Abstract
Few studies exist investigating the brain-behavior relations of event-based prospective memory (EB-PM) impairments following traumatic brain injury (TBI). To address this, children with moderate-to-severe TBI performed an EB-PM test with two motivational enhancement conditions and underwent concurrent diffusion tensor imaging (DTI) at 3 months post-injury. Children with orthopedic injuries (OI; n = 37) or moderate-to-severe TBI (n = 40) were contrasted. Significant group differences were found for fractional anisotropy (FA) and apparent diffusion coefficient for orbitofrontal white matter (WM), cingulum bundles, and uncinate fasciculi. The FA of these WM structures in children with TBI significantly correlated with EB-PM performance in the high, but not the low motivation condition. Regression analyses within the TBI group indicated that the FA of the left cingulum bundle (p = 0.003), left orbitofrontal WM (p < 0.02), and left (p < 0.02) and right (p < 0.008) uncinate fasciculi significantly predicted EB-PM performance in the high motivation condition. We infer that the cingulum bundles, orbitofrontal WM, and uncinate fasciculi are important WM structures mediating motivation-based EB-PM responses following moderate-to-severe TBI in children.
Keywords: cingulum bundles, diffusion tensor imaging, event-based prospective memory, incentive, motivation, pediatrics orbitofrontal white matter, traumatic brain injury, uncinate fasciculus
Introduction
Prospective memory (PM) involves a combination of memorial and executive processes that allow one to form intentions that are executed at a later time. Event-based PM (EB-PM) tasks are triggered by an external cue (Einstein and McDaniel, 1990, 1996) such as telling a friend of an upcoming party when they are seen next or giving school permission slips to a parent to sign. Everyday life for children and adults alike is replete with instrumental activities, such as PM tasks, to the extent that PM is an essential ability to effectively manage the challenges of daily living (Harris, 1984; Meacham and Dumitru, 1976; Winograd, 1988). Neurologic insults such as traumatic brain injury (TBI) have been shown to result in impairments of PM performance in adults (Cockburn, 1995; Fortin et al., 2002; Groot et al., 2002; Hannon et al., 1995; Henry et al., 2007; Kinsella et al., 1996; Kliegel et al., 2004; Knight et al., 2005, 2006; Louda et al., 2007; Mathias and Mansfield, 2005; Roche et al., 2002, 2007; Shum et al., 1999) and children (McCauley and Levin, 2004; McCauley et al., 2009, 2010a, in press; Ward et al., 2004, 2007). Although brain-behavior relations of episodic (i.e., retrospective) memory have been frequently studied in children with TBI (Anderson and Catroppa, 2007; Catroppa and Anderson, 2002; Catroppa et al., 2008; Di Stefano et al., 2000; Donders, 1993; Donders and Hoffman, 2002; Lowther and Mayfield, 2004; Roman et al., 1998; Salorio et al., 2005), little corresponding neuroimaging data currently exists concerning PM functioning in children and adolescents with TBI. One recent neuroimaging study addressing this gap (McCauley et al., 2010b), found that 3 months following moderate-to-severe TBI, areas of cortical thinning significantly correlated with EB-PM performance included regions in the left (e.g., dorsolateral and inferior prefrontal cortex, anterior and posterior cingulate, temporal lobe, fusiform, and parahippocampal gyri), and right hemispheres (e.g., dorsolateral, inferior, and medial prefrontal cortex, cingulate, and temporal lobe). While this study of cortical gray matter thickness was one of the first to investigate brain-behavior relations between TBI and EB-PM in children, it did not investigate brain regions that may be specifically related to motivation-based EB-PM behavior.
Lesion studies have supported the supposition that damage to medial prefrontal areas, temporal cortex (Burgess et al., 2003; Daum and Ackermann, 1994; Daum and Mayes, 2000; Palmer and McDonald, 2000), and even parts of the thalamus (Cheng et al., 2008) impairs PM functioning. Positron emission tomography (PET) studies have revealed the special roles of the bilateral frontal and right dorsolateral prefrontal cortices, and right inferior parietal/precuneus play in PM functioning (Burgess et al., 2001, 2003; Okuda et al., 1998). More recent PET work by Okuda and colleagues has implicated bilateral frontal poles, left lingual gyrus, right gyrus rectus, and the anterior cingulate gyrus in subserving EB-PM. Functional magnetic resonance imagine (fMRI) studies have suggested that structures including right middle and inferior prefrontal, parietal/precuneus, bilateral cingulate, and frontal poles (including the functional dichotomy of medial versus lateral BA 10), left middle and inferior prefrontal, and middle and inferior/posterior temporal, and fusiform and parahippocampal gyri to be important areas facilitating PM functioning (Eschen et al., 2007; Gilbert et al., 2009; Poppenk et al., 2010; Reynolds et al., 2009; Simons et al., 2006). Other imaging modalities have yet to be deployed to further our understanding of neural mechanisms underlying PM and the neuropathological processes that impair PM.
Diffusion tensor imaging
Diffusion tensor imaging (DTI) is a relatively new imaging technique that incorporates specific pulsed magnetic field gradients into a standard MRI sequence increasing sensitivity to the diffusion of water molecules (Lazar et al., 2006; Mori et al., 1999). In normal brain tissue, there are physical boundaries restricting water diffusion in white matter (WM), resulting in greater water movement parallel to axons with less movement perpendicular to axons. This diffusion restriction is referred to as fractional anisotropy (FA; the ratio of anisotropy to isotropy); FA ranges from 0 to 1, where values nearer to 0 are representative of isotropy or increased diffusion (e.g., as a result of injury or disease). Conversely, values closer to 1 represent water diffusion more parallel to WM fiber tracts in normal brain tissue. Isotropic water diffusion is also commonly measured by the apparent diffusion coefficient (ADC), which is the mean diffusivity across eigenvalues. Generally speaking, measures of diffusivity such as ADC are inversely related to FA; however, while both ADC and FA reflect changes in WM microstructure, the association between these two indices is complex and incompletely understood. Previous studies have shown that FA and ADC metrics are sensitive to pathological changes such as demyelination, axonal damage, and other microstructural WM changes following pediatric TBI (Ewing-Cobbs et al., 2008; Lee et al., 2003; Levin et al., 2008; Oni et al., 2010; Wang et al., 2008; Wilde et al., 2006a, 2006b, 2008, 2010; Wozniak et al., 2007; Wu et al., 2010). Typically, higher FA and lower ADC are associated with preserved WM tract integrity. The relation of FA and ADC to WM integrity may be influenced by dynamic processes during childhood and adolescent development, making it a particularly useful imaging tool in detecting neuropathological changes that occur in this population (Yung et al., 2007).
Functional roles of specific regions of interest
Three regions of interest (ROIs) were selected for this study based on the following criteria: (1) white matter tracts or regions that are amenable to quantitative tractography using DTI, and (2) white matter underlying structures with known involvement in either memory functioning or reward processing. Based on these criteria, the cingulum bundle, orbitofrontal white matter, and uncinate fasciculi were selected. The hippocampus was not selected as it is primarily a gray matter structure that is difficult to assess reliably using DTI tractography.
Cingulum bundle
The cingulum bundle is a critical fiber pathway coursing the cingulate gyrus, possessing myriad connections linking the prefrontal cortex and parietal areas (i.e., dorsal component) and the amygdala, nucleus accumbens, hypothalamus, and anterior insula (i.e., ventral component) (Bush et al., 2000), terminating posteriorly in the parahippocampal gyrus and hippocampus (Kobayashi and Amaral, 2007); the cingulum bundle, therefore, forms a significant confluent pathway of the Papez circuit (Papez, 1995). The anterior cingulate cortex has been linked with emotion, cognition, memory, and motor functions while the posterior aspect has been specifically associated with memory and pain perception (Burianova et al., 2010; Fang et al., 2009; Kim et al., 2007; Leube et al., 2008; Maddock and Buonocore, 1997; McDonald et al., 2001, Nielson and Bryant, 2005; Nyberg et al., 2003; Paus et al., 1993; Riha et al., 2008; Valenstein et al., 1987; Wang et al., 2005). Moreover, non-human primate (Amiez et al., 2006; McCoy et al., 2003; Pearson et al., 2009) and human experiments (Fujiwara et al., 2009; Nieuwenhuis et al., 2005) suggest that anterior and posterior cingulate cortex plays a significant role in mediating reward processing. Of particular interest to this study is the function of the anterior cingulate in responding to monetary incentives (Akitsuki et al., 2003; Bush et al., 2002) and modulating levels of motivation (Bush et al., 2000; Clark et al., 2009; Pessoa, 2009; Simoes-Franklin et al., 2009). Significant atrophy and dysfunction of the cingulate has been reported after TBI (Yount et al., 2002) as have fMRI and PET studies in patients with cognitive impairments following TBI (Fontaine et al., 1999; Scheibel et al., 2009; Soeda et al., 2005). In addition to supporting general cognitive flexibility and allowing both shifting and focused attention, the cingulum bundle is important in learning and memory in adolescents with mild TBI (Wu et al., 2010). Advanced neuroimaging techniques, such as DTI (Fellgiebel et al., 2005), have demonstrated that decreased FA of the posterior cingulate cortex is associated with poorer memory performance in patients with diagnoses of either mild cognitive impairment or dementia.
Orbitofrontal white matter
The orbitofrontal region (and orbitofrontal-amygdala system) has been shown to be involved in a number of functions including emotion and motivation in non-human primate (Barbas, 2007a, 2007b; Schultz et al., 1998, 2000) and human studies (Elliott et al., 2003; Rolls, 2004; Schultz, 2004, 2006) and, of more specific interest to this study, processing of reward value (O'Doherty et al., 2001; Rolls, 2004). Functional neuroimaging and brain lesion studies have revealed lateralized functions of the orbital frontal in that the left orbital frontal is involved in positive or reward-related affect whereas the right orbital frontal is involved in negative or punishment-related affect (Eddington et al., 2009; O'Doherty et al., 2001; Ogai et al., 2005; Schutter and van Honk, 2006; Thut et al., 1997; Tranel et al., 2002; Wang et al., 2005). Recent work supports the concept that motivation can alter top-down modulation of executive functions (Gazzaley et al., 2007; Krawczyk et al., 2007); connections between the orbital frontal and dorsolateral prefrontal cortex play a pivotal role in this modulation of cognitive control as found in studies of non-human primates (Hikosaka and Watanabe, 2000) and working memory paradigms in humans (Gilbert and Fiez, 2004; Pochon et al., 2002). In a recent fMRI study, Szatkowska and colleagues (2008) reported that verbal working memory performance (n-back paradigm) was modulated by the orbital frontal cortex; structural equation modeling revealed that under the low motivation (no money) condition, the left and right orbital frontal positively increased the activation of the dorsolateral prefrontal cortex, but in the high motivation condition (monetary incentive), the right orbital frontal cortex exerted a strong negative influence on the activation of the left dorsolateral prefrontal cortex. This suggests that the orbital frontal mediates motivational influences on cognitive control processes such as working memory and perhaps other facets of executive function/cognitive control.
