Abstract
Context
Patients on investigational clinical trials and their caregivers experience poor quality of life (QOL), which declines as the disease progresses.
Objective
To examine the effect of a standardized cognitive–behavioral problem-solving educational intervention on the QOL of patients enrolled on investigational clinical trials and their caregivers.
Design
Prospective, multi-institution, randomized trial. QOL was measured repeatedly over 6 months.
Participants
Patients were simultaneously enrolled onto phase 1, 2, or 3 Institutional Review Board (IRB)-approved cancer clinical trials.
Intervention
Intervention arm dyads participated in three conjoint educational sessions during the first month, learning the COPE problem solving model. Nonintervention arm dyads received usual care.
Outcome Measures
Global QOL was measured by the City of Hope Quality of Life Instruments for Patients or Caregivers; problem solving skills were measured by the Social Problem Solving Inventory-Revised.
Results
The results are reported using the CONSORT statement. The analytic data set included 476 dyads including 1596 patient data points and 1576 care giver data points. Patient QOL showed no significant difference in the rate of change between the intervention and usual care arms (p = 0.70). Caregiver QOL scores in the intervention arm declined, but at less than half the rate in the control arm (p = 0.02).
Conclusions
The COPE intervention enabled the average caregiver to come much closer to stable QOL over the 6-month follow-up. Future studies should enroll subjects much earlier in the cancer illness trajectory, a common patient/caregiver theme. The maximum effect was seen in caregivers who completed the 6-month follow-up, suggesting that the impact may increase over time.
Introduction
Patients with advanced cancer and their families experience significant distress in four domains: physical,1,2 psychological, social, and spiritual.3–5 These domains are often summarized by the term “quality of life.” High levels of distress are associated with increased health care utilization.6 Quality of life (QOL) and reduction of distress are recognized as important goals of cancer care.7–9 A systematic review emphasized the need for increased research to yield evidence-based strategies to guide end-of-life care.10
The detrimental effects of cancer extend to the patient's family and friends and may be particularly burdensome for primary caregivers. Psychological distress is well documented in patients with a wide variety of cancer types, stages and sites as well as in their caregivers.11–19 Patients' perception of distress may reflect problems in multiple domains.20,21 Patients with cancer report that quality of life may be related to treatment as well as psychosocial factors such as external stress and perceived support.22,23
Several barriers have been identified that impair efforts to improve the quality of life of patients with advanced cancer on investigational clinical trials and their caregivers: (1) patients and their physicians often focus on disease-directed investigational or ad hoc therapy, utilizing palliative care late in the disease trajectory or never24,25; (2) patients and families have widely variable problem-solving skills and face diverse emotional and situational challenges26; (3) QOL assessment and/or intervention is not universally integrated into practice or clinical research.10
Patients, families, and some health professionals experience cognitive dissonance when treatment focuses only on disease-directed therapy for cancer that is refractory to curative treatment while minimal attention is devoted to palliative care.24 Models integrating palliative care with disease-directed therapy reduce this cognitive dissonance.27,28 Houts and colleagues29 have described a cognitive/behavioral problem solving approach summarized by the acronym COPE (Creativity, Optimism, Planning and Expert information). A trial of the COPE intervention reported improvement in symptom management in the hospice setting.
We initiated a multisite randomized controlled trial of COPE, “Simultaneous Care Educational Intervention (SCEI): Linking Palliation and Clinical Trials.” Global QOL was the primary end point of the study. We measured problem-solving abilities as a secondary end point.
Study Design, Patient Population, and Statistics
Health educators were trained by an expert in the COPE model (M.L.). Sessions were videotaped and reviewed with the expert and educators between sites to increase intersite consistency of the intervention. Adults with relapsed, refractory, or recurrent solid tumors or lymphoma enrolled onto phase 1 or 2, or phase 3 trials that compared therapy for advanced cancer were eligible. These patients are among the sickest and most distressed cancer patients; clinical trial participation usually follows depleting conventional therapies or because few effective therapies exist for a given diagnosis. Advanced disease carries a higher symptom burden for patients and greater distress for caregivers. Although each of the participating cancer centers had a unique roster of eligible trials, the criteria for a qualifying phase 1, 2, or 3 clinical trial were the same across institutions. The eligible patient pool was stable (refractory, recurrent or metastatic disease) despite the variety of clinical trials; patients who went off their clinical trial continued to have advanced illness with high symptom burden, and were continued on the SCEI study.
Exclusion criteria included patients: receiving concomitant chemotherapy and radiation; on adjuvant phase III studies; with hematopoietic malignancies; with primary brain tumors; not fluent in English; less than 18 years of age or lacking a willing caregiver.
Patients designated one caregiver as their coparticipant. “Caregiver” was defined as an adult regularly involved with the patient and their care. Patients and caregivers signed informed consent to participate in SCEI. Participation stopped with the death of the patient, when a patient or caregiver requested or at the completion of study follow-up (6 months).
SCEI was presented as a complementary study to the therapeutic clinical trial, which acted as the standardized threshold for entry onto SCEI. Randomization assignments to SCEI or the control group were generated by the biostatistics office, using a three-to-one weighted randomization scheme, blocked by site. Randomization only occurred after consents were signed; the operations office was contacted to procure a unique patient identifier and determine intervention/control assignment.
