Abstract
Background
Children with food allergies often have concurrent asthma.
Objective
The authors aimed to determine the prevalence of asthma in children with food allergies and the association of specific food allergies with asthma.
Methods
Parental questionnaire data regarding food allergy, corroborated by allergic sensitization were completed for a cohort of 799 children with food allergies. Multivariate regression analysis tested the association between food allergy and reported asthma.
Results
In this cohort, the prevalence of asthma was 45.6%. After adjusting for each food allergy, environmental allergies, and family history of asthma, children with egg allergy (odds ratio [OR] = 2.0; 95% confidence interval [CI] = 1.3–3.2; P < .01) or tree nut allergy (OR = 2.0; 95% CI = 1.1–3.6; P = .02) had significantly greater odds of report of asthma.
Conclusion
There is a high prevalence of asthma in the food-allergic pediatric population. Egg and tree nut allergy are significantly associated with asthma, independent of other risk factors.
Keywords: asthma, food allergy, food hypersensitivity, nut allergy, nut hypersensitivity, egg allergy, egg hypersensitivity, pediatrics, allergy, asthma epidemiology
Introduction
The past 3 decades have seen a dramatic rise in the prevalence of allergic diseases, including asthma and food allergies.1–5 Current estimates of clinically relevant food hypersensitivity range from 1% to 3.2% in children and adults in the general population.6–9 Children tend to be allergic to milk, eggs, wheat, and peanuts,10 whereas adults primarily react to peanuts, tree nuts, and seafood.1,6 Asthma prevalence has mirrored this increase, and it is currently estimated that 8.5% of children suffer from asthma.3 For those with food allergy, the prevalence of asthma is as high as 29%.2
Many studies have shown the prevalence and influence of allergic phenotypes in cohorts of patients with asthma.11–14 These studies suggest that patients with food allergies are at higher risk for asthma diagnosis15 and asthma morbidity.12–14,16 However, few studies have focused on asthma outcomes in cohorts of children with food allergy.17
Milk, egg,18 and wheat sensitization has been associated with the development of asthma in very young children.19–21 However, clinically relevant food allergy in children spanning all ages and the relationship to asthma has been less well described.
The aims of this study were to define the prevalence of asthma in a large cohort of children with food allergies and determine if individual food allergies were specifically associated with asthma. We hypothesized that there is a high prevalence of asthma in the food-allergic population and that individual food allergies differentially associate with asthma risk.
Methods
Study Participants
This study was a cross-sectional analysis of 799 children with skin prick test or specific immunoglobulin E (sIgE) corroborating parental report of food allergy from a cohort of 1413 children with physician-diagnosed food allergy. Throughout, we have used the term parent to include all primary caregivers and subject to refer to the food-allergic child in question. The study included children with Allergy Program visits at Children’s Hospital Boston or 2 suburban pediatric allergy practices. If parental report was positive for the subject having a clinical history of allergic reaction to one or more foods, then, the parent was asked to participate in the study. Parents were excluded if they were not intimately familiar with the child’s conception and birth because the primary purpose of this cohort was to evaluate prenatal factors predicting childhood food allergy. Data for siblings of the primary subject were not included in the analysis.
After obtaining written informed consent from the subject’s parent, a questionnaire regarding the subject’s food allergy history, environmental allergies, family history, and demographics was distributed. Data were collected prospectively over a 4-year period from May 2005 through October 2009. The study was approved by the institutional review board of the Children’s Hospital Boston.
Definition of Predictor Variables
Parental report of food allergy was determined by answering questionnaire items phrased, “The following questions pertain to your [child’s] food allergy history,” with response options, “never ate this food,” “no,” or “yes.” Listed foods were “eggs,” “dairy/milk products,” “seafood,” “tree nuts (tree nuts include walnuts, macadamia nuts, almonds, pistachios, cashews, pecans, brazil nuts, hazelnuts),” “peanuts/peanut butter,” and “sesame seeds.” Food allergy was defined in this study as report of allergy and evidence of sensitization by skin prick test or sIgE. Those with report of food allergy not corroborated by evidence of allergic sensitization, when treated as a separate group, did not affect the relationship of food allergy to asthma. As these subjects were possibly misclassified and statistically not informative, they were not included in the final analysis. Report of “never trying the food” or “not being allergic” was analyzed as such.