Uncinate fasciculus
The uncinate fasciculus is a principal white matter tract connecting the temporal pole and other anterior temporal areas to the inferior region of the frontal lobe (Bracht et al., 2009; Burgel et al., 2006; Catani et al., 2002; Catani and Thiebaut de Schotten, 2008; Ebeling and von Cramon, 1992; Highley et al., 2002; Mori et al., 2002; Petrides and Pandya, 2007; Schmahmann et al., 2007; Sedat and Duvernoy, 1990; Ungerleider et al., 1989). The uncinate fasciculus has been identified as a critical part of the temporal-neocortical memory system (Squire and Zola-Morgan, 1991). Reduced structural integrity of the uncinate fasciculus has been related to episodic and working memory performance in healthy adults (Charlton et al., 2010; Niogi et al., 2008a; Sasson et al., 2010; Voineskos et al., 2010), adults with mild traumatic brain injury (Geary et al., 2010; Niogi et al., 2008a, 2008b), temporal lobe epilepsy (Diehl et al., 2008; McDonald et al., 2008), chronic (Nestor et al., 2004, 2008), recent-onset schizophrenia (Szeszko et al., 2008), and schizotypal personality disorder (Nakamura et al., 2005). The integrity of the uncinate fasciculus is also positively related to performance on verbal- and visual-based tasks of episodic memory in healthy children and adolescents (Mabbott et al., 2009).
Despite considerable evidence of shared and unique neural networks involved in PM, very few studies to date have explored the relation between EB-PM performance and neuropathological changes following TBI in children and adolescents. To address this gap, we investigated EB-PM in children and adolescents with moderate-to-severe TBI and concurrent MRI at 3 months post-injury in a prospective cohort using the same experimental methodology as that reported previously (McCauley et al., 2009). In our study of the neural correlates of EB-PM, motivation was varied using two levels of monetary incentive (i.e., dollars versus pennies) where participants exchanged points for money based on their performance. Given our previous work on EB-PM in children with chronic TBI, we posited three hypotheses: (1) DTI metrics of the three ROIs would be significantly poorer in children 3 months after moderate-to-severe TBI compared to those of controls; (2) DTI metrics of the three ROIs would be correlated with EB-PM performance (under high and low motivation conditions); and (3) DTI metrics of the ROIs would independently predict EB-PM performance (under high and low motivation conditions).
Methods
Participants
TBI severity was assessed by the lowest post-resuscitation Glasgow Coma Scale (GCS) (Teasdale and Jennett, 1974) score in the first 24 h post-injury. Our sample included children ranging in ages 7 to 16 years: 40 children with moderate-to-severe TBI (post-resuscitation GCS ≤8 [severe] and either post-resuscitation GCS 13–15 with trauma-related intracranial abnormalities on CT scan of the head at hospital admission [Williams et al., 1990] or GCS of 9–12 irrespective of CT results), and 37 children who sustained orthopedic injuries (OI) not involving the head, but requiring emergency room treatment. The OI participants were included to control for risk factors predisposing children to traumatic injury and to equate for other nonspecific factors (e.g., stress, anxiety) associated with trauma and hospitalization. All participants were fluent in English, the product of full-term births (i.e., ≥37 weeks of gestation and >2500 g), without pre-existing major psychiatric (e.g., schizophrenia, bipolar disorder) or pervasive developmental disorder (e.g., autism spectrum disorder), and no previous hospitalization for head injury. As part of the design of the larger study, children were assessed at 3 months (±2 weeks) post-injury. Socioeconomic status was measured by the Socioeconomic Composite Index (SCI) (Yeates et al., 1997).
Informed consent was obtained from the parent/guardian through a process approved by the Institutional Review Boards of Baylor College of Medicine, the University of Texas at Dallas, the University of Texas Southwestern Medical Center, and the University Miami School of Medicine. Child assent was obtained in accordance with current federal regulations. Participants were recruited prospectively from consecutive admissions to American College of Surgeons Level-1 trauma centers in Houston, Dallas, and Miami as part of a longitudinal study of the neurobehavioral outcomes of children following moderate-to-severe TBI.
MRI acquisition
All participants underwent magnetic resonance imaging (MRI) without sedation on Philips 1.5 T Intera scanners (Philips, Cleveland, OH) at Texas Children's Hospital (Houston), the Rogers MRI Center, University of Texas Southwestern Medical Center (Dallas), Jackson Memorial Hospital (Miami), and Miami Children's Hospital using identical protocols and comparable platforms and software. Regular quality assurance testing was performed on all three scanners including American College of Radiology (ACR) phantom and Weisskoff testing (Weisskoff, 1996) for echo planar imaging (EPI) sequences. All scanners were in a similar range on Weisskoff testing and this was considered acceptable through consensus of the neuroradiologists on the study.
DTI fiber tracking analysis
Transverse multislice spin echo, single shot, echo planar imaging (EPI) sequences were used (10150.5 ms TR, 90 ms TE, 2.7 mm slices, 0 mm gap). A 256 FOV (RFOV = 100%) was used with a measured voxel size of 2.67 × 2.69 × 2.70 mm. Diffusion was measured along 15 directions (no. of b-value = 2, low b-value = 0, high b-value = 860 sec/mm2). To improve the signal-to-noise ratio, each high b-value image was acquired twice and averaged, with the low b image acquired once. The acquisition time for 55 slices was approximately 5:45 min.
DTI analysis
All data were carefully inspected for any artifacts or irregularities that may have compromised its accuracy or reliability. The Philips PRIDE-registration tool (Netsch, 2001) was used to remove shear and eddy current distortion and head motion prior to calculating FA maps with Philips fiber tracking 4.1v3 Beta 2 software (Cleveland, OH). ROIs were created using established protocols detailed in previous publications and described briefly below. After ROIs were created, automated Philips three-dimensional fiber tracking tool (Hoogenraad, 2002) was utilized to determine fiber tracks passing through the cingulum bundle, orbital frontal white matter, and uncinate fasciculus. The algorithm for fiber tracking is based upon the fiber assignment by continuous tracking (FACT) method (Mori et al., 1999). Tracking terminated if the FA in the voxels decreased to <0.2 or if the angle between adjacent voxels along the track was >7°.
Regions of interest and interoperator reliability
Two experienced raters independently measured FA twice for the right and left sides of the three target structures in a subset of 11 participants with TBI and 11 participants with OI. Intra- and inter-rater reliabilities were calculated using Shrout-Fleiss reliability statistics (Shrout and Fleiss, 1979) to obtain intra-class correlation coefficients (ICCs) that were all above 0.94 (range, 0.94 to 0.99; mean, 0.975 ± 0.02) for the target structures.
Cingulum bundle
Our methods for defining the cingulum bundle have been presented previously (Wilde et al., 2010). In brief, the ROI originated from the cingulum bundle and was created on FA color maps in the left and right parasagittal planes. Using the automatic 3D ROI algorithm function, two to three seed points were placed linearly along the cingulum bundle in the right and parasagittal planes separately. The multi-ROI fiber tracking function was used to select fibers that were common to the three ROIs, and the software provided the statistics including the mean FA and ADC for the selected fibers comprising the cingulum bundle.
Orbitofrontal white matter
Methods used by our group to measure the orbital frontal and interrater reliability coefficients have been previously presented (Levin et al., 2008). In short, ROIs were drawn in the coronal plane on a slice just anterior to the first slice where the genu of the corpus callosum was visible. A line extending from the interhemispheric fissure (at a point just inferior to the corpus callosum) to the lateral orbital sulci formed the superior boundary of the orbital frontal region (all WM inferior to this line). Right and left sides were estimated separately and all WM within boundaries was included for calculating FA and ADC.
Uncinate fasciculus
We calculated FA and ADC in the right and left uncinate fasciculus using multiple ROIs applied in previously published DTI protocols (Wakana et al., 2007). In brief, our method isolated fibers traversing the two ROIs that comprise the uncinate fasciculus. The first ROI was demarcated on the coronal slice just anterior to the first visible slice of the genu of the corpus callosum and incorporated all WM in the cerebral hemisphere. The second ROI was delineated on a coronal slice at the level of anterior commissure to include WM in the temporal lobes.
Design and procedure
Details of the EB-PM task design have been published previously (McCauley et al., 2009, 2010a, in press; McCauley and Pedroza, 2010), but will be briefly described here. The study used a crossover design with two motivation conditions, each lasting one hour (with no wash-out interval between motivation conditions). Briefly, the extrinsic motivation conditions involved the monetary units of either dollars (high motivation) or pennies (low motivation); participants were told that all payments would occur after the task was completed. A randomization table was used to randomize motivation condition order (sequence) across participants. Instructions for each condition were administered at the beginning of the first and second hours (periods) of testing. While performing other tasks during the experimental and neuropsychological battery (a standard battery order for all participants was used to control for difficulty level of the ongoing tasks), the child was asked to respond, “Please give me three points,” each time the examiner said, “Let's try something different.” This EB-PM cue was presented every 15–20 min, with three PM cue presentations in each of the motivation conditions; each motivation block (period) required one hour. The target PM response window was 5 sec, but no child in any group made PM responses (correct or incorrect) outside this window. The scoring algorithm for the EB-PM task was: 2 points for realizing the delayed intention (PM component) and 2 additional points for recalling the correct phrase (retrospective memory component or RM). Correct responses were awarded 4 points, and responses with incorrect RM content (e.g., “Please give me five points” or “Please give me some points”) were awarded 2 points. A maximum of 12 points was available in each of the two conditions. Perfect performance in both conditions resulted in a maximum reward payment of $12.12. No performance feedback was given until after both conditions had been completed. Cash payments were made at the end of the testing session, well after completing the EB-PM task.
Data analysis
Statistical significance was defined as α = 0.05 for all analyses unless otherwise specified. Planned comparisons were analyzed holding significance at α = 0.05 and all post-hoc comparisons were adjusted using the Bonferroni correction for multiple comparisons. All analyses were conducted with SAS software for Windows v9.2. Spearman ρ correlations were used to assess brain-behavior relations, and the DTI data were analyzed using a linear mixed model with either FA or ADC as the dependent variables. The participant variable was treated as a random effect to account for correlation between multiple measures within the same participant. Other main effects of interest included age-at-test, socioeconomic composite index, gender, group (TBI vs. OI), laterality (left vs. right hemisphere), and group × laterality interaction. Linear regression was used to determine the relative contribution of each of the ROIs to EB-PM performance.
Results
Sample characteristics
There was a positive trend for a significant difference by age-at-test (p = 0.10) as the TBI group was older than the OI group (Table 1). No other between-group comparisons for continuous variables were significant, including the socioeconomic composite index. The TBI and OI groups differed significantly by mechanism of injury as the OI group sustained more low-velocity injuries (e.g., sports/play) compared to the greater proportion of high-velocity injuries sustained by both TBI groups (e.g., motor vehicle accident, auto-pedestrian). The groups did not differ significantly by race/ethnicity or gender.
Table 1.
