Abstract
It is well known that many of the actions of 17β-estradiol (E2) in the central nervous system are mediated via intracellular receptor/transcription factors that interact with steroid response elements on target genes. However, there is compelling evidence for membrane steroid receptors for estrogen in hypothalamic and other brain neurons. Yet, it is not well understood how estrogen signals via membrane receptors, and how these signals impact not only membrane excitability but also gene transcription in neurons that modulate GnRH neuronal excitability. Indeed, it has been known for sometime that E2 can rapidly alter neuronal activity within seconds, indicating that some cellular effects can occur via membrane delimited events. In addition, E2 can affect second messenger systems including calcium mobilization and a plethora of kinases to alter cell signaling. Therefore, this review will consider our current knowledge of rapid membrane-initiated and intracellular signaling by E2 in hypothalamic neurons critical for reproductive function.
Keywords: 17β-estradiol (E2), estrogen receptor-α (ERα), membrane E2 receptor (mER), Proopiomelanocortin (POMC) and γ-aminobutyric acid (GABA) neurons
It has been known for decades that 17β-estradiol (E2) has acute, rapid actions in CNS neurons including GnRH neurons (Kelly, et al., 1976; Lagrange, et al., 1995; Kelly and Rønnekleiv 2002; Kelly and Lagrange, 1998; Rønnekleiv and Kelly, 2005; Bryant, et al., 2006; Micevych and Mermelstein, 2008; Kelly and Rønnekleiv, 2009). Although the molecular mechanisms underlying these actions are still under intense investigation, it is now generally accepted that these rapid actions of E2 cannot be attributed to the classical nuclear-initiated steroid signaling of ERα or ERβ. One potential mode of rapid signaling is via ERα and ERβ signaling complexes within lipid rafts that activate multiple signaling pathways (Razandi, et al., 1999; Boulware, et al., 2005; Pedram, et al., 2006; Szegõ, et al., 2006; Dewing, et al., 2007; Micevych and Mermelstein, 2008). But it is also apparent that E2 can activate G protein-coupled receptors to modulate GnRH neuronal excitability through both pre- and postsynaptic mechanisms (Gu, et al., 1999; Toran-Allerand, 2004; Toran-Allerand, 2005; Qiu, et al., 2003; Qiu, et al., 2006b; Qiu, et al., 2008; Noel, et al., 2009; Zhang, et al., 2010). This review will concentrate on the rapid presynaptic actions since other chapters have focused on postsynaptic mechanisms of E2 signaling in GnRH neurons.
1. Rapid E2 signaling via a Gαq-mER in POMC and GABA neurons
POMC neurons are the “command” neurons of the hypothalamus. POMC neurons synapse directly on and modulate the excitability of neurosecretory neurons including GnRH, dopamine, corticotropin releasing hormone (CRH), oxytocin and vasopressin neurons (Khachaturian, et al., 1985; Zheng, et al., 2005). The opioid peptide β-endorphin (βEND), a major posttranslational product of POMC neurons, is released into the synaptic cleft and binds to the μ-opioid receptor postsynaptically to activate G protein-coupled, inwardly-rectifying K+ channels (GIRK) in these neurosecretory neurons to inhibit their output (North, 1986). In women, naloxone infusion (i.v.) during the late follicular phase increases serum LH release and advances ovulation (Rossmanith, et al., 1988; Genazzani, et al., 1993). In fact, one of the the most efficacious treatments of hypothalamic amenorrhea is daily treatment with the opioid receptor antagonist naltrexone, which restores normal ovulatory cyclicity in these women (Wildt, et al., 1993). Therefore, there is evidence for tonic opioidergic control of GnRH neurons, which can go awry in pathological states. In addition to controlling neurosecretory neuronal activity, POMC (βEND) neurons also project to hypothalamic and extrahypothalamic nuclei to modulate motivated (natural reward) behaviors such as sex and maternal behavior (Koob, 1992).
So what are the physiological consequences of μ-opioid and GABAB receptor input to GnRH neurons? GnRH neurons express a number of channels that underlie burst firing activity and are only activated at membrane potentials below their resting membrane potential of −63 mV (Zhang, et al., 2007; Zhang, et al., 2009a). These channels include the hyperpolarization-activated non-selective cation channel (HCN1-4) and T-type calcium channels (Cav3.1-3.3) (see Rønnekleiv et al., chapter). In order to reach potentials at which these channels are recruited, activation of K+ (e.g., Kir) channels is critical (Hille 2001). GIRK channels expressed in GnRH neurons fulfill this role. A number of other neurotransmitters are also Gαi,o coupled to GIRK channels in GnRH and in melanin-concentrating hormone (MCH) and NPY neurons (Wu, et al., 2009; Xu, et al., 2009).
Interestingly, μ-opioid and GABAB receptors are coupled to the same population of GIRK channels in POMC, GABA, dopamine and GnRH neurons that produce a pronounced hyperpolarization when activated (Loose, et al., 1990; Kelly, et al., 1992; Lagrange, et al., 1994; Wagner, et al., 2001a; Zhang, et al., 2009b). However, in POMC and GABA neurons, which are presynaptic to GnRH neurons, a relative short (<20 min) exposure to E2 or BSA-E2 in vitro causes a four-fold decrease in the potency of μ-opioid and GABAB receptor agonists to inhibit those neurons through a heterologous desensitization via activation of a membrane estrogen receptor (mER) -initiated signaling cascade (Lagrange, et al., 1994; Lagrange, et al., 1996; Qiu, et al., 2003). Therefore, it is apparent that E2, via a putative mER-initiated signaling pathway, can rapidly increase POMC and GABA neuronal excitability that can subsequently hyperpolarize and recruit the channels underlying burst firing (e.g., T-type calcium channels) in GnRH neurons (Zhang, et al., 2009b).