Measurement of Subject’s Sensitization to Food Allergens
The subjects’ charts were reviewed retrospectively for allergen skin prick test and sIgE results. Skin prick test to commercially available extracts was performed on the back or volar surface of the forearm using standard methods. Skin prick tests were recorded as wheal and flare measurements and/or positive/negative for each item tested. A wheal 3 mm greater than the negative control at 15 minutes was considered positive. Patients were excluded if they had a negative histamine control. The following skin prick test allergens were used: dairy (milk, casein), egg (egg white), tree nut (almond, brazil nut, cashew, hazelnut, pecan, pistachio, walnut), peanut, sesame seed, seafood (clam, codfish, crab, flounder, lobster, salmon, scallop, shrimp, trout, tuna). sIgE was measured by ImmunoCAP assay (Phadia US Inc, Portage, MI). Results of Immuno-CAP testing were deemed positive for values ≥0.35 kUA/L. All tests were performed at a CLIA-certified laboratory. When available, the following sIgE tests were ordered for the food groups: dairy (milk, α lactalbumin, casein, whey), egg (egg white), tree nut (almond, cashew, hazelnut, pecan, pistachio, walnut), peanut, sesame seed, seafood (codfish, crab, lobster, haddock, salmon, shrimp). When skin prick or sIgE tests were repeated, aggregate outcomes were used as “ever positive,” “never positive,” or “never tested” for each allergen.
Definition of Other Predictor Variables
Potential confounders of the relationship between predictor variables and asthma were considered for inclusion in the multivariate model. Variables related to allergy other than food allergy included pet allergy, pollen allergy, and eczema. Variables related to the family history of allergic disease were obtained for the subject’s mother and father and included asthma, hayfever/pollen allergy, and eczema. Previously described factors associated with asthma, such as age, gender, and level of maternal education, were also included. The highest level of maternal education was recorded as “some high school,” “high school degree,” “associates degree or technical school,” “bachelor’s degree,” “master’s degree,” or “professional degree (PhD, MD, JD).”
Definition of Outcome Variable
The primary outcome of interest in this study was parental report of asthma. On the questionnaire, parents were instructed to answer questions “pertaining to [the child’s] non-food allergy history.” Presence or absence of asthma was indicated by checkbox: “yes” or “no”. Those who answered yes to this question were categorized as having asthma.
Statistical Methods
Prevalence of asthma was determined by simple statistical analysis incorporating all cases who answered the question about asthma. For each food group, 3 categories— allergic, not allergic, and never tried—were individually analyzed for association with asthma by incorporating the 3 categories into a logistic regression with “not allergic” as the reference group and adjusting for the “never tried” group. Univariate analysis of the relationship between binary covariates, such as environmental allergies, gender, parental history of asthma, eczema and hayfever/pollen allergy, and the outcome of asthma used 2 × 2 contingency tables. In each situation, odds ratios (ORs) were used to measure the magnitude of effect, and a χ2 test was used to determine statistical significance. Each covariate was evaluated for appropriate use in the analysis. Maternal education was grouped into a 3-category variable: less than or equal to high school diploma, bachelor’s or technical degree, and master’s or doctorate degree. For all analyses, maternal education less than or equal to high school diploma was used as the reference group. Age was found to have a nonlinear relationship with the outcome and was therefore categorized by quartiles for further analysis, with the youngest age group as reference.
We used multivariate logistic regression to study the relationship between each individual food allergy and asthma while controlling for potential confounders. A forward selection regression model included all food allergies and candidate covariates. Significant associations from the multivariate analysis were defined as those with 2-sided P value ≤.05. No corrections for multiple tests were made. SAS version 9.2 (SAS Institute, Cary, NC) and SPSS version 17.0.2 (SPSS Inc, Chicago, IL) were used for analysis.
A post hoc analysis was performed to determine the effect of the number of food allergies on the prevalence of asthma. The number of food allergies was calculated as the sum of any positive food allergies, as defined above, for an individual subject. For analysis, groups were categorized as 1, 2, 3, and greater than or equal to 4 of these 6 food allergies because there were few subjects with 5 allergies and no subjects allergic to all 6 tested foods. The contingency table was analyzed by a χ2 test and Cochrane-Armitage trend test to examine the significance of the trend.