Demographic and Injury Variables of the Sample
| Variable | OI (n = 37) | TBI (n = 40) | Statistical comparison |
|---|---|---|---|
| Age-at-Test (yr), mean (SD) | 12.1 (2.3) | 13.1 (3.0) | F(1,75) = 2.82, p = 0.10 |
| Gender (female-male) | 10:27 | 14:26 | χ2(2) = 0.57, p = 0.45 |
| SCI, mean (SD) | 0.2 (0.9) | 0 (0.7) | F(1,75) = 1.33, p = 0.25 |
| Race/ethnicity, n (%) | |||
| African American | 11 (29.7) | 3 (7.5) | p = 0.07* |
| Asian | 1 (2.7) | 0 (0) | |
| Biracial | 1 (2.7) | 1 (2.5) | |
| European American | 12 (32.4) | 17 (42.5) | |
| Hispanic | 12 (32.4) | 19 (47.5) | |
| Time post-injury (days), mean (SD) | 124.4 (28.7) | 125.3 (36.7) | F(1,75) = 0.01, p = 0.91 |
| GCS (lowest in first 24 h) | 15.0 (0) | 8.7 (4.5) | N/A |
| Mechanism of injury, n (%) | |||
| MVA | 2 (5.4) | 16 (40.0) | p < 0.0001* |
| MCA/scooter/moped | 4 (10.8) | 6 (15.0) | |
| RV | 1 (2.7) | 4 (10.0) | |
| Bicycle | 2 (5.4) | 2 (5.0) | |
| Fall | 6 (16.2) | 7 (17.5) | |
| Hit by falling object | 2 (5.4) | 0 (0) | |
| Sports/play | 18 (48.7) | 2 (5.0) | |
| Hit by motor vehicle | 1 (2.7) | 3 (7.5) | |
| Other | 1 (2.7) | 0 (0) | |
Fisher's exact test.
SCI, Socioeconomic Composite Index; GCS, Glasgow Coma Scale score; MVA, motor vehicle accident; MCA, motorcycle accident; RV, recreational or other off-road vehicle.
Event-based prospective memory performance
Results of the EB-PM task itself (in a subsample partially overlapping the group in this study) at the same time point have been previously presented (McCauley et al., in press), but are briefly summarized here. There was a significant age effect in that older children performed more robustly than younger children; there was no Age-at-Test × Group interaction for EB-PM performance. Children with OI performed better than children with moderate or severe TBI on the EB-PM task and children with moderate TBI outperformed those with severe TBI. EB-PM performance was significantly increased in the high versus low motivation conditions for the children with OI and moderate TBI, but not for children with severe TBI.
DTI metrics
Using a mixed model, parallel analyses were conducted within each ROI for FA and ADC. Main effects of Age-at-Test, Socioeconomic Composite Index, Gender, Group, and Laterality were included in all models. In the initial models for each ROI, Age-at-Test × Group and Laterality × Group interactions were also included, but neither was significant; the models were then re-estimated without these interactions. Least-squares means (LS-means) and standard errors (SE) are presented.
Cingulum bundles
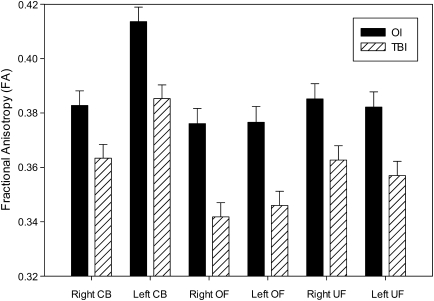
In the cingulum bundle analysis for FA, significant main effects were found for Age-at-Test, Group, and Laterality (all p < 0.05; Table 2). Older Age-at-Test was associated with higher FA. The FA of both the right and left cingulum bundles was significantly lower in the TBI group compared to the OI group (Fig. 1), and FA was higher in the left relative to the right CB in both the TBI and OI groups. In the ADC analyses, Group was significant (p < 0.04), as was a positive trend for Gender (p = 0.07; males had higher ADC). ADC was higher in the TBI group (LS-mean = 0.785, SE = 0.008) compared to the OI group (LS-mean = 0.762, SE = 0.007).
Table 2.
Type III Tests of Fixed Effects for DTI Metrics
| |
Cingulum bundles |
Orbitofrontal white matter |
Uncinate fasciculi |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | df | F | p value | df | F | p value | df | F | p value |
| FA | |||||||||
| Age-at-Test | 1, 72 | 6.73 | <0.02 | 1, 72 | 0.97 | 0.33 | 1, 69 | 0.03 | 0.87 |
| SCI | 1, 72 | 0.17 | 0.68 | 1, 72 | 0.68 | 0.41 | 1, 69 | 0.31 | 0.58 |
| Gender | 1, 72 | 1.88 | 0.17 | 1, 72 | 0.24 | 0.63 | 1, 69 | 1.19 | 0.28 |
| Group | 1, 72 | 13.84 | 0.0004 | 1, 72 | 12.23 | 0.0008 | 1, 69 | 22.53 | <0.0001 |
| Laterality | 1, 76 | 62.53 | <0.0001 | 1, 76 | 1.99 | 0.16 | 1, 62 | 0.57 | 0.47 |
| ADC | |||||||||
| Age-at-Test | 1, 72 | 1.32 | 0.26 | 1, 72 | 0.57 | 0.45 | 1, 69 | 0.01 | 0.94 |
| SCI | 1, 72 | 0.16 | 0.69 | 1, 72 | 0.02 | 0.88 | 1, 69 | 0.01 | 0.94 |
| Gender | 1, 72 | 3.28 | 0.07 | 1, 72 | 0.49 | 0.49 | 1, 69 | 0.25 | 0.62 |
| Group | 1, 72 | 4.71 | <0.04 | 1, 72 | 11.58 | 0.001 | 1, 69 | 9.40 | 0.003 |
| Laterality | 1, 76 | 2.38 | 0.13 | 1, 76 | 3.79 | 0.06 | 1, 62 | 0.73 | 0.40 |
p-values ≤.05 are shown in bold.
FA, fractional anisotropy; ADC, apparent diffusion coefficient; SCI, Socioeconomic Composite Index.
FIG. 1.
Although the Group × Laterality interaction was not significant, fractional anisotropy (FA) for the regions of interest in OI and TBI groups (least-squares means after accounting for Age-at-Test, SCI, and Gender) are shown for comparison. CB, cingulum bundles; OF, orbitofrontal white matter; UF, uncinate fasciculus. Error bars depict standard errors.
Orbitofrontal white matter
In the orbital frontal analysis for FA, a significant main effect was found for Group (p = 0.0008; Table 2); the FA of both the right and left orbital frontal was significantly lower in the TBI group compared to the OI group (Fig. 1). In the ADC analyses, the Group main effect was significant (p = 0.001); the ADC of the total orbital frontal was higher in the TBI group (LS-mean = 0.867, SE = 0.008) compared to the OI group (LS-mean = 0.828, SE = 0.009).
Uncinate fasciculi
In FA analysis for uncinate fasciculus, the main effect of Group was significant (p < 0.0001; Table 2); FA in the right and left uncinate fasciculus combined was significantly lower in the TBI group compared to the OI group (Fig. 1). ADC analyses reveled a significant main effect for Group (p = 0.003); the ADC of the total uncinate fasciculus was higher in the TBI group (LS-mean = 0.851, SE = 0.009) relative to the OI group (LS-mean = 0.813, SE = 0.009).
Although the Group × Laterality interaction was not significant in any ROI for FA or ADC (although there was an expected laterality effect for cingulum bundle FA), LS-means and SE are shown in Figure 1 to illustrate between-group differences. FA in each of the ROIs in both hemispheres was significantly reduced in the TBI group compared to the OI group.
Brain-behavior relations
Spearman ρ correlations were calculated between the EB-PM scores (separately for high/low motivation conditions) and DTI metrics (FA and ADC) within each of the ROIs (cingulum bundle, orbital frontal, uncinate fasciculus). In the cingulum bundles, significant correlations with FA and ADC were found in the TBI group under the high motivation condition for the left cingulum bundle (Table 3). The positive correlation with FA indicates higher FA is related to higher EB-PM scores and similarly for the ADC, a negative correlation means lower ADC is related to higher EB-PM scores. Significant correlations were found in the TBI group under the high motivation condition for the right and left orbital frontal white matter with FA (positive), and the left orbital frontal with ADC (negative). Finally, a significant correlation was found in the TBI group for FA in the right uncinate fasciculus under the high and low motivation conditions. After adjusting for the number of correlations performed within each motivation condition (i.e., p < 0.0083), only the correlations for the FA of the ROIs under the high motivation condition (left cingulum bundle and orbital frontal and right uncinate fasciculus) and the ADC for the left orbital frontal remained significant (Table 3). No correlations were found significant at the p ≤ 0.10 level in the OI group for any ROI under either motivation condition.
Table 3.
Correlations between DTI Metrics of ROIs and Prospective Memory Performance (Partialling for Age-at-Test) within TBI Group
| |
|
EB-PM condition |
|||
|---|---|---|---|---|---|
| |
|
High motivation |
Low motivation |
||
| ROI | DTI metric | ρ | p value | ρ | p value |
| FA | |||||
| Right CB | FA | 0.01 | ns | 0.02 | ns |
| Left CB | FA | 0.55 | 0.0003 | 0.23 | ns |
| Right OF | FA | 0.27 | ns | 0.13 | ns |
| Left OF | FA | 0.42 | <0.008 | 0.19 | ns |
| Right UF | FA | 0.45 | <0.007 | 0.43 | <0.009 |
| Left UF | FA | 0.37 | <0.04 | 0.14 | ns |
| ADC | |||||
| Right CB | ADC | −0.25 | ns | −0.25 | ns |
| Left CB | ADC | −0.36 | <0.03 | −0.28 | ns |
| Right OF | ADC | −0.16 | ns | −0.28 | ns |
| Left OF | ADC | −0.48 | 0.002 | −0.29 | ns |
| Right UF | ADC | −0.20 | ns | −.031 | ns |
| Left UF | ADC | −0.35 | <0.05 | −0.28 | ns |
Spearman ρ correlation coefficients and p values ≤0.05 are shown in bold. There were no significant correlations (all p > 0.10) within the OI group.
PM, prospective memory; ROI, region of interest; CB, cingulum bundle; OF, orbitofrontal white matter; UF, uncinate fasciculus.
Contributions of ROIs to EB-PM performance
To determine the relative contribution of each of the ROIs to EB-PM performance, three linear regression models were performed in the TBI group; models did not include the OI group given the absence of significant brain-behavior correlations. Although our intention was to include the three ROIs (left and right hemispheres) simultaneously in the models, FA and ADC metrics of the ROIs were significantly collinear (e.g., orbital frontal and uncinate fasciculus FA, ρ = 0.80; orbital frontal and uncinate fasciculus ADC, ρ = 0.89; cingulum bundle and uncinate fasciculus ADC, ρ = 0.71). Given this, we explored a set of models with the EB-PM score as the dependent variable with Age-at-Test as a covariate under the two motivation conditions separately; parallel analyses were conducted for FA and ADC. Under the motivated condition using FA, the right (p < 0.008) and left uncinate fasciculus (p < 0.02) significantly predicted EB-PM as did the left orbital frontal (p < 0.02) and left cingulum bundle (p = 0.003; Table 4). The only significant model under the unmotivated condition was the right uncinate fasciculus (t(38) = 2.56, p < 0.02). For ADC, no model was significant under either motivation condition.
Table 4.