Intervention
Intervention arm dyads received a copy of The Home Care Guide for Cancer.30 Each book chapter addresses a problem known to affect patients with cancer including physical symptoms (pain or nausea), psychological symptoms (anxiety or depression), or issues related to resources or relationships, including communicating with one's health care team or getting support or services from family, friends, and community organizations. Each chapter follows the same problem-solving formula.
Each educational session included the trained educator, the patient, and their designated caregiver. The first educational session was conducted up to 7 days prior to or on the day the patient started their investigational clinical trial. The first session focused on becoming familiar with the guide and the COPE problem-solving model, using COPE to address a patient or caregiver-identified problem. The two additional conjoint instructional sessions were conducted within the first 30 days, reinforcing this learning by focusing on two additional patient or caregiver-identified problems. Dyads could use one of the problems in the book's chapters, or identify another problem and apply the model. In either event, the instructors facilitated this process of using the Guide and the COPE model, being careful not to solve the problem for them. Following each session, the educator documented the problem and recorded process notes.
Outcome measures
The primary measurement tools were the City of Hope (COH) Quality of Life (QOL) Instruments for Patients or Caregivers31 and the Social Problem Solving Inventory-Revised.32,33
Patients and caregivers completed baseline psychometric evaluation and demographic information. Follow-up data collection was scheduled at 30, 60, 90, 120, and 180 days after randomization. The patient and caregiver were asked to complete the instruments independently.
The COH QOL Instrument (Cancer Patient/Cancer Survivor Version) is a 41-item ordinal scale that measures global QOL and four subdomains: physical, psychological, social, and spiritual well-being.34 The Family Version is a 37-item ordinal instrument that measures the QOL of a family member caring for a patient with cancer, adapted from the patient QOL tool, revised, and tested with 219 family caregivers of patients with cancer.35
The SPSI-R is a 52-item multidimensional measure of social problem-solving ability derived from a factor analysis of the original theory-driven Social Problem-Solving Inventory.32,36,37 In addition to a total score, it consists of five scales that measure two constructive dimensions (Positive Problem Orientation, Rational Problem Solving) and three dysfunctional dimensions (Negative Problem Orientation, Impulsivity/Carelessness Style, Avoidance Style). Tables for age standardization are included in the scoring manual. The SPSI-R has strong internal consistency, test-retest reliability and strong structural, concurrent, predictive, convergent, and discriminant validity.32
Data were verified at entry and at analysis, and audited for accuracy and Institutional Review Board (IRB) compliance. Summary scores for instruments and subscales were calculated according to the instructions for each instrument. Quality of life scores were rescaled from 0 (minimum possible) to 100 (maximum possible) to allow direct comparison of patients and caregivers.
This study was approved by the IRB at UC Davis and underwent IRB review at all collaborative sites.
Statistical analysis
We summarized baseline patient and caregiver data with univariate descriptive measures, using frequency tables for categorical variables and mean, standard deviation and percentiles for quantitative data. We compared the usual care group to SCEI patients and caregivers to ensure that randomization produced similar groups. All participants with at least one assessment were included in outcome analysis, using intent-to-treat criteria. We compared baseline QOL and problem solving scores for those who completed at least one follow-up with those who completed only baseline, to assess the effects of early attrition. To compare longitudinal change in outcome measures, we used random effects regression models,32 which differ from standard repeated measures analysis of variance (ANOVA), allowing the inclusion of participants with incomplete data.
The primary analysis fitted a linear trend over time for the usual care group (rate of change per month in QOL or problem solving) and tested whether there was an effect of the intervention to increase or decrease the rate of change (interaction between treatment and rate of change, comparison of slopes). To adjust for differences in baseline levels, we included a random effect for baseline score but not for rate of change, as the person-to-person variation in rate of change was not found to exceed the variation expected based on within-person variation. A secondary approach allowed for a post intervention difference that was constant over the follow-up period, rather than increasing with time since baseline. Each model was validated using residual diagnostics. Secondary analyses examined subscales of QOL and of problem solving. Additional analyses explored the effect of possible predictors such as age, relationship of caregiver, and gender. All hypothesis tests were two-sided at level 0.05, and all analyses were carried out using R38 and SAS/STAT® software.39
Initial sample size calculations were designed to have 80% power to detect a standardized difference between the SCEI group and controls of 0.22 standard deviations. We expected to enroll 1152 patients over a 4-year period, and assumed that 80% would complete at least one follow-up. With our actual accrual and drop-out before first follow-up, our power to detect a 0.22 effect size was between 0.48 and 0.51 for patients and caregivers, and to detect an effect size of 0.35 exceeded 80% for both groups.
A data safety monitoring committee (DSMC) met regularly over the 5 years of the study. Only one investigator, the statistician (Dr. Beckett) viewed the interim data with the DSMC.
