,29 Several research groups have reported that skeletal muscle-derived cells have the capacity to differentiate into cardiomyocytes or cardiac muscle lineages in vitro and in vivo,29,30,31,32,33,34 providing a promising cell source for stem cell-based therapies for the treatment of cardiovascular disease. Wnt11 has been shown to induce the differentiation of cells toward a cardiac lineage probably through the activation of a calcium-dependent protein kinase C-mediated Wnt/Ca2+ pathway and Dsh-dependent JNK-mediated planar cell polarity pathway.10,11,12 To augment this cardiomyocytic differentiation capacity, we delivered the Wnt11 gene via a feline leukemia virus-based lentiviral vector to the MDSCs and then examined the inducible expression of cardiac-specific markers to confirm Wnt11's differentiation effect. We found that Wnt11 transduction resulted in elevated expression of NKx2.5 and GATA4 in the transduced MDSCs and also the protein's translocation to the nucleus. In accordance with existing studies showing that cardiac differentiation is driven by the nuclear translocation of Nkx2.5 and GATA4,22 we further observed the increased gene expression of α-MHC, β-MHC, and BNP as well as the protein expression of MHC and TnI; however, we could not detect mRNA expression of ANP, MLC2a, or CaV1.2 in Lv-Wnt11-transduced MDSCs. Is has been reported that in murine bone marrow-derived stem cell populations, Wnt11 induced the expression of GATA4, NKx2.5, β-MHC, TnT, and BNP; however, the expression of α-MHC and ANP were not detected.9 In mouse unfractionated bone marrow mononuclear cells, Wnt11 was sufficient to induce GATA4, NKx2.5, α-MHC, β-MHC, TnT, Cx43, and ANP.10 In combination with our findings, we can see that there are somewhat different effects produced by Wnt11 on cardiac differentiation among different types of postnatal progenitor/stem cells; however, the timing of analysis post-transplantation and the level of Wnt11 expression may have an influence on these observations.
NKx2.5 and GATA4 are two critical transcription factors expressed when cells undergo a cardiac lineage commitment. It is known that Nkx2.5, α-MHC, β-MHC, and TnI are downstream cardiac lineage genes of GATA4 and that BNP is a downstream gene of NKx2.5.35 A recent study demonstrated that Nkx2.5 upregulates GATA4 expression but suppresses β-catenin expression,36 the latter is likely to increase noncanonical Wnt signaling activity which is necessary for differentiation. Afouda et al., also found that GATA4 directly induces Nkx2.5 and Wnt11 expression and that GATA4 promotes myocardial differentiation largely via the Wnt11 pathway.37 Nagy et al., revealed that the expression of the GATA4, Nkx2.5, and BNP genes are downregulated in the hearts of mice deficient for Wnt11 relative to normal control mice.38 Therefore, during Wnt11-induced cardiomyocytic commitment, there seems be a positive feedback loop from GATA4 to Wnt11 to Nkx2.5 and from GATA4 to Nkx2.5 to Wnt11.
JNK signaling functions downstream of the noncanonical Wnt pathway2,27 and JNK has been suggested to regulate cell apoptosis, differentiation, and migration depending on the cell type and stimulus that influences it in the microenvironment.27,39,40 Consistent with these results, we found that Lv-Wnt11-transduced cells obviously enhanced JNK activity in the cultured MDSCs compared to the Lv-GFP transduced MDSCs and facilitated the survival and differentiation of the transplanted cells in infracted myocardium (Figures 5 and 6). A recent report showed that Wnt11 promotes neuroendocrine-like differentiation, survival, and migration of prostate cancer cells via protein kinase A and the inhibition of caspase 9 activity,41 respectively, which further supports our in vivo observations (Figure 6b,c). Two previous studies also indicated that Wnt11 promotes cell migration.42,43 In our cell transplantation experiment, no significant difference in scar size was observed between the implanted Lv-Wnt11 cell group and the control Lv–GFP cell group. One reason for this is probably due to the incomplete cardiac differentiation of the transduced MDSCs, as some of the tested cardiac markers in our experiments were absent during differentiation. The limited number of MDSC-derived myocytes and the electric coupling between these cells and the existing host cardiomyocytes in the infarcted myocardium were also insufficient to block myocardial necrobiosis, which may also explain why there was no difference in the area of scar tissue formation between the two experimental groups.
