SUMMARY
BACKGROUND
Fine-needle aspiration biopsy (FNAB) is a simple, safe and effective method for investigating suspected mycobacterial lymphadenitis in children. Fluorescence microscopy can provide rapid mycobacterial confirmation. Light-emitting diodes (LEDs) provide a cheap and robust excitation light source, making fluorescence microscopy feasible in resource-limited settings.
OBJECTIVE
To compare the diagnostic performance of LED fluorescence microscopy on Papanicolaou (PAP) stained smears with the conventional mercury vapour lamp (MVL).
METHODS
FNAB smears routinely collected from palpable lymph nodes in children with suspected mycobacterial disease were PAP-stained and evaluated by two independent microscopists using different excitatory light sources (MVL and LED). Mycobacterial culture results provided the reference standard. A manually rechargeable battery-powered LED power source was evaluated in a random subset.
RESULTS
We evaluated 182 FNAB smears from 121 children (median age 31 months, interquartile range 10–67). Mycobacterial cultures were positive in 84 of 121 (69%) children. The mean sensitivity with LED (mains-powered), LED (rechargeable battery-powered) and MVL was respectively 48.2%, 50.0% and 51.8% (specificity 78.4%, 86.7% and 78.4%). Inter-observer variation was similar for LED and MVL (κ = 0.5).
CONCLUSION
LED fluorescence microscopy provides a reliable alternative to conventional methods and has many favourable attributes that would facilitate improved, decentralised diagnostic services.
Keywords: LED fluorescence microscopy, fine-needle aspiration biopsy, children, mycobacteria, tuberculosis
CHILDHOOD MYCOBACTERIAL DISEASE is a significant health problem in developing countries. Tuberculosis (TB) is a major cause of disease and death in children in TB-endemic areas, where they carry a significant proportion of the TB disease burden.1–3 Disseminated disease caused by Mycobacterium bovis bacille Calmette-Guérin (BCG) is a serious concern in human immunodeficiency virus (HIV) infected infants who receive BCG vaccination.4 Non-tuberculous mycobacteria (NTM) seems to be uncommon in countries with a high prevalence of TB, although localised and/or disseminated disease may occur.5
The rapid and accurate diagnosis of mycobacterial disease in children remains a challenge due to the low specificity of signs and symptoms, especially in HIV-infected children, difficulties in obtaining bacteriological specimens, the inherent low sensitivities of commonly used diagnostic tests and the fact that children rarely develop sputum smear-positive disease.6 Children with pulmonary TB have extrathoracic disease manifestations in 10–30% of cases. The most common form of extrathoracic disease in children is TB lymphadenitis, which is responsible for up to 50% of all extrathoracic TB.7,8 Palpable peripheral lymph nodes provide an important source of diagnostic material, although it is not always recognised as such.9
Fine-needle aspiration biopsy (FNAB) is a simple, safe and effective method for investigating suspected mycobacterial lymphadenitis in children.9 The conventional Ziehl-Neelsen (ZN) stain for acid-fast bacilli (AFB) is the most widely available method to demonstrate mycobacteria in FNABs; however, its low sensitivity (20–43%) is a major disadvantage.10 Sophisticated new technology such as polymerase chain reaction (PCR) based tests offer exciting opportunities, but these methods are currently too complex and expensive for routine use in low-income countries. Fluorescence microscopy is simple to use and can demonstrate mycobacteria (as well as toxoplasma, Pneumocystis jirovecii, fungi and certain bacteria) in Papanicolaou (PAP) stained smears.11–13 Fluorescent mycobacteria show up as thin, slightly curved brilliant yellow rods, often with a beaded appearance more prominent at the ends. PAP staining is widely and routinely used in most diagnostic cytopathology laboratories, and therefore this technique does not require any additional stains, making it an inexpensive and rapid diagnostic modality.14 Detailed evaluation of cytological abnormalities using light microscopy, combined with direct identification of the organisms mentioned using fluorescence microscopy, provides a powerful diagnostic tool to identify/exclude malignant and/or infectious causes of a persistent lymph node mass. Fluorescence microscopy using the PAP stain was reported to have a diagnostic performance similar to that of the ZN stain for detecting AFB in FNABs.15 Auramine staining may add diagnostic value, as fluorescence microscopy using auramine staining proved to be 10% more sensitive than ZN staining for detecting AFB in sputum specimens;16 however, it does require additional staining over and above the routine PAP stain and excludes subsequent evaluation of cell morphology. Fluorescence microscopy using a modified auramine-rhodamine stain has also been reported to be more sensitive than light microscopy using ZN staining in lymph node aspirates.17
Previously, the high maintenance cost and energy requirements of the mercury vapour lamp (MVL) excitatory light source limited its use in resource-limited settings. This changed with the discovery of ultra-bright light-emitting diodes (LEDs). LED light sources are cheap, robust and extremely durable. Furthermore, they have minimal energy requirements and can be operated from a simple rechargeable battery system to make them completely mains-power independent. LED and MVL light sources offer equivalent performance for sputum fluorescence microscopy using auramine staining.18,19 To date, no studies have been performed on PAP-stained lymph node aspirate biopsies. We compared the diagnostic performance of LED and MVL fluorescence microscopy using routinely collected PAP-stained lymph node aspirate biopsies in children.
