Abstract
AIM: To assess whether glutamate plays a similar role to glutamine in preserving gut wall integrity.
METHODS: The effects of glutamine and glutamate on induced hyperpermeability in intestinal cell lines were studied. Paracellular hyperpermeability was induced in Caco2.BBE and HT-29CL.19A cell lines by adding phorbol-12,13-dibutyrate (PDB) apically, after which the effects of glutamine and glutamate on horseradish peroxidase (HRP) diffusion were studied. An inhibitor of glutamate transport (L-trans-pyrrolidine-2,4-dicarboxylic acid: trans-PDC) and an irreversible blocker (acivicin) of the extracellular glutamine to glutamate converting enzyme, γ-glutamyltransferase, were used.
RESULTS: Apical to basolateral HRP flux increased significantly compared to controls not exposed to PDB (n = 30, P < 0.001). Glutamine application reduced hyperpermeability by 19% and 39% in the respective cell lines. Glutamate application reduced hyperpermeability by 30% and 20%, respectively. Incubation of HT29CL.19A cells with acivicin and subsequent PDB and glutamine addition increased permeability levels. Incubation of Caco2.BBE cells with trans-PDC followed by PDB and glutamate addition also resulted in high permeability levels.
CONCLUSION: Apical glutamate -similar to glutamine- can decrease induced paracellular hyperpermeability. Extracellular conversion of glutamine to glutamate and subsequent uptake of glutamate could be a pivotal step in the mechanism underlying the protective effect of glutamine.
Keywords: Apical, Basolateral, Flux, Glutamate, Glutamine, Gut protection, Gut wall integrity, Intestine, Permeability
INTRODUCTION
Intestinal hyperpermeability, whether cause or effect, seems to be related to the occurrence of sepsis, bacteraemia, and multiple organ failure[1,2].
Managing this change in gut physiology might contribute to substantial health improvement. The semi-essential amino acid, glutamine, is thought to improve clinical outcome in these situations. It has been ascribed several properties that are supportive of intestinal cell function and relevant to cell survival[3]. Additionally, plasma and muscle glutamine concentrations drop dramatically in critically ill patients[4-6]. In vivo experiments, however, have not yet provided definitive evidence to support the claim that glutamine supplementation has a beneficial effect on gut permeability[7]. In contrast, in vitro experiments do show a positive influence of glutamine. Kouznetsova et al[8] induced hyperpermeability in the intestinal HT-29Cl.19A cell line and found that glutamine significantly reduced this increased permeability. Furthermore, Le Bacquer et al[9] demonstrated that glutamine helps to preserve adequate paracellular permeability levels in nutritionally deprived intestinal Caco-2 cells. The precise mechanisms underlying these findings remain to be clarified. Glutamate might play a pivotal role in the effects of glutamine therapy considering the metabolic fate of glutamine: it is mostly converted to glutamate, either intra- or extracellularly[10]. Welbourne et al[11] provide support for this theory by demonstrating that blocking the extracellular glutamine to glutamate converting enzyme γ-glutamyltransferase (γ-GT) and blocking glutamate uptake, both increase paracellular permeability in the proximal tubulelike LLC-PK1-F+ cells.
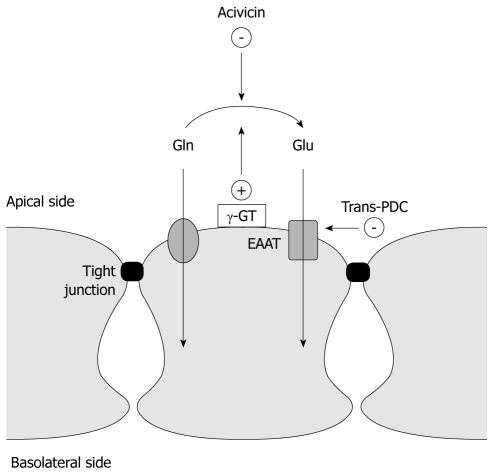
The aim of this study was to assess whether glutamate might play a similar role in the intestine (Figure 1). To do so, an experimental model that allows differentiation between the effects of glutamine and glutamate on induced hyperpermeability in intestinal cell lines was used.
Figure 1.
Model illustrating intestinal cell lineage with tight junctions and EAAT transporter. The extracellular enzymatic conversion of glutamine to glutamate by γ-glutamyltransferase (γ-GT) is shown on the apical side. The experimental design of the study using the γ-GT blocking enzyme, acivicin, and the blocker of the glutamate transporter, EAAT L-trans-pyrrolidine-2,4-dicarboxylic, is included in the figure.