Regression Analyses for Fractional Anisotropy and Event-Based Prospective Memory Performance in TBI Group Under High Motivation Condition
| Variable | β | SE β | t-value | p value | Standardized β | R2/adj. R2 |
|---|---|---|---|---|---|---|
| Model 1: F (2,35) = 3.99, p < 0.03 | 0.19/0.14 | |||||
| Age-at-Test | −0.02 | 0.26 | −0.07 | 0.95 | −0.01 | |
| Right UF | 53.98 | 19.11 | 2.82 | <0.008 | 0.43 | |
| Model 2: F (2,35) = 3.49, p < 0.05 | 0.17/0.12 | |||||
| Age-at-Test | 0.36 | 0.29 | 1.22 | 0.23 | 0.19 | |
| Left UF | 56.0 | 21.87 | 2.56 | <0.02 | 0.39 | |
| Model 3: F (5,34) = 5.10, p < 0.002 | 0.43/0.35 | |||||
| Age-at-Test | 0.01 | 0.23 | 0.04 | 0.97 | 0.01 | |
| Right OF | −32.1 | 23.1 | −1.39 | 0.17 | −0.23 | |
| Left OF | 60.7 | 23.2 | 2.62 | <0.02 | 0.44 | |
| Right CB | −3.95 | 22.41 | −0.18 | 0.86 | −0.03 | |
| Left CB | 85.51 | 26.98 | 3.17 | 0.003 | 0.50 |
p-values ≤.05 are shown in bold.
SE, standard error; CB, cingulum bundle; OF, orbitofrontal white matter; UF, uncinate fasciculus.
There was substantial colinearity of the OI group DTI metrics so the same series of regression models were conducted as in the TBI group. None of the overall F-tests of the models using FA were significant. Analyses were then conducted using ADC and, again, no model produced a significant overall F-test.
Discussion
Our study is the first to examine the neural correlates (specifically of WM structures) mediating motivation-based responses to EB-PM following TBI in children. We found support for our first hypothesis in that children with TBI had significantly lower FA in the three ROIs (cingulum bundles, orbitofrontal WM, and uncinate fasciculi) than those in the OI group, and the ADC of the TBI group also was significantly higher than that of the OI group. This is consistent with prior studies that found similar patterns of DTI metrics in the cingulum bundle (Kraus et al., 2007; Niogi et al., 2008a; Rutgers et al., 2008; Wilde et al., 2010; Wu et al., 2010), orbital frontal and prefrontal WM (Greenberg et al., 2008; Kennedy et al., 2009; Levin et al., 2008; Oni et al., 2010; Wozniak et al., 2007), and uncinate fasciculus (Niogi et al., 2008a; Singh et al., 2009) in children and adults with TBI severity ranging from mild to severe.
We found partial support for our second hypothesis that the DTI metrics of the three ROIs would be correlated with EB-PM performance under both high and low motivation conditions; brain-behavior relations using FA were only significant under the high motivation condition, and only one ROI (left orbital frontal) was significant using ADC. All other ROIs failed to survive correction for multiple correlations (p < 0.0083). These results suggest that WM tract integrity becomes crucial when the individual is motivated to increase effort and that the loss of WM integrity may present an upper limit in terms of potentially achievable performance levels.
The lack of relation between DTI metrics and EB-PM performance in the OI group was not entirely unexpected. In contrast to the wide range of values and greater variability for both DTI metrics and EB-PM performance in the TBI group, there may be a comparative restriction of range (i.e., smaller standard deviation/error) on both of these variables that may have attenuated the correlation between them in the OI group. Our primary goal in this study was to determine whether behavior was related to FA and ADC in specific regions in a population of TBI patients with different degrees of cognitive dysfunction and WM alteration in these areas rather than to undertake this investigation in neurologically intact participants, which would require more subjects and would reach beyond the scope of the current study.
Partial support also was found for our third hypothesis, namely, that the DTI metrics of the ROIs would contribute independently to EB-PM under both high and low motivation conditions. Of the models assessed, only FA under the high motivation condition had a significant overall F-test; further, only FA of the right uncinate fasciculus was significant under the low motivation condition. While not initially anticipated, this is not inconsistent given brain-behavior correlations addressing our first hypothesis, which only reached significance under the high motivation condition. These results also suggest that the integrity of the fiber tracts is most crucial under high task demand conditions created as a response to the high level of extrinsic reward for performance. The significant role played by the left cingulum bundle in mediating the response to motivators (monetary incentives, in particular) has been clearly demonstrated (Akitsuki et al., 2003; Bush et al., 2000, 2002; Clark et al., 2009; Fujiwara et al., 2009; Nieuwenhuis et al., 2005; Pessoa, 2009; Simoes-Franklin et al., 2009), in addition to its function in maintaining a sustained response to PM task demands (Reynolds et al., 2009). Similarly, the function of the orbital frontal in motivated behavior is well-known (Elliott et al., 2003; Rolls, 2004) and includes more recent findings, which extend the orbital frontal's function to the top-down modulation of cognitive control processes in response to changes in motivational status (Gazzaley et al., 2007; Gilbert and Fiez, 2004; Krawczyk et al., 2007; Pochon et al., 2002; Szatkowska et al., 2008). Our results are consistent with previous research demonstrating the greater role of the left relative to the right orbital frontal white matter in mediating positive or reward-related affect and emotion-related learning (Eddington et al., 2009; O'Doherty et al., 2001; Ogai et al., 2005; Schutter and van Honk, 2006; Thut et al., 1997; Tranel et al., 2002; Wang et al., 2005) and is also consistent with others who have reported activation of the anterior cingulate and orbital frontal in response to positive reward (Linke et al., 2010) or anticipation of reward (Ko et al., 2009; Risinger et al., 2005), especially when reward was in the form of a monetary incentive (Kirsch et al., 2003; Staudinger et al., 2009).
These results demonstrate that WM structures known to be important in PM and episodic memory (i.e., retrospective memory) functioning and reward processing are (1) significantly damaged following moderate-to-severe TBI in children, (2) the structural integrity of these structures is related to motivation-based EB-PM performance, and (3) specific WM structures make a significantly unique contribution to motivation-based EB-PM performance, including the left cingulum bundle, left orbitofrontal WM, and bilateral uncinate fasciculi.
While there are no published studies directly evaluating the role of the uncinate fasciculus in PM, its function in episodic and working memory processes is well-established in both healthy and neurologically impaired populations (Charlton et al., 2010; Diehl et al., 2008; Geary et al., 2010; McDonald et al., 2008; Niogi et al., 2008a; Sasson et al., 2010; Voineskos et al., 2010), and its neuroanatomical locus connecting the inferior frontal cortex and the anterior temporal lobe make it a credible structure for facilitating motivation-based EB-PM processes. Our results lend supporting evidence to this speculation given the significant contribution that the right and left uncinate fasciculi make to high-motivated EB-PM functioning, and even the role the right uncinate fasciculus plays in low motivation performance.
Regarding the effects of demographic variables on EB-PM performance, no gender effect was found, which is consistent with studies of normal adults (Einstein and McDaniel, 1990; Kidder et al., 1997; Maujean et al., 2003), typically developing children (Kerns, 2000; Kerns and Price, 2001), children with TBI (McCauley et al., 2009, 2010a), and children with sickle cell disease (McCauley and Pedroza, 2010). Although socioeconomic status (SES) is a well-known moderator of outcome in children with TBI (Taylor, 2004; Taylor et al., 1999, 2002; Yeates et al., 1997), no significant effect of SES on EB-PM performance was found. This is similar to findings of McCauley and colleagues (McCauley et al., 2009, 2010a).
There are some limitations in this study that should be addressed. Retrospective memory was not assessed due to the design of the longitudinal study. This was unfortunate as it could have demonstrated the degree to which impaired retrospective memory abilities may have accounted for impaired EB-PM performance in these children. Studies have found that PM and retrospective memory are clearly dissociable (Maylor et al., 2002; Palmer and McDonald, 2000; West and Craik, 2001; West and Krompinger, 2005) and are not strongly related in adults and healthy elderly (Brandimonte and Passolunghi, 1994; Driscoll et al., 2005; Einstein and McDaniel, 1990; Huppert and Beardsall, 1993; Kidder et al., 1997; Kvavilashvili, 1987; Maylor, 1990; McDaniel and Einstein, 1993; Salthouse et al., 2004), typically developing children (Kvavilashvili et al., 2001), children with chronic TBI (McCauley et al., 2009), or children with sickle cell disease (McCauley and Pedroza, 2010). The close relation of these two memory categories has been demonstrated in early development, but they reportedly diverge very rapidly thereafter (Guajardo and Best, 2000; Ruther and Best, 1993). However, studies in adult TBI have produced contradictory findings concerning the relation between retrospective memory and PM (Groot et al., 2002; Henry et al., 2007; Mathias and Mansfield, 2005). Future work will be required to determine to what extent PM in children with TBI relies on intact retrospective memory capabilities; therefore, generalizing discrepant adult TBI results to children with TBI is not advised. The inclusion of formal assessment of retrospective memory and advanced neuroimaging or fMRI paradigms in children with TBI is recommended to elucidate the effects of TBI on EB-PM and the extent to which retrospective memory performs a contributory role to EB-PM performance.
Acknowledgments
This work was supported by the National Center for Medical Rehabilitation Research (grant no. K23 HD-40896 to S.R.M) and the National Institute Neurological Disorders and Stroke (grant no. NS-21889 to H.S.L). The information in this article has never been published before, either electronically or in print. I would like to extend my personal appreciation to Drs. Mark McDaniel and Harvey Levin, who graciously served as mentors on a K-23 mentored patient-oriented research career development award. We also thank the participants and their families for their interest and willingness to take part in this research.
Author Disclosure Statement
None of the authors have financial or other relationships that could be construed as a conflict of interest with respect to the content of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Medical Rehabilitation Research or the National Institutes of Health.
