Abstract
Background
The amount of cholesterol per LDL particle is variable and related in part to particle size, with smaller particles carrying less cholesterol. This variability causes concentrations of LDL cholesterol (LDL-C) and LDL particles (LDL-P) to be discordant in many individuals.
Methods
LDL-P measured by nuclear magnetic resonance (NMR) spectroscopy, calculated LDL-C, and carotid intima-media thickness (IMT) were assessed at baseline in the Multi-Ethnic Study of Atherosclerosis (MESA), a community-based cohort of 6814 persons free of clinical CVD at entry and followed for CVD events (n=319 during 5.5-year follow-up). Discordance, defined as values of LDL-P and LDL-C differing by ≥ 12 percentile units to give equal-sized concordant and discordant subgroups, was related to CVD events and to carotid IMT in models predicting outcomes for a 1 SD difference in LDL-C or LDL-P, adjusted for age, sex and race.
Results
LDL-C and LDL-P were associated with incident CVD overall: hazard ratios (HR [95% CI]) 1.20 [1.08, 1.34] and 1.32 [1.19, 1.47], respectively, but for those with discordant levels, only LDL-P was associated with incident CVD (HR: 1.45 [1.19, 1.78]) (LDL-C HR: 1.07 [0.88, 1.30])). IMT also tracked with LDL-P rather than LDL-C, i.e., adjusted mean IMT of 958, 932, and 917 μm in the LDL-P > LDL-C discordant, concordant, and LDL-P < LDL-C discordant subgroups, respectively, with the difference persisting after adjustment for LDL-C (p=0.002) but not LDL-P (p=0.60).
Conclusions
For individuals with discordant LDL-C and LDL-P levels, the LDL-attributable atherosclerotic risk is better indicated by LDL-P.
Keywords: LDL particle number, LDL cholesterol, cardiovascular disease risk, NMR, lipoproteins
Low-density lipoprotein (LDL) is conventionally quantified in terms of the mass of cholesterol carried by these particles. LDL cholesterol (LDL-C) has been the standard measure of LDL and LDL-attributable cardiovascular disease (CVD) risk for so long that “LDL” and “LDL-C” tend to be used interchangeably. However, the two terms are not synonymous because the cholesterol content of LDL particles varies more than 2-fold among individuals.1,2 One person may have large, more cholesterol-rich LDL while a second may have smaller cholesterol-poor LDL particles. At the same LDL-C concentration, the second person will have higher numbers of LDL particles.
A priori, it is not clear whether the cholesterol in LDL (LDL-C) or the number of LDL particles would be the more informative marker of LDL-attributable atherosclerotic risk. On the one hand, a more cholesterol-rich LDL particle deposits more cholesterol in the artery wall and from this perspective may be considered more atherogenic than a cholesterol-poor particle. On the other hand, the probability that a particle’s cholesterol will be delivered to an atheroma depends largely on particle number: how many LDL particles enter the artery wall, become oxidized, and are finally taken up by macrophage foam cells.3
Most studies comparing LDL-C and LDL particle number have used plasma apolipoprotein B (apoB) levels for estimation of LDL particle concentration, and have consistently shown apoB to be more strongly associated with CVD than LDL-C.4 However, because the apoB measurement assesses total numbers of LDL plus very-low-density lipoprotein (VLDL) particles, it is uncertain whether the stronger clinical associations of apoB are attributable to LDL particles or to VLDL particles or both. Quantifying LDL particle number (LDL-P) by nuclear magnetic resonance (NMR) spectroscopy5 can help resolve this ambiguity.
LDL-P measured by NMR has, like apoB, been associated more strongly than LDL-C with both preclinical6,7 and clinical2,8-11 atherosclerotic outcomes. The clinical significance to individual patients of these modest population differences in disease association has been unclear. As pointed out recently,12 the conventional approach to comparing the utility of two diagnostic tests by comparing their disease associations in a given population is insensitive if the two tests perform equivalently in a large subset of that population. This problem can be overcome by specifically comparing the two tests in cases in which they disagree - that is, in which they give discordant results.12 With regard to assessment of LDL-attributable risk, clinical significance would accrue only to patients with discordant levels of LDL-C and LDL-P, since individuals with concordant levels should be comparably well served by either analytic measure of LDL.
We used data from the Multi-Ethnic Study of Atherosclerosis (MESA) to examine differences between LDL-C and LDL-P as they relate prospectively to incident CVD events among individuals with concordant and discordant levels. The findings were supplemented by cross-sectional associations of LDL-C and LDL-P in the same population with carotid intimamedia thickness (IMT), an indicator of anatomical atherosclerosis.
METHODS
Study population
Study participants were enrolled in MESA, a multi-center cohort initiated by the National Heart, Lung, and Blood Institute to characterize subclinical atherosclerosis and its progression.13 Eligible participants were 6814 community-based men and women, ages 45-84 years of age and free of self-reported cardiovascular disease, recruited from 4 diverse racial/ethnic groups (African American, Hispanic, White, and Chinese American) at 6 centers in the United States. For examining LDL characteristics and relations with incident CVD, we excluded participants who did not provide informed consent for this ancillary study, those with triglycerides >400 mg/dL or with missing lipid, NMR, or covariate information, leaving 5598 participants for these analyses. For the cross-sectional carotid IMT analyses, we additionally excluded participants on any lipid-lowering medication and those with missing IMT measurements, resulting in a study population of 4499 subjects.
All data, other than incident events, were collected at the first MESA examination (2000-2002).13 The study was approved by the institutional review boards of the participating institutions.