MATERIALS AND METHODS
Study design
We created an experimental set-up using two intestinal cell lines: Caco2.BBE and HT-29CL.19A (both human colon adenocarcinoma derived cell lines). In culture, both cell lines exhibit polarity and apical brush-border membranes, similar to in vivo structure[12,13]. Cells were therefore placed in a bicameral system to simulate a physiological situation in which they are exposed to distinct apical and basolateral compartments, and thereby allowing permeability experiments.
Paracellular hyperpermeability was induced by adding phorbol-12,13-dibutyrate (PDB) to the apical compartment after which the effects of glutamine and glutamate on horseradish peroxidase (HRP) diffusion were studied. To differentiate between the effect of glutamine and glutamate on permeability, an inhibitor of glutamate transport (L-trans-pyrrolidine-2,4-dicarboxylic acid: trans-PDC) and an irreversible blocker (acivicin) of the extracellular glutamine to glutamate converting enzyme, γ-GT, were used.
Cell culture
The HT29Cl.19A cell line, passage number 14-35 and the Caco2.BBE cell line, passage number 34-62, were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum. The medium contained penicillin 40 mg/L, ampicillin 8 mg/L and streptomycin 9 mg/L. The cells were seeded in 12 cm2 culture flasks which were placed in an incubator with a humidified atmosphere of 5% CO2 and 95% O2. The cells were subcultured on transparent filters (12 mm diameter; Falcon, Micronic, Lelystad, The Netherlands) for 14 (HT29Cl.19A) and 21 (Caco2.BBE) days to form confluent monolayers. The medium contained glutamine (2 mmol/L) and was replaced every other day. During the last 2 d before the experiments, the cells were cultured in glutamine-free medium.
Flux experiments
The culture medium was discarded after cell cultivation and filters were rinsed with Ringer’s solution (containing 117.5 mmol/L NaCl, 5.7 mmol/L KCl, 25 mmol/L NaHCO3, 1.2 mmol/L NaH2PO4, 2.5 mmol/L CaCl2, 1.2 mmol/L MgSO4 and 27.8 mmol/L mannitol, kept at pH 7.4). Filters were placed in a bicameral system with 300 μL of Ringer’s solution added to the apical chamber and 700 μL to the basolateral chamber. The bicameral setup with filters was then placed in an incubator with humidified gas (5% CO2, 95% O2) where a temperature of 37°C was maintained. After an equilibration period of 30 min, HRP (type IV; Sigma Chemical Co., St Louis, MO, USA) dissolved in Ringer’s solution was added apically to reach a final concentration of 10-5 mol/L. For the next 4 h, basolateral samples of 5 μL were taken, in triplicate, each hour and replaced by oxygenated Ringer’s solution. The appearance of HRP in these samples was measured enzymatically. To this end, samples were mixed with 180 μL citrate buffer (0.1 mol/L citrate + 0.1 mol/L citric acid at pH 5.5) containing 3.6 μL bovine serum albumin (BSA 20 μL/mL). Three samples of 25 μL were taken from the resulting mixture and were added to 200 μL substrate. Substrate was prepared by adding 340 μL TMB stock (3.3-5.5 tetramethylbenzidine) (6 mg/mL in H2O) and 200 μL 0.3% H2O2 to 20 mL citrate buffer.
Samples were then incubated for 30 min at normal room temperature, after which the positive samples were blue in colour. The reaction was stopped by adding 50 μL HCl (2 mol/L). The samples were read at 450 nm by a spectrophotometer. Data were recorded using Microplate Manager 5 Software, Bio-Rad Laboratories Ltd., UK.
Experiments with PKC-mediated hyperpermeability were conducted by simultaneously adding 1 μmol/L of PDB and HRP to the apical chamber. The effects of L-glutamine (0.6 mmol/L) and L-glutamate (0.6 mmol/L) were (separately) studied by apical application with simultaneous PDB and HRP application. Acivicin experiments were conducted by incubating the cells with 10 μL of the following solution: 1.7 mg acivicin, dissolved in 50 μL HCl (2.0 mol/L) and 50 μL DES (buffering the medium). Inhibition of glutamate transporters EAAT 1-5 was achieved by pre-incubating the cells with 1 mmol/L trans-PDC.