Because of the critical role of POMC and GABA neurons in modulating neurosecretory (GnRH) neuronal activity (Lagrange, et al., 1995), a substantial effort has been exerted to elucidate the mER-mediated signaling pathway (s) using both sharp electrode intracellular and whole cell recording from guinea pig and mouse hypothalamic slices (Lagrange, et al., 1994; Lagrange, et al., 1997; Qiu, et al., 2003; Qiu, et al., 2006b). Indeed, these studies have established that E2 acts stereospecifically with a physiologically-relevant concentration (EC50 = 8 nM) to attenuate the potency of μ-opioid and GABAB agonists in activating GIRK channels (Lagrange, et al., 1997; Qiu, et al., 2003). [Note: The EC50 is the concentration of agonist required to produce fifty percent of the maximum effect in an experimental preparation. The value is obtained from a theoretical curve fitted to experimental data points. The potency (i.e., EC50) is dependent on the binding affinity, the efficacy of agonist, the receptor reserve in the tissue, and the ability of the agonist to penetrate to the site of action (Furchgott, 1978).] Furthermore, estrogenic modulation of μ-opioid and GABAB agonists potency is mimicked by stimulation of adenylyl cyclase with forskolin or by direct protein kinase A (PKA) activation with Sp-cAMP, in a concentration-dependent manner (Lagrange, et al., 1997; Qiu, et al., 2003). On the other hand, selective PKA antagonists such as KT5720 and Rp-cAMP can block the effects of E2. The activation of PKA is downstream in a signaling cascade that is initiated by a Gαq-coupled membrane ER that is linked to activation of phospholipase C (PLC)-protein kinase C (PKC)-PKA (Qiu, et al., 2003; Qiu, et al., 2006b). Hence, this receptor appears to be a Gαq-coupled mER. E2 does not compete for the μ-opioid (or GABAB) receptor or alter the binding affinities of selective ligands with their receptors (Cunningham, et al., 1998). Most importantly, the ER antagonists ICI 164,384 and ICI 182,780 blocked the actions of E2 with subnanomolar affinity that is similar to ICI’s affinity (Ki) for ERα (Weatherill, et al., 1988; Lagrange, et al., 1997), which is the sine qua non for establishing an ER-mediated mechanism. However although ICI antagonized this rapid response in POMC neurons, this Gq-coupled mER is not ERα or ERβ since it can be activated by a diphenylacrylamide compound, STX, that does not bind to ERα or ERβ (Qiu, et al., 2003; Qiu, et al., 2006b). STX (and the endogenous ligand E2) selectively target this Gαq-coupled PLC-PKC-PKA pathway in female guinea pig and mouse POMC neurons and can activate this Gαq-mER signaling pathway in mice deficient in ERα, ERβ and GPR30 (Qiu, et al., 2006b; Qiu, et al., 2008). However, identification (cloning) of the gene encoding the receptor is necessary for definitive characterization of this Gαq-mER.
2. Rapid E2 signaling in GnRH neurons via Gαq-mER
Besides the presynaptic actions of E2 to modulate POMC and GABA input onto GnRH neurons there are a number of direct effects of the steroid on GnRH neuronal excitability (see Rønnekleiv et al., chapter). However, one of the key actions that we identified some years ago was a rapid, direct hyperpolarization of guinea pig GnRH neurons by nanomolar concentrations of E2 via activation of inwardly rectifying K+ channels (Kelly, et al., 1984; Condon, et al., 1989; Lagrange, et al., 1995). In addition to expression of Gαi,o receptors coupled to GIRK channels, GnRH neurons also express inwardly-rectifying K-ATP (Kir 6.2 and SUR1) channels (Zhang, et al., 2007). In fact, the K-ATP channel activity is increased by two-fold in E2-treated animals in synaptically isolated GnRH neurons (Zhang, et al., 2007). Recently, we have identified that both E2 and STX rapidly activate the Gαq-mediated signaling cascade, coupled to PLC-PKC-PKA signaling pathways, to activate K-ATP channels and hyperpolarize GnRH neurons (Zhang, et al., 2010). In addition to the synaptic activation of GIRK channels, this membrane hyperpolarization would also facilitate the recruitment of conductances critical for bursting activity (see Rønnekleiv et al., chapter).
3. Interaction of ER with Insulin-like Growth Factor-1 receptor and reproduction
Systemic administration of E2 in ovariectomized rats activates IGF-I receptors and induces the association between Insulin-like Growth Factor-1 (IGF-I) receptors and ERα in the hypothalamus (Quesada and Etgen, 2001; Cardona-Gómez, et al., 2002; Mendez, et al., 2003). Similar to what has been described in cortical neurons, there is an interaction (complex formation) between the p85 subunit of phosphatidylinositol 3-kinase (PI3K) and ERα within 1–3 h, which leads to activation of protein kinase B/Akt, a serine/threonine kinase that has multiple downstream targets (Cardona-Gomez et al 2002, Mendez et al 2003) (Cardona-Gómez, et al., 2002; Mendez, et al., 2003). Also, the E2-induced activation of insulin growth factor 1 (IGF-I) receptors augments α1-adrenergic receptor signaling, which is important for reproductive functions (Quesada and Etgen, 2001). On the other hand, blockade of IGF-I receptors during E2 priming prevents E2-induced increases in α1-adrenergic receptor binding density as well as IGF-I enhancement of noradrenergic receptor signaling (Quesada and Etgen, 2002). Collectively, these findings support functional interactions between E2 and IGF-I. Therefore, these actions of E2 on the IGF-I receptor signaling pathway may be a key mechanism by which estrogen affects synaptic remodeling and neuronal plasticity during the estrous cycle. Moreover, intracerebroventricular (i.c.v.) infusion of JB-1, a selective competitive antagonist of IGF-1 autophosphorylation, inhibits the estrogen-induced LH surge and sexual behavior in ovariectomized rats (Quesada and Etgen, 2002). In addition, co-administration (i.c.v.) of inhibitors of PI3K (wortmannin) and mitogen-activated protein kinase (MAPK) (PD98059) inhibit the long-term (48 h) effects of E2 to induce the LH surge and facilitate lordosis behavior (Etgen and Acosta-Martinez, 2003). Therefore, facilitation of female sexual behavior by E2 appears to involve activation of both PI3K and MAPK signal transduction pathways. The importance of growth factors for female sexual behavior is further highlighted by observations that epidermal growth factor (EGF) and also IGF-I can, in the absence of estrogen and progesterone (within 1–4 h of i.c.v. administration), induce mating behavior in rats and mice, in part, through an ERα-dependent mechanism (Apostolakis, et al., 2000). This relatively rapid, ligand-independent ER action is in striking contrast to the well established finding that estrogen priming over a period of at least 24 h is needed for progesterone induction of female reproductive behavior (Etgen, et al., 2001). The cross-talk between estrogen signaling and membrane-initiated growth factor signaling in the hypothalamus is particularly interesting, although it is currently not well understood. The ability of both IGF-I and E2 to induce female sexual behavior may involve complex interactions between ERα, the IGF-1 receptor and the PI3K p85 subunit.
4. Cross-talk between ER and metabotropic glutamate receptors
In addition to ER cross talk with IGF-1 receptors, there is evidence for the interaction between ERα and metabotropic glutamate receptor 1a (mGluR1a) signaling in hippocampal and hypothalamic neurons (Boulware, et al., 2005; Dewing, et al., 2007; Micevych and Mermelstein, 2008). The cellular components were originally elucidated in hippocampal neurons and then subsequently studied in hypothalamic neurons (Micevych and Mermelstein, 2008). In hippocampal CA1 neuronal cultures, E2 rapidly stimulates MAPK-dependent cAMP-responsive element binding protein (CREB) phosphorylation (Boulware, et al., 2005). This effect of E2 is mimicked by the membrane-impermeable E2-BSA analog, blocked by ICI 182, 780 and inhibited by the selective mGluR1a antagonist LY367385 ((S)-(+)-α-amino-4carboxy-2-methlybenzeneacetic acid). Because transfection of hippocampal neurons with the mutant ERα abrogates the downstream activation of CREB while the ER agonist propylpyrazoletriol (PPT) stimulates CREB activation, this membrane-localized receptor in the hippocampus is thought to be ERα (Harrington, et al., 2003; Boulware, et al., 2005; Boulware, et al., 2007).