CVD follow-up
The cohort was followed for incident CVD events for a mean of 5.5 years (maximum, 7.0 years). Details of CVD event ascertainment and classification in MESA have been described.14 For this report, incident CVD included myocardial infarction, coronary heart disease death, angina, stroke, stroke death, or other atherosclerotic or CVD death.
Carotid IMT assessment
High-resolution B-mode ultrasound was used to measure carotid IMT. We used the mean of 8 measurements of maximal IMT, which included overt atherosclerotic plaque (right and left, near and far walls, common and internal carotid).15
Risk factor and lipoprotein measurements
Diabetes status was defined as normal, impaired fasting glucose 100 to 125 mg/dL, untreated diabetes mellitus (fasting glucose >125 mg/dL), and treated diabetes mellitus (use of antidiabetic medication). HOMA-IR (homeostasis model assessment of insulin resistance) was calculated as insulin (mU/L) x (glucose [mg/dL] x 0.055)/22.5. Metabolic syndrome was defined according to the revised ATPIII criteria.16
Plasma concentrations of total cholesterol, HDL cholesterol, and triglycerides were measured using blood samples obtained after a 12-hour fast using CDC-standardized methods. Measurements were performed on frozen (−70 deg C) EDTA plasma generally within 2 weeks of blood collection. The Friedewald equation was used to calculate LDL-C.17 LDL-P concentrations (nmol/L) of frozen EDTA plasma specimens were measured by NMR spectroscopy using the LipoProfile-3 algorithm at LipoScience, Inc. (Raleigh, NC). LDL (including intermediate-density lipoprotein) subclasses of different size were quantified from the amplitudes of their spectroscopically distinct lipid methyl group NMR signals.5 LDL-P is the sum of the particle concentrations of the respective LDL subclasses. Inter-assay reproducibility of LDL-P determined from replicate analyses of plasma pools was <4% .
Statistical methods
All analyses were performed using SAS 9.2 (SAS Institute, Cary, NC). Means (±SD) adjusted for age, sex, and race as well as proportions were used to summarize the characteristics of the study sample. Percentile distributions of LDL-C and LDL-P were calculated and their associations with clinical and laboratory characteristics estimated using Spearman rank correlation coefficients. To examine subgroups with concordant (similar) or discordant (dissimilar) levels of LDL-C and LDL-P, we defined “discordance” as values differing by ≥12 percentile units, so that approximately equal numbers of participants would be classified as concordant or discordant. Subgroup differences were evaluated using a χ2 test for categorical variables or an independent-groups t test for continuous variables. Relations of LDL-C and LDL-P with incident CVD events were examined using multivariable Cox proportional hazards regression, adjusting for age, gender, and race/ethnicity (4 groups). Some analyses were adjusted additionally for systolic blood pressure, hypertension treatment, smoking status, body mass index, and diabetes status. Hazard ratios (HRs) and their 95% confidence intervals were determined for a 1-SD increment of each LDL measure. The assumption of proportionality of hazards was confirmed by examining interactions of covariates and survival time in Cox models. Since a substantial proportion of MESA participants were on lipid lowering medication (17.4%), we also repeated all analyses excluding these subjects or adjusting the regression models additionally for lipid medication use. Multiple linear regression analyses were used to investigate relations of LDL-P and LDL-C with carotid IMT, adjusting for age, gender, and race. Additional adjustment for systolic blood pressure was also explored. Coefficients are given as the IMT difference in microns (μm) associated with a 1-SD increment of the LDL measure. Least squares mean IMT values were calculated for subgroups defined by LDL-P or LDL-C tertile. P values were two-tailed and values <0.05 were considered significant.
RESULTS
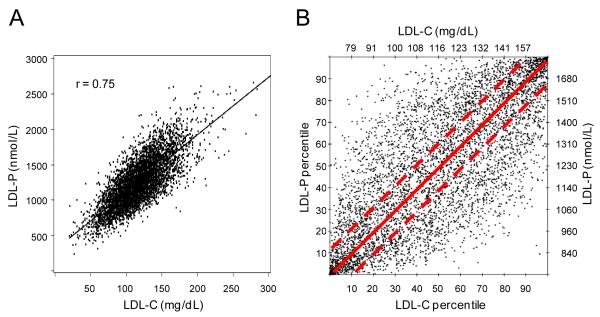
The study population was ethnically-diverse (39% white, 13% Chinese American, 25% African American, 23% Hispanic), with a mean age (±SD) of 62 (±10) years and 51% women. LDL-C and LDL-P levels were highly correlated (Figure 1A; r=0.75) but often discordant. The prevalence and magnitude of this discordance can be seen in Figure 1B, which displays the percentile rank values corresponding to the LDL-C and LDL-P concentrations of each study participant. Although many individuals had concordant levels of LDL-C and LDL-P (points near the diagonal), many others with low LDL-C percentile rank had much higher LDL-P, and vice versa.
Figure 1.
Relations between LDL-C and LDL-P among 5598 MESA participants. (A) Relation of LDL-C and LDL-P concentrations. (B) Relation of LDL-C and LDL-P levels given in percentile units. The dashed lines bracket concordant LDL-C and LDL-P values defined as those within ±12 percentile units.
