Abstract
Background
When a patient is expected to die, the ideal plan of care focuses on comfort. Prior investigation of application of one institution's end-of-life symptom management order (ESMO) protocol suggested that comfort measures were often instituted too late and sometimes not at all. We studied patient factors associated with missed opportunities for use of an ESMO protocol and protocol adherence in order to identify areas for quality improvement.
Methods
We abstracted the terminal hospitalization medical record for all adult decedents hospitalized for at least 3 days between April 2005 and April 2006 (n = 496) at a university medical center. Detailed information was collected about ESMO use and opiate titration at the end of life. Among patients expected to die, we used multivariate logistic regression to evaluate factors associated with whether patients were placed on the ESMO protocol prior to death.
Results
Half of patients who died received ESMO protocol care (n = 248). All had documentation of a do-not-resuscitate (DNR) order (a requirement of the protocol). An opiate drip was used for 95% of patients placed on the ESMO protocol and it was titrated up at least once for 67% of those patients. Patients had a mean of 4 opiate titrations, but for only a mean of 2.2 was a justification documented (symptom documentation is required for each titration per the protocol). In a multivariable regression accounting for other demographic, clinical and provider variables, uninsured patients (risk ratio [RR] 0.25, 95% confidence interval [CI] 0.06–0.62), patients admitted from a nursing home (RR 0.57, 95% CI 0.30–0.99), and patients considered for transplant (RR 0.60, 95% CI 0.40–0.85) were significantly less likely to be placed on the ESMO protocol prior to death.
Conclusions
Evaluation of implementation of a standardized order set can identify areas for quality improvement and missed opportunities for use.
Introduction
Patients dying in hospitals have significant unmet needs for symptom management and communication.1 In response to detected deficiencies, our institution developed and implemented an end-of-life symptom management order (ESMO) protocol in 2004 to improve symptom management at the very end of life2 for patients receiving comfort-oriented care. Evaluation of the implementation of the ESMO protocol by interviewing physicians and nurses suggested that comfort measures were instituted late one quarter of the time and most patients had a life expectancy of less than 1 day.3
When cure is no longer possible and patients are anticipated to be close to death, the best plan of care often focuses on comfort. Unfortunately, there are many barriers to the transition from curative to comfort care.4 Breaking bad news and end-of-life communication are challenging technical skills that are time consuming and can be emotionally difficult.5–8 Physicians also are often concerned about taking away hope from a patient with terminal illness.9 In addition, physicians often struggle with providing an accurate prognosis and evidence suggests that prognoses are systematically overestimated.10
Patients, like clinicians, are also often uncomfortable bringing up end-of-life issues. Often when these difficult decisions and clinical transitions are needed, the patient is quite sick and lacks the capacity to participate in end-of-life decision-making.11–13 In these situations, physicians and surrogate decision makers may not be aware of patient preferences, making decisions more difficult.14,15
Some findings suggest that the withholding and withdrawal of life-sustaining therapies is correlated with physician predictions of survival.16,17 However, it is not known what factors are related to whether patients who are expected to die receive comfort oriented care before death. We hypothesized that comfort-oriented care was less likely to be provided for certain vulnerable patient populations. For example, patients and families may be more resistant to make this transition among younger patients.
In order to identify quality improvement targets, we studied a decedent sample using medical record abstraction to describe use of the protocol (including protocol adherence, timing of the protocol initiation, and dosing of opiates) and to identify patient and provider characteristics associated with potential missed opportunities for use of the ESMO protocol.
Methods
We abstracted the complete medical record (including the hard copy record, a partial electronic medical record and a nursing electronic record) pertaining to the terminal hospitalization for all adult patients who died over 1 year (April 2005 to April 2006) in a tertiary academic medical center who were hospitalized for at least 3 days. We did not include hospitalizations that were fewer than 3 days to exclude patients who died quickly upon admission with limited time to transition to comfort measures. Of 586 adults who died, 86 died less than 3 days after admission. Of the 500 adult decedents who died after 3 or more days, complete terminal hospitalization medical records were available for 496 (99%), who constitute the study sample.