A similar scenario has been proposed for the rapid E2-induced activation and internalization of μ-opioid receptors in the medial preoptic area (mPOA) associated with induction of female sex behavior (Dewing, et al., 2007; Bondar, et al., 2009). The Micevych lab has elucidated a series of events critical for sexual receptivity in the female rodent (Micevych, et al., 2003; Mills, et al., 2004). Sexual receptivity appears to be dependent on NPY Y1 receptor activation of arcuate POMC neurons that project to the medial preoptic area (mPOA), further highlighting the critical role of POMC neurons in reproduction (Mills, et al., 2004). Excitation of POMC neurons releases β-endorphin in the mPOA that upon binding to μ-opioid receptors causes activation, homologous desensitization and internalization (Arttamangkul, et al., 2000). 17β-estradiol and E2-conjugated to biotin (to limit membrane permeability) infused into the arcuate nucleus will initiate μ-opioid receptor activation and internalization in the mPOA and induction of full sexual receptivity in 17β-estradiol (benzoate)-primed, ovariectomized females (Dewing, et al., 2007). These actions of E2 are antagonized by ICI 182,780 and by a mGluR1a antagonist infused into the arcuate nucleus preceding E2 application (Dewing, et al., 2007). Thus, there appears to be an ER/mGluR1 signaling complex coupled to PKCθ that is responsible for the membrane-initiated E2 signaling involved in reproductive behavior (Dewing, et al., 2008). Although ERα and mGluR1a have been co-immunoprecipitated from transfected HEK cells, (Dewing, et al., 2007), the signaling complex has not been fully elucidated, but it is thought to require caveolin proteins (Boulware et al., 2007).
In other studies, E2 has been shown to rapidly activate multiple intracellular kinase cascades including MAPK, PI3K, PKA and PKC pathways relevant to reproduction (Gu, et al., 1996; Watters, et al., 1997; Bi, et al., 2001; Cato, et al., 2002; Yang, et al., 2003). In fact, the time course (15–30 min) is congruent with a membrane-initiated signaling cascade as has been revealed with electrophysiological studies (Lagrange, et al., 1994; Lagrange, et al., 1997; Qiu, et al., 2003; Qiu, et al., 2006b). In hypothalamic (mouse) GnRH neurons, the rapid phosphorylation of CREB, as measured by immunostaining of pCREB following E2 treatment, is observed within 15 min, which fits with the downstream signaling of a Gαq-mER initiated pathway in GnRH neurons (Abraham, et al., 2003; Zhang, et al., 2010). Therefore, there are multiple signaling pathways rapidly activated by E2 in arcuate (POMC) and preoptic (GABA) neurons that can modulate reproductive function.
5. Cross-talk between Gαq-mER and leptin receptor
Leptin plays a key role in energy homeostasis and reproduction and has an important role in the reproductive adaptation to starvation (Chan and Mantzoros, 2005). Serum concentrations of leptin convey nutrient information to the hypothalamic-pituitary gonadal axis (Chan and Mantzoros, 2005), and mutations in leptin or its receptor are associated with profound metabolic and physiological abnormalities such as obesity and infertility (Montez, et al., 2005). Leptin signals via its cognate receptors, leptin receptors (LRs), and there are several isoforms as a result of alternate splicing (Myers, Jr., 2004). The long isoform (LRb) is expressed abundantly in the hypothalamic arcuate, ventromedial and dorsomedial nuclei, and it is the predominant signaling form of the receptor (Bjørbæk, et al., 1997; Elmquist, et al., 1998). In fact, LRb has been localized in POMC, NPY and kisspeptin neurons but not in GnRH neurons (Håkansson, et al., 1998; Meister and Håkansson, 2001; Smith, et al., 2006; Quennell, et al., 2009). Leptin binding to its receptor causes activation of (phosphorylation) Janus Kinase-2 (Jak2) that signals via several pathways. Auto-phosphorylation of LRb allows the docking and subsequent activation of signal transducer and activator of transcription 3 (STAT3), which turns on transcription (Fig. 1) (Bates, et al., 2008; Gao, et al., 2004). The major metabolic effects of leptin are believed to be mediated by STAT3 because neuronal depletion of STAT3 results in an obese phenotype similar to leptin receptor deficient mice (Bates, et al., 2008; Gao, et al., 2004). In addition, the anorexigenic effects of E2 are thought to depend, in part, on STAT3 signaling in POMC neurons since the anorectic effects of E2 are abrogated by STAT3 knockout in female mice (Gao, et al., 2006). However, the PI3K pathway, also activated by LRb, is important for the excitatory actions of leptin since in POMC neurons the leptin-mediated depolarization/excitation is abolished by PI3K inhibition (Hill, et al., 2008; Qiu, et al., 2010). Activation of PI3K generates phosphatidylinositol-3,4,5-triphosphate (PIP3), which appears to contribute to the translocation and activation of PLCγ at the plasma membrane (Fig. 1) (Bae, et al., 1998). Recently, we have identified a potential point of convergence between the Gαq-mER and leptin receptor (LRb) signaling in POMC neurons (Fig. 1) (Qiu, et al., 2010). PI3K and associated proteins are targets for gene regulation by both E2 and STX (Malyala, et al., 2008). 17β-estradiol up-regulates PI3K p85α expression in the dorsomedial portion of the ventromedial hypothalamic nuclei and PI3K p55γ expression in the arcuate nucleus (Malyala, et al., 2008). STX increases the expression of phosphatidylinositol transfer protein β (PITPβ) in the arcuate nucleus (Roepke, et al., 2008). PITPβ transports lipids (phosphatidylinositols) from their site of synthesis (endoplasmic reticulum) to the cellular membrane where they are the preferred substrates for the lipid kinases such as PI3K (Cockcroft and Carvou, 2007). PITPβ activity is required for PI3K signaling and is also necessary for PLC-mediated signaling (Thomas, et al., 1993). In addition, activation of GIRK channels requires permissive levels of membrane phosphatidylinositol (4,5) biphosphate (PIP2), which are controlled by all of these enzymes. GIRK channel activity is enhanced by Gβγ-mediated stabilization of PIP2-GIRK binding (Huang, et al., 1998; Zhang, et al., 1999). Since PI3K- and PLC-mediated signaling are implicated in the membrane-mediated effects of E2, leptin and insulin, changes in the expression of PI3K subunits and transfer proteins may be another indirect mechanism for E2 to augment the excitability of POMC neurons. Therefore, since LRb is expressed in POMC and kisspeptin neurons but not in GnRH neurons (Balthasar, et al., 2004; Smith, et al., 2006; Qiu, et al., 2010), the cross-talk between leptin and E2 to regulate fertility are probably mediated by synaptic inputs onto GnRH neurons via POMC and kisspeptin neurons (Smith, et al., 2006; Gottsch, et al., 2009; Qiu, et al., 2010). Indeed, we have identified the direct excitatory effects of leptin on guinea pig kisspeptin neurons (Qiu et al, unpublished findings).