To explore the origins and potential clinical implications of dissimilarities between these 2 measures of LDL, we examined concordant and discordant subgroups separately, defining discordance as a difference of ≥ 12 percentile units to make half the population “concordant” (points between the dashed lines in Figure 1B). As shown in Table 1, individuals with discordant LDL-P and LDL-C by this definition were divided almost equally into those with relatively cholesterol-poor LDL particles for whom LDL-P was higher than LDL-C percentile rank and those with cholesterol-rich LDL particles for whom LDL-P percentile rank was lower than that of LDL-C. The subgroup with LDL-P > LDL-C discordance, compared to the concordant subgroup, comprised fewer women (43%), had higher prevalence of diabetes, fewer African American and more Hispanic individuals, and also had multiple traits associated with the metabolic syndrome and other known markers of CVD risk: small LDL size, low HDL-C, and elevated triglycerides, glucose, insulin resistance and obesity measures. 54% of this subgroup met the ATPIII definition of metabolic syndrome.16 The other discordant subgroup with LDL-P < LDL-C had the opposite phenotype: more women (62%), less diabetes, and lipid and metabolic characteristics associated with greater insulin sensitivity and lower CVD risk. The correlation coefficients in Table 1 indicate that, with the exception of triglycerides, none of the traits that define the metabolic syndrome were associated with LDL-C, whereas all 5 were significantly associated with LDL-P.
Table 1.
Characteristics of Study Participants with Concordant or Discordant Concentrations of LDL Cholesterol and LDL Particles
| Concordant/Discordant Subgroups | LDL Correlations | ||||
|---|---|---|---|---|---|
| Discordant | Concordant | Discordant | |||
| LDL-P > LDL-C | LDL-P ≈ LDL-C | LDL-P < LDL-C | LDL-C | LDL-P | |
| Number of subjects (%) | 1407 (25) | 2775 (50) | 1416 (25) | --- | --- |
| Age, y | 62 ± 10 | 62 ± 10 | 63 ± 10 | --- | --- |
| Women, % | 43c | 50 | 62c | ||
| Race/ethnicity, % | |||||
| White | 41 | 39 | 39 | --- | --- |
| Chinese American | 11 | 13 | 16a | --- | --- |
| African American | 21b | 25 | 28 | --- | --- |
| Hispanic | 27b | 23 | 17c | --- | --- |
| Hypertension, % | 48a | 44 | 41a | --- | --- |
| Current smoking, % | 14 | 13 | 11 | --- | --- |
| Diabetes, % | 19c | 14 | 9c | --- | --- |
| Metabolic syndrome, % | 54c | 33 | 16c | --- | --- |
| Body mass index, kg/m2 | 29.0 ± 5.3c | 27.8 ± 5.5 | 26.7 ± 5.4c | 0.05b | 0.17c |
| Waist circumference, cm | 100 ± 14c | 97 ± 14 | 94 ± 14c | 0.03 | 0.16c |
| Systolic BP, mmHg | 128 ± 20b | 126 ± 21 | 126 ± 23 | 0.02 | 0.04b |
| Glucose, mg/dL | 111 ± 36c | 106 ± 31 | 101 ± 22c | 0.02 | 0.13c |
| HOMA-IR | 2.4 ± 2.8c | 1.8 ± 1.8 | 1.5 ± 1.8c | 0.02 | 0.21c |
| Triglycerides, mg/dL | 165 ± 73c | 126 ± 62 | 100 ± 50c | 0.10c | 0.39c |
| HDL-C, mg/dL | 44 ±12c | 50 ± 14 | 57 ± 14c | −0.02 | −0.35c |
| LDL-C, mg/dL | 104 ± 20c | 117 ± 37 | 130 ± 23c | --- | 0.75c |
| LDL-P, nmol/L | 1372 ± 240c | 1249 ± 395 | 1117 ± 201c | 0.75c | --- |
| LDL size, nm | 20.3 ± 0.5c | 20.7 ± 0.5 | 21.1 ± 0.4c | 0.04b | −0.39c |
| Cholesterol per LDL* | 1967 ± 200c | 2433 ± 266 | 3039 ± 303c | 0.32c | −0.33c |
| Carotid IMT, μm** | 957 ± 359a | 931 ± 337 | 916 ± 324 | 0.11c | 0.15c |
Values are mean (±SD) adjusted for age, sex, and race, or percentage distribution. P values for comparison of percentages by χ2 tests or means by t test for comparison with concordant subgroup:
P<0.05
P<0.01
P<0.0001.
LDL correlations are Spearman correlation coefficients. Concordant concentrations of LDL-P and LDL-C are defined as those within 12 percentile units; discordant concentrations differ by =12 percentile.
Estimate of the number of cholesterol molecules per LDL particle, calculated by dividing LDL-C (mmol/L) by LDL-P (nmol/L).
Mean IMT values are from the subpopulation (n=4499) not on lipid-lowering drugs: LDL-P > LDL-C discordant (n=1126); concordant (n=2246); LDL-P < LDL-C discordant (n=1127).
Abbreviations: LDL-C, LDL cholesterol; LDL-P, LDL particle number; BP, blood pressure; HOMA-IR, homeostasis model assessment of insulin resistance index; HDL-C, HDL cholesterol.
Relations with incident CVD events
There were 319 CVD events during the mean follow-up of 5.5 years. Baseline levels of LDL-P and LDL-C were both positively associated with future CVD (Table 2). Hazard ratios (95% CI) were 1.20 (1.08, 1.34) for LDL-C (p=0.0009) and 1.32 (1.19, 1.47) for LDL-P (p<0.0001) in models adjusted for age, gender, and race. Additional adjustment for blood pressure, hypertension treatment, smoking, body mass index, and diabetes status did not markedly change these associations: 1.28 (1.15, 1.43) for LDL-C and 1.35 (1.21, 1.50) for LDL-P (p<0.0001 for both).