Medical record abstraction form and ESMO protocol evaluation
A medical record abstraction tool was designed to collect detailed information about the use of the ESMO protocol as well as patient and provider characteristics for the decedent sample. For each patient, we collected whether the ESMO protocol was used. If it was used, we collected the timing of initiation of the protocol, whether an opiate continuous infusion was used and titrated for symptoms, and the highest dose of the continuous opiate infusion. In addition, we evaluated adherence to the ESMO protocol components. Prior to initiation of the ESMO, the protocol requires that a patient have a do-not-resuscitate (DNR) order and documentation that the plan of comfort-oriented care is consistent with the patient's prognosis and goals of care. Each titration of the opiate drip must be justified by symptom documentation.
We also collected patient characteristics including demographics (age, gender, race, ethnicity, primary insurance, religion), social support variables (marital status, preadmission living arrangement), and clinical variables (presence of end-stage disease, presence of dementia, mental status on admission, consideration for transplant, history of outpatient opiate use). Because we were unable to identify individual physicians responsible for a specific patient due to the team-based approach to inpatient care, we collected the patient's primary service during the hospitalization. Presence of preference discussions about end-of-life issues and information about advance directives and surrogate decision-makers also was collected. Last, we collected whether there was medical record documentation that a patient's death was expected. We defined “expected death” as any physician documentation that the patient was terminal, had a grave prognosis, was receiving hospice care, had imminently life-threatening disease in the context of a poor prognosis, or was dying. The specific criteria were reliably abstracted from the medical record (reabstraction κ 0.67, 91% agreement).
Medical record abstraction
Experienced nurse abstractors were trained to use the abstraction tool following a previously used method, which includes intensive training, tandem abstraction and comparison, and reliable abstraction of five testing charts.18 Abstractors participated in bimonthly meetings with discussion of questions and updating of guidelines. A list of frequently asked questions and checklist for efficient abstraction guided abstraction. We completed a 10% reabstraction to assess interrater reliability. The pooled κ statistic for the 14 variables that were abstracted from the medical record and included in the final model was 0.86 suggesting very good agreement. Race and ethnicity were obtained from administrative data.
Statistical analysis
Among all patients identified as dying an expected death, we first compared patients receiving the ESMO to those who did not using bivariate logistic regression and then in a multivariate hypothesis-driven model controlling for other variables to identify patient characteristics and provider service associated with use and nonuse of the protocol. Forward and backward selection multivariate models found identical significant variables. We tested for prespecified interactions between age and all other variables, race and insurance status, ethnicity and insurance status, nursing home and insurance status, presence of dementia and mental status on admission, and consideration of transplant and presence of end-stage disease/end-stage cancer on admission. In order to test the robustness of the results, we performed a sensitivity analysis repeating the model using a different time cutoff for the definition of expected death, and including a variable indicating that a patient had a discussion about preferences within 48 hours of admission documented in the medical record and a variable indicating that a patient had a preference for less aggressive care documented in the medical record within 48 hours of admission. We calculated risk ratios for the final model and used bootstrapping methods (1000 replicates) to calculate bias-corrected empirical 95% confidence intervals.
Results
Among the 496 persons in the decedent sample, 424 patients died an expected death and the ESMO protocol was employed for 236 (56%) of these patients. The mean time between initiation of the ESMO protocol and death was 1.1 days (standard deviation [SD] 2.1; range, 0–21].
The mean age for decedents dying an expected death was 62 years (SD 18). Forty-seven percent of the sample were female and 60% were married. Seventy-eight percent of the sample was white and the majority of the sample was white, non-Hispanic (62%) and had Medicare or private insurance (86%). Fifty-five percent of the sample had end stage disease on admission. Twenty-five percent of the sample was considered for transplant at some point during the terminal hospitalization.