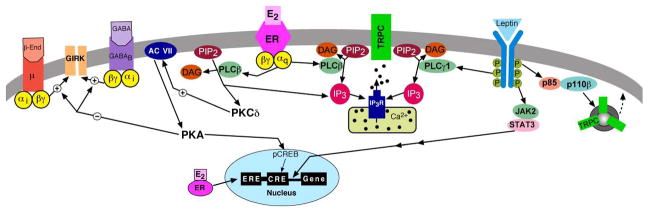
Figure 1. Cross-talk between estrogen and leptin signaling in POMC neurons.
Through a putative GPCR (mER), E2 (and STX) activates a PLCβ-PKCδ-AC-PKA pathway in POMC neurons that attenuates the coupling of μ-opioid and GABAB receptors to GIRK channels (heterologous desensitization) and triggers the release of Ca2+ from intracellular stores. This novel pathway also activates PKA-pCREB mediated transcription and in tandem with ERE-mediated transcription will alter the mRNA expression of relevant genes (channels and signaling molecules, etc.). Leptin binds to LRb to activate PI3 kinase and PLCγ1 to augment TRPC-channel currents and POMC neuronal excitability. Leptin will also activate JAK2-STAT3 transcriptional pathways. Note: The guinea pig, similar to the primate, has all of the key cellular elements in the mediobasal hypothalamus for controlling reproduction and energy homeostasis.
6. E2 signaling via Gαq-mER leads to new gene transcription
More recently, multiple studies have documented the cross-talk between rapid membrane-initiated and long-term nuclear-initiated steroid actions (Wagner, et al., 2001b; Vasudevan, et al., 2001; Kow and Pfaff, 2004; Malyala, et al., 2005; Qiu, et al., 2006b; Qiu, et al., 2006a; Roepke, et al., 2007). For example, it has been found that both acute effects of E2 and the transcriptional changes alter excitability of hypothalamic neurons (Kelly, et al., 2003; Malyala, et al., 2005; Qiu, et al., 2006a). In addition, the E2-induced membrane actions in the ventromedial nucleus (VMH) of the hypothalamus can potentiate its genomic effects on lordosis behavior (Kow and Pfaff, 2004). Moreover, this membrane effect in the VMH appears to be mediated by signaling pathways involving PKC and PKA (Kow and Pfaff, 2004). These findings are particularly intriguing in view of other findings that Gq-mER is coupled to PLC, PKCδ and PKA in POMC/GABA neurons (Qiu, et al., 2003; Qiu, et al., 2006b). Ultimately, E2 via both nuclear-initiated (ERα/β) and membrane-initiated signaling (Gq-mER) regulates the expression of a plethora of channels, channel binding proteins, signaling molecules and neuropeptides, as determined by custom gene microarray analysis of arcuate tissue from E2- and STX-treated female guinea pigs coupled with quantitative real-time PCR (Malyala, et al., 2004; Malyala, et al., 2005; Roepke, et al., 2008). The analysis of gene expression with STX has been used to evaluate the membrane-initiated versus membrane- and nuclear-initiated signaling of E2.
Numerous channels and signaling molecules that are involved in the modulation of channel activity are transcriptional target for ERα, ERβ and Gαq-mER receptors (Malyala, et al., 2005; Roepke, et al., 2007; Roepke, et al., 2008). 17β-estradiol treatment induces the expression of the Ca2+ channel subunit Cav3.1 in the arcuate nucleus of ovariectomized female guinea pigs (Roepke, et al., 2008), which increases the peak T-type Ca2+ current by two-fold in arcuate (POMC/GABA) neurons (Qiu, et al., 2006a). Furthermore, the E2-induced Cav3.1 mRNA expression in the arcuate is abrogated in αERKO mice (Bosch, et al., 2009), indicating that the expression is under the control of an ERα-dependent mechanism. However, STX also up-regulates the Cav3.1 subunit in arcuate neurons in ovariectomized female guinea pigs (Roepke, et al., 2008), which would suggest that the Gq-mER and ERα-mediated transcriptional effects converge on the Cav3.1 gene (pCREB and ERE sites, Fig. 1). The increase in the T-type Ca2+ current augments burst firing and increases neurotransmitter (e.g., β-endorphin) release since burst firing of hypothalamic neurons is facilitated by the actions of T-type Ca2+ channels (Erickson, et al., 1993). Ultimately, E2 up-regulates the expression of β-endorphin in POMC neurons in ovariectomized female guinea pigs (Thornton, et al., 1994; Bethea, et al., 1995) and this increase is correlated with the increased expression of POMC mRNA (Roepke, et al., 2008). The increased POMC (and GABA) neuronal input would provide a hyperpolarizing stimulus via activation of GIRK channels in GnRH neurons (see Section 1).
Also, genes involved in calcium signaling pathways are regulated by both E2 and STX treatment. Long term (24 h and longer) treatment with E2 increases the expression of calmodulin-1, but STX treatment increases calmodulin-dependent kinase, CaM kinase II (CaMKII) in the arcuate nucleus. CaMK II is a modulator of multiple ion channels (Ca2+, K+, Na+) and is required for the Ca2+-sensitive production of long-term potentiation (LTP) in hippocampal and hypothalamic neurons (Fukunaga, et al., 2002; Pitt, 2007). The regulation of these calcium signaling molecules may have multiple effects on gene expression, neuronal excitability and synaptic neurotransmitter release. Finally, E2 down-regulates A-kinase anchoring protein (AKAP) 11 (aka AKAP220) expression, but STX increases the expression of AKAP11 in the arcuate nucleus (Roepke, et al., 2007; Roepke, et al., 2008). AKAPs are critical for scaffolding kinases (e.g., PKA, PKC) and phosphatases (e.g., phosphatase 1) close to ion channels for rapidly modulating their activity (Scott and McCartney, 1994; Wong and Scott, 2004; Hoshi, et al., 2010). Therefore, the membrane initiated signaling by E2 via Gαq-mER can have multiple consequences both in the short term to affect neuronal excitability that can ultimately lead to new gene transcription that can impact multiple synaptic inputs onto GnRH neurons.