MATERIAL AND METHODS
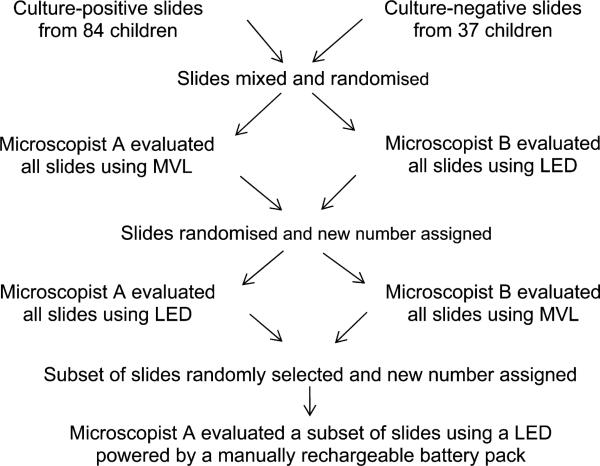
We conducted a retrospective laboratory-based study at Tygerberg Hospital, Cape Town, Western Cape Province, South Africa. All mycobacterial culture-positive FNAB smears from palpable peripheral lymph nodes in children (aged 0–15 years) between 2002 and 2007 were requested from the archive (n = 110). Fifty culture-negative FNAB smears from palpable peripheral lymph nodes in children during the same period were randomly selected. Twenty-six cases from the culture-positive group and 13 cases from the culture-negative group were excluded due to missing PAP-stained slides or lack of cellular material on the slides. All the FNABs were initially performed to rule out mycobacterial infection in children with enlarged lymph nodes. The slides from both groups were mixed and labelled with a random study number to conceal any identifying information (Figure). All slides were independently evaluated for the presence of mycobacteria by two microscopists blinded to all clinical and laboratory information. In the first round, all slides were evaluated by microscopist A using a Zeiss Axiophot (Zeiss, Jena, Germany) conventional fluorescence microscope, while microscopist B used an in-house modified Olympus BX41 (Olympus, Tokyo, Japan) fitted with a royal blue LED light source (peak wave length ±450 nm, 350 mA, 1W, Royal Blue Luxeon emitter, Philips Lumileds Lighting Company, San Jose, CA, USA). The microscopists swapped microscopes for the second round and all the slides were again evaluated for the presence of mycobacteria. Slides were randomly re-assorted and relabelled between rounds, and separate data capture sheets were used for each modality to eliminate the possibility of the first reading influencing the second. All slides were examined using 400× magnification (10× eye piece and 40× objective). Mains electricity was used as power source in the first two rounds. A minimum of 100 high-power fields (HPF) per slide were examined, and slides were graded as 0 (no AFB), 1+ (1–9 AFB/100 HPF), 2+ (1–9 AFB/10 HPF), 3+ (1–9 AFB/HPF) or 4+ (>9 AFB/HPF). In addition, one microscopist evaluated a randomly selected subset of slides using a manually rechargeable battery as power source for the LED microscope.
Figure.
Overview of evaluation procedures. MVL = mercury vapour lamp; LED = light-emitting diode.
Anonymous unlinked data were entered into an Excel spreadsheet (Microsoft, Redmonds, WA, USA). Descriptive and comparative data analysis was performed using Stata 10 (Stata Statistical Software, Release 10, Statacorp 2007, College Station, TX, USA). Sensitivity and specificity and inter-reader agreement were compared among all modalities, using culture as the reference standard. Material for culture was obtained at the time of the initial FNAB according to a standard protocol. The needle and syringe used to prepare the initial cytological smears were rinsed by withdrawing an aliquot of liquid growth media into the syringe and discharging the contents back into a Mycobacterial Growth Indicator Tube (MGIT; BD Diagnostic Systems, Sparks, MD, USA). The inoculated MGIT tubes were incubated for 42 days. Positive cultures were speciated by PCR amplification of the TBD1 (M. tuberculosis deletion 1), regions of difference 10 (RD10) and RD1, as necessary. Sensitivity and specificity were calculated per case (if one of two slides from the same patient was positive the case was regarded as positive), but intra- and inter-observer agreement were calculated on a slide by slide basis.