Comparing patients who received and did not receive the ESMO protocol prior to death showed in bivariate analyses that those not placed on the ESMO protocol were more often younger, minority race, considered for transplant during the hospitalization, had end-stage disease on admission, had Medicaid insurance or were uninsured, and were admitted to the surgery service (Table 1).
Table 1.
Description of Patients Who Died an Expected Death, by Receipt of End-of-Life Symptom Management Protocola
| n = 424 | ESMO (n = 236) | No ESMO (n = 188) |
|---|---|---|
| Age in years, mean (standard deviation) | 65.3b (16.9) [range 19–97] | 58.5b (17.8) [range 24–105] |
| Male | 52% | 53% |
| White | 81%b | 73%b |
| Hispanic | 14% | 19% |
| Married | 61% | 59% |
| Nursing home prior to admin | 5% | 9% |
| Primary insurance | ||
| Private | 47% | 44% |
| Any Medicare | 44% | 36% |
| Medicaid only | 6%b | 12%b |
| Uninsured | 2%b | 6%b |
| Other | 1% | 3% |
| Religion | ||
| Christian | 27% | 27% |
| Catholic | 29% | 36% |
| Jewish | 11% | 8% |
| None | 21% | 15% |
| Other | 12% | 14% |
| Transplant considered | 14%b | 39%b |
| AD within 48 hours of admission | 19% | 12% |
| Outpatient opiates | 24% | 19% |
| Conditions on admission | ||
| End-stage cancer | 25% | 18% |
| Dementia | 5% | 5% |
| Other end-stage disease | 27%b | 48%b |
| Coma | 15% | 10% |
| Service on admission | ||
| Medicine service | 59% | 57% |
| Surgery service | 20%b | 35%b |
| Neuro service | 22%b | 9%b |
Of 496 decedents, 424 died an expected death.
p < 0.05 in bivariate logistic regression.
ESMO, End-of-Life Symptom Management Protocol; AD, advance directive.
In multiple logistic regression, younger patients were less likely to receive the ESMO protocol prior to death—each year of age is associated with a 2% increase in the likelihood of receiving the ESMO protocol—(RR 1.02, 95% CI 1.00–1.04), patients being considered for transplant during hospitalization were 40% less likely (compared to patients who were not; RR 0.60, 95% CI 0.40–0.85) and uninsured patients were 75% less likely (compared to patients with private insurance; RR 0.25, 95% CI 0.06–0.62) to receive the ESMO protocol prior to death. Living in a nursing home prior to admission to the hospital also was associated with not receiving the ESMO protocol prior to death. Patients admitted to the hospital from a nursing home were 43% less likely to receive the protocol compared to those who were not, controlling for the other covariates (RR 0.57, 95% CI 0.30–0.99). If admitted to a neurological service, most commonly the neurology critical care unit, a patient was more likely to receive the ESMO protocol compared to those admitted to the Medicine service (RR 1.48, 95% CI 1.04–2.01; Table 2).
Table 2.
Logistic Regression Predicting Patient Placed on End-of-Life Symptom Management Protocol
| n = 424 | Relative risk | Bias corrected 95% confidence interval |
|---|---|---|
| Age in years | 1.019a | (1.003–1.040) |
| Male | 0.985 | (0.768–1.181) |
| Non-white | 0.844 | (0.620–1.122) |
| Hispanic | 1.025 | (0.735–1.395) |
| Married | 1.067 | (0.858–1.352) |
| Nursing home admit | 0.571a | (0.298–0.989) |
| Transplant considered | 0.603a | (0.402–0.852) |
| Primary insurance | ||
| Private (reference) | ||
| Uninsured | 0.249a | (0.064–0.623) |
| Service on Admission | ||
| Medicine (reference) | ||
| Surgery service | 0.952 | (0.721–1.300) |
| Neuro service | 1.477a | (1.037–2.010) |
p < 0.05 in Logistic Regression. Hosmer and Lemeshow Goodness of Fit Test (χ2 8.8, df 8, p value 0.36), ROC = 0.751, Somers' d = 0.502, γ = 0.503.