Results
Accrual and demographics
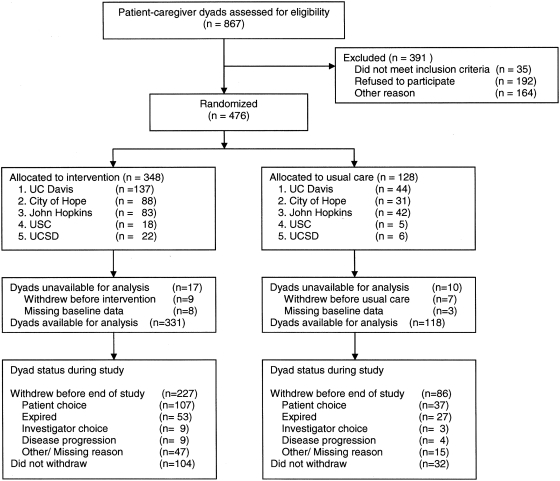
Accrual of patient/caregiver dyads began in February 2003 and was ended by the DSMC on October 1, 2007. The results are reported using the CONSORT statement40 (Fig. 1). Of 476 patient–caregiver dyads randomized to treatment (55% of screened dyads), 94% (449 dyads) had at least one participant complete an assessment, allowing inclusion in the primary outcome analysis. Thus 444 patients contributed a total of 1596 data time points (mean 3.6 data points per person); 165 patients completed data through the 6-month visit follow-up. Data were available on 446 caregivers with a total of 1576 observations (mean 3.5 data points per person; 162 caregivers completed the 6-month visit). The primary reasons for failure to complete the entire follow-up period were patient or caregiver withdrawal of consent (30% of usual care, 31% in SCEI) and death (21% of usual care, 15% in SCEI).
FIG. 1.
Study flow diagram. Numbers in individual analyses may differ because dyads with data available for analysis may not have had data on both patient and caregiver, or on both quality of life and problem-solving scales.
Patient (Table 1) and caregiver (Table 2) demographics are shown along with tumor type. (Table 3).
Table 1.
Demographics
| n = 441 Dyads Intervention 324 (73.5%) Control 117 (26.5%) |
|
|
|
|
|---|---|---|---|---|
| |
Patients |
Caregivers |
||
| Number | Percentage | Number | Percentage | |
| Gender | ||||
| Male | 196 | 44% | 302 | 68% |
| Female | 243 | 55% | 131 | 31% |
| Mean age | 61.5 years | 61.4 years | ||
| Race/ethnicity | ||||
| African American | 20 | 5% | 23 | 6% |
| Asian/Pacific Islander | 29 | 7% | 32 | 8% |
| Caucasian/Other | 381 | 88% | 338 | 85% |
| Native American/Indigenous | 3 | 1% | 5 | 1% |
| Hispanic (any race) | 41 | 9% | 31 | 8% |
| Education | ||||
| (K–12) | 155 | 35% | 142 | 32% |
| College | 170 | 39% | 209 | 47% |
| Graduate School | 105 | 24% | 82 | 19% |
| Unknown | 11 | 2% | 8 | 2% |
| Household income? | ||||
| <10K / year | 32 | 7% | ||
| 10–50 K | 188 | 41% | ||
| 50–80 K | 83 | 18% | ||
| >80 K | 104 | 29% | ||
| Missing data | 35 | 8% | ||
Table 2.
Caregiver Characteristics
| Relationship to patient | Number | Percentage |
| Spouse | 308 | 70%% |
| Child | 71 | 16% |
| Parent | 12 | 3% |
| Unrelated | 18 | 4% |
| Other | 24 | 5% |
| Employment status | Number | Percentage |
| Working full or part time | 117 | 49% |
| Retired | 136 | 31% |
| Not working at all | 79 | 20% |
| Hours of care per day/week | Number | Percentage |
| 1 hour | 133 | 31% |
| 2–4 hours | 105 | 24% |
| 5–9 hours | 57 | 13% |
| 10–14 hours | 29 | 7% |
| 15–20 hours | 14 | 3% |
| >20 hours | 90 | 21% |
| Born in the United States | Number | Percentage |
| Yes | 350 | 80% |
| No | 83 | 19% |
Table 3.
Cancer by Site
| Gastrointestinal | 120 | 28% |
| Genito-urinary | 113 | 27% |
| Thoracic | 88 | 21% |
| Breast | 41 | 10% |
| Gynecologic | 28 | 7% |
| Sarcoma | 6 | 1% |
| Melanoma | 5 | 1% |
| Other | 24 | 6% |
Baseline psychometric results
Baseline quality of life and problem solving skills were similar in the two study arms (Table 4). Mean QOL levels were about 8 percentage points higher for patients who had at least one follow-up than for those who did not complete any follow-ups (p < 0.001, analysis of variance), but did not differ significantly for caregivers. The QOL scores were slightly higher for patients than for their caregivers (p = 0.04) but varied widely on a standardized 0–100 point scale, with one quarter of patient scores above 75% and one quarter below 50%, with some extreme scores below 25% for patients and 30% for caregivers. Baseline problem solving skills did not differ for patients and caregivers, and were comparable to the general population.33
Table 4.