Table 2.
Associations of LDL Cholesterol and LDL Particle Number with Incident CVD in Participants with Concordant or Discordant Levels
| Model 1 | Model 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Levels (SD) | LDL-C | LDL-P | LDL-C | LDL-P | ||||||||
| Subgroup | n | CVD Events |
LDL-C | LDL-P | HR (95% CI) |
p | HR (95% CI) |
p | HR (95% CI) |
p | HR (95% CI) |
p |
| Overall | 5598 | 319 | 117 (31) |
1249 (333) |
1.20 (1.08, 1.34) |
0.0009 | 1.32 (1.19, 1.47) |
<0.0001 | 1.28 (1.15, 1.43) |
<0.0001 | 1.35 (1.21, 1.50) |
<0.0001 |
| Concordant | 2775 | 160 | 117 (37) |
1252 (395) |
1.27 (1.12, 1.44) |
0.0003 | 1.27 (1.12, 1.44) |
0.0002 | 1.32 (1.16, 1.50) |
<0.0001 | 1.30 (1.14, 1.48) |
<0.0001 |
| Discordant* | 2823 | 159 | 117 (25) |
1246 (259) |
1.07 (0.88, 1.30) |
0.52 | 1.45 (1.19, 1.78) |
0.0003 | 1.17 (0.96, 1.42) |
0.13 | 1.41 (1.15, 1.75) |
0.001 |
Estimates reported are from multivariable Cox regression analyses. Model 1 was adjusted for age, gender and race. Model 2 was adjusted additionally for systolic blood pressure, hypertension treatment, smoking, body mass index, and diabetes status. Hazards ratios (95% confidence intervals) are for a 1-SD increment of LDL-P or LDL-C, using the SD values in the overall population of 333 nmol/L and 31.4 mg/dL, respectively.
This subgroup comprises the combined LDL-P > LDL-C and LDL-P < LDL-C discordant subgroups in Table 1.
Abbreviations: LDL-C, LDL cholesterol; LDL-P, LDL particle number.
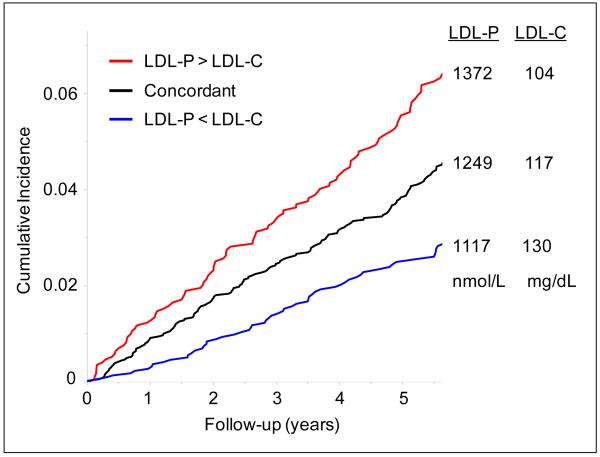
As might have been anticipated based on the subgroup characteristics in Table 1, the participants in the concordant and discordant subgroups differed in CVD risk (Figure 2). During follow-up, 160 CVD events were experienced by individuals with concordant LDL-C and LDL-P (event rate of 10.1 per 1000 person-years, adjusted for age, gender, and race), compared to 101 and 58 events (adjusted rates of 12.5 and 7.3 per 1000 person-years, respectively; p=0.0025) for those with LDL-P > LDL-C and LDL-P < LDL-C discordance, respectively. Mean levels of LDL-P in the 3 subgroups tracked positively with risk, whereas LDL-C levels were inversely related to risk. As a consequence (Table 2), LDL-C was only weakly associated with incident CVD among the 50% of individuals in the combined discordant subgroups (hazard ratio (95% CI) 1.07 (0.88, 1.30); p=0.52 adjusted for age, gender, and race), whereas LDL-P in this subgroup retained a risk association comparable to that of individuals with concordant LDL-P and LDL-C (hazard ratio (95% CI) 1.45 (1.19, 1.78); p=0.0003). These analyses were repeated excluding subjects on lipid lowering medication or including lipid lowering treatment as a covariate in the regression models and the findings were not appreciably altered.
Figure 2.
Cumulative incidence of cardiovascular events in subgroups with concordant or discordant levels of LDL-C and LDL-P, from proportional hazards models adjusted for age, gender, and race. The 3 subgroups are the same as in Table 1; mean levels of LDL-P and LDL-C are adjusted for age, gender, and race.
In fully adjusted models that included LDL-P (same covariates as Model 2 in Table 2), the discordant subgroups did not differ from the concordant group with respect to risk of CVD. When compared with the concordant group, the LDL-P > LDL-C (HR: 1.02 [0.79, 1.32]) and the LDL-P < LDL-C (HR: 0.87 [0.64, 1.19]) groups had similar risk of CVD. However, in analogous models including LDL-C instead of LDL-P, the LDL-P > LDL-C (HR: 1.28 [0.99, 1.67]) group had somewhat higher risk of CVD and the LDL-P < LDL-C (HR: 0.68 [0.50, 0.92]) group had lower risk than the concordant group.