7. Conclusions
Since the first cloning of the estrogen receptor/transcription factor ERα in 1986 (Greene, et al., 1986), it has become abundantly clear that there are not only multiple receptors (ERα, ERβ, GPR30, etc.) but also multiple modes of estrogen signaling via membrane and intracellular shuttling of these receptors. Also, this diversity of signaling allows the pleiotropic actions of E2 in the CNS, where the steroid can collaborate with neurotransmitters, growth factors and transcription factors. The actions of E2 span from rapid (seconds) to long term (hours) time frame to not only affect reproduction but other homeostatic functions that are critical for reproductive function. Certainly the recognition of the importance of membrane initiated signaling is long overdue, and its importance will only continue to escalate as we identify new tools to probe these actions.
Acknowledgments
The authors thank colleagues who contributed to the work described herein, especially Drs. Oline K. Rønekleiv, Chunguang Zhang, Troy A. Roepke, Anna Malyala and Ms. Martha A. Bosch. Drs. Oline K. Ronnekleiv and Troy A. Roepke provided helpful comments on earlier drafts of the manuscript. The work described herein was supported by PHS grants NS 43330, NS 38809 and DK 68098.
Abbreviations
- CREB
cAMP-responsive element binding protein
- Gαq-mER
mER that is Gαq-coupled to activation of phospholipase Cβ
- GIRK
G protein-coupled inwardly rectifying K+
- IGF-1
Insulin-like Growth Factor-1
- JAK2
Janus Kinase-2
- LRb
Leptin receptor b
- MAPK
mitogen-activated protein kinase
- mGluR1a
metabotropic glutamate receptor 1a
- PKA
protein kinase A
- PKB
protein kinase B also known as Akt
- PKC
protein kinase C
- PI3K
phosphatidylinositol 3-kinase
- PLC
phospholipase C
- PIP2
phosphatidylinositol (4,5) biphosphate
- PIP3
phosphatidylinositol-3,4,5-triphosphate
- PITPβ
phosphatidylinositol transfer protein β (PITPβ)
- TRPC
Transient receptor potential canonical type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham IM, Han SK, Todman MG, Korach KS, Herbison AE. Estrogen receptor beta mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolakis EM, Garai J, Lohmann JE, Clark JH, O’Malley BW. Epidermal growth factor activates reproductive behavior independent of ovarian steroids in female rodents. Mol Endo. 2000;14:1086–1098. doi: 10.1210/mend.14.7.0490. [DOI] [PubMed] [Google Scholar]
- Arttamangkul S, Alvarez-Maubecin V, Thomas G, Williams JT, Grandy DK. Binding and internalization of fluorescent opioid peptide conjugates in living cells. mol pharmacol. 2000;58:1570–1580. doi: 10.1124/mol.58.6.1570. [DOI] [PubMed] [Google Scholar]
- Bae YS, Cantley LG, Chen C-S, Kim S-R, Kwon K-S, Rhee SG. Activation of phospholipase C-γ by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:4465–4469. doi: 10.1074/jbc.273.8.4465. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, Lowell BB. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Bates SH, Dundon TA, Seifert M, Carlson M, Maratos-Flier E, Myers MG., Jr LRb-STAT3 signaling is required for the neuroendocrine regulation of energy expenditure by leptin. Diabetes. 2008;53:3067–3073. doi: 10.2337/diabetes.53.12.3067. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Hess DL, Widmann AA, Henningfeld JM. Effects of progesterone on prolactin, hypothalamic beta-endorphin, hypothalamic substance P, and midbrain serotonin in guinea pigs. Neuroendo. 1995;61:695–703. doi: 10.1159/000126897. [DOI] [PubMed] [Google Scholar]
- Bi R, Foy MR, Vouimba RM, Thompson RF, Baudry M. Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc Natl Acad Sci U S A. 2001;98:13391–13395. doi: 10.1073/pnas.241507698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørbæk C, Uotani S, da Silva B, Flier JS. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem. 1997;272:32686–32695. doi: 10.1074/jbc.272.51.32686. [DOI] [PubMed] [Google Scholar]
- Bondar G, Kuo J, Hamid N, Micevych P. Estradiol-induced estrogen receptor-α trafficking. J Neurosci. 2009;29:15323–15330. doi: 10.1523/JNEUROSCI.2107-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch MA, Hou J, Fang Y, Kelly MJ, Rønnekleiv OK. 17β-estradiol regulation of the mRNA expression of T-type calcium channel subunits: role of estrogen receptor α and estrogen receptor β. J Comp Neuro. 2009;512:347–358. doi: 10.1002/cne.21901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci. 2007;27:9941–9950. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DN, Sheldahl LC, Marriott LK, Shapiro RA, Dorsa DM. Multiple pathways transmit neuroprotective effects of gonadal steroids. Endocrine. 2006;29:199–207. doi: 10.1385/ENDO:29:2:199. [DOI] [PubMed] [Google Scholar]
- Cardona-Gómez GP, Mendez P, Garcia-Segura LM. Synergistic interaction of estradiol and insulin-like growth factor-I in the activation of PI3K/Akt signaling in the adult rat hypothalamus. Mol Brain Res. 2002;107:80–88. doi: 10.1016/s0169-328x(02)00449-7. [DOI] [PubMed] [Google Scholar]
- Cato ACB, Nestl A, Mink S. Rapid actions of steroid receptors in cellular signaling pathways. Science’s STKE. 2002:re9–re21. doi: 10.1126/stke.2002.138.re9. [DOI] [PubMed] [Google Scholar]
- Chan JL, Mantzoros CS. Role of leptin in energy-deprivation states: normal human physiology and clinical implications for hypothalamic amenorrhoea and anorrexia nervosa. Lancet. 2005;366:74–85. doi: 10.1016/S0140-6736(05)66830-4. [DOI] [PubMed] [Google Scholar]
- Cockcroft S, Carvou N. Biochemical and biological functions of class I phosphatidylinositol transfer proteins. Biochim Biophys Acta. 2007;1771:677–691. doi: 10.1016/j.bbalip.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Condon TP, Rønnekleiv OK, Kelly MJ. Estrogen modulation of the α1-adrenergic response of hypothalamic neurons. Neuroendo. 1989;50:51–58. doi: 10.1159/000125201. [DOI] [PubMed] [Google Scholar]
- Cunningham MJ, Fang Y, Selley DE, Kelly MJ. μ-opioid agonist-stimulated [35S]GTPgammaS binding in guinea pig hypothalamus: Effects of estrogen. Brain Res. 1998;791:341–346. doi: 10.1016/s0006-8993(98)00201-7. [DOI] [PubMed] [Google Scholar]
- Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor-α interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewing P, Christensen A, Bondar G, Micevych P. Protein kinase C signaling in the hypothalamic arcuate nucleus regulates sexual receptivity in female rats. Endocrinology. 2008;149:5934–5942. doi: 10.1210/en.2008-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–547. [PubMed] [Google Scholar]