Baseline Quality of Life (on 0–100 scale) and Problem-Solving Skills
| |
Mean score (SD) |
p value for difference |
||
|---|---|---|---|---|
| Outcome measure | Usual care | SCEI intervention | Usual care vs SCEI | Patients vs. caregivers |
| QOL (0–100 scale) | ||||
| Patients | 64.4 (15.6) | 61.7 (15.2) | p = 0.11 | |
| Caregivers | 61.1 (13.5) | 62.6 (13.8) | p = 0.33 | p = 0.04 (usual care and SCEI combined) |
| Problem solving | ||||
| Patients | 106.2 (15.5) | 104.4 (13.7) | p = 0.37 | |
| Caregivers | 104.9 (13.6) | 106.8 (13.5) | p = 0.65 | p = 0.14 (usual care and SCEI combined) |
SD, standard deviation; SCEI, Simultaneous Care Educational Intervention.
Results of primary outcome analysis
QOL analysis
In random effects regression models, patients showed a modest but significant decline in overall QOL (based on estimated slope) of about half a percentage point per month on the 0–100 percent QOL scale (p < 0.001, Table 5), corresponding to approximately a 5-point (0.2 standard deviation [SD]) drop in QOL over 6 months compared to the mean level at baseline. There was no significant difference in the rate of change between the intervention and the usual care arms (p = 0.70).
Table 5.
Estimates of Effects of SCEI Intervention on Quality of Life and Problem-Solving Skills Based on Random Effects Models Assuming Constant Change per Month that May Differ Between Treatment Groups (primary hypothesis)
| Outcome measure | Model-based estimate | Standard error | p value: for parameter = 0 |
|---|---|---|---|
| Quality of life (0–100 scale) | |||
| Patients | |||
| Baseline level | 63.4 | 0.70 | <0.001 |
| Change per month in QOL, usual care group | −0.53 | 0.23 | 0.02 |
| Effect of SCEI on change/moa | −0.10 | 0.27 | 0.70 |
| Caregivers | |||
| Baseline level | 61.7 | 0.64 | <0.001 |
| Change per month in QOL, usual care group | −0.87 | 0.19 | <0.001 |
| Effect of SCEI on change/moa | 0.50 | 0.22 | 0.02 |
| Problem-solving skills | |||
| Patients | |||
| Baseline level | 104.6 | 0.69 | <0.001 |
| Change per month in problem solving, usual care | −0.53 | 0.21 | 0.01 |
| Effect of SCEI on change/moa | −0.04 | 0.24 | 0.86 |
| Caregivers | |||
| Baseline level | 106.5 | 0.65 | <0.001 |
| Change per month in problem solving, usual care | −0.23 | 0.21 | 0.28 |
| Effect of SCEI on change/moa | −0.30 | 0.24 | 0.21 |
Models allow for different baseline levels and length of follow-up.
Estimates of difference between rate of change per month under usual care and rate of change under SCEI intervention. Positive values indicate higher (more favorable) levels for the SCEI intervention, with slower decline in the outcome, while negative values indicate faster decline in the outcome compared to usual care.
SCEI, Simultaneous Care Educational Intervention; QOL, quality of life.
Caregivers also showed a significant decline in overall QOL, with scores that declined almost one point per month in the usual care arm (p < 0.001, Table 5). This would correspond to a drop of approximately 8.5% (0.4 SD) of baseline mean level over the six-month follow-up period. Caregiver QOL scores in the intervention arm also declined, but at less than half the rate of the control arm, a statistically significant difference (p = 0.02) with a predicted decline from baseline QOL of only 3.6 points at 6 months. Secondary analysis assuming a one-time impact on QOL rather than a difference in rate of decline showed a difference but did not find it statistically significant, suggesting that the impact may increase over time as our primary model proposed. Secondary analyses did not find evidence of outcome differences between centers, or of violation of the assumptions of normality of the residuals.
SPSI-R problem solving
Patients showed a decline in problem solving skills of about half a point per month (p = 0.01, Table 5). Caregivers did not show any significant changes in problem solving skills over the course of the study in either the usual care or intervention arms (Table 5).
Secondary end points
QOL subdomains
Subscale analysis for the patients showed significant declines in the psychological and social domains but no significant changes in physical or spiritual domains (Table 6). There were no differences between the intervention and the usual care arms.
Table 6.
Estimates of Effects of SCEI Intervention on Quality of Life Subscales Based on Random Effects Models Assuming Constant Change per Month that May Differ Between Treatment Groups (primary hypothesis)
| Outcome: quality of life subscales | Model-based estimate | Standard error | p value: for parameter = 0 |
|---|---|---|---|
| Psychological | |||
| Change per month, patients, usual care group | −0.27 | 0.08 | 0.001 |
| Effect of SCEI on patient changea | 0.02 | 0.09 | 0.82 |
| Change/mo, caregivers, usual care | −0.79 | 0.37 | 0.03 |
| Effect of SCEI on caregiver changea | 0.59 | 0.42 | 0.16 |
| Social | |||
| Change/mo, patients, usual care | −0.15 | 0.06 | 0.01 |
| Effect of SCEI on patient changea | −0.02 | 0.07 | 0.81 |
| Change/mo, caregivers, usual care | −1.30 | 0.24 | <0.001 |
| Effect of SCEI on caregiver changea | 0.46 | 0.27 | 0.09 |
| Physical | |||
| Change/mo. patients, usual care | −0.04 | 0.10 | 0.72 |
| Effect of SCEI on patient change | −0.00 | 0.11 | 0.97 |
| Change/mo. caregivers, usual care | −0.23 | 0.16 | 0.14 |
| Effect of SCEI on caregiver changea | 0.09 | 0.18 | 0.61 |
| Spiritual | |||
| Change/mo, patients, usual care | −0.05 | 0.08 | 0.54 |
| Effect of SCEI on patient changea | −0.06 | 0.09 | 0.47 |
| Change/mo, caregivers, usual care | −0.91 | 0.18 | <0.001 |
| Effect of SCEI on caregiver changea | 0.76 | 0.21 | <0.001 |
Models allow for different baseline levels and length of follow-up.