References
- Akitsuki Y. Sugiura M. Watanabe J. Yamashita K. Sassa Y. Awata S. Matsuoka H. Maeda Y. Matsue Y. Fukuda H. Kawashima R. Context-dependent cortical activation in response to financial reward and penalty: an event-related fMRI study. Neuroimage. 2003;19:1674–1685. doi: 10.1016/s1053-8119(03)00250-7. [DOI] [PubMed] [Google Scholar]
- Amiez C. Joseph J.P. Procyk E. Reward encoding in the monkey anterior cingulate cortex. Cereb. Cortex. 2006;16:1040–1055. doi: 10.1093/cercor/bhj046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson V. Catroppa C. Memory outcome at 5 years post-childhood traumatic brain injury. Brain Inj. 2007;21:1399–1409. doi: 10.1080/02699050701785070. [DOI] [PubMed] [Google Scholar]
- Barbas H. Flow of information for emotions through temporal and orbitofrontal pathways. J. Anat. 2007a;211:237–249. doi: 10.1111/j.1469-7580.2007.00777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H. Specialized elements of orbitofrontal cortex in primates. Ann. NY Acad. Sci. 2007b;1121:10–32. doi: 10.1196/annals.1401.015. [DOI] [PubMed] [Google Scholar]
- Bracht T. Tuscher O. Schnell S. Kreher B. Rusch N. Glauche V. Lieb K. Ebert D. Il'yasov K.A. Hennig J. Weiller C. van Elst L.T. Saur D. Extraction of prefronto-amygdalar pathways by combining probability maps. Psychiatry Res. 2009;174:217–222. doi: 10.1016/j.pscychresns.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Brandimonte M.A. Passolunghi M.C. The effect of cue-familiarity, cue-distinctiveness, and retention interval on prospective remembering. Q. J. Exp. Psychol. A. 1994;47:565–587. doi: 10.1080/14640749408401128. [DOI] [PubMed] [Google Scholar]
- Burgel U. Amunts K. Hoemke L. Mohlberg H. Gilsbach J.M. Zilles K. White matter fiber tracts of the human brain: three-dimensional mapping at microscopic resolution, topography and intersubject variability. Neuroimage. 2006;29:1092–1105. doi: 10.1016/j.neuroimage.2005.08.040. [DOI] [PubMed] [Google Scholar]
- Burgess P.W. Quayle A. Frith C.D. Brain regions involved in prospective memory as determined by positron emission tomography. Neuropsychologia. 2001;39:545–555. doi: 10.1016/s0028-3932(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Burgess P.W. Scott S.K. Frith C.D. The role of the rostral frontal cortex (area 10) in prospective memory: a lateral versus medial dissociation. Neuropsychologia. 2003;41:906–918. doi: 10.1016/s0028-3932(02)00327-5. [DOI] [PubMed] [Google Scholar]
- Burianova H. McIntosh A.R. Grady C.L. A common functional brain network for autobiographical, episodic, and semantic memory retrieval. Neuroimage. 2010;49:865–874. doi: 10.1016/j.neuroimage.2009.08.066. [DOI] [PubMed] [Google Scholar]
- Bush G. Luu P. Posner M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Bush G. Vogt B.A. Holmes J. Dale A.M. Greve D. Jenike M.A. Rosen B.R. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc. Natl. Acad. Sci. USA. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M. Howard R.J. Pajevic S. Jones D.K. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Catani M. Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44:1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Catroppa C. Anderson V. Recovery in memory function in the first year following TBI in children. Brain Inj. 2002;16:369–384. doi: 10.1080/02699050110104444. [DOI] [PubMed] [Google Scholar]
- Catroppa C. Anderson V. Ditchfield M. Coleman L. Using magnetic resonance imaging to predict new learning outcome at 5 years after childhood traumatic brain injury. J. Child Neurol. 2008;23:486–496. doi: 10.1177/0883073807309773. [DOI] [PubMed] [Google Scholar]
- Charlton R.A. Barrick T.R. Lawes I.N. Markus H.S. Morris R.G. White matter pathways associated with working memory in normal aging. Cortex. 2010;46:474–489. doi: 10.1016/j.cortex.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Cheng H.-D. Wang K. Xi C.-H. Niu C.-S. Fu X.-M. Prefrontal cortex involvement in the event-based prospective memory: evidence from patients with lesions in the prefrontal cortex. Brain Inj. 2008;22:697–704. doi: 10.1080/02699050802263035. [DOI] [PubMed] [Google Scholar]
- Clark L. Lawrence A.J. Astley-Jones F. Gray N. Gambling near-misses enhance motivation to gamble and recruit win-related brain circuitry. Neuron. 2009;61:481–490. doi: 10.1016/j.neuron.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn J. Task interruption in prospective memory: a frontal lobe function? Cortex. 1995;31:87–97. doi: 10.1016/s0010-9452(13)80107-4. [DOI] [PubMed] [Google Scholar]
- Daum I. Ackermann H. Frontal-type memory impairment associated with thalamic damage. Int. J. Dev. Neurosci. 1994;77:187–198. doi: 10.3109/00207459408986030. [DOI] [PubMed] [Google Scholar]
- Daum I. Mayes A.R. Memory and executive function impairments after frontal or posterior cortex lesions. Behav. Neurol. 2000;12:161–173. doi: 10.1155/2000/327304. [DOI] [PubMed] [Google Scholar]
- Di Stefano G. Bachevalier J. Levin H.S. Song J.X. Scheibel R.S. Fletcher J.M. Volume of focal brain lesions and hippocampal formation in relation to memory function after closed head injury in children. J. Neurol. Neurosurg. Psychiatry. 2000;69:210–216. doi: 10.1136/jnnp.69.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl B. Busch R.M. Duncan J.S. Piao Z. Tkach J. Luders H.O. Abnormalities in diffusion tensor imaging of the uncinate fasciculus relate to reduced memory in temporal lobe epilepsy. Epilepsia. 2008;49:1409–1418. doi: 10.1111/j.1528-1167.2008.01596.x. [DOI] [PubMed] [Google Scholar]
- Donders J. Memory functioning after traumatic brain injury in children. Brain Inj. 1993;7:431–437. doi: 10.3109/02699059309029686. [DOI] [PubMed] [Google Scholar]
- Donders J. Hoffman N.M. Gender differences in learning and memory after pediatric traumatic brain injury. Neuropsychology. 2002;16:491–499. doi: 10.1037//0894-4105.16.4.491. [DOI] [PubMed] [Google Scholar]
- Driscoll I. McDaniel M.A. Guynn M.J. Apolipoprotein E and prospective memory in normally aging adults. Neuropsychology. 2005;19:28–34. doi: 10.1037/0894-4105.19.1.28. [DOI] [PubMed] [Google Scholar]
- Ebeling U. von Cramon D. Topography of the uncinate fascicle and adjacent temporal fiber tracts. Acta Neurochir. 1992;115:143–148. doi: 10.1007/BF01406373. [DOI] [PubMed] [Google Scholar]
- Eddington K.M. Dolcos F. McLean A.N. Krishnan K.R. Cabeza R. Strauman T.J. Neural correlates of idiographic goal priming in depression: goal-specific dysfunctions in the orbitofrontal cortex. Soc. Cogn. Affect. Neurosci. 2009;4:238–246. doi: 10.1093/scan/nsp016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein G.O. McDaniel M.A. Normal aging and prospective memory. J. Exp. Psychol. Learn. Mem. Cogn. 1990;16:717–726. doi: 10.1037//0278-7393.16.4.717. [DOI] [PubMed] [Google Scholar]
- Einstein G.O. McDaniel M.A. Retrieval processes in prospective memory: theoretical approaches and some new empirical findings. In: M.A. Brandimonte., editor; G.O. Einstein., editor; M.A. McDaniel., editor. Prospective Memory: Theory and Applications. Lawrence Erlbaum: Mahwah, NJ; 1996. pp. 115–142. [Google Scholar]
- Elliott R. Newman J.L. Longe O.A. Deakin J.F. Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: a parametric functional magnetic resonance imaging study. J. Neurosci. 2003;23:303–307. doi: 10.1523/JNEUROSCI.23-01-00303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschen A. Freeman J. Dietrich T. Martin M. Ellis J. Martin E. Kliegel M. Motor brain regions are involved in the encoding of delayed intentions: a fMRI study. Int. J. Psychophysiol. 2007;64:259–268. doi: 10.1016/j.ijpsycho.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Ewing-Cobbs L. Prasad M.R. Swank P. Kramer L. Cox C.S., Jr. Fletcher J.M. Barnes M. Zhang X. Hasan K.M. Arrested development and disrupted callosal microstructure following pediatric traumatic brain injury: relation to neurobehavioral outcomes. Neuroimage. 2008;42:1305–1315. doi: 10.1016/j.neuroimage.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J. Jin Z. Wang Y. Li K. Kong J. Nixon E.E. Zeng Y. Ren Y. Tong H. Wang P. Hui K.K. The salient characteristics of the central effects of acupuncture needling: limbic-paralimbic-neocortical network modulation. Hum. Brain Mapp. 2009;30:1196–1206. doi: 10.1002/hbm.20583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellgiebel A. Muller M.J. Wille P. Dellani P.R. Scheurich A. Schmidt L.G. Stoeter P. Color-coded diffusion-tensor-imaging of posterior cingulate fiber tracts in mild cognitive impairment. Neurobiol. Aging. 2005;26:1193–1198. doi: 10.1016/j.neurobiolaging.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Fontaine A. Azouvi P. Remy P. Bussel B. Samson Y. Functional anatomy of neuropsychological deficits after severe traumatic brain injury. Neurology. 1999;53:1963–1968. doi: 10.1212/wnl.53.9.1963. [DOI] [PubMed] [Google Scholar]
- Fortin S. Godbout L. Braun C. Strategic sequence planning and prospective memory impairments in frontally lesioned head trauma patients performing activities of daily living. Brain Cogn. 2002;48:361–365. [PubMed] [Google Scholar]
- Fujiwara J. Tobler P.N. Taira M. Iijima T. Tsutsui K. Segregated and integrated coding of reward and punishment in the cingulate cortex. J. Neurophysiol. 2009;101:3284–3293. doi: 10.1152/jn.90909.2008. [DOI] [PubMed] [Google Scholar]
- Gazzaley A. Rissman J. Cooney J. Rutman A. Seibert T. Clapp W. D'Esposito M. Functional interactions between prefrontal and visual association cortex contribute to top-down modulation of visual processing. Cereb. Cortex. 2007;17(Suppl. 1):125–135. doi: 10.1093/cercor/bhm113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary E.K. Kraus M.F. Pliskin N.H. Little D.M. Verbal learning differences in chronic mild traumatic brain injury. J. Int. Neuropsychol. Soc. 2010;16:506–516. doi: 10.1017/S135561771000010X. [DOI] [PubMed] [Google Scholar]
- Gilbert A.M. Fiez J.A. Integrating rewards and cognition in the frontal cortex. Cogn. Affect Behav. Neurosci. 2004;4:540–552. doi: 10.3758/cabn.4.4.540. [DOI] [PubMed] [Google Scholar]
- Gilbert S.J. Gollwitzer P.M. Cohen A.-L. Oettingen G. Burgess P.W. Separable brain systems supporting cued versus self-initiated realization of delayed intentions. J. Exp. Psychol. Learn. Mem. Cogn. 2009;35:905–915. doi: 10.1037/a0015535. [DOI] [PubMed] [Google Scholar]
