Abstract
Adoptive T-cell transfer showed promising efficacy in recent trials raising interest in T cells with redirected specificity against tumors. T cells were engineered with a chimeric antigen receptor (CAR) with predefined binding and CD3ζ signaling to initiate T-cell activation. CD28 costimulation provided by a CD28-CD3ζ signaling CAR moreover improved T cell activation and persistence; however, it failed to meet the expectations with respect to mounting attacks against solid tumors infiltrated with regulatory T (Treg) cells. We revealed that a CD28 CAR–redirected T-cell attack is accompanied by higher numbers of Treg cells infiltrating the tumor and is less efficient against cancer cells in presence of Treg cells than a CD3ζ CAR T-cell attack. Deletion of the lck binding moiety in the CD28 CAR endodomain, however, improved redirected anti-tumor activity in presence of Treg cells without impairing interferon-γ (IFN-γ) secretion, proliferation, and cytolysis. CD28 modification abrogated interleukin-2 (IL-2) induction upon CAR engagement which in turn is no longer available to sustain Treg cell persistence. CARs with the modified CD28 endodomain thereby expedite the implementation of adoptive T-cell therapy in patients with a variety of cancer types that are heavily infiltrated by Treg cells.
Introduction
Adoptive T-cell transfer has shown significant efficacy in the treatment of malignancies and can be curative in patients with chronic myeloid leukemia1 or Epstein–Barr virus-associated malignancies.2 Trials targeting metastatic melanoma demonstrated significant success in the treatment of solid tumors using ex vivo expanded tumor infiltrating lymphocytes with preselected tumor specificity.3 Limitations in the isolation of tumor infiltrating T cells are overcome by genetically engineering T cells with a chimeric antigen receptor (CAR) which recognizes a predefined, tumor-associated antigen. Recent trials using ex vivo engineered and amplified T cells particularly demonstrate the feasibility and therapeutic success of the strategy.4,5 CAR-engineered T cells have the advantage of recognizing target antigen through their antibody-derived binding domain in a major histocompatibility complex–independent fashion and stimulating T-cell activation through intracellular CD3ζ.6 Second-generation CARs provide costimulation, in addition to the primary CD3ζ-signal, aimed at improving the redirected effector functions, inhibiting the activation-induced cell death and prolonging T-cell persistence in vivo.7,8,9 A CD28-CD3ζ CAR thereby delivers CD28 costimulation to the engineered T cell in the absence of agonistic CD28 ligands such as B7.1 and B7.2 which are frequently lacking in tumor cells.
Although CAR-engineered T cells infiltrate the tumor tissue and persist in the periphery over long periods,10 tumor reduction is frequently transient, which is likely due to tumor-associated immune repression. The latter is mediated, at least in part, by regulatory T (Treg) cells which heavily infiltrate solid tumor lesions. This is corroborated by the clinical observation that patients with increased Treg numbers carry a poor prognosis, particularly in advanced stages of the disease, despite the presence of tumor-specific T cells.11,12,13,14,15,16 Several mechanisms are supposed to be involved in Treg-mediated effector T-cell repression, including suppressive cytokines such as interleukin-10 (IL-10) and transforming growth factor-β, IL-2 consumption, granzyme B-mediated apoptosis, as well as cell surface receptor interactions resulting in cell cycle arrest.17,18 Effector T cells, on the other hand, contribute to the network through secretion of IL-2 which sustains proliferation and suppression by Treg cells.19 The central role of IL-2 is additionally highlighted by the observation that the number of Treg cells increased in circulation after treatment with IL-2.20 Tremendous efforts are currently underway to eliminate Treg cells in tumor tissue or to make the redirected effector T-cell response Treg cell-resistant.21,22
In this study we show that a redirected T-cell antitumor attack is less effective in presence of Treg cells when mediated by a CD28-CD3ζ than by a CD3ζ CAR. Modification of the CAR CD28 endodomain which ceased IL-2 induction improved the antitumor response in presence of Treg cells. Data provide a strategy for the adoptive T-cell therapy of solid tumors which are notoriously infiltrated by Treg cells.
Results
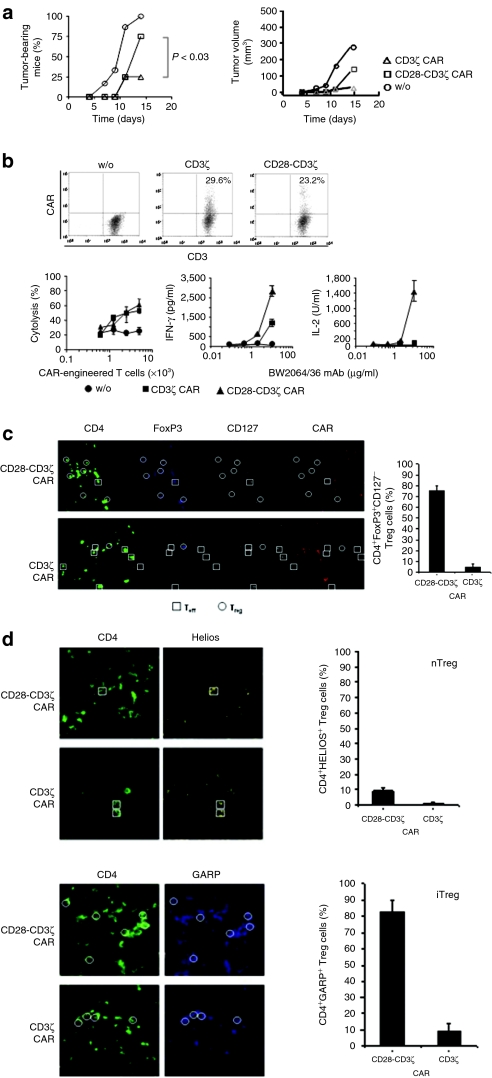
We explored the antitumor response of cytolytic T cells redirected via the CD3ζ or the CD28-CD3ζ signaling CAR, both specific for carcinoembryonic antigen (CEA), in immune competent mice under conditions which closely mimic the immunological situation of a long-term cancer patient. CEA+ C15A3 tumor cells, which lack CD28 agonistic ligands, were subcutaneously transplanted into CEA-transgenic mice23 which are tolerant for CEA. T cells were engineered with a CEA-specific CAR with either CD28-CD3ζ or CD3ζ signaling domain, each CAR expressed on same levels, and adoptively transferred by intravenous injection into mice with established tumors. As summarized in Figure 1a tumor progression was less repressed by CD28-CD3ζ than by CD3ζ CAR–redirected T cells.