Estimates of difference between rate of change per month under usual care and rate of change under SCEI intervention. Positive values indicates (more favorable) levels for the SCEI intervention, with slower decline in the outcome, while negative values indicate faster decline in the outcome compared to usual care.
SCEI, Simultaneous Care Educational Intervention.
Subscale analysis for the caregivers showed significant decline in psychological, social, and spiritual domains, with declines of about a point per month in each area, but no change in physical well-being (Table 6). The decline of almost a point per month for spiritual well-being in the usual care group (p < 0.001) was nearly completely canceled out in the SCEI group (p < 0.001). Psychological well-being declined approximately 0.8 points per month in the usual care group (p = 0.03), compared to a decline of 0.2 points per month in the SCEI group (p = 0.16). Social well-being declined over a point per month in the usual care group (p < 0.001); SCEI participants scored about one third higher, with a trend for significance (p = 0.09). In secondary models hypothesizing a one-time effect of the SCEI intervention rather than a continuous gain over time, the caregivers in the SCEI group showed significant impact for psychological, social, and spiritual QOL.
Problem-solving subscales
In patients, positive and rational problem solving approaches decreased and avoidance approaches showed a trend for increasing over time (p = 0.009, 0.01, 0.053, respectively, Table 7). Caregivers showed a decrease in rational problem solving skills (p = 0.04, Table 7). There were no significant differences between arms, suggesting that the relatively better psychosocial QOL trajectories for caregivers in the COPE intervention arm are likely not a direct result of improved problem solving skills.
Table 7.
Estimates of Effects of SCEI Intervention on SPSI-R Subscale Measures During Follow-Up, Based on Random Effects Models Assuming Constant Change per Month that may Differ between Treatment Groups (primary hypothesis)
| Outcome: problem solving subscales | Parameter estimate | Standard error | p value: for parameter = 0 |
|---|---|---|---|
| Positive approaches | |||
| Change/mo, patients, usual care | −0.79 | 0.30 | 0.009 |
| Intervention effect on patient changea | −0.09 | 0.34 | 0.80 |
| Change/mo, caregivers, usual care | −0.45 | 0.30 | 0.13 |
| Intervention effect on caregiver changea | −0.47 | 0.24 | 0.17 |
| Rational approaches | |||
| Change/mo, patients, usual care | −0.68 | 0.27 | 0.01 |
| Intervention effect on patient changea | 0.05 | 0.31 | 0.88 |
| Change/mo, caregivers, usual care | −0.56 | 0.27 | 0.04 |
| Intervention effect on caregiver changea | −0.30 | 0.31 | 0.33 |
| Avoidance | |||
| Change/mo, patients, usual care | 0.41 | 0.21 | 0.053 |
| Intervention effect on patient changeb | −0.07 | 0.24 | 0.77 |
| Change/mo, caregivers, usual care | 0.02 | 0.21 | 0.91 |
| Intervention effect on caregiver changeb | 0.38 | 0.24 | 0.12 |
| Impulsivity | |||
| Change/mo, patients, usual care | 0.17 | 0.22 | 0.44 |
| Intervention effect on patient changeb | 0.09 | 0.25 | 0.72 |
| Change/mo, caregivers, usual care | 0.37 | 0.22 | 0.09 |
| Intervention effect on caregiver changeb | −0.31 | 0.25 | 0.22 |
| Negative approaches | |||
| Change/mo, patients, usual care | −0.08 | 0.21 | 0.72 |
| Intervention effect on patient changeb | 0.10 | 0.24 | 0.68 |
| Change/mo, caregivers, usual care | −0.31 | 0.21 | 0.14 |
| Intervention effect on caregiver changeb | 0.12 | 0.24 | 0.62 |
Models allow for different baseline levels and length of follow-up.
Positive values indicate higher (more favorable) levels for the SCEI intervention.
Positive values indicate less favorable levels (more dysfunctional problem solving) for the SCEI intervention.
SCEI, Simultaneous Care Educational Intervention; SPSI-R, Social Problem Solving Inventory-Revised.