Ethics approval was granted by the Committee for Human Research, University of Stellenbosch, Tygerberg, South Africa (Protocol N08/06/177).
RESULTS
A total of 182 FNAB slides from 121 children were evaluated. All 182 slides were evaluated with LED and MVL fluorescence microscopy using mains electricity as power source. A random subset of 45 slides was evaluated with LED fluorescence microscopy using a manually rechargeable battery pack as power source.
Patient demographics and sample characteristics are shown in Tables 1 and 2. Compared to mycobacterial culture as the reference standard, the diagnostic performance of all three modalities was comparable (Table 3). The mean sensitivity obtained with fluorescence microscopy compared to culture was respectively 48.2%, 50.0% and 51.8% with LED (mains-powered), LED (rechargeable battery-powered) and MVL, when a positive smear was regarded as ≥1+; mean specificity 78.4%, 86.7% and 78.4%. Specificity increased to 85.1%, 100% and 89.2%, while sensitivity decreased to 31.5%, 42.7% and 34.5% when a positive smear was defined as ≥2+. The inter-observer variation was identical for LED and MVL with a kappa (κ) value of 0.5 (moderate agreement). This improved for both LED (κ = 0.6, moderate agreement) and MVL (κ = 0.7, substantial agreement) when a positive smear was defined as ≥2+. In three smears from two children, both microscopists visualised abundant mycobacteria, although the culture was negative. The differences in culture positivity and FNAB positivity in relation to HIV status are shown in Table 4. The mean time spent reading a negative smear was 3.2 and 3.9 min with LED and MVL, respectively.
Table 1.
Demographics and sample characteristics of children in whom routine fine needle aspiration biopsies were performed (n = 121)
| Demographics | n (%) |
|---|---|
| Age, years | |
| ≤1 | 39 (32) |
| 1–5 | 51 (42) |
| 5–10 | 21 (18) |
| 10–15 | 10 (8) |
| Sex | |
| Male | 64 (53) |
| HIV status | |
| Positive | 29 (24) |
| Negative | 36 (30) |
| Unknown | 56 (46) |
| Sample characteristics | |
| Site of lymph node | |
| Cervical | 64 (53) |
| Axillary | 29 (24) |
| Other | 9 (7) |
| Not specified | 19 (16) |
| Mycobacterial cultures | |
| Positive | 84 (69) |
| Negative | 37 (31) |
HIV = human immunodeficiency virus.
Table 2.
Mycobacteria isolated on culture, age distribution and site of FNAB
| Site of FNAB |
|||||||
|---|---|---|---|---|---|---|---|
| Mycobacteria isolated | n/N (%) | Median age (IQR) months | Cervical node | Axillary node* | Inguinal node | Other | Site not given |
| All | 84/121 (69) | 42 | 24 | 2 | 5 | 11 | |
| M. tuberculosis | 54/84 (64) | 44.5 (25–72) | 35 | 4 | 2 | 4 | 9 |
| M. bovis BCG | 23/84 (28) | 4 (3.6–6) | 1 | 19 | 0 | 1 | 2 |
| M. tuberculosis complex, NOS | 6/84 (7) | 32 (14.8–63.5) | 5 | 1 | 0 | 0 | 0 |
| Mycobacterial species NOS | 1/84 (1) | 11† | 1 | 0 | 0 | 0 | 0 |
Side not specified.
One case only.
FNAB = fine-needle aspirate biopsy; IQR = interquartile range; BCG = bacilli Calmette-Guérin; NOS = not otherwise specified.
Table 3.