Chemicals
Falcon filters were obtained from Micronic (Lelystad, The Netherlands), penicillin/streptomycin from Boehringer Mannheim (Almere, The Netherlands) and ampicillin from Sigma-Aldrich Chemie BV (Zwijndrecht, the Netherlands). All other cell culture materials were obtained from Gibco (Breda, the Netherlands). Chemicals used for the Ringer’s solution were obtained from Merck (Merck Nederland BV, Amsterdam). PDB, L-glutamate, L-glutamine and trans-PDC were obtained from Sigma-Aldrich Chemie BV (Zwijndrecht, The Netherlands).
Statistical analysis
Statistical analyses of differences between groups were performed by one way ANOVA and the Tukey-Kramer test. A P-value < 0.05 was considered significant. HRP flux results are presented graphically as percentages of total flux. Total flux is defined by the HRP + PDB groups, which therefore represent the 100% mark. Graphpad Prism 3.03 for Windows® (GraphPad Software Inc., California, USA) was used for analyses and graphical output.
RESULTS
Hyperpermeability was successfully induced by PDB stimulation: apical to basolateral HRP flux increased significantly in the HT29Cl.19A and the Caco2.BBE cell line (with a maximum after 4 h) compared to controls not exposed to PDB (n = 30, P < 0.001). Cells in the PDB group defined the 100% mark, and all values were composed of triplicate measurements per group per experiment and were repeated 3-11 times.
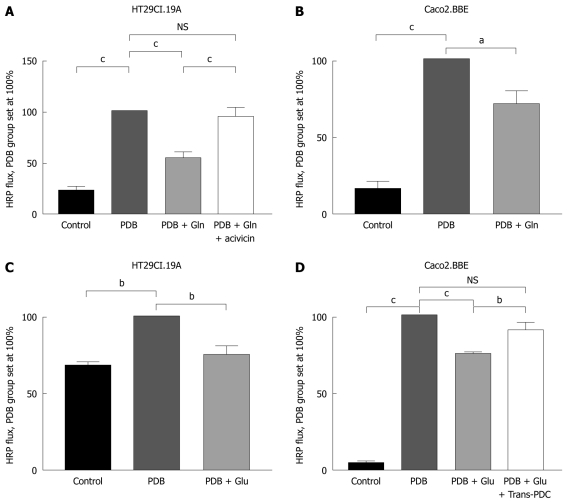
In HT29Cl.19A cells, glutamine application reduced hyperpermeability by 45% (n = 11, P < 0.001) (Figure 2A). In the Caco2.BBE cell line, glutamine application reduced hyperpermeability by 30% (n = 3, P < 0.05) (Figure 2B).
Figure 2.
Effects of glutamine and glutamate on phorbol-12,13-dibutyrate-induced permeability in the two intestinal cell lines. A: In the HT29Cl.19A cell line, glutamine addition resulted in a 45% decrease in permeability. Acivicin nullified this effect, n = 6; B: In the Caco2.BBE cell line, glutamine addition resulted in a 30% decrease in permeability, n = 3; C: In the HT29Cl.19A cell line, glutamate addition resulted in a 25% decrease in permeability, n = 3; D: In the Caco2.BBE cell line, glutamate addition resulted in a 25% decrease in permeability. Trans-PDC nullified this effect, n = 4. aP < 0.05, bP < 0.01, cP < 0.01.
Glutamate application reduced hyperpermeability by 25% in the HT29Cl.19A cell line (n = 3, P < 0.01) (Figure 2C) and by 25% in the Caco2.BBE cell line (n = 4, P < 0.001) (Figure 2D).
Incubation of HT29CL.19A cells with acivicin and subsequent PDB and glutamine addition resulted in high permeability levels which were not significantly different from the PDB group (n = 11, Figure 2A).
Incubation of Caco2.BBE cells with trans-PDC and subsequent PDB and glutamate addition also resulted in high permeability levels, once again not significantly different from the PDB group (n = 4, Figure 2D).
Control experiments revealed that acivicin and trans-PDC did not alter HRP permeability (results not shown).
DISCUSSION
We found that both glutamine and glutamate can reduce an induced form of hyperpermeability in human colon derived cell lines. The effect of glutamine could be nullified by blocking the extracellular converting enzyme, γ-GT, whereas the effect of glutamate could be nullified by blocking the glutamate transporters EAAT 1-5.
These results lead to two suggestions: firstly, the conversion of glutamine to glutamate is essential for its beneficial effect on permeability. Secondly, transport of glutamate into the cell is essential for the beneficial effect of glutamate on permeability.