Intercellular electric coupling is essential for normal cardiac contraction. We observed an increased expression of the gap junction protein Cx43 in the Lv-Wnt11-transduced MDSCs and spontaneous beating by some of the cells; however, our electrophysiological analyses demonstrate that Wnt11-expressing MDSCs do not behave as fully differentiated cardiomyocytes (data not shown). Additional measurements of calcium transport and the action potential of the Lv-Wnt11-transduced MDSCs would provide better evidence that the cardiomyocyte differentiated MDSCs were electrophysiologically functional and we believe that future studies in this area are required. Future studies that track the cellular fates of Lv-Wnt11-transduced MDSCs after their transplantation into infracted myocardium and observing improvements in cardiac function will yield useful information on the therapeutic application of autologous cellular cardiomyoplasty using MDSCs.
Materials and Methods
Mouse skeletal MDSCs. MDSCs were isolated from the skeletal muscle of 7-day-old C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) as previously described using a modified version of the preplate technique14,16 and cultured in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum, 10% horse serum, and 0.5% chicken embryo extract. All the flasks used for MDSC cell culture were coated with type I collagen.
Lentiviral production and transduction. The feline leukemia virus-based lentiviral vectors carrying the Wnt11 cDNA and the GFP reporter gene (Cat# Ex-T3437-Lv19) and vector bearing only the GFP reporter gene (Cat# Ex-EGFP-Lv19) were purchased from GeneCopoeia (Germantown, MD). Expression plasmids were expanded in the stabl3 bacterial strain (Invitrogen, Carlsbad, CA) and purified using CsCl gradient. Lentiviral particles were produced by performing transient co-transfection involving a three-plasmid expression system in HEK293T cells according to user's manual provided by the company, and concentrated through ultracentrifugation, if needed. At about 60% confluence, MDSCs seeded in 6-well plates were transduced overnight with 1-ml nondiluted viral supernatant in the presence of polybrene (5 ng/ml medial), and subcultured for MDSC amplification. Transduced cells subsequently underwent GFP sorting using a FACSAria dual-laser fluorescence cell sorter (Becton Dickinson, Franklin Lakes, NJ) to select cells which can express Wnt11 and/or the GFP gene.
Semiquantitative RT-PCR analysis. RNA samples were isolated from cultured cells seeded in 6-well plates using Trizol reagent (Invitrogen) and first strand cDNA synthesis was performed using a reverse transcription kit from Promega (Madison, WI). PCR was performed using a PCR kit from Promega for 35 cycles, with each cycle ramping to 94 °C for 30 seconds, 58 °C for 50 seconds, and 72 °C for 40 seconds. The primers used in PCR amplification are available upon request.
Real-time quantitative PCR. A 20-µl reaction mixture containing 10 µl SYBR green PCR master mix (Applied Biosystems, Foster, CA) and 0.25 µmol/l of forward and reverse primers was applied in a 96-well plate running on a 7900HT FAST Real-Time-PCR system (Applied Biosystems). β-Actin was used as an internal control for amplification of α, β-MHC and BNP. Gene expression signals were analyzed using SDS2.2.2 software (Applied Biosystems) as follows: Expression = 2−ΔCt, where ΔCt = (Ctgene – Ctβactin); relative expression of B to A = 2−ΔΔCt, where ΔΔCt = (Ctgene B – Ctgene A) and Ct is the threshold cycle.44
Immunocytochemistry. Cells grown in a 12-well plate were fixed in −20 °C methanol or in 2% paraformaldehyde, permeabilized with 0.1% Triton X-100, blocked with 10% donkey serum in phosphate-buffered saline, and incubated over night at 4 °C with antibodies against Wnt11, Nkx2.5, GATA4, Cx43, TnI (all from Santa Cruz, Santa Cruz, CA), anti-α-actinin (Sigma, St Louis, MO) and cardiac MHC (Novus Biologicals, Littleton, CO). The following day, the cells were incubated with secondary antibodies(donkey anti-rabbit or goat IgG conjugated to AlexaFluor-594; Molecular Probes, Carlsbad, CA), washed three times with phosphate-buffered saline, and visualized using a Leica DMIRB fluorescence microscope (Leica, Deerfield, IL) equipped with a Retiga 1300 digital camera (QImaging, Burnaby, British Columbia, Canada). All images were acquired with Northern Eclipse software (version 6.0; Empix Imaging, Mississauga, Ontario, Canada). The percentage of the cells staining positive for Nkx2.5, GATA4, and Cx43 was calculated from images of four to six fields obtained with a light microscope (×10).Cell nuclei were counterstained with DAPI. To analyze the of BMP4-induced MLC2a expression, mouse MDSCs seeded on 12-well plate were treated with 50 ng/ml BMP4 for 6 days and subsequently cultured under normal medium for additional 8 days. The cells were fixed in −20 °C methanol for 5 minutes and immunostained using an antibody against MLC2a (Santa Cruz).