Figure 1.
A CD28-CD3ζ chimeric antigen receptor (CAR) redirected T cell response is accompanied by an increase in regulatory T (Treg) cell infiltration. (a) CEA+ C15A3 tumor cells (5 × 105 cells/mouse) were subcutaneously inoculated in immunocompetent, carcinoembryonic antigen (CEA)-transgenic C57BL6 mice (five mice per group). Spleen T cells engineered by retroviral transduction with a CEA-specific, CD3ζ, and CD28-CD3ζ signaling CAR, respectively, were applied by intravenous injection at day 0 (5 × 105 CAR T cells per mouse). Mock-engineered T cells without CAR (w/o) served as control. Tumor growth was daily monitored. (b) CAR expression by engineered T cells was recorded by flow cytometry using a FITC-conjugated anti-CD3 antibody and a PE-conjugated anti-immunoglobulin G1 (IgG1) antibody which recognizes the CAR extracellular IgG1 CH2CH3 domain. CAR expressing T cells were adjusted to same numbers before use in functional assays. Engineered or mock-modified, preactivated T cells (w/o) were coincubated (104 CAR expressing T cells per well) with CEA+ tumor cells (2.5 × 104 tumor cells/well), and tumor cell lysis was recorded. Anti-CD3/CD28 preactivated T cells without CAR modification used as controls exhibit spontaneous cytotoxicity of about 20%. To record cytokine secretion, engineered T cells (104 CAR T cells per well) as well as the mock-modified T cells (w/o) were incubated on plates coated with increasing concentrations of the BW2064/36 monoclonal antibody (mAb) which binds to the anti-CEA single-chain variable fragment CAR domain. Interferon-γ (IFN-γ) and IL-2 secreted by the redirected T cells into the culture supernatants were determined by enzyme-linked immunosorbent assay. Plates coated with an isotype-matched IgG mAb did not induce IFN-γ or IL-2 secretion (data not shown). (c) Sections of biopsies from C15A3 tumors of mice (n = 5) treated with CD8+ T cells engineered with the CEA-specific CD28-CD3ζ and CD3ζ CAR, respectively, were stained for CD4 (green), FoxP3 (blue), CD127 (white), and for CAR (red) which is detected by an anti-mouse IgG γ-chain antibody directed toward the CAR extracellular IgG1 CH2CH3 domain. Multicolor staining was analyzed by laser-scan microscopy. Host effector T (Teff) cells are CD4+FoxP3−CD127+ and Treg cells are CD4+FoxP3+CD127−. Some examples of Treg cells are shown by circles; squares indicate effector CD4+ T cells for comparison. Note that CAR-engineered CD8+ T cells were adoptively transferred and the host CD4+ T cells were CAR-negative. The number of Treg cells per CD4+ T cells was recorded. A minimum of 100 cells per slide was counted. The data represent the mean + standard error of the mean of five tumors of each group. (d) Sections of biopsies from C15A3 tumors of mice (n = 5) treated with CD3+ T cells engineered with the CEA-specific CD28-CD3ζ and CD3ζ CAR, respectively, were stained for CD4 (green) and Helios (yellow) to record natural Treg (nTreg) cells, and alternatively for CD4 (green) and GARP (blue) to record induced Treg (iTreg) cells. Some examples of iTreg cells are shown by circles; squares indicate nTreg cells for comparison. The number of GARP+ Treg cells and Helios+ Treg cells, respectively, per Treg cell (%) were recorded. A minimum of 100 cells per slide was counted. The data represent the mean + standard error of the mean of five tumors of each group.
Lower antitumor activity of CD28-CD3ζ CAR–redirected T cells was an unexpected finding since same numbers of CAR expressing T cells were transferred, both CARs were expressed at similar levels and redirected cytolysis was similarly efficient by CD3ζ and CD28-CD3ζ CAR–redirected T cells in vitro (Figure 1b). The CD4:CD8 T-cell ratio was not altered upon transduction and was routinely 1:1–2:1. CD28 costimulation was exclusively provided by the CD28-CD3ζ CAR since C15A3 tumor cells lack agonistic CD28 ligands. T cells redirected by the CD28-CD3ζ CAR secreted higher amounts of interferon- γ (IFN-γ) compared to T cells with the CD3ζ CAR, whereas IL-2 secretion was only observed upon CD28-CD3ζ CAR signaling (Figure 1b) which is in accordance with the previous reports.24 However, immune histological analyses revealed that tumors of mice treated with CD28-CD3ζ CAR engineered CD8+ T cells were more heavily infiltrated by CD4+FoxP3+CD127− Treg cells than tumors of mice treated with CD3ζ CAR T cells (Figure 1c). Since we adoptively transferred CD8+ T cells, infiltrating CD4+ Treg cells are unlikely to be generated by conversion of CAR engineered T cells. To record the Treg cell phenotype in the tumor tissue, we adoptively transferred of CAR engineered CD3+ Tells. Treg cells induced in the periphery highly express GARP25 whereas natural Treg cells express Helios, an Ikaros transcription factor family member.26 Immunostainings revealed that the majority of Treg cells are induced Treg cells indicated by GARP expression and the minority are Helios+ natural Treg cells (Figure 1d). We assumed that reduced efficiency of CD28-CD3ζ CAR redirected T cells in the antitumor attack may be due to increased numbers of Treg cells infiltrating the tumor lesion.