Other covariates in this model that were not significant include advance directive within 48 hours of admission, dementia on admission, end-stage cancer on admission, other end-stage disease on admission, use of outpatient opiates, mental status on admission, Medicare insurance and Medicaid insurance, and religion.
Relative risk estimates were calculated using Stata v. 9 and bias corrected 95% confidence intervals were calculated using bootstrapping methods.
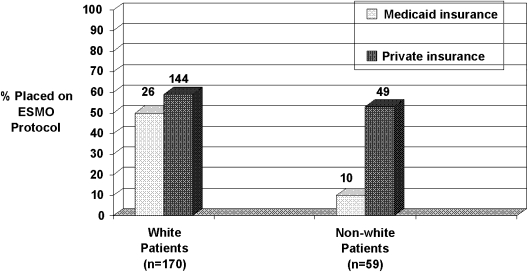
None of the tested interactions was significant in the model, except race and Medicaid insurance. The difference in ESMO protocol use between Medicaid and private insurance is much lower for white patients than non-white patients (Fig. 1). It should be noted that there were only 10 non-white patients who were expected to die and had Medicaid insurance; only one of them was placed on the ESMO protocol. Inclusion of this interaction in the model did not substantively affect the point estimates or p values for the variables in the model. In an additional analysis, we excluded four influential observations, which did not substantively change the model.
FIG. 1.
Interaction between race and Medicaid insurance: the difference in end-of-life symptom management order (ESMO) protocol use between Medicaid and private insurance is much lower for white patients than non-white patients. Of 26 white patients who have Medicaid insurance, 13 were placed on the EMSO protocol. Of 144 white patients who have private insurance, 85 were placed on the EMSO protocol. Of 10 non-white patients who have Medicaid insurance, only 1 was placed on the EMSO protocol. Of 49 non-white patients who have private insurance, 26 were placed on the EMSO protocol.
Sensitivity analyses
Among the 424 decedents identified as having an expected death at some point prior to death, 235 (47%) had documentation of an expected death at least 3 days prior to death. Repeating the multivariable model in this smaller sample found similar results, although some findings were no longer statistically significant due to restricted sample size.
Whether or not a patient had a preference discussion documented within 48 hours of admission also was evaluated. Although patients who were eventually placed on the ESMO protocol were more likely to have a preference discussion documented early in the hospitalization (39% versus 28%, p < 0.05), this variable was not significant in multivariate regression and did not change the odds ratios or significance levels for other variables in the model.
Similarly, we tested inclusion of early documentation of a preference for less aggressive medical care (general aggressiveness, or decision not to receive resuscitation) in the multivariable model. Patients who were eventually placed on the ESMO protocol were more likely to have a preference for less aggressive medical care early in the hospitalization (26% versus 18%, p < 0.05), however, this variable was not significant when added to the multivariate regression model and did not have an effect on the odds ratios or significance levels for other variables in the model.
Adherence to the ESMO protocol
All of the patients who were placed on the ESMO protocol had a DNR order (a requirement of the protocol). Documentation of a goals of care discussion, required by the ESMO protocol, between the physician and the patient and/or patient's family resulting in a comfort-oriented care plan occurred prior to initiation of the protocol for 94% of the patients. Among the 16 patients who did not satisfy this criterion, 5 had documented family discussions but the last documented discussion indicated that aggressive care was desired; four had a discussion documented after the day of initiation of the protocol; 6 had records indicating plans for a discussion in the near future; and one had a nursing note indicating a planned discussion.