Results
A total of 1522 questionnaires were distributed among the 3 participating sites to be filled out in waiting rooms or returned by mail. Of the 1413 (93%) questionnaires returned, 1330 (87%) subjects answered the question regarding the presence of asthma. Of these, 799 (60%) had skin prick test or sIgE available to corroborate the report of food allergy. Only these subjects were included in the final analysis.
Table 1 summarizes the distribution of predictor variables and covariates among subjects with and without report of asthma. It also reports the results of the univariate analysis for each predictor and covariate. The subjects were predominantly male and in their early school age years. Peanut was the most common food allergy reported (53%), followed by egg (44%), dairy (38%), and tree nut (24%). Eczema was reported in three fourths of subjects, and hayfever and pet allergy were each present in nearly half of this cohort. Asthma was reported in 365 subjects (45.6%).
Table 1.
Demographic and Univariate Analysis of Allergic Phenotype and Association With Asthma
| No Asthma (n = 434) | Asthma (n = 365) | Odds Ratio (95% Confidence Interval) | P Value | |
|---|---|---|---|---|
| Egg allergya | 166 | 185 | 1.4 (1.1–1.8) | .01 |
| Dairy allergya | 154 | 146 | 1.2 (0.9–1.5) | .28 |
| Seafood allergya | 27 | 40 | 1.6 (1.0–2.7) | .06 |
| Tree nut allergya | 77 | 113 | 2.3 (1.6–3.5) | <.01 |
| Peanut allergya | 223 | 198 | 1.1 (0.8–1.6) | .40 |
| Sesame allergya | 18 | 29 | 1.7 (0.9–3.0) | .10 |
| Pollen allergy/Hayfever | 141 | 237 | 4.3 (3.2–5.9) | <.01 |
| Pet allergy | 125 | 212 | 3.5 (2.6–4.7) | <.01 |
| Eczema | 313 | 278 | 1.3 (1.0–1.8) | .08 |
| Female gender | 181 | 122 | 0.7 (0.5–0.9) | .02 |
| Maternal asthma | 74 | 107 | 2.0 (1.4–2.8) | <.01 |
| Paternal asthma | 53 | 91 | 2.4 (1.6–3.4) | <.01 |
| Maternal hayfever/Pollen allergy | 179 | 190 | 1.6 (1.2–2.1) | <.01 |
| Paternal hayfever/Pollen allergy | 168 | 181 | 1.6 (1.2–2.1) | <.01 |
| Maternal eczema | 93 | 93 | 1.3 (0.9–1.8) | .16 |
| Paternal eczema | 50 | 68 | 1.8 (1.2–2.7) | <.01 |
| Maternal education | ||||
| High school diploma or less | 33 | 40 | Reference | — |
| Bachelor’s or technical degree | 234 | 204 | 0.7 (0.4–1.2) | .2 |
| Master’s or doctorate degree | 156 | 114 | 0.6 (0.4–1.0) | .06 |
| Age (quartiles) | ||||
| First quartile (0–2.9 years) | 155 | 31 | Reference | — |
| Second quartile (2.9–4.9 years) | 121 | 81 | 3.3 (2.1–45.4) | <.01 |
| Third quartile (4.9–7.7 years) | 93 | 111 | 6.0 (3.7–9.6) | <.01 |
| Fourth quartile (7.7–20.1 years) | 59 | 138 | 11.7 (7.2–19.1) | <.01 |
| Number of food allergies | <.001b | |||
| 1 | 280 | 170 | ||
| 2 | 86 | 94 | ||
| 3 | 17 | 42 | ||
| ≥4 | 4 | 15 | ||
Reference group is those with no allergy, adjusted for those who reported never trying the food in question.
χ2 For contingency table.
For each food allergy, after adjusting for those who had “never tried” the food, only children with egg allergy and tree nut allergy had significantly higher odds of having asthma than those without the allergy. As expected, several of the covariates were also positively and significantly associated with asthma, such as hayfever, pet allergy, and family history of allergic diseases. The risk of asthma increased sequentially by quartile of age compared with the youngest quartile. Besides the effect of increasing age, hayfever and pet allergy had the strongest associations with asthma. Univariate analysis of gender showed that female gender was negatively associated with asthma.