- Greenberg G. Mikulis D.J. Ng K. DeSouza D. Green R.E. Use of diffusion tensor imaging to examine subacute white matter injury progression in moderate to severe traumatic brain injury. Arch. Phys. Med. Rehabil. 2008;89:S45–50. doi: 10.1016/j.apmr.2008.08.211. [DOI] [PubMed] [Google Scholar]
- Groot Y.C.T. Wilson B.A. Evans J. Watson P. Prospective memory functioning in people with and without brain injury. J. Int. Neuropsychol. Soc. 2002;8:645–654. doi: 10.1017/s1355617702801321. [DOI] [PubMed] [Google Scholar]
- Guajardo N.R. Best D.L. Do preschoolers remember what to do? Incentive and external cues in prospective memory. Cogn. Dev. 2000;15:75–97. [Google Scholar]
- Hannon R. Adams P. Harrington S. Fries-Dias C. Gipson M.T. Effects of brain injury and age on prospective memory self-rating and performance. Rehabil. Psychol. 1995;40:289–298. [Google Scholar]
- Harris J.E. Remembering to do things: a forgotten topic, in: Everyday Memory: Actions and Absent-mindedness. In: M.M. Gruneberg., editor; P.E. Morris., editor; R.N. Sykes., editor. Academic Press; New York: 1984. pp. 71–92. [Google Scholar]
- Henry J.D. Phillips L.H. Crawford J.R. Kliegel M. Theodorou G. Summers F. Traumatic brain injury and prospective memory: influence of task complexity. J. Clin. Exp. Neuropsychol. 2007;29:457–466. doi: 10.1080/13803390600762717. [DOI] [PubMed] [Google Scholar]
- Highley J.R. Walker M.A. Esiri M.M. Crow T.J. Harrison P.J. Asymmetry of the uncinate fasciculus: a post-mortem study of normal subjects and patients with schizophrenia. Cereb. Cortex. 2002;12:1218–1224. doi: 10.1093/cercor/12.11.1218. [DOI] [PubMed] [Google Scholar]
- Hikosaka K. Watanabe M. Delay activity of orbital and lateral prefrontal neurons of the monkey varying with different rewards. Cereb. Cortex. 2000;10:263–721. doi: 10.1093/cercor/10.3.263. [DOI] [PubMed] [Google Scholar]
- Hoogenraad F. Multi-center evaluation of in-vivo fibertracking. Philips Medical Systems; Cleveland: 2002. [Google Scholar]
- Huppert F.A. Beardsall L. Prospective memory impairment as an early indicator of dementia. J. Clin. Exp. Neuropsychol. 1993;15:805–821. doi: 10.1080/01688639308402597. [DOI] [PubMed] [Google Scholar]
- Kennedy M.R. Wozniak J.R. Muetzel R.L. Mueller B.A. Chiou H.H. Pantekoek K. Lim K.O. White matter and neurocognitive changes in adults with chronic traumatic brain injury. J. Int. Neuropsychol. Soc. 2009;15:130–136. doi: 10.1017/S1355617708090024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns K.A. The CyberCruiser: an investigation of development of prospective memory in children. J. Int. Neuropsychol. Soc. 2000;6:62–70. doi: 10.1017/s1355617700611074. [DOI] [PubMed] [Google Scholar]
- Kerns K.A. Price K.J. An investigation of prospective memory in children with ADHD. Child Neuropsychol. 2001;7:162–171. doi: 10.1076/chin.7.3.162.8744. [DOI] [PubMed] [Google Scholar]
- Kidder D.P. Park D.C. Hertzog C. Morrell R.W. Prospective memory and aging: the effects of working memory and prospective memory task load. Aging Neuropsychol. Cogn. 1997;4:93–112. [Google Scholar]
- Kim J.H. Park K.Y. Seo S.W. Na D.L. Chung C.S. Lee K.H. Kim G.M. Reversible verbal and visual memory deficits after left retrosplenial infarction. J. Clin. Neurol. 2007;3:62–66. doi: 10.3988/jcn.2007.3.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella G. Murtagh D. Landry A. Homfray K. Hammond M. O'Beirne L. Dwyer L. Lamont M. Ponsford J. Everyday memory following traumatic brain injury. Brain Inj. 1996;10:499–507. doi: 10.1080/026990596124214. [DOI] [PubMed] [Google Scholar]
- Kirsch P. Schienle A. Stark R. Sammer G. Blecker C. Walter B. Ott U. Burkart J. Vaitl D. Anticipation of reward in a nonaversive differential conditioning paradigm and the brain reward system: an event-related fMRI study. Neuroimage. 2003;20:1086–1095. doi: 10.1016/S1053-8119(03)00381-1. [DOI] [PubMed] [Google Scholar]
- Kliegel M. Eschen A. Thöne-Otto A.I. Planning and realization of complex intentions in traumatic brain injury and normal aging. Brain Cogn. 2004;56:43–54. doi: 10.1016/j.bandc.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Knight R.G. Harnett M. Titov N. The effects of traumatic brain injury on the predicted and actual performance of a test of prospective remembering. Brain Inj. 2005;19:27–38. doi: 10.1080/02699050410001720022. [DOI] [PubMed] [Google Scholar]
- Knight R.G. Titov N. Crawford M. The effects of distraction on prospective remembering following traumatic brain injury assessed in a simulated naturalistic environment. J. Intl. Neuropsychol. Soc. 2006;12:8–16. doi: 10.1017/S1355617706060048. [DOI] [PubMed] [Google Scholar]
- Ko C.H. Liu G.C. Hsiao S. Yen J.Y. Yang M.J. Lin W.C. Yen C.F. Chen C.S. Brain activities associated with gaming urge of online gaming addiction. J. Psychiatr. Res. 2009;43:739–747. doi: 10.1016/j.jpsychires.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y. Amaral D.G. Macaque monkey retrosplenial cortex: III. Cortical efferents. J. Comp. Neurol. 2007;502:810–833. doi: 10.1002/cne.21346. [DOI] [PubMed] [Google Scholar]
- Kraus M.F. Susmaras T. Caughlin B.P. Walker C.J. Sweeney J.A. Little D.M. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007;130:2508–2519. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- Krawczyk D.C. Gazzaley A. D'Esposito M. Reward modulation of prefrontal and visual association cortex during an incentive working memory task. Brain Res. 2007;1141:168–177. doi: 10.1016/j.brainres.2007.01.052. [DOI] [PubMed] [Google Scholar]
- Kvavilashvili L. Remembering intention as a distinct form of memory. Br. J. Psychol. 1987;78:507–518. [Google Scholar]
- Kvavilashvili L. Messer D.J. Ebdon P. Prospective memory in children: the effects of age and task interruption. Dev. Psychol. 2001;37:418–430. doi: 10.1037//0012-1649.37.3.418. [DOI] [PubMed] [Google Scholar]
- Lazar M. Alexander A.L. Thottakara P.J. Badie B. Field A.S. White matter reorganization after surgical resection of brain tumors and vascular malformations. Am. J. Neuroradiol. 2006;27:1258–1271. [PMC free article] [PubMed] [Google Scholar]
- Lee Z.I. Byun W.M. Jang S.H. Ahn S.H. Moon H.K. Chang Y. Diffusion tensor magnetic resonance imaging of microstructural abnormalities in children with brain injury. Am. J. Phys. Med. Rehabil. 2003;82:556–559. doi: 10.1097/01.PHM.0000073830.15643.6A. [DOI] [PubMed] [Google Scholar]
- Leube D.T. Weis S. Freymann K. Erb M. Jessen F. Heun R. Grodd W. Kircher T.T. Neural correlates of verbal episodic memory in patients with MCI and Alzheimer's disease—a VBM study. Int. J. Geriatr. Psychiatry. 2008;23:1114–1118. doi: 10.1002/gps.2036. [DOI] [PubMed] [Google Scholar]
- Levin H.S. Wilde E.A. Chu Z. Yallampalli R. Hanten G.R. Li X. Chia J. Vasquez A.C. Hunter J.V. Diffusion tensor imaging in relation to cognitive and functional outcome of traumatic brain injury in children. J. Head Trauma Rehabil. 2008;23:197–208. doi: 10.1097/01.HTR.0000327252.54128.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke J. Kirsch P. King A.V. Gass A. Hennerici M.G. Bongers A. Wessa M. Motivational orientation modulates the neural response to reward. Neuroimage. 2010;49:2618–2625. doi: 10.1016/j.neuroimage.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Louda J. Loseva D. Mielke R. Prospective memory in patients with traumatic brain injury: an overview. Zeit. Neuropsychol. 2007;18:91–99. [Google Scholar]
- Lowther J.L. Mayfield J. Memory functioning in children with traumatic brain injuries: a TOMAL validity study. Arch. Clin. Neuropsychol. 2004;19:105–118. doi: 10.1016/s0887-6177(02)00222-6. [DOI] [PubMed] [Google Scholar]
- Mabbott D.J. Rovet J. Noseworthy M.D. Smith M.L. Rockel C. The relations between white matter and declarative memory in older children and adolescents. Brain Res. 2009;1294:80–90. doi: 10.1016/j.brainres.2009.07.046. [DOI] [PubMed] [Google Scholar]
- Maddock R.J. Buonocore M.H. Activation of left posterior cingulate gyrus by the auditory presentation of threat-related words: an fMRI study. Psychiatry Res. 1997;75:1–14. doi: 10.1016/s0925-4927(97)00018-8. [DOI] [PubMed] [Google Scholar]
- Mathias J.L. Mansfield K.M. Prospective and declarative memory problems following moderate and severe traumatic brain injury. Brain Inj. 2005;19:271–282. doi: 10.1080/02699050400005028. [DOI] [PubMed] [Google Scholar]
- Maujean A. Shum D. McQueen R. The effect of cognitive demand on prospective memory in individuals with traumatic brain injury. Brain Impair. 2003;4:135–145. [Google Scholar]
- Maylor E.A. Age and prospective memory. Q. J. Exp. Psychol. A. 1990;42:471–493. doi: 10.1080/14640749008401233. [DOI] [PubMed] [Google Scholar]
- Maylor E.A. Smith G. Della Sala S. Logie R.H. Prospective and retrospective memory in normal aging and dementia: an experimental study. Mem. Cogn. 2002;30:871–884. doi: 10.3758/bf03195773. [DOI] [PubMed] [Google Scholar]
- McCauley S.R. Levin H.S. Prospective memory in pediatric traumatic brain injury: a preliminary study. Dev. Neuropsychol. 2004;25:5–20. doi: 10.1080/87565641.2004.9651919. [DOI] [PubMed] [Google Scholar]
- McCauley S.R. McDaniel M.A. Pedroza C. Chapman S.B. Levin H.S. Incentive effects on event-based prospective memory performance in children and adolescents with traumatic brain injury. Neuropsychology. 2009;23:201–209. doi: 10.1037/a0014192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley S.R. Pedroza C. Event-based prospective memory in children with sickle cell disease: effect of cue distinctiveness. Child Neuropsychol. 2010;16:293–312. doi: 10.1080/09297041003601470. [DOI] [PubMed] [Google Scholar]
- McCauley S.R. Pedroza C. Chapman S.B. Cook L.G. Hotz G. Vasquez A.C. Levin H.S. Event-based prospective memory performance during subacute recovery following moderate to severe traumatic brain injury in children: effects of monetary incentives. J. Int. Neuropsychol. Soc. 2010a;16:335–341. doi: 10.1017/S135561770999138X. [DOI] [PubMed] [Google Scholar]
- McCauley S.R. Pedroza C. Chapman S.B. Cook L.G. Vásquez A.C. Levin H.S. Monetary incentive effects on event-based prospective memory three months after traumatic brain injury in children. J. Clin. Exp. Neuropsychol. In press. [DOI] [PMC free article] [PubMed]