Diagnostic performance of fluorescence microscopy using different light and/or power sources compared to mycobacterial culture as the reference standard
| Mercury vapour lamp |
LED, mains electricity |
LED, battery-operated |
||||
|---|---|---|---|---|---|---|
| Diagnostic performance | n/N | % (95%CI) | n/N | % (95%CI) | n/N | % (95%CI) |
| Sensitivity* | ||||||
| ≥1+ positive | 43.5/84 | 51.8 (40.2–60.1) | 40.5/84 | 48.2 (38.4–59.3) | 14/28 | 50.0 (32.6–67.4) |
| ≥2+ positive | 29/84 | 34.5 (25.2–45.2) | 26.5/84 | 31.5 (23.1–42.7) | 12/28 | 42.9 (26.5–60.9) |
| Specificity* | ||||||
| ≥1+ positive | 29/37 | 78.4 (62.8–88.6) | 29/37 | 78.4 (62.8–88.6) | 13/15 | 86.7 (62.1–96.3) |
| ≥2+ positive | 33/37 | 89.2 (75.3–95.7) | 31.5/37 | 85.1 (68.7–92.4) | 15/15 | 100.0 (79.6–100) |
| PPV, %† | ||||||
| ≥1+ positive | 66.2 | 64.6 | 75.4 | |||
| ≥2+ positive | 72.3 | 63.5 | 100 | |||
| NPV, %† | ||||||
| ≥1+ positive | 66.5 | 64.9 | 67.9 | |||
| ≥2+ positive | 62.5 | 60.3 | 68.1 | |||
| κ value inter-observer variation | ||||||
| ≥1+ positive | 0.5 (0.37–0.63) | 0.5 (0.37–0.63) | NA | |||
| ≥2+ positive | 0.7 (0.59–0.82) | 0.6 (0.49–0.75) | NA | |||
Average for the two readers combined.
Calculated by using Bayes’ theorem; prior prevalence has been estimated at 45% from a previous prospective study.15
LED = light-emitting diode; CI = confidence interval; PPV = positive predictive value; NPV = negative predictive value; NA = not applicable; performed on a subgroup and read by a single reader.
Table 4.
Culture and FNAB positivity in relation to HIV status*
| HIV-positive n/N (%) | HIV-negative n/N (%) | P value | |
|---|---|---|---|
| Culture-positive | 25/29 (86.2) | 27/36 (75) | 0.263 |
| FNAB-positive† | 19/29 (65.5) | 9/36 (25) | 0.001 |
HIV status unknown: culture-positive 32/56 (57.1%); FNAB-positive 6/56 (10.7%).
Positive defined as graded ≥1+ on more occasions than not by both reviewers.
FNAB = fine-needle aspirate biopsy; HIV = human immunodeficiency virus.
DISCUSSION
No statistically significant differences were noted in the diagnostic performance of PAP-stained fluorescence microscopy using LED and MVL (both mains electricity) as a light source, or LED using a manually rechargeable battery pack. This demonstrates that LED technology provides a reliable alternative light source for fluorescence microscopy, with many other added advantages over conventional fluorescent light sources.20 Ultra-bright LEDs are much cheaper than conventional light sources and extremely durable, with an expected lifespan of 30 000–50 000 hours. Its user-friendliness is further enhanced, as no warm-up period is required, minimal heat is produced and good image quality is obtained even if used in a room that is not completely darkened.21 Portable manually rechargeable battery operation or solar power are feasible power source alternatives due to its minimal energy requirements, which makes it ideally suited to resource-limited settings where a dependable power supply may be lacking. In addition, LEDs do not contain any environmentally hazardous material.
HIV-positive children were more likely to have positive FNAB smears than HIV-negative children, although the culture-positive proportion was similar in both groups (Table 4). This can be explained by a higher bacillary load in lymph nodes of immune-compromised patients (as opposed to a lower bacillary load in sputum due to a lack of cavitatory disease). Unfortunately, the results are limited by small numbers and a relatively large percentage of cases with unknown HIV status.
This is the first study that compared LED fluorescence microscopy and conventional fluorescence microscopy using routinely collected PAP-stained lymph node aspirate biopsies. Previous studies that compared LED with conventional MVL microscopy used auramine stained sputum smears and also found the diagnostic performance of the two methods to be comparable.18,19 The main advantage of using PAP rather than auramine staining on FNAB smears is that no additional staining is required (PAP stain being the routine stain in most cytopathology laboratories), resulting in a rapid and inexpensive method. Another major advantage is that cell morphology is preserved, which allows simultaneous or subsequent evaluation for malignancy (e.g., Hodgkin's lymphoma) and other cytopathological diagnoses.