Because the effect of trans-PDC on the protective action of glutamine was not studied, and similarly, the effect of acivicin on the protective action of glutamine was not studied, further research will be necessary to confirm these suggestions. Not all of the experiments were performed with both cell lines due to inherent differences between the two cell lineages. The HT29Cl.19A cell line proved unstable during later experiments compared to the Caco2.BBE cell line. Additionally, the Caco2.BBE cell line has been shown to possess EAATs[14], making it the favourable cell line for trans-PDC related experiments. Moreover, these inconsistencies might account for the observed differences in the reduction of induced hyperpermeability between the cell lines[15]. However, our study design was not focussed or powered on cell line comparison.
Glutamine and glutamate seem to reduce this hyperpermeability by acting on the paracellular permeability (tight junction) as opposed to transcellular permeability (endocytosis). PDB, induces a Protein Kinase C (PKC)-mediated hyperpermeability. This signal transduction pathway is also activated by clinically relevant mediators, including lipopolysaccharides[16]. PKC is thought to regulate tight junction (TJ) permeability via tightening and loosening of the cell’s perijunctional actomyosin ring (PAMR)[17-19]. Furthermore, rinsing the PDB from the cells restored permeability levels to control values, indicating that the effect of PDB is not due to cell destruction (results not shown). In such, PDB addition creates a paracellular hyperpermeability which can be monitored by HRP diffusion from apical to basolateral compartments.
HRP needs to remain enzymatically active to be measured. Approximately 97% of the HRP that reaches the basolateral compartment via the transcellular pathway is degraded[20,21] and loses its enzyme activity. The detection of enzymatically active HRP in this study therefore verified that we measured paracellular permeability.
Our results suggest that glutamine needs transamination to glutamate to exert its effect. In a broader scope, it would be interesting to quantify the transamination by glutaminase intracellularly. However, since blocking γ-GT extracellularly immediately showed a decrease in the effect of glutamine, the extracellular conversion seems important independent of intracellular mechanisms.
The protective effect of glutamine on gut mucosa is often thought to result from cell proliferation and attenuation of apoptosis[22]. Our study indicates that this is probably not the sole reason. HRP flux was inhibited within the four hour window of this study. Enterocyte proliferation, however, takes more than 4 h, thus cell proliferation can not (completely) explain the observed favourable effect. To exclude indirect effects of glutamine and glutamate metabolism, the measurement of metabolites by HPLC could pinpoint such effects.
Proliferation and maintaining the integrity of enterocytes requires an adequate supply of glutamine. Hence, plasma levels are normally maintained around 0.6 mmol/L[23,24]. This physiological concentration was therefore used in the present study. For easier comparison the glutamate concentration was also set at 0.6 mmol/L, even though its physiological concentration approaches 24-80 μmol/L[23,24].
The 0.6 mmol/L of glutamine and glutamate were applied to the apical chamber. In vivo, however, luminal concentrations of glutamine and glutamate commonly exceed 0.6 mmol/L after protein-rich meals[25]. It is, therefore, interesting to see that this concentration can already elicit advantageous effects. Future studies comparing different concentrations of glutamine and glutamate should be performed to optimally quantify dosage effects. To allow a comparison with catabolic patients, it would also be interesting to detect a minimum dose of glutamine and glutamate which still elicits a protective effect on hyperpermeability.
In summary, we have shown that apical glutamate-similar to glutamine can decrease an induced paracellular hyperpermeability in two human colon derived cell lines. Because of the nature of the permeability inducing agent, PDB, glutamine and glutamate probably exert their effect through interaction with tight junctions. Furthermore, the extracellular conversion of glutamine to glutamate and the subsequent uptake of glutamate could be a pivotal step in the mechanism underlying the protective effect of glutamine. Yet, to certify this mechanism, the focus should be on different concentrations of apically applied glutamine and glutamate in different cell lines or in co-cultured cell lines, in parallel with research on intracellular conversion.
COMMENTS
Background
Intestinal hyperpermeability seems to be related to the occurrence of sepsis, bacteraemia, and multiple organ failure. The semi-essential amino acid, glutamine, is thought to improve clinical outcome in these situations. Glutamate might play a pivotal role in the effects of glutamine therapy considering the metabolic fate of glutamine: it is mostly converted to glutamate.
Research frontiers
The authors found that both glutamine and glutamate can reduce an induced form of hyperpermeability in colon cell lines.
Innovations and breakthroughs
Glutamine and glutamate seem to reduce this hyperpermeability by acting on paracellular permeability as opposed to transcellular permeability.