- Erickson KR, Rønnekleiv OK, Kelly MJ. Role of a T-type calcium current in supporting a depolarizing potential, damped oscillations, and phasic firing in vasopressinergic guinea pig supraoptic neurons. Neuroendo. 1993;57:789–800. doi: 10.1159/000126438. [DOI] [PubMed] [Google Scholar]
- Etgen AM, Acosta-Martinez M. Participation of growth factor signal transduction pathways in estradiol facilitation of female reproductive behavior. Endocrinology. 2003;144:3828–3835. doi: 10.1210/en.2003-0157. [DOI] [PubMed] [Google Scholar]
- Etgen AM, Ansonoff MA, Quesada A. Mechanisms of ovarian steroid regulation of norepinephrine receptor-mediated signal transduction in the hypothalamus: Implications for female reproductive physiology. Horm Behav. 2001;40:169–177. doi: 10.1006/hbeh.2001.1676. [DOI] [PubMed] [Google Scholar]
- Fukunaga K, Horikawa K, Shibata S, Takeuchi Y, Miyamoto E. Ca2+/ calmondulin-dependent protein kinase II-dependent long-term potentiation in the rat suprachiasmatic nucleus and its inhibition by melatonin. J Neurosci Res. 2002;70:799–807. doi: 10.1002/jnr.10400. [DOI] [PubMed] [Google Scholar]
- Furchgott RF. Pharmacological characterization of receptors: Its relation to radioligand-binding studies. Federation Proc. 1978;37:115–120. [PubMed] [Google Scholar]
- Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, Leranth C, Toran-Allerand D, Priest CA, Roberts JL, Gao X-B, Mobbs C, Shulman GI, Diano S, Horvath TL. Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nature Med. 2006;13:89–94. doi: 10.1038/nm1525. [DOI] [PubMed] [Google Scholar]
- Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI, Fu X-Y. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci USA. 2004;101:4661–4666. doi: 10.1073/pnas.0303992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genazzani AR, Genazzani AD, Volpogni C, Pianazzi F, Li GA, Surico N, Petraglia F. Opioid control of gonadotropin secretion in humans. Hum Reprod. 1993;8:151–153. doi: 10.1093/humrep/8.suppl_2.151. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Navarro VM, Zhao Z, Glidewell-Kenney C, Weiss J, Jameson JL, Clifton DK, Levine JE, Steiner RA. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci. 2009;29:9390–9395. doi: 10.1523/JNEUROSCI.0763-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene GL, Gilna P, Waterfield M, Baker A, Hort Y, Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science. 1986;231:1150–1153. doi: 10.1126/science.3753802. [DOI] [PubMed] [Google Scholar]
- Gu G, Rojo AA, Zee MC, Yu J, Simerly RB. Hormonal regulation of CREB phosphorylation in the anteroventral periventricular nucleus. J Neurosci. 1996;16:3035–3044. doi: 10.1523/JNEUROSCI.16-09-03035.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Korach KS, Moss RL. Rapid action of 17beta-estradiol on kainate-induced currents in hippocampal neurons lacking intracellular estrogen receptors. Endocrinology. 1999;140:660–666. doi: 10.1210/endo.140.2.6500. [DOI] [PubMed] [Google Scholar]
- Håkansson ML, Brown H, Ghilardi N, Skoda RC, Meister B. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J Neurosci. 1998;18:559–572. doi: 10.1523/JNEUROSCI.18-01-00559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington WR, Sheng S, Barnett DH, Petz LN, Katzenellenbogen JA, Katzenellenbogen BS. Activities of estrogen receptor alpha-and beta-selective ligands at diverse estrogen responsive gene sites mediating transactivation or transrepression. Mol Cell Endocrinol. 2003;206:13–22. doi: 10.1016/s0303-7207(03)00255-7. [DOI] [PubMed] [Google Scholar]
- Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signalng in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Potassium channels and chloride channels. In: Hille B, editor. Ion channels of excitable membranes. Sinaur; Sunderland, MA: 2001. pp. 131–165. [Google Scholar]
- Hoshi N, Langeberg LK, Gould CM, Newton AC, Scott JD. Interaction with AKAP79 modifies the cellular pharmacology of PKC. Molecular Cell. 2010;37:541–550. doi: 10.1016/j.molcel.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C-L, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Lagrange AH. Nontranscriptional effects of estradiol in neuropeptide neurons. Current Opinion in Endocrinology and Diabetes. 1998;5:66–72. [Google Scholar]
- Kelly MJ, Loose MD, Rønnekleiv OK. Estrogen suppresses μ-opioid and GABAB-mediated hyperpolarization of hypothalamic arcuate neurons. J Neurosci. 1992;12:2745–2750. doi: 10.1523/JNEUROSCI.12-07-02745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Moss RL, Dudley CA. Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Res. 1976;114:152–157. doi: 10.1016/0006-8993(76)91017-9. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Qiu J, Wagner EJ, Rønnekleiv OK. Rapid effects of estrogen on G protein-coupled receptor activation of potassium channels in the central nervous system (CNS) J Steroid Biochem Mol Biol. 2003;83:187–193. doi: 10.1016/s0960-0760(02)00249-2. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Rønnekleiv OK. Control of CNS neuronal excitability by estrogens via membrane-initiated signaling. Mol Cell Endocrinol. 2009;308:17–25. doi: 10.1016/j.mce.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Rønnekleiv OK. Rapid membrane effects of estrogen in the central nervous system. In: Pfaff DW, editor. Hormones, Brain and Behavior. Academic Press; San Diego: 2002. pp. 361–380. [Google Scholar]
- Kelly MJ, Rønnekleiv OK, Eskay RL. Identification of estrogen-responsive LHRH neurons in the guinea pig hypothalamus. Brain Res Bull. 1984;12:399–407. doi: 10.1016/0361-9230(84)90112-6. [DOI] [PubMed] [Google Scholar]
- Khachaturian H, Lewis ME, Shafer MKH, Watson SJ. Anatomy of the CNS opioid systems. Trends Neurosci. 1985;8:111–119. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: Anatomy, pharmacology and function of reward pathways. Trends Pharm Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Kow LM, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. Proc Natl Acad Sci U S A. 2004;101:12354–12357. doi: 10.1073/pnas.0404889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange AH, Rønnekleiv OK, Kelly MJ. The potency of μ-opioid hyperpolarization of hypothalamic arcuate neurons is rapidly attenuated by 17β-estradiol. J Neurosci. 1994;14:6196–6204. doi: 10.1523/JNEUROSCI.14-10-06196.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange AH, Rønnekleiv OK, Kelly MJ. Estradiol-17β and μ-opioid peptides rapidly hyperpolarize GnRH neurons: A cellular mechanism of negative feedback? Endocrinology. 1995;136:2341–2344. doi: 10.1210/endo.136.5.7720682. [DOI] [PubMed] [Google Scholar]