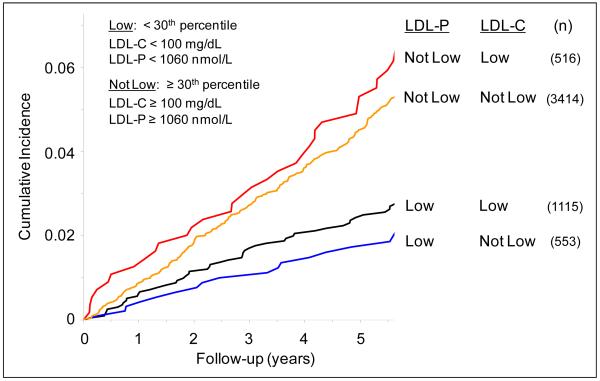
Since low LDL-C levels are used clinically as LDL treatment goals, we next examined the prevalence and clinical consequences of LDL-P discordance among MESA participants with low LDL-C (<100 mg/dL; <30th percentile) or equivalently low LDL-P (<1060 nmol/L; <30th percentile). As shown in Figure 3, 1115 (68%) of 1631 participants with LDL-C <100 mg/dL had equivalently low LDL-P. 50 CVD events were experienced in this subgroup during follow-up, corresponding to an age- and gender-adjusted event rate of 8.2 per 1000 person-years. Among the 516 (32%) individuals with low LDL-C, but discordantly higher LDL-P, there were 33 events (adjusted rate of 11.3 per 1000 person-years) compared to only 18 among participants with low LDL-P, but discordantly higher LDL-C (adjusted rate of 6.2 per 1000 person-years; p=0.055).
Figure 3.
Cumulative incidence of cardiovascular events in subgroups with low LDL-C and/or low LDL-P, from proportional hazards models adjusted for age and gender. Low LDL-C and LDL-P values were defined as < 100 mg/dL and <1060 nmol/L, respectively (<30th percentile).
Relations with carotid IMT
We restricted these cross-sectional analyses to 4499 participants not taking lipid-altering drugs, so that measured LDL values would more closely reflect long-term exposures. In this population, LDL-C and LDL-P were both significantly associated with carotid IMT (p<0.0001). Beta-coefficients from linear regression analyses adjusted for age, gender, and race were 33.2 and 41.5 μm per 1-SD increment of LDL-C and LDL-P, respectively. Additional adjustment for systolic blood pressure modestly attenuated these associations (31.4 vs 38.3 μm). As shown in Table 1, adjusted mean values of carotid IMT trended similarly to CVD event rates in the 3 concordant/discordant subgroups: 958, 932, and 917 μm in the LDL-P > LDL-C discordant, concordant, and LDL-P < LDL-C discordant subgroups, respectively.
In fully adjusted models that included LDL-C, IMT in the LDL-P > LDL-C (25.8 μm [4.2, 47.3]) and the LDL-P < LDL-C (−22.0 μm [−43.7, −0.4]) subgroups differed from that in the concordant group (p= 0.0017). But in analogous models containing LDL-P, the discordant subgroups did not differ from the concordant group with respect to IMT (p= 0.60). When compared with the concordant group, the LDL-P > LDL-C (−3.6 μm [−25.1, 18.0]) and the LDL-P < LDL-C (9.1 μm [−12.4, 30.6]) discordant groups had similar IMT.
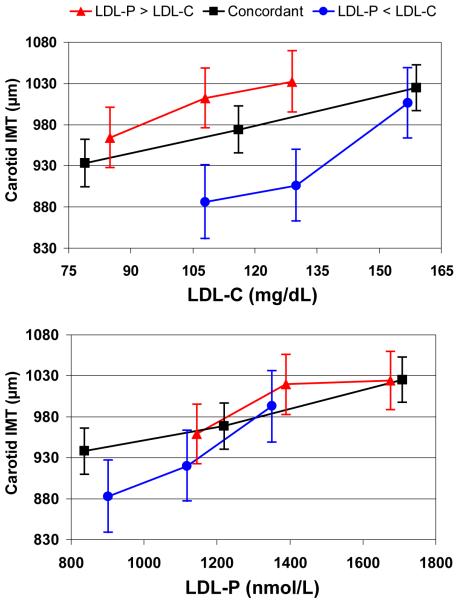
These differences are presented graphically in Figure 4 in terms of adjusted mean IMT values by tertile of LDL-C or LDL-P in the concordant and discordant subgroups. For LDL-P (bottom panel), there was a fairly consistent relationship between LDL-P concentration and increased carotid IMT. Irrespective of the subgroup examined, and despite the marked differences between subgroups in lipid and metabolic characteristics, a given LDL-P level corresponded to approximately the same IMT value. In contrast, LDL-C relations with IMT differed strikingly between the subgroups (top panel). For example, in the LDL-P > LDL-C discordant subgroup, individuals in the 2nd tertile with a mean LDL-C of 108 mg/dL had a mean (95% CI) IMT value of 1012 (975, 1049) μm, whereas those in the 1st tertile of the LDL-P < LDL-C discordant subgroup with exactly the same mean LDL-C of 108 mg/dL had a much lower IMT value of 886 (841, 932) μm.
Figure 4.
Carotid IMT in μm by tertile of LDL-C (top) or LDL-P (bottom) in 3 subgroups with concordant or discordant LDL levels. Least squares mean IMT and 95% confidence intervals are from multiple linear regression models adjusted for age, gender, race, systolic blood pressure, hypertension treatment, smoking, body mass index, and diabetes status. Subgroups analyzed: LDL-P > LDL-C discordant; n=1126 ( ), concordant; n=2246 (
), concordant; n=2246 ( ), LDL-P < LDL-C discordant; n=1127 (
), LDL-P < LDL-C discordant; n=1127 ( ).
).