The sensitivity in this study was lower than previous reports using PAP-stained fluorescence microscopy in adults.15 As this was a retrospective study, it is possible that some of the slides in the current study had been stained by the rapid PAP method (which in our experience is not effective in rendering bacilli fluorescent). Two passes are routinely performed in our clinic; it is possible that the slide with the best material was used for the standard ZN stain and the poorer quality slide was available for the study. A minimum of a hundred HPF were screened to simulate normal working conditions in a busy cytopathology laboratory. In everyday practice, the clinical information and cytomorphology would probably influence the pathologist to scan more fields in a case where there is a strong clinical or cytomorphological suspicion for mycobacterial infection.
An important obstacle was the presence of small, naturally fluorescent particles in some smears. These particles resembled AFB in some instances, creating the potential for false-positive results. In smears with a low density of bacilli, the presence of these particles may have created enough doubt in the microscopist's mind to disregard one or two true bacilli (false-negatives). These problems were also raised in a study that utilised auramine staining on lymph node aspirates.17 The effect of this can be seen in the improvement in inter- and intra-observer correlation when a cut-off of 2+ rather than 1+ was used to define a positive case.
Similarly, a number of slides had poor quality coverslips containing fluorescent particles, which interfered with the ability to screen the smears. In everyday practice, these smears would have been returned to the laboratory for remounting, and the coverslips replaced. Fading of the stain may also have impacted negatively on the sensitivity as there was a delay between staining and evaluating the slides.
The strength of this method is that fluorescence microscopy can be used as a rapid and inexpensive first-round test on lymph node aspirates. Aspirates with 1–9 AFB/10 HPF or more can confidently be diagnosed as positive without the need for further staining, thereby reducing workload and cost. Results can theoretically be available within 1 h from the time of FNAB, and can thus shorten diagnostic and treatment delay in a subset of patients with a high bacillary load. The use of a rapid/ultrafast PAP stain can further reduce the turnaround time; however, in our experience this may reduce sensitivity compared with standard PAP staining. Aspirates with a low load of bacilli or no bacilli on fluorescence require further investigation with additional staining, such as ZN or auramine.
Our results are limited by the fact that this was a retrospective study. Prospective studies are needed to evaluate novel methods to further optimise diagnostic utility, including the optimal wavelength at which mycobacteria in a PAP stain fluoresce, and the specific ingredient or combination of ingredients that renders the organisms fluorescent. Mycobacterial culture was used as the reference standard. Three smears from two children, in which abundant mycobacteria were seen but where the mycobacterial cultures were persistently negative, in all likelihood represent false-negative cultures. M. tuberculosis was cultured in subsequent gastric aspirates from one of these children.
The current study confirms that LED provides a reliable alternative light source for fluorescence microscopy, but, more importantly, that this can be achieved using routine PAP staining allowing simultaneous cytopathology evaluation. LED has many favourable attributes that would facilitate improved, decentralised diagnostic services in even the most r esource-limited settings.
Acknowledgements
This study was supported by SATBAT—a South African/US research training collaboration (National Institutes of Health [NIH]/Fogarty International Center [FIC] award nos. 1U2RTW007373 and 5U2RTW007370). The content is solely the responsibility of the authors and does not necessarily represent the official views of the FIC or the NIH.
References
- 1.Donald PR. Childhood tuberculosis: out of control? Curr Opin Pulm Med. 2002;8:178–182. doi: 10.1097/00063198-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Chintu C, Mudenda V, Lucas S, et al. Lung diseases at necropsy in African children dying from respiratory illnesses: a descriptive necropsy study. Lancet. 2002;360:985–990. doi: 10.1016/S0140-6736(02)11082-8. [DOI] [PubMed] [Google Scholar]