- Lagrange AH, Rønnekleiv OK, Kelly MJ. Modulation of G protein-coupled receptors by an estrogen receptor that activates protein kinase A. Mol Pharmacol. 1997;51:605–612. doi: 10.1124/mol.51.4.605. [DOI] [PubMed] [Google Scholar]
- Lagrange AH, Wagner EJ, Rønnekleiv OK, Kelly MJ. Estrogen rapidly attenuates a GABAB response in hypothalamic neurons. Neuroendo. 1996;64:114–123. doi: 10.1159/000127106. [DOI] [PubMed] [Google Scholar]
- Loose MD, Rønnekleiv OK, Kelly MJ. Membrane properties and response to opioids of identified dopamine neurons in the guinea pig hypothalamus. J Neurosci. 1990;10:3627–3634. doi: 10.1523/JNEUROSCI.10-11-03627.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malyala A, Kelly MJ, Rønnekleiv OK. Estrogen modulation of hypothalamic neurons: Activation of multiple signaling pathways and gene expression changes. Steroids. 2005;70:397–406. doi: 10.1016/j.steroids.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Malyala A, Pattee P, Nagalla SR, Kelly MJ, Rønnekleiv OK. Suppression subtractive hybridization and microarray identification of estrogen regulated hypothalamic genes. Neurochem Res. 2004;29:1189–1200. doi: 10.1023/b:nere.0000023606.13670.1d. [DOI] [PubMed] [Google Scholar]
- Malyala A, Zhang C, Bryant D, Kelly MJ, Rønnekleiv OK. PI3K signaling effects in hypothalamic neurons mediated by estrogen. J Comp Neurol. 2008;506:895–911. doi: 10.1002/cne.21584. [DOI] [PubMed] [Google Scholar]
- Meister B, Håkansson ML. Leptin receptors in hypothalamus and circumventricular organs. Clin Exp Pharmacol Physiol. 2001;28:610–617. doi: 10.1046/j.1440-1681.2001.03493.x. [DOI] [PubMed] [Google Scholar]
- Mendez P, Azcoitia I, Garcia-Segura LM. Estrogen receptor alpha forms estrogen-dependent multimolecular complexes with insulin-like growth factor receptor and phospatidylinositol 3-kinase in the adult rat brain. Mol Brain Res. 2003;112:170–176. doi: 10.1016/s0169-328x(03)00088-3. [DOI] [PubMed] [Google Scholar]
- Micevych PE, Mermelstein PG. Membrane estrogen receptors acting through metabotropic glutamate receptors: an emerging mechanism of estrogen action in brain. Mol Neurobiol. 2008;38:66–77. doi: 10.1007/s12035-008-8034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych PE, Rissman EF, Gustafsson JA, Sinchak K. Estrogen receptor-alpha is required for estrogen-induced mu-opioid receptor internalization. J Neurosci Res. 2003;71:802–810. doi: 10.1002/jnr.10526. [DOI] [PubMed] [Google Scholar]
- Mills RH, Sohn RK, Micevych PE. Estrogen-induced mu-opioid receptor internalization in the medial preoptic nucleus is mediated via neuropeptide Y-Y1 receptor activation in the arcuate nucleus of female rats. J Neurosci. 2004;24:947–955. doi: 10.1523/JNEUROSCI.1366-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montez JM, Soukas A, Asilmaz E, Fayzikhodjaeva G, Fantuzzi G, Friedman JM. Acute leptin deficiency, leptin resistance, and the physiologic response to leptin withdrawal. Proc Natl Acad Sci USA. 2005;102:2537–2542. doi: 10.1073/pnas.0409530102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG., Jr Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res. 2004;59:287–304. doi: 10.1210/rp.59.1.287. [DOI] [PubMed] [Google Scholar]
- Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. Involvement of G-protein couple receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Mol Endo. 2009;3:349–359. doi: 10.1210/me.2008-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA. Membrane conductances and opioid receptor subtypes. Natl Inst Drug Abuse Res Monograph Series. 1986;71:81–88. [PubMed] [Google Scholar]
- Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endo. 2006;20:1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- Pitt GS. Calmodulin and CaMKII as molecular switches for cardiac ion channels. Cardiovasc Res. 2007;73:641–647. doi: 10.1016/j.cardiores.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Jamali K, Xue C, Kelly MJ, Rønnekleiv OK. Estrogen upregulates T-type calcium channels in the hypothalamus and pituitary. J Neurosci. 2006a;26:11072–11082. doi: 10.1523/JNEUROSCI.3229-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Rønnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Krust A, Graham S, Murphy S, Korach KS, Chambon P, Scanlan TS, Rønnekleiv OK, Kelly MJ. A G protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci. 2006b;26:5649–5655. doi: 10.1523/JNEUROSCI.0327-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Fang Y, Rønnekleiv OK, Kelly MJ. Leptin exceites proopiomelanocortin neurons via activation of TRPC channels. J Neurosci. 2010;30:1560–1565. doi: 10.1523/JNEUROSCI.4816-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Rønnekleiv OK, Kelly MJ. Modulation of hypothalamic neuronal activity through a novel G-protein coupled estrogen membrane receptor. Steroids. 2008;73:985–991. doi: 10.1016/j.steroids.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quennell JH, Mulligan AC, Tups A, Liu X, Phipps SJ, Kemp CJ, Herbison AE, Grattan DR, Anderson GM. Leptin indirectly reulates gonadotropin-releasing hormone neuronal function. Endocrinology. 2009;150:2805–2812. doi: 10.1210/en.2008-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada A, Etgen AM. Insulin-like growth factor-1 regulation of α1-adrenergic receptor signaling is estradiol dependent in the preoptic area and hypothalamus of female rats. Endocrinology. 2001;142:599–607. doi: 10.1210/endo.142.2.7946. [DOI] [PubMed] [Google Scholar]
- Quesada A, Etgen AM. Functional interactions between estrogen and insulin-like growth factor-I in the regulation of α1B-adrenoceptors and female reproductive function. J Neurosci. 2002;22:2401–2408. doi: 10.1523/JNEUROSCI.22-06-02401.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: Studies of ERα and ERβ expressed in Chinese hamster ovary cells. Mol Endo. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- Roepke TA, Malyala A, Bosch MA, Kelly MJ, Rønnekleiv OK. Estrogen regulation of genes important for K+ channel signaling in the arcuate nucleus. Endocrinology. 2007;148:4937–4951. doi: 10.1210/en.2007-0605. [DOI] [PubMed] [Google Scholar]