Western blot analysis. Laemmli sample buffer (Bio-Rad Laboratories, Hercules, CA) was used to collect proteins from live cells. After being prepared via standard procedures, protein samples were separated on 10–20% sodium dodecyl sulfate-polyacrylamide electrophoresis gels (Minigels; Bio-Rad Laboratories) and were transferred to nitrocellulose membranes that then were blocked for at least 1 hours at room temperature with 5% (wt./vol.) nonfat milk in Tris buffer saline with Tween-20 (0.1 mol/l Tris-base, pH 7.5, 0.15 mol/l NaCl, and 0.1% Tween-20). Rabbit anti-NKx2.5 and rabbit anti-Cx43, mouse anti-JNK1 (Santa Cruz) and rabbit anti-phosphor-JNK (Cell Signaling, Danvers, MA) were applied as primary antibodies, and mouse anti-β-actin (1:8,000; Sigma) was used as an internal control. Horse-radish peroxidase-conjugated secondary antibodies (Pierce, Rockford, IL) were applied at a dilution of 1:5,000 and then blots were developed using SuperSignal West Pico chemiluminescent substrate (Pierce) and positive bands were visualized on X-ray film.
Acute myocardial infarction and intramyocardial cell transplantation. All animal experiments and surgical procedures were approved by the institutional animal care and use committee of the University of Pittsburgh (Pittsburgh, PA) (Protocol # 0912973). Acute myocardial infarction was induced in six nonobese diabetic-severe combined immunodeficient (NOD-SCID) mice (male, age 8 weeks, 25–30 g, NOD.CB17-PrkdcSCID/J; Jackson Laboratory). Immediately after ligation, 3 × 105 cells in a solution of 30 µl of phosphate-buffered saline per heart were injected directly into the ischemic region, as previously describe.18 Two weeks post-transduction, Lv-Wnt11 and Lv-GFP transduced cells were injected into the infracted myocardium of three mice. The mice were sacrificed 3 weeks after cell transplantation for immunohistochemical evaluation of the heart tissue.
Tissue processing and immunohistochemical staining. Mice were euthanized, and their hearts were harvested and frozen in 2-methylbutane precooled in liquid nitrogen, and serially cryosectioned into 8-µm thick sections (from the apex to the base). The heart sections of the three mouse cohort were used for analysis. To detect whether the transplanted MDSCs differentiated toward a cardiomyocytic lineage, the sections were detected using a cardiac-specific monoclonal mouse antibody, anti-TnI (1:200; Santa Cruz), which has no crossreactivity with skeletal muscle. The sections were incubated at room temperature for 2 hours with the primary antibody which was detected using a donkey anti-mouse IgG antibody conjugated with AlexaFluor-594 (1:200; Molecular Probes, Eugene, OR). The sections were then counterstained using goat anti-GFP conjugated with fluorescein isothiocyanate (1:200; Abcam, Cambridge, MA) to track the engraftment and survival of the transplanted cells. The sections were finally counterstained with the nuclear stain DAPI. Two to three typical sections from the same location in each heart were immunostained and positively stained cells were enumerated. Cells staining positive for GFP alone or with both GFP and TnI within the infarct area were counted using images obtained from five to eight fields using a fluorescence microscope (×10).
Statistical analysis. Data are expressed as mean ± SEM. Unpaired two-tailed Student's t-test was used for the comparison between two groups based on the original data. A P value of <0.05 was considered statistically significant.