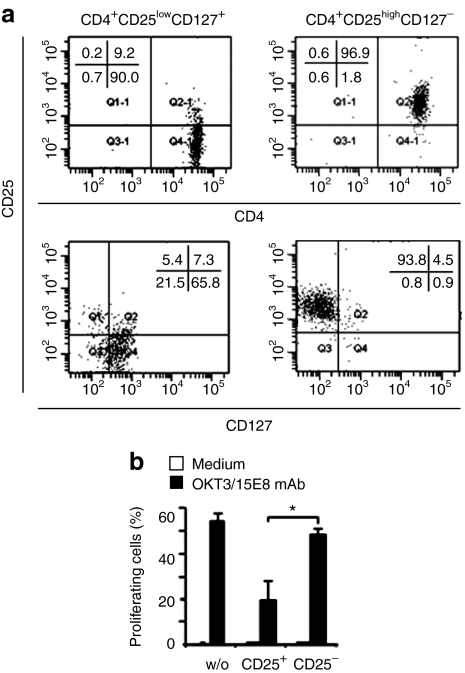
We therefore ventured to explore the impact of Treg cells on the antitumor cell activity of the CAR-redirected effector T cells in an experimental setting which allows a close control of the number of effector and Treg cells. Human natural Treg cells with the CD4+CD25highCD127− phenotype and autologous CD4+CD25lowCD127+ effector T cells as controls were isolated to high purities (Figure 2a). The isolated Treg cells were verified by a standard in vitro suppressor assay indicating suppressed proliferation of allogeneic effector T cells in the presence of Treg cells but not in the presence of control CD4+CD25− T cells (Figure 2b). To record the redirected cytolytic response of effector T cells in the presence of Treg cells, anti-CEA CAR-engineered effector T cells were coinoculated with autologous Treg cells, or CD4+CD25− T cells as control, by subcutaneous application together with CEA+ C15A3 tumor cells into CD1−/− nu/nu mice (Figure 3a). The assay design allows for recording the efficacy of the redirected tumor cell lysis by the engineered cytotoxic T lymphocytes in the presence of a defined number of Treg cells in a sensitive fashion without the confounding effects of different migratory capacities and accumulation of the respective cell types. As shown in Figure 3b tumors rapidly expanded and killed the host within 25 days in the absence of effector T cells. T cells engineered with the CEA-specific CD3ζ CAR prevented tumor formation in the presence of Treg cells with the same efficiency as in the presence of CD4+CD25− control T cells (Figure 3c). The CD28-CD3ζ CAR–redirected T-cell antitumor cell response, on the other hand, were substantially reduced in presence of Treg cells, indicated by a more progressive tumor outgrowth. This was unexpected since the in vitro cytolytic efficacies of T cells redirected by the CD3ζ and CD28-CD3ζ CAR in a short-term cytotoxicity assay were similar (cf. Figure 1b).
Figure 2.
Isolation of human regulatory T (Treg) cells. (a) CD4+CD25high Treg cells were isolated from peripheral blood lymphocytes by magnetic cell sorting procedures and recorded for CD4, CD25, and CD127 by flow cytometry. (b) CD4+CD25high Treg cells as well as CD4+CD25− T cells as control were monitored for their suppressive activity by coincubation with proliferating 5-carboxylfluorescein diacetate succinimidyl ester-labeled effector T cells in the presence of the agonistic anti-CD3 monoclonal antibody (mAb) OKT3 and anti-CD28 mAb 15E8. Incubations of effector T cells without additional cells (w/o) and coincubations without agonistic mAbs (medium) served as controls. Proliferating effector T cells were recorded by flow cytometry as described in Materials and Methods. *P < 0.05.
Figure 3.
Tumor cell lysis by the CD28-CD3ζ chimeric antigen receptor (CAR) redirected cytotoxic T lymphocytes (CTLs) is reduced in presence of regulatory T (Treg) cells. (a) A schematic diagram depicting the experimental design. (b) CEA+ C15A3 tumor cells were subcutaneously inoculated into CD1−/− nu/nu mice (5 × 105 cells/mouse). Tumor formation is shown; lines represent data from individual mice, the bold line indicates the mean. (c) CD3+ T cells were engineered to express the carcinoembryonic antigen (CEA)-specific CD3ζ or the CD28-CD3ζ CAR, respectively, and subcutaneously coinoculated with C15A3 tumor cells and autologous CD4+CD25highFoxP3high Treg cells into CD1−/− nu/nu mice (5 × 105 cells each/mouse). Coinoculation with CD4+CD25− T cells served as a control. Tumor growth was daily monitored. Tumor volumes in each individual mouse are shown; the bold line represents the mean. Tumor volumes at day 28 were compared using Student's t-test (*P < 0.05; n.s., not significant). At least three different blood donors were used for this study, a representative experiment is shown.
Since CD28-CD3ζ CAR–redirected T cells, in contrast to the CD3ζ CAR T cells, secreted IL-2 upon CAR engagement, we wished to explore whether reduced antitumor response in presence of Treg cells may be ascribed to IL-2 which sustains Treg cell accumulation and function. Hence, we deleted by mutation the lck binding domain in the CAR CD28 moiety to eliminate the signals for IL-2 induction. Upon CD28δ-CD3ζ CAR engagement of antigen, no IL-2 was secreted by the engineered T cells which stands in contrast to T cells with the wild-type CD28-CD3ζ CAR (Figure 4a). IFN-γ, however, was secreted in similar amounts by both types of engineered T cells, indicating selective knockout of the IL-2 inducing capacity. Interestingly, the in vitro cytolytic activity toward CEA+ tumor cells and CAR driven proliferation remained unaltered compared to the wild-type CD28-CD3ζ CAR. Compared to the CD3ζ CAR, the CD28D-CD3ζ CAR has the advantage of increased proliferation and IFN-γ secretion. In vivo coinoculation of the engineered T cells revealed that in the presence of Treg cells, the cytolytic activity of CD28δ-CD3ζ CAR–redirected T cells was the same as in the presence of CD4+CD25− control T cells (Figure 4b). This is in contrast to effector T cells redirected by the wild-type CD28-CD3ζ CAR (cf. Figure 3c).
Figure 4.