DISCUSSION
The present study confirms in a large multi-ethnic cohort the wide variability of the cholesterol content of LDL particles. The consequence of this variability is that LDL-C levels either over-represent or under-represent the concentration of LDL particles (LDL-P) in many people. Since it is not obvious from a pathophysiologic standpoint which of the 2 LDL measures would be expected to have a closer link with atherosclerotic risk, we assessed prospective associations with CVD events and cross-sectional associations with carotid IMT separately in individuals with concordant or discordant levels of LDL-C and LDL-P. The results indicate that when the cholesterol and particle measures of LDL disagree, the clinical and subclinical outcomes track with LDL-P more so than with LDL-C. The same conclusion was reached in a prospective study of CVD risk in the Framingham Offspring Study.2
The reasons why the amount of cholesterol per LDL particle varies >2-fold between individuals are well understood mechanistically. The variation is strongly related to triglyceride levels and responsive to metabolic circumstances and lipid-altering treatments.1,2,18,19 LDL size differences are one reason for cholesterol compositional variability, with smaller cholesterol-poor LDL particles predominating when triglycerides are elevated.20 Independent of LDL size, LDL particles can contain more or less cholesterol ester in the particle core.1
Owing to the linkage between triglyceride levels and the size and cholesterol content of LDL particles, many lipid and metabolic variables associated with elevated triglycerides, such as low HDL-C, insulin resistance, diabetes, and obesity, are related to a reduced cholesterol content per LDL particle and hence to LDL-P > LDL-C discordance. Individuals with these lipid and metabolic characteristics unquestionably have enhanced CVD risk. It remains uncertain whether the mechanism(s) responsible for this risk are related primarily to elevations of LDL-P or whether the other variables associated with LDL-P>LDL-C discordance are more relevant than LDL-P from an etiologic perspective. The results of this study support the speculation that this risk is not as independent of LDL as studies equating LDL-C with “LDL” have suggested. Furthermore, it is plausible that elevated LDL particle concentrations might identify in a more straightforward manner those patients likely to benefit from LDL-lowering treatment. This hypothesis should be tested in future trials.
Other clinical implications of our findings require consideration that LDL-C is used for not just one, but several, purposes. These are discussed separately below.
Risk assessment
Although LDL-C is universally recognized as a major CVD risk factor, ATP III guidelines recommend the use of total cholesterol rather than LDL-C for Framingham 10-year risk scoring even though prediction algorithms employing LDL-C are equally discriminating.21 Discordance between LDL-C and LDL-P thus has no direct impact on primary risk stratification conducted with Framingham risk scoring. Nor does LDL-P appreciably improve the performance of a multivariable risk model including LDL-C, HDL-C, triglycerides, and non-lipid risk factors.11 However, ATP III also invoked the concept of metabolic syndrome as a “risk enhancer” because of evidence that, at any given LDL-C level, coronary risk is higher when a patient has metabolic syndrome.21 Whether this enhanced risk comes exclusively from sources “beyond LDL” as commonly assumed, or is related to LDL-C levels in metabolic syndrome patients under-representing LDL (particle) concentrations and LDL-associated risk, is a key question for future research. Our results are consistent with Framingham data indicating that LDL-P > LDL-C discordance is strongly linked to all 5 metabolic syndrome markers.22 It is thus possible that much of the enhanced risk of patients with metabolic syndrome comes from unrecognized LDL-P elevations, with less risk than generally believed coming from the metabolic syndrome components themselves.
Risk management
Once a patient’s coronary risk level has been assessed, guidelines prescribe corresponding LDL-C treatment initiation thresholds and LDL-C goals as the primary focus of lipid-lowering therapy.21 Because LDL-C is used to assess whether LDL-lowering treatment has been successful (goal achievement implying the patient’s risk has been acceptably lowered), any deficiency of LDL-C to accurately reflect LDL concentration and LDL-attributable risk might translate into suboptimal risk management. Patients with cholesterol-poor LDL particles who achieve recommended LDL-C goals will not have achieved correspondingly low LDL-P levels and, as a consequence, may be subject to “residual risk”.18,23,24 In contrast, patients with relatively cholesterol-rich LDL may have adequately low LDL-P despite having LDL-C levels above goal, and therefore may gain little from additional LDL therapy. Our results in Figure 3 support these conjectures, as do those from the Treating to New Targets (TNT) study showing that more intensive LDL-lowering treatment only benefited the subgroup of patients with metabolic syndrome (and inferred LDL-P>LDL-C discordance), not those without metabolic syndrome.25 ATP III included additional recommendations for management of non-HDL-C for patients with elevated triglycerides. These recommendations might mitigate, at least in part, the deficiency of LDL-C to accurately reflect LDL-attributable risk in patients with the metabolic syndrome, but whether this approach is superior to treatment guided by LDL-P is not known and should be the focus of future trials.
CVD epidemiology
Atherosclerotic disease has a complex etiology and other risk factors besides LDL play important causal roles. Our current understandings about the contributions and importance of traditional and “novel” risk factors have been shaped by epidemiologic studies in which LDL-C was used in multivariable models to account for the risk attributable to LDL. Although the ability to form strong inferences regarding biological mechanisms from epidemiologic studies may be questioned by some, it is evident that to the extent LDL-C does not provide a full accounting for LDL-related risk, incorrect conclusions may have been drawn regarding the potential importance of certain “novel” risk factors. One example is small LDL size, which is associated with atherosclerotic risk independently of LDL-C,20 but not LDL-P.11 The former observation led to the belief that small LDL particles are inherently more atherogenic than large ones, a conclusion not supported by recent analyses.7,9,10 Our findings suggest that any risk marker associated with discordance between LDL-C and LDL-P will potentially improve the discrimination of multivariable models containing LDL-C, even if they do not actually contribute to risk independently of LDL (particles). Future studies should take this possibility into consideration, particularly when addressing the potential clinical benefits of treatments targeting non-LDL risk markers. If we have been misled about the etiological relevance of non-LDL risk markers by LDL-P>LDL-C discordance, it is likely that therapies influencing these markers may not influence risk unless they also influence LDL-P.