Among patients placed on the protocol, 95% (n = 238) had an opiate drip initiated as part of the comfort care plan. For half of these patients (n = 120) the infusion was initiated in the context of their mechanical ventilator being withdrawn expecting death. The opiate drip was titrated up at least once for 67% of patients as part of the protocol. On average, a patient who had his or her opiate drip increased received four titrations for symptom relief; however symptoms were documented in the medical record, on average, for only 2.2 of the 4 titrations. The highest dose of opiate in morphine equivalents ranged from 0.5 mg/hr to 120 mg/hr with a mean of 9 mg/hr [SD 12].
Discussion
Not all patients dying in the hospital require comfort-oriented care at the very end of life: for some patients efforts were ongoing to save the patient at the time of death and others were comatose or had no distressing symptoms. This study evaluated only patients for whom death was expected and found that fully 46% of these patients died in the hospital without the institution's end of life symptom management order protocol in place. The predictors of dying an expected death without this protocol were not clinical variables suggesting lack of need, but instead provider and patient characteristics that suggest inadequate attention to comfort at the end of life. A patient considered for transplant was associated with being 40% less likely to die with this protocol, indicating barriers in the transition from aggressive care to comfort. Variation between clinical services suggests that practice patterns play a role. These findings identify quality improvement targets for care at the end of life in the hospital.
There are several potential reasons why a patient expected to die in the hospital does not receive a care plan oriented toward comfort care. In the interview study that led to the current chart-based analysis,3 physicians often reported that the ESMO protocol was instituted too late and their explanation for that nearly always was that “the patient (or family) was not ready.” Why weren't these patients and families ready? Likely because the clinicians had not prepared them for this transition through iterative discussion of prognosis and guidance toward comfort as the disease trajectory worsened.19 The predictors of not receiving the ESMO protocol suggest that factors that might make discussion of bad news and comfort-oriented decisions more difficult—younger patients considered for transplant—are an important obstacle to a comfortable dying process among those dying an expected death.20 Work is needed to better integrate prognostic trajectory into decision making for patients aiming for aggressive care who nevertheless are approaching death.
Further exploration is needed to understand why lack of insurance was associated with lower likelihood of receiving the ESMO protocol. Might this relate to lack of trust—and thus unwillingness to agree to less than full aggressive treatment – among persons who perennially had inadequate access to care? Or perhaps unavailability of proxy decision makers plays a role, since many of the patients without insurance at the studied facility are homeless and more likely to be unbefriended. The relationship between the effects of being under-insured and of non-white race—an a posteriori finding for hypothesis generation—suggests that this group might be at risk for poorer end of life care in the hospital and merits thorough evaluation.
Nursing home status may be a marker for patients for whom end of life decision-making is particularly challenging. Most deaths among nursing home patients occur in the nursing facility and not in the hospital.21 Although this evaluation was carried out during a time period in which Physician Orders for Life-Sustaining Treatment forms were not yet available in California, it may be that nursing home residents expected to die were transferred to the hospital only if there was pressure to maintain survival, selecting a small group for whom there was likely to be a mismatch between aggressiveness of care and prognosis. This nursing home effect is seen even after the addition of dementia and presence of a surrogate decision maker into the model. It may be more difficult to communicate with the proxies of nursing home residents (for example, there may be more conservators involved with care of nursing home residents who may be more difficult to contact than family members). Exploration of obstacles to early discussions among residents transferred from nursing homes to the hospital is needed.
This study has several limitations. First, only patients who died in the hospital are included in the analysis and we may be missing patients who were expected to die but were discharged, for example, to hospice. We should note however, that this sampling method identified nearly all of the patients who receive ESMO protocol care at the studied institution. A prospective study found that of 127 patients treated with the ESMO protocol, 120 died in the hospital and only 7 were discharged from the hospital.3 Also, the analysis is cross-sectional; the associations detected cannot be assumed to be causal. However, we are able to identify subgroups of patients that have low rates of ESMO protocol use that identifies potential targets for quality improvement. In addition, we were limited to data that were available in the medical record. Furthermore, we studied only a single hospital. This analysis should be performed in other sorts of hospitals and in other regions. Also, it should not be assumed that patients who were not prescribed the ESMO protocol died in an uncomfortable manner. Only 7% of patients who were expected to die received cardiopulmonary resuscitation (CPR) at the time of death. However, the protocol is accepted as the standard for comfort-oriented care at the end of life at the studied institution. It is also important to note that some patients—even if fully informed—might prefer aggressive modalities rather than comfort-oriented care at the very end of life.