- McCauley S.R. Wilde E.A. Merkley T.L. Schnelle K.P. Bigler E.D. Hunter J.V. Chu Z. Vasquez A.C. Levin H.S. Patterns of cortical thinning in relation to event-based prospective memory performance three months after moderate to severe traumatic brain injury in children. Dev. Neuropsychol. 2010b;35:318–332. doi: 10.1080/87565641003696866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy A.N. Crowley J.C. Haghighian G. Dean H.L. Platt M.L. Saccade reward signals in posterior cingulate cortex. Neuron. 2003;40:1031–1040. doi: 10.1016/s0896-6273(03)00719-0. [DOI] [PubMed] [Google Scholar]
- McDaniel M.A. Einstein G.O. The importance of cue familiarity and cue distinctiveness in prospective memory. Memory. 1993;1:23–41. doi: 10.1080/09658219308258223. [DOI] [PubMed] [Google Scholar]
- McDonald C.R. Ahmadi M.E. Hagler D.J. Tecoma E.S. Iragui V.J. Gharapetian L. Dale A.M. Halgren E. Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology. 2008;71:1869–1876. doi: 10.1212/01.wnl.0000327824.05348.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald C.R. Crosson B. Valenstein E. Bowers D. Verbal encoding deficits in a patient with a left retrosplenial lesion. Neurocase. 2001;7:407–417. doi: 10.1076/neur.7.5.407.16250. [DOI] [PubMed] [Google Scholar]
- Meacham J.A. Dumitru J. Prospective remembering and external-retrieval cues, report no. MS 1284. Catalog of Selected Documents in Psychology; Washington, DC: 1976. [Google Scholar]
- Mori S. Crain B.J. Chacko V.P. van Zijl P.C. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann. Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Mori S. Kaufmann W.E. Davatzikos C. Stieltjes B. Amodei L. Fredericksen K. Pearlson G.D. Melhem E.R. Solaiyappan M. Raymond G.V. Moser H.W. van Zijl P.C. Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magn. Reson. Med. 2002;47:215–223. doi: 10.1002/mrm.10074. [DOI] [PubMed] [Google Scholar]
- Nakamura M. McCarley R.W. Kubicki M. Dickey C.C. Niznikiewicz M.A. Voglmaier M.M. Seidman L.J. Maier S.E. Westin C.F. Kikinis R. Shenton M.E. Fronto-temporal disconnectivity in schizotypal personality disorder: a diffusion tensor imaging study. Biol. Psychiatry. 2005;58:468–478. doi: 10.1016/j.biopsych.2005.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor P.G. Kubicki M. Gurrera R.J. Niznikiewicz M. Frumin M. McCarley R.W. Shenton M.E. Neuropsychological correlates of diffusion tensor imaging in schizophrenia. Neuropsychology. 2004;18:629–637. doi: 10.1037/0894-4105.18.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor P.G. Kubicki M. Niznikiewicz M. Gurrera R.J. McCarley R.W. Shenton M.E. Neuropsychological disturbance in schizophrenia: a diffusion tensor imaging study. Neuropsychology. 2008;22:246–254. doi: 10.1037/0894-4105.22.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netsch T. Towards real-time multi-modality 3-d medical image registration. International Conference on Computer Vision; Vancouver: 2001. 2001. pp. 718–725. [Google Scholar]
- Nielson K.A. Bryant T. The effects of non-contingent extrinsic and intrinsic rewards on memory consolidation. Neurobiol. Learn. Mem. 2005;84:42–48. doi: 10.1016/j.nlm.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S. Slagter H.A. von Geusau N.J. Heslenfeld D.J. Holroyd C.B. Knowing good from bad: differential activation of human cortical areas by positive and negative outcomes. Eur. J. Neurosci. 2005;21:3161–3168. doi: 10.1111/j.1460-9568.2005.04152.x. [DOI] [PubMed] [Google Scholar]
- Niogi S.N. Mukherjee P. Ghajar J. Johnson C.E. Kolster R. Lee H. Suh M. Zimmerman R.D. Manley G.T. McCandliss B.D. Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain. 2008a;131:3209–3221. doi: 10.1093/brain/awn247. [DOI] [PubMed] [Google Scholar]
- Niogi S.N. Mukherjee P. Ghajar J. Johnson C. Kolster R.A. Sarkar R. Lee H. Meeker M. Zimmerman R.D. Manley G.T. McCandliss B.D. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. Am. J. Neuroradiol. 2008b;29:967–973. doi: 10.3174/ajnr.A0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L. Marklund P. Persson J. Cabeza R. Forkstam C. Petersson K.M. Ingvar M. Common prefrontal activations during working memory, episodic memory, and semantic memory. Neuropsychologia. 2003;41:371–377. doi: 10.1016/s0028-3932(02)00168-9. [DOI] [PubMed] [Google Scholar]
- O'Doherty J. Kringelbach M.L. Rolls E.T. Hornak J. Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat. Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Ogai M. Iyo M. Mori N. Takei N. A right orbitofrontal region and OCD symptoms: a case report. Acta Psychiatr. Scand. 2005;111:74–76. doi: 10.1111/j.1600-0447.2004.00395.x. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- Okuda J. Fujii T. Yamadori A. Kawashima R. Tsukiura T. Fukatsu R. Suzuki K. Ito M. Fukuda H. Participation of the prefrontal cortices in prospective memory: evidence from a PET study in humans. Neurosci. Lett. 1998;253:127–130. doi: 10.1016/s0304-3940(98)00628-4. [DOI] [PubMed] [Google Scholar]
- Oni M.B. Wilde E.A. Bigler E.D. McCauley S.R. Wu T.C. Yallampalli R. Chu Z. Li X. Hunter J.V. Vasquez A.C. Levin H.S. Diffusion tensor imaging analysis of frontal lobes in pediatric traumatic brain injury. J. Child Neurol. 2010;25:976–984. doi: 10.1177/0883073809356034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer H. McDonald S. The role of frontal and temporal lobe processes in prospective remembering. Brain Cogn. 2000;44:103–107. [Google Scholar]
- Papez J.W. A proposed mechanism of emotion. J. Neuropsychiatry Clin. Neurosci. 1995;1937;7:103–112. doi: 10.1176/jnp.7.1.103. [DOI] [PubMed] [Google Scholar]
- Paus T. Petrides M. Evans A.C. Meyer E. Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: a positron emission tomography study. J. Neurophysiol. 1993;70:453–469. doi: 10.1152/jn.1993.70.2.453. [DOI] [PubMed] [Google Scholar]
- Pearson J.M. Hayden B.Y. Raghavachari S. Platt M.L. Neurons in posterior cingulate cortex signal exploratory decisions in a dynamic multioption choice task. Curr. Biol. 2009;19:1532–1537. doi: 10.1016/j.cub.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. How do emotion and motivation direct executive control? Trends Cogn. Sci. 2009;13:160–166. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Pandya D.N. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J. Neurosci. 2007;27:11573–1186. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochon J.B. Levy R. Fossati P. Lehericy S. Poline J.B. Pillon B. Le Bihan D. Dubois B. The neural system that bridges reward and cognition in humans: an fMRI study. Proc. Natl. Acad. Sci. USA. 2002;99:5669–5674. doi: 10.1073/pnas.082111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk J. Moscovitch M. McIntosh A.R. Ozcelik E. Craik F.I. Encoding the future: successful processing of intentions engages predictive brain networks. Neuroimage. 2010;49:905–913. doi: 10.1016/j.neuroimage.2009.08.049. [DOI] [PubMed] [Google Scholar]
- Reynolds J.R. West R. Braver T. Distinct neural circuits support transient and sustained processes in prospective memory and working memory. Cereb. Cortex. 2009;19:1208–1221. doi: 10.1093/cercor/bhn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riha P.D. Rojas J.C. Colorado R.A. Gonzalez-Lima F. Animal model of posterior cingulate cortex hypometabolism implicated in amnestic MCI and AD. Neurobiol. Learn. Mem. 2008;90:112–124. doi: 10.1016/j.nlm.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risinger R.C. Salmeron B.J. Ross T.J. Amen S.L. Sanfilipo M. Hoffmann R.G. Bloom A.S. Garavan H. Stein E.A. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. Neuroimage. 2005;26:1097–1108. doi: 10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Roche N.L. Fleming J.M. Shum D.H. Self-awareness of prospective memory failure in adults with traumatic brain injury. Brain Inj. 2002;16:931–945. doi: 10.1080/02699050210138581. [DOI] [PubMed] [Google Scholar]
- Roche N.L. Moody A. Szabo K. Fleming J.M. Shum D.H.K. Prospective memory in adults with traumatic brain injury: an analysis of perceived reasons for remembering and forgetting. Neuropsychol. Rehabil. 2007;17:314–334. doi: 10.1080/09602010600831004. [DOI] [PubMed] [Google Scholar]
- Rolls E.T. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55:11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- Roman M.J. Delis D.C. Willerman L. Magulac M. Demadura T.L. de la Pena J.L. Loftis C. Walsh J. Kracun M. Impact of pediatric traumatic brain injury on components of verbal memory. J. Clin. Exp. Neuropsychol. 1998;20:245–258. doi: 10.1076/jcen.20.2.245.1168. [DOI] [PubMed] [Google Scholar]
- Rutgers D.R. Toulgoat F. Cazejust J. Fillard P. Lasjaunias P. Ducreux D. White matter abnormalities in mild traumatic brain injury: a diffusion tensor imaging study. Am. J. Neuroradiol. 2008;29:514–519. doi: 10.3174/ajnr.A0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruther N.M. Best D.L. Development of prospective memory in preschoolers. Meeting of the Southeastern Psychological Association; Atlanta: 1993. [Google Scholar]
- Salorio C.F. Slomine B.S. Grados M.A. Vasa R.A. Christensen J.R. Gerring J.P. Neuroanatomic correlates of CVLT-C performance following pediatric traumatic brain injury. J. Int. Neuropsychol. Soc. 2005;11:686–696. doi: 10.1017/S1355617705050885. [DOI] [PubMed] [Google Scholar]
- Salthouse T.A. Berish D.E. Siedlech K.L. Construct validity and age sensitivity of prospective memory. Mem. Cogn. 2004;32:1133–1148. doi: 10.3758/bf03196887. [DOI] [PubMed] [Google Scholar]
- Sasson E. Doniger G.M. Pasternak O. Assaf Y. Structural correlates of memory performance with diffusion tensor imaging. Neuroimage. 2010;50:1231–1242. doi: 10.1016/j.neuroimage.2009.12.079. [DOI] [PubMed] [Google Scholar]
- Scheibel R.S. Newsome M.R. Troyanskaya M. Steinberg J.L. Goldstein F.C. Mao H. Levin H.S. Effects of severity of traumatic brain injury and brain reserve on cognitive-control related brain activation. J. Neurotrauma. 2009;26:1447–1461. doi: 10.1089/neu.2008.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann J.D. Pandya D.N. Wang R. Dai G. D'Arceuil H.E. de Crespigny A.J. Wedeen V.J. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- Schultz W. Neural coding of basic reward terms of animal learning theory, game theory, microeconomics and behavioural ecology. Curr. Opin. Neurobiol. 2004;14:139–147. doi: 10.1016/j.conb.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annu. Rev. Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Schultz W. Tremblay L. Hollerman J.R. Reward prediction in primate basal ganglia and frontal cortex. Neuropharmacology. 1998;37:421–429. doi: 10.1016/s0028-3908(98)00071-9. [DOI] [PubMed] [Google Scholar]