Surrogate endpoint for CVD
Besides blood pressure, only LDL-C is considered a validated surrogate endpoint by the FDA, meaning that clinical benefit is assumed to result from LDL-C lowering. A potential flaw in this paradigm is that LDL-C changes can result either from changes in LDL (particle) concentration or cholesterol content, or both. Common lipid-altering treatments affect both LDL lipid composition and particle number, causing the magnitude and even direction of changes in LDL-C and LDL-P to differ. Statins reduce LDL particles but also reduce their cholesterol content, thereby reducing LDL-C more than LDL-P.18,23 Hormone replacement therapy in the Women’s Health Initiative had the same effect.26 Treatments that increase LDL size, including niacin, fibrates, glitazones, and therapeutic lifestyle change, will reduce LDL-P more than LDL-C.10,27-29 In light of these findings, evaluation of whether LDL-P might be an even better surrogate CVD endpoint than LDL-C may be warranted.
CONCLUSION
When LDL-P and LDL-C were discordant, LDL-P was more strongly associated with risk of CVD events and with carotid IMT than was LDL-C. This finding has potentially important implications regarding our understanding of the etiology of atherosclerotic cardiovascular disease. The relevance of these findings to the management of risk for cardiovascular disease deserves additional study.
ACKNOWLEDGEMENTS
This research was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute. The NMR lipoprotein particle analyses were donated by LipoScience, Inc. The authors thank the other investigators, staff, and participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. We also thank Dr. Robyn McClelland of the MESA Coordinating Center at the University of Washington for performing independent verification of all statistical analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: This research was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute. NMR lipoprotein particle analyses were donated by LipoScience, Inc. Drs. Otvos and Shalaurova are employees of LipoScience, Inc. Dr. Otvos is also a shareholder of LipoScience, serves as Chief Scientific Officer, and is a member of its Board of Directors.
Contributor Information
James D. Otvos, LipoScience, Inc., Raleigh, NC.
Samia Mora, Center for Cardiovascular Disease Prevention, Department of Medicine, Brigham and Women’s Hospital, and Harvard Medical School, Boston, MA.
Irina Shalaurova, LipoScience, Inc., Raleigh, NC.
Philip Greenland, Departments of Preventive Medicine and Medicine, Feinberg School of Medicine, Northwestern University, Chicago, IL.
Rachel H. Mackey, Department of Epidemiology, University of Pittsburgh, Pittsburgh, PA.
David C. Goff, Jr., Department of Epidemiology and Prevention, Wake Forest University School of Medicine, Winston-Salem, NC.
REFERENCES
- 1.Otvos JD, Jeyarajah EJ, Cromwell WC. Measurement issues related to lipoprotein heterogeneity. Amer J Cardiol. 2002;90(suppl):22i–29i. doi: 10.1016/s0002-9149(02)02632-2. [DOI] [PubMed] [Google Scholar]
- 2.Cromwell WC, Otvos JD, Keyes MJ, Pencina MJ, Sullivan L, Vasan RS, Wilson PWF, D’Agostino RB. LDL particle number and risk for future cardiovascular disease in the Framingham Offspring Study – implications for LDL management. J Clin Lipidol. 2007;1:583–92. doi: 10.1016/j.jacl.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–44. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 4.Brunzell JD, Davidson M, Furberg CD, Goldberg RB, Howard BV, Stein JH, Witzum JL. Lipoprotein management in patients with cardiometabolic risk: consensus conference report from the American Diabetes Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2008;51:1512–24. doi: 10.1016/j.jacc.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 5.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847–70. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Rosenson RS, Otvos JD, Freedman DS. Relations of lipoprotein subclass levels and low-density lipoprotein size to progression of coronary artery disease in the Pravastatin Limitation of Atherosclerosis in the Coronary Arteries (PLAC-I) Trial. Am J Cardiol. 2002;90:89–94. doi: 10.1016/s0002-9149(02)02427-x. [DOI] [PubMed] [Google Scholar]
- 7.Mora S, Szklo M, Otvos JD, Greenland P, Psaty BM, Goff DC, Jr, O’Leary DH, Saad MF, Tsai MY, Sharrett AR. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2007;192:211–7. doi: 10.1016/j.atherosclerosis.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Kuller LH, Arnold A, Tracy R, Otvos J, Burke G, Psaty B, Siscovick D, Freedman DS, Kronmal R. Nuclear magnetic resonance spectroscopy of lipoproteins and risk of coronary heart disease in the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 2002;22:1175–80. doi: 10.1161/01.atv.0000022015.97341.3a. [DOI] [PubMed] [Google Scholar]
- 9.Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009;119:931-9–7. doi: 10.1161/CIRCULATIONAHA.108.816181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otvos JD, Collins D, Freedman DS, Shalaurova I, Schaefer EJ, McNamara JR, Bloomfield HE, Robins SJ. LDL and HDL particle subclasses predict coronary events and are changed favorably by gemfibrozil therapy in the Veterans Affairs HDL Intervention Trial (VA-HIT) Circulation. 2006;113:1556–63. doi: 10.1161/CIRCULATIONAHA.105.565135. [DOI] [PubMed] [Google Scholar]