T cells with a modified CD28δ-CD3ζ chimeric antigen receptor (CAR) are deficient in CAR-mediated interleukin-2 (IL-2) secretion and are less repressed by coinoculated regulatory T (Treg) cells. (a) T cells were engineered with wild-type CD28-CD3ζ CAR and with the modified CD28δ-CD3ζ CAR, respectively, and stimulated via CAR by binding to the immobilized BW2064/36 monoclonal antibody (mAb) which binds to the anti-carcinoembryonic antigen (CEA) single-chain variable fragment CAR domain. Binding to an irrelevant immunoglobulin G1 (IgG1) served as a control. Proliferation during 48 hours incubation was recorded by BrdU incorporation. Interferon-γ (IFN-γ) and IL-2 secreted into the culture supernatant were monitored by enzyme-linked immunosorbent assay. Cytolytic activities of T cells engineered with the wild-type CD28-CD3ζ versus the modified CD28δ-CD3ζ CAR, were recorded by coincubation (0.5–5 × 103 CAR T cells/well) with CEA+ tumor cells (2.5 × 104 cells/well) for 48 hours. Specific cytolysis was recorded using the 2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide salt–based viability assay. Statistical analyses were performed using the Student's t-test. (b) T cells with the modified CD28δ-CD3ζ CAR were coinoculated with CEA+ C15A3 tumor cells and autologous Treg cells into CD1−/− mice (5 × 105 cells each/mouse; six mice per group). Coinoculation with autologous CD4+CD25− T cells served as a control. Tumor growth was daily monitored. Tumor volumes in each individual mouse are shown; the bold line represents the mean. Tumor volumes at day 28 were compared using Student's t-test. CTLs, cytotoxic T lymphocytes; n.s., not significant.
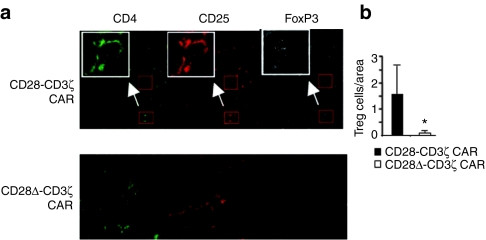
Staining of the respective tumor tissues revealed substantially more Treg cells in tumors treated with the wild-type than with the modified CD28δ-CD3ζ CAR-engineered T cells, despite the fact that the same number of Treg and effector T cells cells were initially applied (Figure 5). The data imply that Treg cells more efficiently survive and/or proliferate in the presence of effector T cells with the wild-type CD28 CAR, which induces IL-2 secretion, than with the modified CAR without IL-2 secretion. The effect is likely mediated by IL-2 which is in accordance to the fact that no other source of IL-2 was present in the CD1−/− nu/nu mouse and IL-2 is needed to sustain both the repressive capacity and survival of Treg cells.27
Figure 5.
Reduced regulatory T (Treg) cell infiltration of tumors treated with CD28δ-CD3ζ chimeric antigen receptor (CAR) T cells. (a) Tissue sections were taken from tumor lesions (Figures 3c and 4b) and stained by Cy2-conjugated monoclonal antibody (mAb) for CD4 (green), Cy3-conjugated mAb for CD25 (red) and Cy5-conjugated mAb for FoxP3 (white). Treg cells with the CD4+CD25+FoxP3+ phenotype are exemplarily shown in larger magnifications (inserts). (b) Treg cells were found in higher numbers in tumors treated with the wild-type CD28-CD3ζ CAR T cells compared to tumors treated with the modified CD28δ-CD3ζ CAR T cells. The number of Treg cells per area, i.e., at 630-fold magnification, was determined in sections of tumors treated with wild-type and modified CAR T cells. A minimum of 20 areas per group were analyzed. *P < 0.05.
Discussion
In the immune competent and CEA-tolerant host, the cytolytic T-cell response toward CEA+ tumors was less efficient when redirected by the CD28-CD3ζ CAR compared to T cells with the CD3ζ CAR. This is an unexpected finding since CD28 costimulation drives full activation of effector T cells increasing cytokine secretion, proliferation, and persistence in vivo. The antitumor response of CD28-CD3ζ CAR–redirected T cells, however, is accompanied by increased infiltration with Treg cells compared to the CD3ζ CAR T-cell response. To assess the situation in a sensitive and quantitative assay, we coinoculated defined numbers of the respective effector and repressor T cells together with tumor cells into CD1−/− nu/nu mice. Confounding effects which will impact quantitative data are thereby avoided including different migratory capacities of the respective cell types and the different accumulation of Treg cells in the presence of CAR-engineered T cells. Tumor cell lysis by coinoculated cytotoxic T cell will result in delayed tumor outgrowth; on the other hand, tumor growth reflects the ability of Treg cells to repress the cytolytic antitumor cell response of the autologous effector cells. Hence, the assay records the balance of repressor and effector activities of a known number of interacting cells. In this study, we used a 1:1:1 ratio of Treg cells-to-effector T cells-to-tumor cells to simulate a tumor lesion heavily infiltrated with Treg cells.
Effector T cells were activated either by a CD3ζ or a combined CD28-CD3ζ signaling CAR upon CEA+ tumor cell engagement. To avoid costimulation independently of CAR signals, we used CEA+ tumor cells which lack stimulatory CD28 ligands. In the presence of Treg cells, tumor cell elimination by CD28-CD3ζ CAR–redirected T cells is less efficient than in presence of CD25− control cells and compared to the effect of CD3ζ CAR T cells (Figure 3c). We assume that a relevant factor in this process is IL-2 since IL-2 is needed by Treg cells to survive and execute repressor functions.27 T cells with a CD28-CD3ζ CAR secrete IL-2 upon CAR engagement and thereby support Treg survival and function in the tumor tissue where CAR signaling occurred. IL-2 secretion is initiated by CD28-mediated lck recruitment and phosphorylation;28 accordingly, deletion of the lck binding motif in the cytoplasmic CD28 CAR domain eliminated IL-2 secretion upon antigen engagement. T cells with the modified CD28δ-CD3ζ CAR, which are deficient in CAR-induced IL-2 secretion, do not support Treg survival. Consequently, the antitumor cell response by those cells was more efficient than the wild-type CD28-CD3ζ CAR T-cell response in presence of coinoculated Treg cells. Our conclusion is sustained by the observation that despite the fact that the same number of Treg cells was initially coinoculated less Treg cells persisted in those tumor lesions which were treated with CD28δ-CD3ζ CAR T cells compared to the corresponding lesions treated with wild-type CD28-CD3ζ CAR T cells.