- Roepke TA, Xue C, Bosch MA, Scanlan TS, Kelly MJ, Rønnekleiv OK. Genes associated with membrane-initiated signaling of estrogen and energy homeostasis. Endocrinology. 2008;149:6113–6124. doi: 10.1210/en.2008-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rønnekleiv OK, Kelly MJ. Diversity of ovarian steroid signaling in the hypothalamus. Front Neuroendo. 2005;26:65–84. doi: 10.1016/j.yfrne.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Rossmanith WG, Mortola JF, Yen SSC. Role of endogenous opioid peptides in the initiation of the midcycle luteinizing hormone surge in normal cycling women. J Clin Endo Metab. 1988;67:695–700. doi: 10.1210/jcem-67-4-695. [DOI] [PubMed] [Google Scholar]
- Scott JD, McCartney S. Localization of A-kinase through anchoring proteins. Mol Endo. 1994;8:5–11. doi: 10.1210/mend.8.1.8152430. [DOI] [PubMed] [Google Scholar]
- Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006;18:298–303. doi: 10.1111/j.1365-2826.2006.01417.x. [DOI] [PubMed] [Google Scholar]
- Szegõ ÉM, Barabás K, Balog J, Szilágyi N, Korach KS, Juhász G, Abrahám IM. Estrogen induces estrogen receptor α-dependent cAMP response element-binding protein phosphorylation via mitogen activated protein kinase pathway in basal forebrain cholinergic neurons In Vivo. J Neurosci. 2006;26:4104–4110. doi: 10.1523/JNEUROSCI.0222-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GMH, Cunningham E, Fensome A, Ball A, Totty NF, Truong O, Hsuan JJ, Cockcroft S. An essential role for phosphatidylinositol transfer protein in phospholipase C-mediated inositol lipid signaling. Cell. 1993;74:919–928. doi: 10.1016/0092-8674(93)90471-2. [DOI] [PubMed] [Google Scholar]
- Thornton JE, Loose MD, Kelly MJ, Rønnekleiv OK. Effects of estrogen on the number of neurons expressing β-endorphin in the medial basal hypothalamus of the female guinea pig. J Comp Neurol. 1994;341:68–77. doi: 10.1002/cne.903410107. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. Minireview: A plethora of estrogen receptors in the brain: where will it end? Endocrinology. 2004;145:1069–1074. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. Estrogen and the brain: beyond ER-α, ER-β and 17β-estradiol. Ann NY Acad Sci. 2005;1052:136–144. doi: 10.1196/annals.1347.009. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Kow L-M, Pfaff DW. Early membrane estrogenic effects required for full expression of slower genomic actions in a nerve cell line. Proc Natl Acad Sci USA. 2001;98:12267–12271. doi: 10.1073/pnas.221449798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner EJ, Rønnekleiv OK, Bosch MA, Kelly MJ. Estrogen biphasically modifies hypothalamic GABAergic function concomitantly with negative and positive control of luteinizing hormone release. J Neurosci. 2001a;21:2085–2093. doi: 10.1523/JNEUROSCI.21-06-02085.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner EJ, Rønnekleiv OK, Kelly MJ. The noradrenergic inhibition of an apamine-sensitive small conductance Ca2+ -activated K+ channel in hypothalamic γ-aminobutyric acid neurons: Pharmacology, estrogen sensitivity and relevance to the control of the reproductive axis. J Pharmacol Exp Ther. 2001b;299:21–30. [PubMed] [Google Scholar]
- Watters JJ, Campbell JS, Cunningham MJ, Krebs EG, Dorsa DM. Rapid membrane effects of steroids in neuroblastoma cells: Effects of estrogen on mitogen activated protein kinase signalling cascade and c-fos immediate early gene transcription. Endocrinology. 1997;138:4030–4033. doi: 10.1210/endo.138.9.5489. [DOI] [PubMed] [Google Scholar]
- Weatherill PJ, Wilson APM, Nicholson RI, Davies P, Wakeling AE. Interaction of the antioestrogen ICI 164,384 with the oestrogen receptor. J Ster Bioc Mol Biol. 1988;30:263–266. doi: 10.1016/0022-4731(88)90103-3. [DOI] [PubMed] [Google Scholar]
- Wildt L, Leyendecker G, Sir-Petermann T, Waibel-Treber S. Treatment with naltrexone in hypothalamic ovarian failure: Induction of ovulation and pregnancy. Hum Reprod. 1993;8:350–358. doi: 10.1093/oxfordjournals.humrep.a138050. [DOI] [PubMed] [Google Scholar]
- Wong W, Scott JD. AKAP signalling complexes focal points in space and time. Nat Rev Mol Cell Biol. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- Wu M, Dumalska I, Morozova E, Van den Pol AN, Alreja M. Melanin-concentrating hormone directly inhibits GnRH neurons and blocks kisspeptin activation, linking energy balance to reproduction. Proc Natl Acad Sci USA. 2009;106:17217–17222. doi: 10.1073/pnas.0908200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kirigiti MA, Cowley MA, Grove KL, Smith MS. Suppression of basal spontaneous gonadotropin-releasing hormone neuronal activity during lactation: role of inhibitory effects of neuropeptide Y. Endocrinology. 2009;150:333–340. doi: 10.1210/en.2008-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S-H, Sharrocks AD, Whitmarsh AJ. Transcriptional regulation by the MAP kinase signaling cascades. Gene. 2003;320:3–21. doi: 10.1016/s0378-1119(03)00816-3. [DOI] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Levine JE, Rønnekleiv OK, Kelly MJ. Gonadotropin-releasing hormone neurons express KATP Channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. J Neurosci. 2007;27:10153–10164. doi: 10.1523/JNEUROSCI.1657-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Rick EA, Kelly MJ, Rønnekleiv OK. 17β-estradiol regulation of T-type calcium channels in gonadotropin-releasing hormone neurons. J Neurosci. 2009a;29:10552–10562. doi: 10.1523/JNEUROSCI.2962-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Rønnekleiv OK, Kelly MJ. GABAB receptor mediated inhibition of GnRH neurons is suppressed by kisspeptin-GPR54 signaling. Endocrinology. 2009b;150:2388–2394. doi: 10.1210/en.2008-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Kelly MJ, Rønnekleiv OK. 17β-estradiol rapidly increases ATP-sensitive potassium channel activity in gonadotropin-releasing hormone neurons via a protein kinase signaling pathway. Endocrinology. 2010;151 doi: 10.1210/en.2010-0177. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, He C, Yan X, Mirshashi T, Logothetis DE. Activation of inwardly rectifying K+ channels by distinct PtdIns(4,5)P2 interactions. Nature Cell Biology. 1999;1:183–188. doi: 10.1038/11103. [DOI] [PubMed] [Google Scholar]
- Zheng SX, Bosch MA, Rønnekleiv OK. Mu-opioid receptor mRNA expression in identified hypothalamic neurons. J Comp Neurol. 2005;487:332–344. doi: 10.1002/cne.20557. [DOI] [PubMed] [Google Scholar]