Applications
These results suggest further research on glutamate in feeding.
Peer review
The paper by Vermeulen et al describes experiments demonstrating that glutamate has a similar protective effect on intestinal hyperpermeability as glutamine. The used two different cell lines and appropriate inhibitors to show that extracellular conversion of glutamine to glutamate and subsequent uptake of glutamate could be a pivotal step in the mechanism underlying the protective action of glutamine. This is the first report showing the protective effect of glutamate itself.
Footnotes
Supported by VU University Medical Center, Amsterdam, The Netherlands
Peer reviewers: Dr. Claudia Zwingmann, PhD, Professor, Department of Medicine, University of Montreal, Centre de Recherche, 264 Rene-Levesque Est, Montreal, QC, H2X 1P1, Canada; Julian Swierczynski, MD, PhD, Professor, Department of Biochemistry, Medical University of Gdansk, 80-211 Gdansk, Poland
S- Editor Wang YR L- Editor Webster JR E- Editor Zheng XM
References
- 1.Soeters PB, Luyer MD, Greve JW, Buurman WA. The significance of bowel permeability. Curr Opin Clin Nutr Metab Care. 2007;10:632–638. doi: 10.1097/MCO.0b013e3282a0780e. [DOI] [PubMed] [Google Scholar]
- 2.Gatt M, Reddy BS, MacFie J. Review article: bacterial translocation in the critically ill--evidence and methods of prevention. Aliment Pharmacol Ther. 2007;25:741–757. doi: 10.1111/j.1365-2036.2006.03174.x. [DOI] [PubMed] [Google Scholar]
- 3.Wischmeyer PE. Glutamine: role in gut protection in critical illness. Curr Opin Clin Nutr Metab Care. 2006;9:607–612. doi: 10.1097/01.mco.0000241672.09676.03. [DOI] [PubMed] [Google Scholar]
- 4.Oudemans-van Straaten HM, Bosman RJ, Treskes M, van der Spoel HJ, Zandstra DF. Plasma glutamine depletion and patient outcome in acute ICU admissions. Intensive Care Med. 2001;27:84–90. doi: 10.1007/s001340000703. [DOI] [PubMed] [Google Scholar]
- 5.Askanazi J, Carpentier YA, Michelsen CB, Elwyn DH, Furst P, Kantrowitz LR, Gump FE, Kinney JM. Muscle and plasma amino acids following injury. Influence of intercurrent infection. Ann Surg. 1980;192:78–85. doi: 10.1097/00000658-198007000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parry-Billings M, Evans J, Calder PC, Newsholme EA. Does glutamine contribute to immunosuppression after major burns? Lancet. 1990;336:523–525. doi: 10.1016/0140-6736(90)92083-t. [DOI] [PubMed] [Google Scholar]
- 7.De-Souza DA, Greene LJ. Intestinal permeability and systemic infections in critically ill patients: effect of glutamine. Crit Care Med. 2005;33:1125–1135. doi: 10.1097/01.ccm.0000162680.52397.97. [DOI] [PubMed] [Google Scholar]
- 8.Kouznetsova L, Bijlsma PB, van Leeuwen PA, Groot JA, Houdijk AP. Glutamine reduces phorbol-12,13-dibutyrate-induced macromolecular hyperpermeability in HT-29Cl.19A intestinal cells. JPEN J Parenter Enteral Nutr. 1999;23:136–139. doi: 10.1177/0148607199023003136. [DOI] [PubMed] [Google Scholar]
- 9.Le Bacquer O, Laboisse C, Darmaun D. Glutamine preserves protein synthesis and paracellular permeability in Caco-2 cells submitted to "luminal fasting". Am J Physiol Gastrointest Liver Physiol. 2003;285:G128–G136. doi: 10.1152/ajpgi.00459.2002. [DOI] [PubMed] [Google Scholar]
- 10.Newsholme P, Procopio J, Lima MM, Pithon-Curi TC, Curi R. Glutamine and glutamate--their central role in cell metabolism and function. Cell Biochem Funct. 2003;21:1–9. doi: 10.1002/cbf.1003. [DOI] [PubMed] [Google Scholar]
- 11.Welbourne TC, Chevalier D, Mu X. Glutamate transport modulation of paracellular permeability across LLC-PK1-F+ monolayers. Am J Physiol. 1996;271:E889–E895. doi: 10.1152/ajpendo.1996.271.5.E889. [DOI] [PubMed] [Google Scholar]