SUPPLEMENTARY MATERIAL Figure S1. Immunostaining of GFP- or Wnt11-transduced MDSCs for α-actinin. The cells were incubated with monoclonal mouse antibodies against alpha-actinin followed by rabbit anti-mouse conjugated to AlexaFluor 594(upper panels) or goat anti-mouse IgG conjugated to AlexaFluor 488(lower panel). Figure S2. MLC2a immunofluorescent staining (red) in Lv-Wnt11-transduced MDSCs following the addition of BMP4 to medium. Nuclei were counterstained with DAPI. Figure S3. Cardiac-specific Troponin I staining (red) in human MDSCs transduced with Lv-Wnt11 or Lv-GFP. Nuclei were counterstained with DAPI (blue). Video S1. Muscle-derived stem cells transduced with a lentivirus encoding for Wnt11 showing spontaneous and rhythmic beating.
Acknowledgments
This work was supported in part by grants awarded to J.H. from the NIH (IU54AR050733-01, HL 069368), the William F. and Jean W. Donaldson Endowed Chair and The Orris C. Hirtzel and Beatrice Dewey Hirtzel Memorial Foundation at Children's Hospital of Pittsburgh, and the Henry J. Mankin Endowed Chair at the University of Pittsburgh. We also thank Dr Bridget Deasy's laboratory for live cell imaging data and Dr Edwin Levitan (Department of Pharmacology and Chemical Biology, University of Pittsburgh) for the electrophysiological analyses.
Supplementary Material
Immunostaining of GFP- or Wnt11-transduced MDSCs for α-actinin. The cells were incubated with monoclonal mouse antibodies against alpha-actinin followed by rabbit anti-mouse conjugated to AlexaFluor 594(upper panels) or goat anti-mouse IgG conjugated to AlexaFluor 488(lower panel).
MLC2a immunofluorescent staining (red) in Lv-Wnt11-transduced MDSCs following the addition of BMP4 to medium. Nuclei were counterstained with DAPI.
Cardiac-specific Troponin I staining (red) in human MDSCs transduced with Lv-Wnt11 or Lv-GFP. Nuclei were counterstained with DAPI (blue).
Muscle-derived stem cells transduced with a lentivirus encoding for Wnt11 showing spontaneous and rhythmic beating.
REFERENCES
- Cohen ED, Tian Y., and, Morrisey EE. Wnt signaling: an essential regulator of cardiovascular differentiation, morphogenesis and progenitor self-renewal. Development. 2008;135:789–798. doi: 10.1242/dev.016865. [DOI] [PubMed] [Google Scholar]
- Katoh M., and, Katoh M. WNT signaling pathway and stem cell signaling network. Clin Cancer Res. 2007;13:4042–4045. doi: 10.1158/1078-0432.CCR-06-2316. [DOI] [PubMed] [Google Scholar]
- Klaus A., and, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- Logan CY., and, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Reya T., and, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- van Amerongen R, Mikels A., and, Nusse R. Alternative wnt signaling is initiated by distinct receptors. Sci Signal. 2008;1:re9. doi: 10.1126/scisignal.135re9. [DOI] [PubMed] [Google Scholar]
- Eisenberg CA., and, Eisenberg LM. WNT11 promotes cardiac tissue formation of early mesoderm. Dev Dyn. 1999;216:45–58. doi: 10.1002/(SICI)1097-0177(199909)216:1<45::AID-DVDY7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Pandur P, Läsche M, Eisenberg LM., and, Kühl M. Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature. 2002;418:636–641. doi: 10.1038/nature00921. [DOI] [PubMed] [Google Scholar]
- Belema Bedada F, Technau A, Ebelt H, Schulze M., and, Braun T. Activation of myogenic differentiation pathways in adult bone marrow-derived stem cells. Mol Cell Biol. 2005;25:9509–9519. doi: 10.1128/MCB.25.21.9509-9519.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty MP, Abdel-Latif A, Li Q, Hunt G, Ranjan S, Ou Q.et al. (2008Noncanonical Wnt11 signaling is sufficient to induce cardiomyogenic differentiation in unfractionated bone marrow mononuclear cells Circulation 1172241–2252. [DOI] [PubMed] [Google Scholar]
- Flaherty MP., and, Dawn B. Noncanonical Wnt11 signaling and cardiomyogenic differentiation. Trends Cardiovasc Med. 2008;18:260–268. doi: 10.1016/j.tcm.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi M, Haendeler J, Badorff C, Brandes RP, Hoffmann J, Pandur P.et al. (2005Non-canonical Wnt signaling enhances differentiation of human circulating progenitor cells to cardiomyogenic cells J Biol Chem 28016838–16842. [DOI] [PubMed] [Google Scholar]