Discussion
This trial showed a moderately statistically significant impact of the COPE intervention on QOL for caregivers, but not for patients. The impact would correspond to about a one-point overall improvement on the measurement scale on most of the psycho-social questions during a 6-month follow-up, which translates into almost no decline in QOL rather than a significant decline in QOL over 6 months. An intervention that responds to the caregiver addresses the reduced confidence in caregiver ability to manage the illness and the perception of decreased support as cancer progresses.41
Is the statistically significant finding clinically significant? One proposal is that clinical significance be equal to ½ SD or more of effect size.42 Our effect size is a decrease of about 0.3 SD (relative to baseline variation) in the 6-month decline in overall QOL for caregivers, with somewhat larger effects in the psychosocial subdomains, consistent with a moderately clinically significant impact. The PROMIS investigators43 are working toward a consensus view on clinical significance in QOL trials.
Poorer problem solving of emotional and situational challenges by patients and family members is associated with diminished QOL.44 Certainly the increased and highly complex burdens that caregivers report20,45,46 and the risks to caregivers47–50 underscore the importance of this group as a target.51 A different dosing of the problem solving intervention or an additional intervention may be necessary for the patients with more dysfunctional problem-solving styles.52
The limitations of this study include slower than anticipated accrual, turnover in trainers that might affect the educational intervention, limited enrollment of ethnic minorities, and the English language requirements.
The impact of COPE in our study increased over time, reinforcing our view that introducing COPE at initial diagnosis has the potential to increase the benefit of the intervention,23 especially before the presence of highly distressing physical symptoms.53,54
Acknowledgments
Supported by National Institutes of Health/National Cancer Institute (NIH/NCI) grant R25 95260 and by grant number UL1 RR024146 from the National Center for Research Resources (NCRR), a component of the NIH, and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bucher JA. Loscalzo M. Zabora J. Houts PS. Hooker C. BrintzenhofeSzoc K. Problem-solving cancer care education for patients and caregivers. Cancer Pract. 2001;9:66–70. doi: 10.1046/j.1523-5394.2001.009002066.x. [DOI] [PubMed] [Google Scholar]
- 2.Portenoy RK. Thaler HT. Kornblith AB. Lepore JM. Friedlander-Klar H. Kiyasu E. Sobel K. Coyle N. Kemeny N. Norton L, et al. The Memorial Symptom Assessment Scale: An instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30A:1326–1336. doi: 10.1016/0959-8049(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 3.Carlson LE. Angen M. Cullum J. Goodey E. Koopmans J. Lamont L. MacRae JH. Martin M. Pelletier G. Robinson J. Simpson JS. Speca M. Tillotson L. Bultz BD. High levels of untreated distress and fatigue in cancer patients. Br J Cancer. 2004;90:2297–2304. doi: 10.1038/sj.bjc.6601887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlson LE. Bultz BD. Benefits of psychosocial oncology care: Improved quality of life and medical cost offset. Health Qual Life Outcomes. 2003;1:8. doi: 10.1186/1477-7525-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zabora J. BrintzenhofeSzoc K. Curbow B. Hooker C. Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10:19–28. doi: 10.1002/1099-1611(200101/02)10:1<19::aid-pon501>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 6.Feuerstein M. Optimizing cancer survivorship. J Cancer Survivorship. 2007;1:1–4. doi: 10.1007/s11764-006-0001-y. [DOI] [PubMed] [Google Scholar]
- 7.Gotay CC. Assessing cancer-related quality of life across a spectrum of applications. J Natl Cancer Inst Monogr. 2004:126–133. doi: 10.1093/jncimonographs/lgh004. [DOI] [PubMed] [Google Scholar]
- 8.Jacobsen PB. Screening for psychological distress in cancer patients: Challenges and opportunities. J Clin Oncol. 2007;25:4526–4527. doi: 10.1200/JCO.2007.13.1367. [DOI] [PubMed] [Google Scholar]
- 9.National Intitutes of Health: National Institutes of Health State-of-the-Science Conference: Statement on Improving End-of-Life Care Paper presented at: State-of-the-Science Conference. Improving End-of-Life Care. 2004.