In conclusion, an institutional ESMO protocol can be widely disseminated throughout a hospital to be used in the care of the majority of expected deaths. Analysis of a palliative intervention of this sort can identify areas in need of improvement in its implementation (i.e., lapses in symptom documentation to justify upward titration of opiates) as well as apparent disparities in its application. These aspects of inpatient care at the end of life should be targeted for improvement.
Acknowledgments
This project was supported by a donation from Mary Kay Farley to RAND Health. Mrs. Farley cares deeply about the topic and encouraged this work. Dr. Walling was supported by a National Research Service Award Training Grant T32 PE19001, the UCLA Specialty Training and Advanced Research Program, the National Institutes of Health (NIH) Loan Repayment Program, and a Career Development Award from the National Palliative Care Research Center. The funding source had no role in the design, execution, analysis or interpretation of the study or on the decision to submit the results for publication. No author reports any conflict of interest with this paper.
Preliminary data presented at the Society of General Internal Medicine Annual National Meeting in Minneapolis, Minnesota on Thursday, April 29, 2010.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Teno JM. Clarridge BR. Casey V. Welch LC. Wetle T. Shield R. Mor V. Family perspectives on end-of-life care at the last place of care. JAMA. 2004;291:88–93. doi: 10.1001/jama.291.1.88. [DOI] [PubMed] [Google Scholar]
- 2.Fineberg IC. Wenger NS. Brown-Saltzman K. Unrestricted opiate administration for pain and suffering at the end of life: Knowledge and attitudes as barriers to care. J Palliat Med. 2006;9:873–883. doi: 10.1089/jpm.2006.9.873. [DOI] [PubMed] [Google Scholar]
- 3.Walling AM. Brown-Saltzman K. Barry T. Quan RJ. Wenger NS. Assessment of implementation of an order protocol for end-of-life symptom management. J Palliat Med. 2008;11:857–863. doi: 10.1089/jpm.2007.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badger JM. Factors that enable or complicate transitions in critical care. Am J Crit Care. 2005;14:513–521. [PubMed] [Google Scholar]
- 5.Back AL. Arnold RM. Baile WF. Tulsky JA. Fryer-Edwards K. Approaching difficult communication tasks in oncology. CA Cancer J Clin. 2005;55:164–177. doi: 10.3322/canjclin.55.3.164. [DOI] [PubMed] [Google Scholar]
- 6.Friedrichson M. Milberg A. Concerns about losing control when breaking bad news to terminally ill patients with cancer. Physician's perspective. J Palliat Med. 2006;9:673–682. doi: 10.1089/jpm.2006.9.673. [DOI] [PubMed] [Google Scholar]
- 7.Baile WF. Glober GA. Lenzi R. Beale EA. Kudelka AP. Discussing disease progression and end-of-life decisions. Oncology. 1999;13:1021–1031. [PubMed] [Google Scholar]
- 8.Hulsman RL. Pranger S. Fabriek M. Fabriek M. Karemaker JM. Smets EM. How stressful is doctor-patient communication? Physiological and psychological stress of medical students in simulated history taking and bad-news consultations. Int J Psychophysiol. 2010;77:26–34. doi: 10.1016/j.ijpsycho.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Phillips KD. Taking away hope. BMJ. 1994;13:6952. doi: 10.1136/bmj.309.6952.478c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glare P. Virik K. Jones M. Hudson M. Eychmuller S. Simes J. Christakis N. A systematic review of physicians' survival predictions in terminally ill cancer patients. BMJ. 2003;327:195–198. doi: 10.1136/bmj.327.7408.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morita T. Tei Y. Inoue S. Impaired communication capacity and agitated delerium in the final week of terminally ill cancer patients: Prevalence and identification of research focus. J Pain Symptom Manage. 2003;26:827–834. doi: 10.1016/s0885-3924(03)00287-2. [DOI] [PubMed] [Google Scholar]