- 11.El Harchaoui K, van der Steeg WA, Stroes ES, Kuivenhoven JA, Otvos JD, Wareham NJ, Hutten BA, Kastelein JP, Khaw K-T, Boekholdt SM. Value of low-density lipoprotein particle number and size as predictors of coronary artery disease in apparently healthy men and women: the EPIC-Norfolk Proscpective Population Study. J Am Coll Cardiol. 2007;49:547–53. doi: 10.1016/j.jacc.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 12.Glasziou P, Irwig L, Deeks JJ. When should a new test become the current reference standard? Ann Intern Med. 2008;149:816–21. doi: 10.7326/0003-4819-149-11-200812020-00009. [DOI] [PubMed] [Google Scholar]
- 13.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacobs DR, Kronmal R, Liu K, Clark Nelson J, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 14.Folsom AR, Kronmal RA, Detrano RC, O’Leary DH, Bild DE, Bluemke DA, Budoff MJ, Liu K, Shea S, Szklo M, Tracy RP, Watson KE, Burke GL. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence. Arch Intern Med. 2008;168:1333–9. doi: 10.1001/archinte.168.12.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Leary DH, Polak JF, Kronmal RA, Savage PJ, Borhani NO, Kittner SJ, Tracy R, Gardin JM, Price TR, Furberg CD. Thickening of the carotid wall. A marker for atherosclerosis in the elderly? Cardiovascular Health Study Collaborative Research Group. Stroke. 1996;27:224–31. doi: 10.1161/01.str.27.2.224. [DOI] [PubMed] [Google Scholar]
- 16.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 17.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 18.Sniderman AD. Differential response of cholesterol and particle measures of atherogenic lipoproteins to LDL-lowering therapy: implications for clinical practice. J Clin Lipidol. 2008;2:36–42. doi: 10.1016/j.jacl.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Sniderman AD, St-Pierre AC, Cantin B, Dagenais GR, Despres J-P, Lamarche B. Concordance/discordance between plasma apolipoprotein B levels and cholesterol indexes of atherosclerotic risk. Am J Cardiol. 2003;91:1173–77. doi: 10.1016/s0002-9149(03)00262-5. [DOI] [PubMed] [Google Scholar]
- 20.Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res. 2002;43:1363–79. doi: 10.1194/jlr.r200004-jlr200. [DOI] [PubMed] [Google Scholar]
- 21.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 22.Kathiresan S, Otvos JD, Sullivan LM, Keyes MJ, Schaefer EJ, Wilson PW, D’Agostino RB, Vasan RS, Robins SJ. Increased small low-density lipoprotein particle number: a prominent feature of the metabolic syndrome in the Framingham Heart Study. Circulation. 2006;113:20–9. doi: 10.1161/CIRCULATIONAHA.105.567107. [DOI] [PubMed] [Google Scholar]
- 23.Rosenson RS, Otvos JD, Hsia J. Effects of rosuvastatin and atorvastatin on low-density and high-density lipoprotein particle concentrations in patients with the metabolic syndrome: a randomized, double-blind, controlled study. Diabetes Care. 2009;32:1087–91. doi: 10.2337/dc08-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cromwell WC, Otvos JD. Heterogeneity of low-density lipoprotein particle number in patients with type 2 diabetes mellitus and low-density lipoprotein cholesterol <100 mg/dL. Am J Cardiol. 2006;98:1599–1602. doi: 10.1016/j.amjcard.2006.07.036. [DOI] [PubMed] [Google Scholar]
- 25.Deedwania P, Barter P, Carmena R, Fruchart J-C, Grundy SM, Haffner S, Kastelein JJP, LaRosa JC, Schacher H, Shepherd J, Waters DD. Reduction of low-density lipoprotein cholesterol in patients with coronary heart disease and metabolic syndrome: analysis of the Treating to New Targets study. Lancet. 2006;368:919–28. doi: 10.1016/S0140-6736(06)69292-1. [DOI] [PubMed] [Google Scholar]
- 26.Hsia J, Otvos JD, Rossouw JE, Wu L, Wassertheil-Smoller S, Hendrix SL, Robinson JG, Lund B, Kuller LH. Lipoprotein particle concentrations may explain the absence of coronary protection in the Women’s Health Initiative hormone trials. Arterioscler Thromb Vasc Biol. 2008;28:1666–71. doi: 10.1161/ATVBAHA.108.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuvin JT, Dave DM, Sliney KA, Mooney P, Patel AR, Kimmelsteil CD, Karas RH. Effects of extended-release niacin on lipoprotein particle size, distribution, and inflammatory markers in patients with coronary artery disease. Am J Cardiol. 2006;98:743–745. doi: 10.1016/j.amjcard.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Goldberg RB, Kendall DM, Deeg MA, Buse JB, Zagar AJ, Pinaire JA, Tan MH, Khan MA, Perez AT, Jacober SJ. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with Type 2 diabetes and dyslipidemia. Diabetes Care. 2005;18:1547–54. doi: 10.2337/diacare.28.7.1547. [DOI] [PubMed] [Google Scholar]
- 29.Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, Bales CW, Henes S, Samsa GP, Otvos JD, Kulkarni KR, Slentz CA. Effects of the amount and intensity of exercise training on plasma lipoproteins. N Engl J Med. 2002;347:1483–92. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]