Compared to CD3ζ CAR T cells, modified CD28δ-CD3ζ CAR T cells have the advantage to secrete higher amounts of IFN-γ and to amplify more vigorously since the other costimulatory functions remained unaltered, including the phosphatidylinositol 3-kinase (PI3K)-Akt pathway which leads to the induction of IFN-γ secretion. In this context, it is worth mentioning that hyperactivity of the PI3K-Akt pathway in effector T cells leads to autoimmunity through resistance to Treg repression.29 Resistance to Treg repression was also described in spontaneous mouse models such as nonobese diabetic and MRL/Mp30,31 as well as in genetically engineered mice including Cbl-b−/−,32 Traf6-δT,33 dnTGF-RII,34 and NFATc2/c3 double-knockout mice.35 While the NFAT pathway is stimulated by lck signaling, activation of the PI3K-Akt pathway is increased in Cbl-b−/− and Traf6-δT mice, implying a role of this pathway as a determinant of resistance to Treg suppression.32 CD28 costimulation activates the PI3K-Akt pathway upon p85α-binding and recruitment into the TCR synapse.36 Under physiologic conditions, antigen engagement does not result in a level of PI3K-Akt activation that by itself is great enough to overcome Treg-mediated repression. Hyperactivation of the PI3K-Akt or hypoactivity of the lck pathway in effector T cells may lead to Treg resistance, tipping the balance to persistently activated effector T-cell responses and autoimmunity. By induction of IL-2 secretion, hyperactivation of the lck signaling pathway, on the other hand, sustains survival of both the effector and Treg cells.
While IL-2 sustains Treg cell survival, Treg repression additionally involves other factors including TGF-β and IL-10. In accordance with the findings by Loskog et al.37 we previously reported repression of redirected T-cell proliferation in vitro in the presence of TGF-β.38 In the absence of Treg cells, T cells redirected by the CD28-CD3ζ CAR more efficiently eradicated TGF-β+ tumor cells compared to CD3ζ CAR–redirected T cells. CD28 costimulation overcomes TGF-β repression of proliferation of effector T cells, thereby amplifying the number of effector T cells.38 In contrast to the in vitro studies by Loskog et al.37 we here recorded substantially reduced antitumor response by CD28-CD3ζ CAR T cell in presence of Treg cells. There are a number of significant experimental differences including the lower effector-to-target cell ratio we used, monitoring the eradication of tumor forming cells by effector T cells in vivo, targeting tumor cells which lack CD28 agonistic CD28 ligands, and recording the T cell-response in the immune competent, syngeneic host as well as in a xenograft model.
Multiple other sources of IL-2 may contribute to sustain the survival of both the redirected effector T cells as well as Treg cells. First, effector T cells may be CAR-independently stimulated to secrete IL-2 by interaction with agonistic B7 molecules which are physiologically provided by antigen-presenting cells. Second, therapeutic IL-2 administration sustains the survival of adoptively transferred T cells and substantially improves their therapeutic efficacy,39 but will sustain the survival of Treg cells as well. This is reflected by the observation that high-dose IL-2 administration in cancer patients increased the number of circulating Treg cells.20 Hence, we suggest that adoptive cancer immunotherapy may benefit from CD28δ CAR-modified effector T cells, along with IL-2 independent expansion of effector T cells through IL-7 and IL-15.40
Materials and Methods
Cell lines and reagents. C15A3 cells (kindly provided by Dr M. Neumaier, Universität Heidelberg-Mannheim, Mannheim, Germany) were derived from mouse MC38 fibrosarcoma cells by transfection with a CEA encoding plasmid. OKT3 (ATCC CRL 8001) is a hybridoma cell line that produces the anti-CD3 monoclonal antibody (mAb) OKT3. 15E8 is a hybridoma line that produces the anti-CD28 mAb 15E8 (kind gift from Dr. R. van Lier, Academic Medical Center, Amsterdam, The Netherlands). BW2064/36 is an internal image anti-idiotypic antibody directed against the anti-CEA single-chain variable fragment (scFv) BW431/26. Identity of hybridoma cultures were tested on a regular basis by specificity controls of the produced antibodies. All cell lines were cultured in RPMI 1640 medium, 10% (v/v) fetal calf serum (FCS) (Life Technologies, Paisley, UK). OKT3, 15E8, and BW2064/36 mAbs were affinity purified from hybridoma supernatants using goat anti-mouse immunoglobulin G1 (IgG1) antibody (Southern Biotechnology, Birmingham, AL) immobilized on N-hydroxysuccinimide ester–activated Sepharose (Amersham Biosciences, Freiburg, Germany). The phycoerythrin (PE)-conjugated and fluorescein isothiocyanate (FITC)-conjugated anti-CD3, anti-CD4, anti-CD8, and anti-CD127 mAbs were purchased from Dako (Hamburg, Germany). The allophycocyanin-conjugated anti-CD4 mAb was purchased from Dako. The PE-conjugated anti-CD25 mAb was purchased from Miltenyi Biotech (Bergisch Gladbach, Germany) and the PE-conjugated F(ab)2-anti-human IgG1 antibody was purchased from Southern Biotechnology. The allophycocyanin-conjugated anti-human FoxP3 mAb PCH101 was purchased from Natutech (Frankfurt a. M., Germany). The goat anti-human IgG antibody and its biotin-, FITC-, and PE-conjugated F(ab′)2 fragment derivatives were purchased from Southern Biotechnology. Matched pair capture and biotinylated detection antibodies for determination of IFN-γ (NIB42, B133), IL-10 (JES3-19F1, JES3-12G8), and IL-2 (JES3-9D7, B33-2), respectively, by enzyme-linked immunosorbent assay were purchased from BD Biosciences (San Diego, CA). Purified CEA was purchased from Calbiotech (Spring Valley, CA), and bovine submaxillary mucin was obtained from Sigma (Deisenhofen, Germany). Recombinant human IL-2 was purchased from Chiron GmbH (Ratingen, Germany).
Magnetic-activated cell sorting. Peripheral blood lymphocytes from healthy donors were isolated by density centrifugation and monocytes were depleted by plastic adherence. Nonadherent lymphocytes were washed with cold phosphate-buffered saline containing 0.5% (w/v) bovine serum albumin, 1% (v/v) FCS, and 2 mmol/l EDTA. CD3+, CD4+CD25+ and CD4+CD25− T cells were isolated by magnetic-activated cell sorting using human CD4+ and CD25+ microbeads, respectively (Miltenyi Biotec, Bergisch Gladbach, Germany). The number of contaminating cells in the isolated T-cell populations was <5%.