- Schultz W. Tremblay L. Hollerman J.R. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb. Cortex. 2000;10:272–284. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Schutter D.J. van Honk J. Increased positive emotional memory after repetitive transcranial magnetic stimulation over the orbitofrontal cortex. J. Psychiatry Neurosci. 2006;31:101–104. [PMC free article] [PubMed] [Google Scholar]
- Sedat J. Duvernoy H. Anatomical study of the temporal lobe. Correlations with nuclear magnetic resonance. J. Neuroradiol. 1990;17:26–49. [PubMed] [Google Scholar]
- Shrout P.E. Fleiss J.L. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Shum D. Valentine M. Cutmore T. Performance of individuals with severe long-term traumatic brain injury on time-, event-, and activity-based prospective memory tasks. J. Clin. Exp. Neuropsychol. 1999;21:49–58. doi: 10.1076/jcen.21.1.49.943. [DOI] [PubMed] [Google Scholar]
- Simoes-Franklin C. Hester R. Shpaner M. Foxe J.J. Garavan H. Executive function and error detection: the effect of motivation on cingulate and ventral striatum activity. Hum. Brain Mapp. 2009;31:458–469. doi: 10.1002/hbm.20879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons J.S. Scholvinck M.L. Gilbert S.J. Frith C.D. Burgess P.W. Differential components of prospective memory? Evidence from fMRI. Neuropsychologia. 2006;44:1388–1397. doi: 10.1016/j.neuropsychologia.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Singh M. Jeong J. Hwang D. Sungkarat W. Gruen P. Novel diffusion tensor imaging methodology to detect and quantify injured regions and affected brain pathways in traumatic brain injury. Magn. Reson. Imaging. 2009;28:22–40. doi: 10.1016/j.mri.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeda A. Nakashima T. Okumura A. Kuwata K. Shinoda J. Iwama T. Cognitive impairment after traumatic brain injury: a functional magnetic resonance imaging study using the Stroop task. Neuroradiology. 2005;47:501–506. doi: 10.1007/s00234-005-1372-x. [DOI] [PubMed] [Google Scholar]
- Squire L.R. Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Staudinger M.R. Erk S. Abler B. Walter H. Cognitive reappraisal modulates expected value and prediction error encoding in the ventral striatum. Neuroimage. 2009;47:713–721. doi: 10.1016/j.neuroimage.2009.04.095. [DOI] [PubMed] [Google Scholar]
- Szatkowska I. Bogorodzki P. Wolak T. Marchewka A. Szeszkowski W. The effect of motivation on working memory: an fMRI and SEM study. Neurobiol. Learn. Mem. 2008;90:475–478. doi: 10.1016/j.nlm.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Szeszko P.R. Robinson D.G. Ashtari M. Vogel J. Betensky J. Sevy S. Ardekani B.A. Lencz T. Malhotra A.K. McCormack J. Miller R. Lim K.O. Gunduz-Bruce H. Kane J.M. Bilder R.M. Clinical and neuropsychological correlates of white matter abnormalities in recent onset schizophrenia. Neuropsychopharmacology. 2008;33:976–84. doi: 10.1038/sj.npp.1301480. [DOI] [PubMed] [Google Scholar]
- Taylor H.G. Research on outcomes of pediatric traumatic brain injury: current advances and future directions. Dev. Neuropsychol. 2004;25:199–225. doi: 10.1080/87565641.2004.9651928. [DOI] [PubMed] [Google Scholar]
- Taylor H.G. Yeates K.O. Wade S.L. Drotar D. Klein S.K. Stancin T. Influences on first-year recovery from traumatic brain injury in children. Neuropsychology. 1999;13:76–89. doi: 10.1037//0894-4105.13.1.76. [DOI] [PubMed] [Google Scholar]
- Taylor H.G. Yeates K.O. Wade S.L. Drotar D. Stancin T. Minich N. A prospective study of short- and long-term outcomes after traumatic brain injury in children: behavior and achievement. Neuropsychology. 2002;16:15–27. doi: 10.1037//0894-4105.16.1.15. [DOI] [PubMed] [Google Scholar]
- Teasdale G. Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Thut G. Schultz W. Roelcke U. Nienhusmeier M. Missimer J. Maguire R.P. Leenders K.L. Activation of the human brain by monetary reward. Neuroreport. 1997;8:1225–1228. doi: 10.1097/00001756-199703240-00033. [DOI] [PubMed] [Google Scholar]
- Tranel D. Bechara A. Denburg N.L. Asymmetric functional roles of right and left ventromedial prefrontal cortices in social conduct, decision-making, and emotional processing. Cortex. 2002;38:589–612. doi: 10.1016/s0010-9452(08)70024-8. [DOI] [PubMed] [Google Scholar]
- Ungerleider L.G. Gaffan D. Pelak V.S. Projections from inferior temporal cortex to prefrontal cortex via the uncinate fascicle in rhesus monkeys. Exp. Brain Res. 1989;76:473–784. doi: 10.1007/BF00248903. [DOI] [PubMed] [Google Scholar]
- Valenstein E. Bowers D. Verfaellie M. Heilman K.M. Day A. Watson R.T. Retrosplenial amnesia. Brain. 1987;110(Pt 6):1631–1646. doi: 10.1093/brain/110.6.1631. [DOI] [PubMed] [Google Scholar]
- Voineskos A.N. Rajji T.K. Lobaugh N.J. Miranda D. Shenton M.E. Kennedy J.L. Pollock B.G. Mulsant B.H. Age-related decline in white matter tract integrity and cognitive performance: a DTI tractography and structural equation modeling study. Neurobiol. Aging. 2010. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- Wakana S. Caprihan A. Panzenboeck M.M. Fallon J.H. Perry M. Gollub R.L. Hua K. Zhang J. Jiang H. Dubey P. Blitz A. van Zijl P. Mori S. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Rao H. Wetmore G.S. Furlan P.M. Korczykowski M. Dinges D.F. Detre J.A. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proc. Natl. Acad. Sci. USA. 2005;102:17804–17809. doi: 10.1073/pnas.0503082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.Y. Bakhadirov K. Devous M.D., Sr. Abdi H. McColl R. Moore C. Marquez de la Plata C.D. Ding K. Whittemore A. Babcock E. Rickbeil T. Dobervich J. Kroll D. Dao B. Mohindra N. Madden C.J. Diaz-Arrastia R. Diffusion tensor tractography of traumatic diffuse axonal injury. Arch. Neurol. 2008;65:619–626. doi: 10.1001/archneur.65.5.619. [DOI] [PubMed] [Google Scholar]
- Ward H. Shum D. Dick B. McKinlay L. Baker-Tweney S. Interview study of the effects of paediatric traumatic brain injury on memory. Brain Inj. 2004;18:471–495. doi: 10.1080/02699050310001646107. [DOI] [PubMed] [Google Scholar]
- Ward H. Shum D. McKinlay L. Baker S. Wallace G. Prospective memory and pediatric traumatic brain injury: effects of cognitive demand. Child Neuropsychol. 2007;13:219–239. doi: 10.1080/09297040600910003. [DOI] [PubMed] [Google Scholar]
- Weisskoff R.M. Simple measurement of scanner stability for functional NMR imaging of activation in the brain. Magn. Reson. Med. 1996;36:643–645. doi: 10.1002/mrm.1910360422. [DOI] [PubMed] [Google Scholar]
- West R. Craik F.I. Influences on the efficiency of prospective memory in younger and older adults. Psychol. Aging. 2001;16:682–696. [PubMed] [Google Scholar]
- West R. Krompinger J. Neural correlates of prospective and retrospective memory. Neuropsychologia. 2005;43:418–433. doi: 10.1016/j.neuropsychologia.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Wilde E.A. Bigler E.D. Haider J.M. Chu Z. Levin H.S. Li X. Hunter J.V. Vulnerability of the anterior commissure in moderate to severe pediatric traumatic brain injury. J. Child Neurol. 2006a;21:769–776. doi: 10.1177/08830738060210090201. [DOI] [PubMed] [Google Scholar]
- Wilde E.A. Chu Z. Bigler E.D. Hunter J.V. Fearing M.A. Hanten G. Newsome M.R. Scheibel R.S. Li X. Levin H.S. Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. J. Neurotrauma. 2006b;23:1412–1426. doi: 10.1089/neu.2006.23.1412. [DOI] [PubMed] [Google Scholar]
- Wilde E.A. McCauley S.R. Hunter J.V. Bigler E.D. Chu Z. Wang Z.J. Hanten G.R. Troyanskaya M. Yallampalli R. Li X. Chia J. Levin H.S. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70:948–955. doi: 10.1212/01.wnl.0000305961.68029.54. [DOI] [PubMed] [Google Scholar]
- Wilde E.A. Ramos M.A. Yallampalli R. Bigler E.D. McCauley S.R. Chu Z. Wu T.C. Hanten G. Scheibel R.S. Li X. Vasquez A.C. Hunter J.V. Levin H.S. Diffusion tensor imaging of the cingulum bundle in children after traumatic brain injury. Dev. Neuropsychol. 2010;35:333–351. doi: 10.1080/87565641003696940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D.H. Levin H.S. Eisenberg H.M. Mild head injury classification. Neurosurgery. 1990;27:422–428. doi: 10.1097/00006123-199009000-00014. [DOI] [PubMed] [Google Scholar]
- Winograd E. Some observations on prospective remembering. In: M.M. Gruneberg., editor; P.E. Morris., editor; R.N. Sykes., editor. Practical Aspects of Memory: Current Research and Issues. John Wiley: Oxford, United Kingdom; 1988. pp. 348–353. [Google Scholar]
- Wozniak J.R. Krach L. Ward E. Mueller B.A. Muetzel R. Schnoebelen S. Kiragu A. Lim K.O. Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: a diffusion tensor imaging (DTI) study. Arch. Clin. Neuropsychol. 2007;22:555–568. doi: 10.1016/j.acn.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T.C. Wilde E.A. Bigler E.D. Yallampalli R. McCauley S.R. Troyanskaya M. Chu Z. Li X. Hanten G. Hunter J.V. Levin H.S. Evaluating the relationship between memory functioning and cingulum bundles in acute mild traumatic brain injury using diffusion tensor imaging. J. Neurotrauma. 2010;27:303–307. doi: 10.1089/neu.2009.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates K.O. Taylor H.G. Drotar D. Wade S.L. Klein S. Stancin T. Schatschneider C. Preinjury family environment as a determinant of recovery from traumatic brain injuries in school-age children. J. Int. Neuropsychol. Soc. 1997;3:617–630. [PubMed] [Google Scholar]
- Yount R. Raschke K.A. Biru M. Tate D.F. Miller M.J. Abildskov T. Gandhi P. Ryser D. Hopkins R.O. Bigler E.D. Traumatic brain injury and atrophy of the cingulate gyrus. J. Neuropsychiatry Clin. Neurosci. 2002;14:416–423. doi: 10.1176/jnp.14.4.416. [DOI] [PubMed] [Google Scholar]
- Yung A. Poon G. Qiu D.Q. Chu J. Lam B. Leung C. Goh W. Khong P.L. White matter volume and anisotropy in preterm children: a pilot study of neurocognitive correlates. Pediatr. Res. 2007;61:732–736. doi: 10.1203/pdr.0b013e31805365db. [DOI] [PubMed] [Google Scholar]