- 3.Marais BJ, Hesseling AC, Gie RP, Schaaf HS, Beyers N. The burden of childhood tuberculosis and the accuracy of community-based surveillance data. Int J Tuberc Lung Dis. 2006;10:259–263. [PubMed] [Google Scholar]
- 4.Hesseling AC, Johnson LF, Jaspan H, et al. Disseminated bacille Calmette-Guerin disease in HIV-infected South African infants. Bull World Health Organ. 2009;87:505–511. doi: 10.2471/BLT.08.055657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zar HJ. Chronic lung disease in human immunodeficiency virus (HIV) infected children. Pediatr Pulmonol. 2008;43:1–10. doi: 10.1002/ppul.20676. [DOI] [PubMed] [Google Scholar]
- 6.Eamranond P, Jaramillo E. Tuberculosis in children: reassessing the need for improved diagnosis in global control strategies. Int J Tuberc Lung Dis. 2001;5:594–603. [PubMed] [Google Scholar]
- 7.Shingadia D, Novelli V. Diagnosis and treatment of tuberculosis in children. Lancet Infect Dis. 2003;3:624–632. doi: 10.1016/s1473-3099(03)00771-0. [DOI] [PubMed] [Google Scholar]
- 8.Cruz AT, Starke JR. Clinical manifestations of tuberculosis in children. Paediatr Respir Rev. 2007;8:107–117. doi: 10.1016/j.prrv.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Wright CA, Warren RM, Marais BJ. Fine-needle aspiration biopsy: an undervalued diagnostic modality in paediatric myco -bacterial disease. Int J Tuberc Lung Dis. 2009;13:1467–1475. [PubMed] [Google Scholar]
- 10.Daniel TM. Rapid diagnosis of tuberculosis: laboratory techniques applicable in developing countries. Rev Infect Dis. 1989;11(Suppl 2):S471–S478. doi: 10.1093/clinids/11.supplement_2.s471. [DOI] [PubMed] [Google Scholar]
- 11.Kupper T, Steffen U, Wehle K, Richartz G, Pfitzer P. Morphological study of bacteria of the respiratory system using fluorescence microscopy of Papanicolaou-stained smears with special regard to the identification of Mycobacteria sp. Cytopathology. 1995;6:388–402. doi: 10.1111/j.1365-2303.1995.tb00486.x. [DOI] [PubMed] [Google Scholar]
- 12.Kupper T, Wehle K, Marzahn S, Pfitzer P. The cytologic diagnosis of Mycobacterium kansasii tuberculosis by fluorescence microscopy of Papanicolaou-stained specimens. Cytopathology. 1995;6:331–338. doi: 10.1111/j.1365-2303.1995.tb00579.x. [DOI] [PubMed] [Google Scholar]
- 13.Zaharopoulos P. Demonstration of parasites in toxoplasma lymphadenitis by fine-needle aspiration cytology: report of two cases. Diagn Cytopathol. 2000;22:11–15. doi: 10.1002/(sici)1097-0339(200001)22:1<11::aid-dc4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 14.Wright CA, van Zyl Y, Burgess SM, Blumberg L, Leiman G. Mycobacterial autofluorescence in Papanicolaou-stained lymph node aspirates: a glimmer in the dark? Diagn Cytopathol. 2004;30:257–260. doi: 10.1002/dc.20009. [DOI] [PubMed] [Google Scholar]
- 15.Wright CA, van der Burg M, Geiger D, Noordzij JG, Burgess SM, Marais BJ. Diagnosing mycobacterial lymphadenitis in children using fine needle aspiration biopsy: cytomorphology, ZN staining and autofluorescence—making more of less. Diagn Cytopathol. 2008;36:245–251. doi: 10.1002/dc.20788. [DOI] [PubMed] [Google Scholar]
- 16.Steingart KR, Henry M, Ng V, et al. Fluorescence versus conventional sputum smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis. 2006;6:570–581. doi: 10.1016/S1473-3099(06)70578-3. [DOI] [PubMed] [Google Scholar]
- 17.Annam V, Kulkarni MH, Puranik RB. Comparison of the modified fluorescent method and conventional Ziehl-Neelsen method in the detection of acidfast bacilli in lymph node aspirates. Cytojournal. 2009;6:13. doi: 10.4103/1742-6413.53887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marais BJ, Brittle W, Painczyk K, et al. Use of light-emitting diode fluorescence microscopy to detect acid-fast bacilli in sputum. Clin Infect Dis. 2008;47:203–207. doi: 10.1086/589248. [DOI] [PubMed] [Google Scholar]
- 19.Trusov A, Bumgarner R, Valijev R, et al. Comparison of Lumin™ LED fluorescent attachment, fluorescent microscopy and Ziehl-Neelsen for AFB diagnosis. Int J Tuberc Lung Dis. 2009;13:836–841. [PubMed] [Google Scholar]
- 20.Minion J, Sohn H, Pai M. Light-emitting diode technologies for TB diagnosis: what is on the market? Expert Rev Med Devices. 2009;6:341–345. doi: 10.1586/erd.09.26. [DOI] [PubMed] [Google Scholar]
- 21.Hung NV, Sy DN, Anthony RM, Cobelens FG, van Soolingen D. Fluorescence microscopy for tuberculosis diagnosis. Lancet Infect Dis. 2007;7:238–239. doi: 10.1016/S1473-3099(07)70059-2. author reply 239–240. [DOI] [PubMed] [Google Scholar]