- 12.Gagnon P. Allard P. Masse B. DeSerres M. Delerium in terminal cancer: A prospective study using daily screening, early diagnosis, and continuous monitoring. J Pain Symptom Manage. 2000;19:412–426. doi: 10.1016/s0885-3924(00)00143-3. [DOI] [PubMed] [Google Scholar]
- 13.Wenger NS. Oye RK. Bellamy PE. Lynn J. Phillips RS. Desbiens NA. Kussin P. Youngner SJ. Prior capacity of patients lacking decision making ability early in hospitalization: Implications for advance directive administration. J Gen Intern Med. 1994;9:539–543. doi: 10.1007/BF02599276. [DOI] [PubMed] [Google Scholar]
- 14.Wenger NS. Phillips RS. Teno JM. Oye RK. Dawson NV. Liu H. Califf R. Layde P. Hakim R. Lynn J. Physician understanding of patient resuscitation preferences: Insights and clinical implications. J Am Geriatr Soc. 2000;48:S44–51. doi: 10.1111/j.1532-5415.2000.tb03140.x. [DOI] [PubMed] [Google Scholar]
- 15.Shalowitz DI. Garrett-Mayer E. Wendler D. The accuracy of surrogate decision makers: A systematic review. Arch Intern Med. 2006;166:493–497. doi: 10.1001/archinte.166.5.493. [DOI] [PubMed] [Google Scholar]
- 16.Cook D. Rocker G. Marshall J. Sjokvist P. Dodek P. Griffith L. Freitag A. Varon J. Bradley C. Levy M. Finfer S. Hamielec C. McMullin J. Weaver B. Walter S. Guyatt G Level of Care Study Investigators and the Canadian Critical Care Trials Group. Withdrawal of mechanical ventilation in anticipation of death in the intensive care unit. N Engl J Med. 2003;349:1123–1132. doi: 10.1056/NEJMoa030083. [DOI] [PubMed] [Google Scholar]
- 17.Keenan SP. Busch KD. Chen LM. McCarthy L. Inman KJ. Sibbald WJ. A retrospective review of a large cohort of patients undergoing the process of withholding or withdrawal of life support. Crit Care Med. 1997;25:13241331. doi: 10.1097/00003246-199708000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Wenger NS. Soloman DH. Roth CP. MacLean CH. Saliba D. Kamberg CJ. Rubenstein LZ. Young RT. Sloss EM. Louie R. Adams J. Chang JT. Venus PJ. Schnelle JF. Shekelle PG. The quality of medical care provided to vulnerable community dwelling Older patients. Ann Intern Med. 2003;139:740–747. doi: 10.7326/0003-4819-139-9-200311040-00008. [DOI] [PubMed] [Google Scholar]
- 19.Walling AM. Asch SM. Lorenz K. Roth CP. Barry T. Kahn KL. Wenger NS. The quality of care provided to hospitalized patients at the end of Life. Arch Intern Med. 2010;170:1057–1063. doi: 10.1001/archinternmed.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larson AM. Integrating palliative care for liver transplant candidates: “Too well for transplant, too sick for life.”. JAMA. 2006;18:2168–2176. doi: 10.1001/jama.295.18.2168. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell SL. Teno JM. Kiely DK. Shaffer ML. Jones RN. Prigerson HG. Volicer L. Givens JL. Hamel MB. The clinical course of advanced dementia. N Engl J Med. 2009;361:1529–1538. doi: 10.1056/NEJMoa0902234. [DOI] [PMC free article] [PubMed] [Google Scholar]