- 10.Lorenz KA. Lynn J. Dy S. Mularski RA. Shugarman LR. Hughes R. Asch SM. Rolon C. Rastegar A. Shekelle PG. Quality measures for symptoms and advance care planning in cancer: A systematic review. J Clin Oncol. 2006;24:4933–4938. doi: 10.1200/JCO.2006.06.8650. [DOI] [PubMed] [Google Scholar]
- 11.Elting LS. Avritscher EBC. Cooksley CD. Cardenas-Turanzas M. Garden AS. Chambers MS. Psychosocial and Economic Impact of Cancer. Dent Clin North Am. 2008;52:231–252. doi: 10.1016/j.cden.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Kim Y. Kashy D. Wellisch D. Spillers R. Kaw C. Smith T. Quality of Life of couples dealing with cancer: Dyadic and individual adjustment among breast and prostate cancer survivors and their spousal caregivers. Ann Behav Med. 2008;35:230–238. doi: 10.1007/s12160-008-9026-y. [DOI] [PubMed] [Google Scholar]
- 13.Eton DT. Lepore SJ. Helgeson VS. Psychological distress in spouses of men treated for early-stage prostate carcinoma. Cancer. 2005;103:2412–2418. doi: 10.1002/cncr.21092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen M-L. Chu L. Chen H-C. Impact of cancer patients' quality of life on that of spouse caregivers. Support Care Cancer. 2004;12:469–475. doi: 10.1007/s00520-004-0636-z. [DOI] [PubMed] [Google Scholar]
- 15.Braun M. Mikulincer M. Rydall A. Walsh A. Rodin G. Hidden morbidity in cancer: Spouse caregivers. J Clin Oncol. 2007;25:4829–4834. doi: 10.1200/JCO.2006.10.0909. [DOI] [PubMed] [Google Scholar]
- 16.Ko CM. Malcarne VL. Varni JW. Roesch SC. Banthia R. Greenbergs HL. Sadler GR. Problem-solving and distress in prostate cancer patients and their spousal caregivers. Support Care Cancer. 2005;13:367–374. doi: 10.1007/s00520-004-0748-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carmack Taylor C. Badr H. Lee J. Fossella F. Pisters K. Gritz ER. Schover L. Lung cancer patients and their spouses: Psychological and relationship functioning within 1 month of treatment initiation. Ann Behav Med. 2008;36:129–140. doi: 10.1007/s12160-008-9062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawes S. Malcarne V. Ko C. Sadler G. Banthuia R. Sherman S. Varni J. Schmidt J. Identifying problems faced by spouses and partners of patients with prostate cancer. Oncol Nurs Forum. 2006;33:807–814. doi: 10.1188/06.ONF.807-814. [DOI] [PubMed] [Google Scholar]
- 19.Northouse LL. Mood D. Templin T. Mellon S. George T. Couples' patterns of adjustment to colon cancer. Soc Sci Med. 2000;50:271–284. doi: 10.1016/s0277-9536(99)00281-6. [DOI] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Distress Management. National Comprehensive Cancer Network. 2009.
- 21.Velikova G. Stark D. Selby P. Quality of life instruments in oncology. Eur J Cancer. 1999;35:1571–1580. doi: 10.1016/s0959-8049(99)00193-8. [DOI] [PubMed] [Google Scholar]
- 22.Lehto US. Ojanen M. Kellokumpu-Lehtinen P. Predictors of quality of life in newly diagnosed melanoma and breast cancer patients. Ann Oncol. 2005;16:805–816. doi: 10.1093/annonc/mdi146. [DOI] [PubMed] [Google Scholar]
- 23.Talcott JA. Prostate cancer quality of life: Beyond initial treatment and the patient. J Clin Oncol. 2007;25:4155–4156. doi: 10.1200/JCO.2007.12.1996. [DOI] [PubMed] [Google Scholar]
- 24.Earle CC. Cancer survivorship research and guidelines: Maybe the cart should be beside the horse. J Clin Oncol. 2007;25:3800–3801. doi: 10.1200/JCO.2007.12.2325. [DOI] [PubMed] [Google Scholar]
- 25.Freireich EJ. Kurzrock R. The role of investigational therapy in management of patients with advanced metastatic malignancy. J Clin Oncol. 2009;27:304–306. doi: 10.1200/JCO.2008.19.6543. [DOI] [PubMed] [Google Scholar]
- 26.Ko CM. Malcarne VL. Varni JW. Roesch SC. Banthia R. Greenbergs HL. Sadler GR. Problem-solving and distress in prostate cancer patients and their spousal caregivers. Support Care Cancer. 2005;13:367–374. doi: 10.1007/s00520-004-0748-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrington SE. Smith TJ. The role of chemotherapy at the end of life: “When is enough, enough?”. JAMA. 2008;299:2667–2678. doi: 10.1001/jama.299.22.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Temel J. Greer J. Muzikansky A. Gallagher ER. Admane S. Jackson VA. Dahlin CM. Blinderman CD. Jacobsen J. Pirl WF. Billings JA. Lynch TJ. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 29.McMillan SC. Interventions to facilitate family caregiving at the end of life. J Palliat Med. 2005;8(Suppl 1):S132–139. doi: 10.1089/jpm.2005.8.s-132. [DOI] [PubMed] [Google Scholar]
- 30.Houts PS, editor. Home Care Guide for Cancer: How to Care for Family and Friends At Home. Philadelphia: American College of Physicians; 1994. [Google Scholar]
- 31.City of Hope Pain & Palliative Care Resource Center: Web-based resources including quality of life measures for patients and caregivers. Jul 9, 2009. http://prc.coh.org/ [Jul 26;2009 ]. http://prc.coh.org/