- Terami H, Hidaka K, Katsumata T, Iio A., and, Morisaki T. Wnt11 facilitates embryonic stem cell differentiation to Nkx2.5-positive cardiomyocytes. Biochem Biophys Res Commun. 2004;325:968–975. doi: 10.1016/j.bbrc.2004.10.103. [DOI] [PubMed] [Google Scholar]
- Gharaibeh B, Lu A, Tebbets J, Zheng B, Feduska J, Crisan M.et al. (2008Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique Nat Protoc 31501–1509. [DOI] [PubMed] [Google Scholar]
- Lee JY, Qu-Petersen Z, Cao B, Kimura S, Jankowski R, Cummins J.et al. (2000Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing J Cell Biol 1501085–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R.et al. (2002Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration J Cell Biol 157851–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B, Zheng B, Jankowski RJ, Kimura S, Ikezawa M, Deasy B.et al. (2003Muscle stem cells differentiate into haematopoietic lineages but retain myogenic potential Nat Cell Biol 5640–646. [DOI] [PubMed] [Google Scholar]
- Oshima H, Payne TR, Urish KL, Sakai T, Ling Y, Gharaibeh B.et al. (2005Differential myocardial infarct repair with muscle stem cells compared to myoblasts Mol Ther 121130–1141. [DOI] [PubMed] [Google Scholar]
- Payne TR, Oshima H, Okada M, Momoi N, Tobita K, Keller BB.et al. (2007A relationship between vascular endothelial growth factor, angiogenesis, and cardiac repair after muscle stem cell transplantation into ischemic hearts J Am Coll Cardiol 501677–1684. [DOI] [PubMed] [Google Scholar]
- Gros J, Serralbo O., and, Marcelle C. WNT11 acts as a directional cue to organize the elongation of early muscle fibres. Nature. 2009;457:589–593. doi: 10.1038/nature07564. [DOI] [PubMed] [Google Scholar]
- Wörsdörfer P, Maxeiner S, Markopoulos C, Kirfel G, Wulf V, Auth T.et al. (2008Connexin expression and functional analysis of gap junctional communication in mouse embryonic stem cells Stem Cells 26431–439. [DOI] [PubMed] [Google Scholar]
- Armiñán A, Gandía C, Bartual M, García-Verdugo JM, Lledó E, Mirabet V.et al. (2009Cardiac differentiation is driven by NKX2.5 and GATA4 nuclear translocation in tissue-specific mesenchymal stem cells Stem Cells Dev 18907–918. [DOI] [PubMed] [Google Scholar]
- Chen D, Zhao M., and, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- Klaus A, Saga Y, Taketo MM, Tzahor E., and, Birchmeier W. Distinct roles of Wnt/β-catenin and Bmp signaling during early cardiogenesis. Proc Natl Acad Sci USA. 2007;104:18531–18536. doi: 10.1073/pnas.0703113104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzen K, Nagai R., and, Komuro I. A role for bone morphogenetic protein signaling in cardiomyocyte differentiation. Trends Cardiovasc Med. 2002;12:263–269. doi: 10.1016/s1050-1738(02)00172-x. [DOI] [PubMed] [Google Scholar]
- Carr LK, Steele D, Steele S, Wagner D, Pruchnic R, Jankowski R.et al. (20081-year follow-up of autologous muscle-derived stem cell injection pilot study to treat stress urinary incontinence Int Urogynecol J Pelvic Floor Dysfunct 19881–883. [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Moriguchi T, Masuyama N, Kusakabe M, Hanafusa H, Takada R.et al. (2002JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates EMBO Rep 369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt BT, Feduska JM, Witt AM., and, Deasy BM. Robotic cell culture system for stem cell assays. Industrial Robot. 2008;35:116–124. [Google Scholar]