Flow cytometry. CAR expression in human T cells was recorded by flow cytometry using a PE-conjugated F(ab′)2 anti-human IgG1 antibody (1 µg/ml), which recognizes the CAR extracellular IgG1 CH2CH3 “spacer”, and the FITC-conjugated anti-CD3 mAb UCHT-1. Murine CAR expression by mouse T cells was detected by the FITC-conjugated anti-CD3 mAb KT3 (Serotec, Raleigh, NC) and the PE-conjugated goat anti-mouse IgG1 antibody (Southern Biotech, Birmingham, AL). Expression of the transcription factor FoxP3 was monitored as follows. magnetic-activated cell sorting-isolated CD4+CD25high Treg cells and CD4+CD25− T cells were labeled with FITC-conjugated anti-CD4 and PE-conjugated anti-CD25 mAbs, fixed, and permeabilized using the “FoxP3 labeling kit” (Natutech) or the “FoxP3 staining buffers” (Miltenyi Biotec). Permeabilized cells were incubated with the allophycocyanin-conjugated anti-FoxP3 mAbs PCH101 or 3G3. Labeled cells were analyzed on a FACSCanto cytofluorometer equipped with the FACSDiva research software (BD Biosciences).
Generation of CARs and transduction of T cells. The generation of the retroviral expression cassettes for the CEA-specific CARs BW431/26scFv-Fc-CD3ζ (#439) and BW431/26scFv-Fc-CD28-CD3ζ (#607) has been recently described in detail.8 The CAR HRS3scFv-Fc-CD28-CD3ζ (#926) is specific for CD30 and has the same structure as the receptor #607. BW431/26scFv-Fc-CD28δ-CD3ζ (#946) was generated by replacing the CD28 binding domain for lck by site directed mutagenesis changing P560 to A560, P563 to A563, and P564 to A564 using the following primer oligonucleotides: S-CD28-DLCK (sense): CCCACCCGCAAGCATTACCAGGCCTATGCCGCCGCACGCGACTTCGCAGCCTAT; AS-CD28- DLCK (antisense): ATAGGCTGCGAA GTCGCGTGCGGCGGCATAGGCCTGGTAATGCTTGCGGGTGGG. Human T cells were retrovirally transduced as described8 using 293T cells (ATCC CRL-11268) as retrovirus producer cells. Murine T cells were transduced according to Pouw et al. 2007.41 CAR expression was monitored by flow cytometry using a PE-conjugated F(ab′)2 anti-human IgG1 antibody (1 µg/ml) and a FITC-conjugated anti-CD3 mAb UCHT-1.
CAR-mediated activation of engineered T cells. T cells were engineered with the anti-CEA CAR, cultivated in RPMI 1640 medium (104 CAR expressing T cells per well), 10% (v/v) FCS, without stimuli for 24 hours, washed, and co-cultivated with tumor cells (2.5 × 104 per well) for 48 hours in 96-well round-bottomed plates. Alternatively, engineered T cells were cultured on 96-well round-bottomed plates in the presence of BW2064/36 mAb for specific engagement of the anti-CEA CAR. Culture supernatants were analyzed for IFN-γ by enzyme-linked immunosorbent assay using the matched pair antibodies NIB42 and B133.5 (BD Biosciences). Briefly, IFN-γ was bound to a solid-phase mAb NIB42 and detected by a biotinylated mAb B133. IL-2 was detected by B33-2 mAb, JES3-9D7 mAb, and by a biotinylated anti-human IL-2. The reaction product was visualized by a peroxidase-streptavidin conjugate and ABTS (both from Roche Diagnostics, Mannheim, Germany) as substrate. Proliferation was recorded by BrdU incorporation. Briefly, T cells were engineered with anti-CEA CARs and cultivated in microtiter plates (Polysorb; Nunc, Roskilde, Denmark) (2.5 × 104 CAR engineered T cells/well) precoated with purified anti-idiotypic mAb BW2064/36 or an IgG1 mAb for control (each 0.01–10 µg/ml). After 48 hours cell proliferation was determined using the “5-bromo-2′-deoxy-uridine Labeling and Detection Kit III” (Roche Diagnostics). BrdU labeled cells were recorded by enzyme-linked immunosorbent assay using peroxidase-labeled anti-BrdU antibody and ABTS as substrate. To monitor the cytolytic activity, CD3+ T cells (104 CAR expressing T cells per well) were co-cultivated with tumor cells (2.5 × 104 cells per well) for 48 hours in 96-well round-bottomed plates. Specific cytotoxicity of receptor-grafted T cells was monitored by a 2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide salt (XTT)-based colorimetric assay (Roche Diagnostics). Reduction of XTT to formazan by viable tumor cells was monitored colorimetrically. Maximal reduction of XTT was determined as the mean of six wells containing tumor cells only and the background as the mean of six wells containing RPMI 1640 medium, 10% (v/v) FCS. Nonspecific formation of formazan due to the presence of effector cells was determined from triplicate wells containing effector cells in the same number as in the corresponding experimental wells. Cytotoxicity was calculated as follows: cytotoxicity [%] = [1 –OD (experimental wells−corresponding number of effector cells)/OD (tumor cells without effector cells−medium)] × 100.
5-carboxylfluorescein diacetate succinimidyl ester (CFSE) labeling. Fresh allogeneic CD4+CD25− responder T cells were labeled with CFSE (Invitrogen, Karlsruhe, Germany) as previously described.42 Briefly, responder T cells were washed twice in phosphate-buffered saline, incubated with CFSE labeling solution (1 mmol/l final concentration) for 5 minutes on ice and washed five times with cold RPMI 1640 medium, 10% (v/v) FCS. CD4+CD25− and CD4+CD25+ T cells (1 × 104 cells per well), respectively, were incubated with CFSE-labeled CD4+CD25− responder T cells (5 × 104 cells per well) in the presence or absence of the anti-CD3 mAb OKT3 (0.5 µg/ml) and anti-CD28 mAb 15E8 (0.5 µg/ml). After 3 days, CFSE dilution was analyzed by flow cytometry and the number of cycling and noncycling CFSE-labeled T cells was determined.