- 12.Peterson MD, Mooseker MS. Characterization of the enterocyte-like brush border cytoskeleton of the C2BBe clones of the human intestinal cell line, Caco-2. J Cell Sci. 1992;102(Pt 3):581–600. doi: 10.1242/jcs.102.3.581. [DOI] [PubMed] [Google Scholar]
- 13.Augeron C, Laboisse CL. Emergence of permanently differentiated cell clones in a human colonic cancer cell line in culture after treatment with sodium butyrate. Cancer Res. 1984;44:3961–3969. [PubMed] [Google Scholar]
- 14.Sambuy Y, De Angelis I, Ranaldi G, Scarino ML, Stammati A, Zucco F. The Caco-2 cell line as a model of the intestinal barrier: influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol Toxicol. 2005;21:1–26. doi: 10.1007/s10565-005-0085-6. [DOI] [PubMed] [Google Scholar]
- 15.Hilgendorf C, Spahn-Langguth H, Regårdh CG, Lipka E, Amidon GL, Langguth P. Caco-2 versus Caco-2/HT29-MTX co-cultured cell lines: permeabilities via diffusion, inside- and outside-directed carrier-mediated transport. J Pharm Sci. 2000;89:63–75. doi: 10.1002/(SICI)1520-6017(200001)89:1<63::AID-JPS7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 16.Wightman PD, Raetz CR. The activation of protein kinase C by biologically active lipid moieties of lipopolysaccharide. J Biol Chem. 1984;259:10048–10052. [PubMed] [Google Scholar]
- 17.Stenson WF, Easom RA, Riehl TE, Turk J. Regulation of paracellular permeability in Caco-2 cell monolayers by protein kinase C. Am J Physiol. 1993;265:G955–G962. doi: 10.1152/ajpgi.1993.265.5.G955. [DOI] [PubMed] [Google Scholar]
- 18.Turner JR, Angle JM, Black ED, Joyal JL, Sacks DB, Madara JL. PKC-dependent regulation of transepithelial resistance: roles of MLC and MLC kinase. Am J Physiol. 1999;277:C554–C562. doi: 10.1152/ajpcell.1999.277.3.C554. [DOI] [PubMed] [Google Scholar]
- 19.Kotsonis P, Funk L, Prountzos C, Iannazzo L, Majewski H. Differential abilities of phorbol esters in inducing protein kinase C (PKC) down-regulation in noradrenergic neurones. Br J Pharmacol. 2001;132:489–499. doi: 10.1038/sj.bjp.0703813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heyman M, Ducroc R, Desjeux JF, Morgat JL. Horseradish peroxidase transport across adult rabbit jejunum in vitro. Am J Physiol. 1982;242:G558–G564. doi: 10.1152/ajpgi.1982.242.6.G558. [DOI] [PubMed] [Google Scholar]
- 21.Heyman M, Darmon N, Dupont C, Dugas B, Hirribaren A, Blaton MA, Desjeux JF. Mononuclear cells from infants allergic to cow's milk secrete tumor necrosis factor alpha, altering intestinal function. Gastroenterology. 1994;106:1514–1523. doi: 10.1016/0016-5085(94)90405-7. [DOI] [PubMed] [Google Scholar]
- 22.Larson SD, Li J, Chung DH, Evers BM. Molecular mechanisms contributing to glutamine-mediated intestinal cell survival. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1262–G1271. doi: 10.1152/ajpgi.00254.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melis GC, Boelens PG, van der Sijp JR, Popovici T, De Bandt JP, Cynober L, van Leeuwen PA. The feeding route (enteral or parenteral) affects the plasma response of the dipetide Ala-Gln and the amino acids glutamine, citrulline and arginine, with the administration of Ala-Gln in preoperative patients. Br J Nutr. 2005;94:19–26. doi: 10.1079/bjn20051463. [DOI] [PubMed] [Google Scholar]
- 24.Cynober LA. Plasma amino acid levels with a note on membrane transport: characteristics, regulation, and metabolic significance. Nutrition. 2002;18:761–766. doi: 10.1016/s0899-9007(02)00780-3. [DOI] [PubMed] [Google Scholar]
- 25.Adibi SA, Mercer DW. Protein digestion in human intestine as reflected in luminal, mucosal, and plasma amino acid concentrations after meals. J Clin Invest. 1973;52:1586–1594. doi: 10.1172/JCI107335. [DOI] [PMC free article] [PubMed] [Google Scholar]