- 32.D'Zurilla T. Nezu A. Maydeu-Olivares A. Social Problem-Solving Inventory—Revised (SPSI-R) North Tonawanda, NY: Multi-Health Systems; 2002. [Google Scholar]
- 33.D'Zurilla T. Nezu A. Maydeu-Olivares A. Social Problem-Solving Inventory - Revised (SPSI-R) SPSI-R homepage. www.mhs.com/ecom/(qcrndheqtanmkqyzvtis4vjk)/product.aspx?RptGrpID = SPS. [Mar 1;2009 ]. www.mhs.com/ecom/(qcrndheqtanmkqyzvtis4vjk)/product.aspx?RptGrpID = SPS
- 34.Ferrell BR. Dow KH. Grant M. Measurement of the quality of life in cancer survivors. Qual Life Res. 1995;4:523–531. doi: 10.1007/BF00634747. [DOI] [PubMed] [Google Scholar]
- 35.Juarez G. Ferrell B. Uman G. Podnos Y. Wagman LD. Distress and quality of life concerns of family caregivers of patients undergoing palliative surgery. Cancer Nurs. 2008;31:2–10. doi: 10.1097/01.NCC.0000305682.13766.c2. [DOI] [PubMed] [Google Scholar]
- 36.D'Zurilla T. Nezu A. Development and preliminary evaluation of the Social Problem-Solving Inventory. Psychol Assess. 1990;2:156–163. [Google Scholar]
- 37.D'Zurilla T. Nezu A. Problem-solving therapy: A positive approach to clinical intervention. www.loc.gov/catdir/toc/ecip0615/2006018590.html. [Mar 1;2009 ]. www.loc.gov/catdir/toc/ecip0615/2006018590.html
- 38.Vienna, Austria: R Foundation for Statistical Computing; 2006. R Foundation for Statistical Computing: A Language and Environment for Statistical Computing [computer program]. Version. [Google Scholar]
- 39.Cary, NC: SAS Institute; 2004. SAS/STAT [computer program]. Version 9.0. [Google Scholar]
- 40.Boutron I. Moher D. Altman DG. Schulz KF. Ravaud P. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008;148:295–309. doi: 10.7326/0003-4819-148-4-200802190-00008. [DOI] [PubMed] [Google Scholar]
- 41.Northouse LL. Mood DW. Montie JE. Sandler HM. Forman JD. Hussain M. Pienta KJ. Smith DC. Sanda MG. Kershaw T. Living with prostate cancer: Patients' and spouses' psychosocial status and quality of life. J Clin Oncol. 2007;25:4171–4177. doi: 10.1200/JCO.2006.09.6503. [DOI] [PubMed] [Google Scholar]
- 42.Sloan JA. Cella D. Hays RD. Clinical significance of patient-reported questionnaire data: Another step toward consensus. J Clin Epidemiol. 2005;58:1217–1219. doi: 10.1016/j.jclinepi.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 43.Garcia SF. Cella D. Clauser SB. Flynn KE. Lad T. Lai JS. Reeve BB. Smith AW. Stone AA. Weinfurt K. Standardizing patient-reported outcomes assessment in cancer clinical trials: A patient-reported outcomes measurement information system initiative. J Clin Oncol. 2007;25:5106–5112. doi: 10.1200/JCO.2007.12.2341. [DOI] [PubMed] [Google Scholar]
- 44.Banthia R. Malcarne VL. Varni JW. Ko CM. Sadler GR. Greenbergs HL. The effects of dyadic strength and coping styles on psychological distress in couples faced with prostate cancer. J Behav Med. 2003;26:31–52. doi: 10.1023/a:1021743005541. [DOI] [PubMed] [Google Scholar]
- 45.Adler N, editor; Page A, editor. Cancer Care for the Whole Patient: Meeting psychosocial health needs. Washington, D.C: National Academies Press; 2008. [PubMed] [Google Scholar]
- 46.Glajchen M. The emerging role and needs of family caregivers in cancer care. J Support Oncol. 2004;2:145–155. [PubMed] [Google Scholar]
- 47.Braun M. Mikulincer M. Rydall A. Walsh A. Rodin G. Hidden morbidity in cancer: Spouse caregivers. J Clin Oncol. 2007;25:4829–4834. doi: 10.1200/JCO.2006.10.0909. [DOI] [PubMed] [Google Scholar]
- 48.O'Rourke N. Tuokko H. The psychological and physical costs of caregiving: The Canadian Study of Health and Aging. J Appl Gerontol. 2000;19:389–404. [Google Scholar]
- 49.Schulz R. Beach SR. Caregiving as a risk factor for mortality: The caregiver health effects study. JAMA. 1999;282:2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 50.Nijboer C. Tempelaar R. Sanderman R. Triemstra M. Spruijt RJ. van den Bos GA. Cancer and caregiving: The impact on the caregiver's health. Psychooncology. 1998;7:3–13. doi: 10.1002/(SICI)1099-1611(199801/02)7:1<3::AID-PON320>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 51.Lutgendorf SK. Laudenslager ML. Care of the caregiver: Stress and dysregulation of inflammatory control in cancer caregivers. J Clin Oncol. 2009;27:2894–2895. doi: 10.1200/JCO.2009.22.1523. [DOI] [PubMed] [Google Scholar]
- 52.Hodges LJ. Humphris GM. Macfarlane G. A meta-analytic investigation of the relationship between the psychological distress of cancer patients and their carers. Soc Sci Med. 2005;60:1–12. doi: 10.1016/j.socscimed.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 53.Feuerstein M. Handbook of Cancer Survivorship. www.loc.gov/catdir/toc/fy0707/2006929207.html. [Mar 1;2009 ]. www.loc.gov/catdir/toc/fy0707/2006929207.html
- 54.McMillan SC. Small BJ. Weitzner M. Schonwetter R. Tittle M. Moody L. Haley WE. Impact of coping skills intervention with family caregivers of hospice patients with cancer: A randomized clinical trial. Cancer. 2006;106:214–222. doi: 10.1002/cncr.21567. [DOI] [PubMed] [Google Scholar]