Assay for tumor growth. CD1−/− nu/nu mice were purchased from Charles River Laboratories (Sulzfeld, Germany). All animal experiments were performed according to the Animal Experiments Committee regulations and approved by the Landesamt für Natur, Umwelt und Verbraucherschutz, Recklinghausen, Germany (K17/35-05). CEA+ C15A3 tumor cells were subcutaneously coinjected together with T cells without or with CAR and with or without Treg cells into CD1−/− nu/nu mice (5 × 105 cells each per mouse; six mice per group). Alternatively, immunocompetent, CEA-transgenic C57BL6 mice with CEA expression in gut epithelia cells23 were used as the host for transplanted CEA+ C15A3 tumor cells (Figures 1 and 5). Tumor volumes were daily recorded; statistical analyses were performed using the Student's t-test.
Immunohistochemistry. Snap-frozen tumor tissue was fixed with 4% (vol/vol) paraformaldehyde and processed for cryostat sections. To detect, human T cells sections were stained overnight in Tris-buffered saline, 0.8% (wt/vol) bovine serum albumin with the mouse anti-CD4 mAb 4B12 (IgG1) (dilution 1:40) (Novocastra, Dossenheim, Germany), anti-CD25 mAb 4C9 (IgG2b) (dilution 1:200) (Novocastra), and polyclonal rabbit anti-FoxP3 Ab ab10563 (dilution 1:800) (Abcam, Eching, Germany), and anti-CD127 mAb eBioRDR5 (IgG1) (dilution 1:10) (eBioscience, Frankfurt/Main, Germany) respectively. Furthermore, the secondary antibodies Cy3-conjugated goat anti-mouse IgG, Cy2-conjugated goat anti-mouse IgG2, Cy2-conjugated goat anti-mouse IgG, Cy5-conjugated goat anti-mouse IgG, and Cy5-conjugated goat antirabbit IgG (Jackson ImmunoResearch, West Grove, PA) were used in a 1:200 dilution. To detect CAR-engineered mouse T cells (Figure 1c,d) tumor sections were stained overnight in Tris-buffered saline, 0.8% (wt/vol) bovine serum albumin with Alexa-488 conjugated anti-mouse CD4 mAb clone RM4-5 (dilution 1:10, green), Pacific-blue–conjugated anti-mouse FoxP3 clone MF-14 (dilution 1:10, blue), Alexa-647 conjugated anti-mouse CD127 mAb clone A7R34 (dilution 1:10, white), Alexa-647 conjugated antimouse CD25 mAb clone PC61 (dilution 1:10, yellow) (all from BioLegend, Eching, Germany), Pacific-blue–conjugated antimouse GARP clone YGIC86 (dilution 1:10, blue) (eBioscience), Alexa-647 conjugated anti-Helios clone 22F6 mAb (dilution 1:10, yellow) (BioLegend), and DyLight-549 conjugated anti-mouse IgG γ-chain (KPL, Hamburg, Germany), respectively. Nuclei were stained by 4′,6-diamidino-2-phenylindole. For detection of multiple antigens, the slide was blocked with Tris-buffered saline, 5% (wt/vol) bovine serum albumin for 1 hour between individual antigen stainings. Finally, slides were dehydrated, transferred into xylole, coverslipped with Entellan (ProSciTec, Thuringowa, Australia), and analyzed using Zeiss Confocal Laser Scanning Microscope LSM 510 (Karl Zeiss, Oberkochen, Germany). Specificity of staining was assayed by incubation with the respective isotype-matched control antibody.
Acknowledgments
We thank Petra Hoffmann, Birgit Hops, Frank Steiger and Samir Tawadros for their technical assistance. D.M.K. received a fellowship from the Center for Molecular Medicine Cologne. Our work was supported by a grant from the Deutsche Krebshilfe, Bonn, Germany, the Else Kröner-Fresenius Stiftung, Bad Homburg, Germany and the CLLGRF Foundation, Houston, TX, USA. The authors declared no conflict of interest.
REFERENCES
- Kolb HJ, Schmid C, Barrett AJ., and, Schendel DJ. Graft-versus-leukemia reactions in allogeneic chimeras. Blood. 2004;103:767–776. doi: 10.1182/blood-2003-02-0342. [DOI] [PubMed] [Google Scholar]
- Gottschalk S, Heslop HE., and, Rooney CM. Adoptive immunotherapy for EBV-associated malignancies. Leuk Lymphoma. 2005;46:1–10. doi: 10.1080/10428190400002202. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA., and, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM.et al. (2006Cancer regression in patients after transfer of genetically engineered lymphocytes Science 314126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS.et al. (2009Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen Blood 114535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshhar Z. The T-body approach: redirecting T cells with antibody specificity. Handb Exp Pharmacol. 2008. pp. 329–342. [DOI] [PubMed]
- Friedmann-Morvinski D, Bendavid A, Waks T, Schindler D., and, Eshhar Z. Redirected primary T cells harboring a chimeric receptor require costimulation for their antigen-specific activation. Blood. 2005;105:3087–3093. doi: 10.1182/blood-2004-09-3737. [DOI] [PubMed] [Google Scholar]
- Hombach A, Wieczarkowiecz A, Marquardt T, Heuser C, Usai L, Pohl C.et al. (2001Tumor-specific T cell activation by recombinant immunoreceptors: CD3 zeta signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3 zeta signaling receptor molecule J Immunol 1676123–6131. [DOI] [PubMed] [Google Scholar]
- Finney HM, Lawson AD, Bebbington CR., and, Weir AN. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol. 1998;161:2791–2797. [PubMed] [Google Scholar]
- Till BG, Jensen MC, Wang J, Chen EY, Wood BL, Greisman HA.et al. (2008Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells Blood 1122261–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL.et al. (2006Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse J Clin Oncol 245373–5380. [DOI] [PubMed] [Google Scholar]
- Wolf D, Wolf AM, Rumpold H, Fiegl H, Zeimet AG, Muller-Holzner E.et al. (2005The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer Clin Cancer Res 118326–8331. [DOI] [PubMed] [Google Scholar]
- Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D.et al. (2009Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer J Clin Oncol 27186–192. [DOI] [PubMed] [Google Scholar]
- Baecher-Allan C., and, Anderson DE. Regulatory cells and human cancer. Semin Cancer Biol. 2006;16:98–105. doi: 10.1016/j.semcancer.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T.et al. (2007FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis Clin Cancer Res 13902–911. [DOI] [PubMed] [Google Scholar]