- Tamaki T, Akatsuka A, Okada Y, Uchiyama Y, Tono K, Wada M.et al. (2008Cardiomyocyte formation by skeletal muscle-derived multi-myogenic stem cells after transplantation into infarcted myocardium PLoS ONE 3e1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Latif A, Zuba-Surma EK, Case J, Tiwari S, Hunt G, Ranjan S.et al. (2008TGF-β1 enhances cardiomyogenic differentiation of skeletal muscle-derived adult primitive cells Basic Res Cardiol 103514–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsic N, Mamaeva D, Lamb NJ., and, Fernandez A. Muscle-derived stem cells isolated as non-adherent population give rise to cardiac, skeletal muscle and neural lineages. Exp Cell Res. 2008;314:1266–1280. doi: 10.1016/j.yexcr.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Invernici G, Cristini S, Madeddu P, Brock S, Spillmann F, Bernasconi P.et al. (2008Human adult skeletal muscle stem cells differentiate into cardiomyocyte phenotype in vitro Exp Cell Res 314366–376. [DOI] [PubMed] [Google Scholar]
- Nomura T, Ueyama T, Ashihara E, Tateishi K, Asada S, Nakajima N.et al. (2008Skeletal muscle-derived progenitors capable of differentiating into cardiomyocytes proliferate through myostatin-independent TGF-β family signaling Biochem Biophys Res Commun 365863–869. [DOI] [PubMed] [Google Scholar]
- Winitsky SO, Gopal TV, Hassanzadeh S, Takahashi H, Gryder D, Rogawski MA.et al. (2005Adult murine skeletal muscle contains cells that can differentiate into beating cardiomyocytes in vitro PLoS Biol 3e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher CJ, Diman NY, McDermott DA., and, Basson CT. Transcription factor cascades in congenital heart malformation. Trends Mol Med. 2003;9:512–515. doi: 10.1016/j.molmed.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Riazi AM, Takeuchi JK, Hornberger LK, Zaidi SH, Amini F, Coles J.et al. (2009NKX2-5 regulates the expression of β-catenin and GATA4 in ventricular myocytes PLoS ONE 4e5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afouda BA, Martin J, Liu F, Ciau-Uitz A, Patient R., and, Hoppler S. GATA transcription factors integrate Wnt signalling during heart development. Development. 2008;135:3185–3190. doi: 10.1242/dev.026443. [DOI] [PubMed] [Google Scholar]
- Nagy II, Railo A, Rapila R, Hast T, Sormunen R, Tavi P.et al. (2010Wnt-11 signalling controls ventricular myocardium development by patterning N-cadherin and β-catenin expression Cardiovasc Res 85100–109. [DOI] [PubMed] [Google Scholar]
- Yu C, Minemoto Y, Zhang J, Liu J, Tang F, Bui TN.et al. (2004JNK suppresses apoptosis via phosphorylation of the proapoptotic Bcl-2 family protein BAD Mol Cell 13329–340. [DOI] [PubMed] [Google Scholar]
- Coogan TP, Squibb KS, Motz J, Kinney PL., and, Costa M. Distribution of chromium within cells of the blood. Toxicol Appl Pharmacol. 1991;108:157–166. doi: 10.1016/0041-008x(91)90279-n. [DOI] [PubMed] [Google Scholar]
- Uysal-Onganer P, Kawano Y, Caro M, Walker MM, Diez S, Darrington RS.et al. (2010Wnt-11 promotes neuroendocrine-like differentiation, survival and migration of prostate cancer cells Mol Cancer 955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouko L, Ziegler TR, Gu LH, Eisenberg LM., and, Yang VW. Wnt11 signaling promotes proliferation, transformation, and migration of IEC6 intestinal epithelial cells. J Biol Chem. 2004;279:26707–26715. doi: 10.1074/jbc.M402877200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, Saúde L, Concha ML, Geisler R.et al. (2000Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation Nature 40576–81. [DOI] [PubMed] [Google Scholar]
- Livak KJ., and, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔ C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immunostaining of GFP- or Wnt11-transduced MDSCs for α-actinin. The cells were incubated with monoclonal mouse antibodies against alpha-actinin followed by rabbit anti-mouse conjugated to AlexaFluor 594(upper panels) or goat anti-mouse IgG conjugated to AlexaFluor 488(lower panel).
MLC2a immunofluorescent staining (red) in Lv-Wnt11-transduced MDSCs following the addition of BMP4 to medium. Nuclei were counterstained with DAPI.
Cardiac-specific Troponin I staining (red) in human MDSCs transduced with Lv-Wnt11 or Lv-GFP. Nuclei were counterstained with DAPI (blue).
Muscle-derived stem cells transduced with a lentivirus encoding for Wnt11 showing spontaneous and rhythmic beating.