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P.et al. (2004Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival Nat Med 10942–949. [DOI] [PubMed] [Google Scholar]
- Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Tang Q., and, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenburg S, Takahashi T, de la Rosa M, Janke M, Karsten G, Muzzulini T.et al. (2008IL-2 induces in vivo suppression by CD4(+)CD25(+)Foxp3(+) regulatory T cells Eur J Immunol 381643–1653. [DOI] [PubMed] [Google Scholar]
- Ahmadzadeh M., and, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P.et al. (2009Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients Clin Cancer Res 152148–2157. [DOI] [PubMed] [Google Scholar]
- Beyer M, Kochanek M, Darabi K, Popov A, Jensen M, Endl E.et al. (2005Reduced frequencies and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine Blood 1062018–2025. [DOI] [PubMed] [Google Scholar]
- Eades-Perner AM, van der Putten H, Hirth A, Thompson J, Neumaier M, von Kleist S.et al. (1994Mice transgenic for the human carcinoembryonic antigen gene maintain its spatiotemporal expression pattern Cancer Res 544169–4176. [PubMed] [Google Scholar]
- Hombach A, Sent D, Schneider C, Heuser C, Koch D, Pohl C.et al. (2001T-cell activation by recombinant receptors: CD28 costimulation is required for interleukin 2 secretion and receptor-mediated T-cell proliferation but does not affect receptor-mediated target cell lysis Cancer Res 611976–1982. [PubMed] [Google Scholar]
- Wang R, Kozhaya L, Mercer F, Khaitan A, Fujii H., and, Unutmaz D. Expression of GARP selectively identifies activated human FOXP3+ regulatory T cells. Proc Natl Acad Sci USA. 2009;106:13439–13444. doi: 10.1073/pnas.0901965106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y.et al. (2010Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells J Immunol 1843433–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton AM, Donovan EE, Piccirillo CA., and, Shevach EM. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol. 2004;172:6519–6523. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- Wong NK, Lai JC, Birkenhead D, Shaw AS., and, Johnson P. CD45 down-regulates Lck-mediated CD44 signaling and modulates actin rearrangement in T cells. J Immunol. 2008;181:7033–7043. doi: 10.4049/jimmunol.181.10.7033. [DOI] [PubMed] [Google Scholar]
- Wohlfert EA., and, Clark RB. ‘Vive la Résistance!'–the PI3K-Akt pathway can determine target sensitivity to regulatory T cell suppression. Trends Immunol. 2007;28:154–160. doi: 10.1016/j.it.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Monk CR, Spachidou M, Rovis F, Leung E, Botto M, Lechler RI.et al. (2005MRL/Mp CD4+,CD25- T cells show reduced sensitivity to suppression by CD4+,CD25+ regulatory T cells in vitro: a novel defect of T cell regulation in systemic lupus erythematosus Arthritis Rheum 521180–1184. [DOI] [PubMed] [Google Scholar]
- You S, Belghith M, Cobbold S, Alyanakian MA, Gouarin C, Barriot S.et al. (2005Autoimmune diabetes onset results from qualitative rather than quantitative age-dependent changes in pathogenic T-cells Diabetes 541415–1422. [DOI] [PubMed] [Google Scholar]
- Wohlfert EA, Callahan MK., and, Clark RB. Resistance to CD4+CD25+ regulatory T cells and TGF-beta in Cbl-b-/- mice. J Immunol. 2004;173:1059–1065. doi: 10.4049/jimmunol.173.2.1059. [DOI] [PubMed] [Google Scholar]
- King CG, Kobayashi T, Cejas PJ, Kim T, Yoon K, Kim GK.et al. (2006TRAF6 is a T cell-intrinsic negative regulator required for the maintenance of immune homeostasis Nat Med 121088–1092. [DOI] [PubMed] [Google Scholar]
- Fahlén L, Read S, Gorelik L, Hurst SD, Coffman RL, Flavell RA.et al. (2005T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells J Exp Med 201737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bopp T, Palmetshofer A, Serfling E, Heib V, Schmitt S, Richter C.et al. (2005NFATc2 and NFATc3 transcription factors play a crucial role in suppression of CD4+ T lymphocytes by CD4+ CD25+ regulatory T cells J Exp Med 201181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang D., and, Liu YC. Proteolysis-independent regulation of PI3K by Cbl-b-mediated ubiquitination in T cells. Nat Immunol. 2001;2:870–875. doi: 10.1038/ni0901-870. [DOI] [PubMed] [Google Scholar]
- Loskog A, Giandomenico V, Rossig C, Pule M, Dotti G., and, Brenner MK. Addition of the CD28 signaling domain to chimeric T-cell receptors enhances chimeric T-cell resistance to T regulatory cells. Leukemia. 2006;20:1819–1828. doi: 10.1038/sj.leu.2404366. [DOI] [PubMed] [Google Scholar]
- Koehler H, Kofler D, Hombach A., and, Abken H. CD28 costimulation overcomes transforming growth factor-beta-mediated repression of proliferation of redirected human CD4+ and CD8+ T cells in an antitumor cell attack. Cancer Res. 2007;67:2265–2273. doi: 10.1158/0008-5472.CAN-06-2098. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA, Restifo NP, Yang JC, Morgan RA., and, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S, Mastaglio S, Bondanza A, Ponzoni M, Sanvito F, Aldrighetti L.et al. (2009IL-7 and IL-15 allow the generation of suicide gene-modified alloreactive self-renewing central memory human T lymphocytes Blood 1131006–1015. [DOI] [PubMed] [Google Scholar]
- Pouw NM, Westerlaken EJ, Willemsen RA., and, Debets R. Gene transfer of human TCR in primary murine T cells is improved by pseudo-typing with amphotropic and ecotropic envelopes. J Gene Med. 2007;9:561–570. doi: 10.1002/jgm.1047. [DOI] [PubMed] [Google Scholar]
- Venken K, Thewissen M, Hellings N, Somers V, Hensen K, Rummens JL.et al. (2007A CFSE based assay for measuring CD4+CD25+ regulatory T cell mediated suppression of auto-antigen specific and polyclonal T cell responses J Immunol Methods 3221–11. [DOI] [PubMed] [Google Scholar]