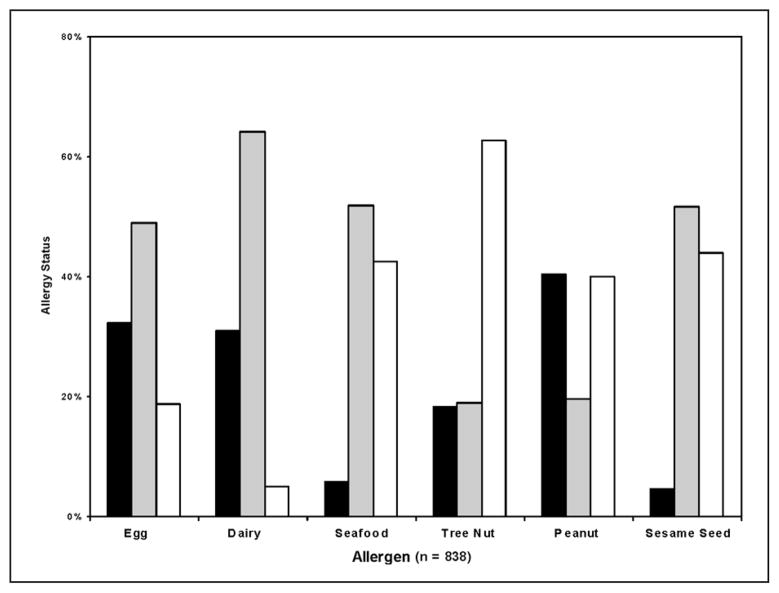
Figure 1 shows the prevalence of each food allergy reported. Nearly half of the subjects had never tried seafood, peanuts, or sesame seeds, more than 60% had never tried tree nuts. When the “never tried” group is removed from each food allergy, there were more peanut-allergic subjects than non-peanut-allergic subjects, and for tree nuts, the proportion of allergic and not allergic subjects was nearly equal.
Figure 1.
Prevalence of food allergy status: bar chart illustrating report of food allergy response to each allergen: black, allergic; gray, not allergic; white, never tried
Table 2 summarizes the results of the multivariate analysis of food allergy and asthma in this cohort of food-allergic children. A total of 718 subjects were included in the final model. After adjusting for each food allergy and those who had “never tried” each potential allergen as well as for environmental allergies, eczema, age, and parental history of asthma, those with egg allergy or tree nut allergy had twice the odds of having asthma (egg, OR = 2.0, 95% confidence interval [CI] = 1.3–3.2, P < .01; tree nut allergy, OR = 2.0, 95% CI = 1.1–3.6, P = .02). Other known risks for asthma, such as environmental allergies, parental history, and increasing age also proved to be independently associated with asthma, whereas female gender remained negatively associated with asthma. A regression model that included those with report of food allergy not corroborated by allergic sensitization as a separate group (because this group’s allergic status is unclear and is highly subject to misclassification) demonstrated similar results (n = 1133; egg, OR = 1.6, P = .01; tree nut, OR = 2.1, P = .005).
Table 2.
Multivariate Analysis of Allergic Phenotype and Association With Asthma
| Asthmaa |
||
|---|---|---|
| Odds Ratio (95% Confidence Interval) | P Value | |
| Egg allergyb | 2.0 (1.3–3.2) | <.01 |
| Dairy allergyb | 1.0 (0.8–2.8) | .93 |
| Seafood allergyb | 1.0 (0.4–2.2) | .94 |
| Tree nut allergyb | 2.0 (1.1–3.6) | .02 |
| Peanut allergyb | 1.5 (0.9–2.6) | .1 |
| Sesame allergyb | 1.4 (0.6–3.3) | .5 |
| Pet allergy | 2.0 (1.4–2.9) | <.01 |
| Pollen allergy | 2.5 (1.7–3.7) | <.01 |
| Paternal asthma | 2.0 (1.2–3.1) | <.01 |
| Maternal asthma | 1.6 (1.0–2.4) | .04 |
| Female gender | 0.7 (0.5–0.9) | .02 |
| Age (quartiles) | ||
| First quartile | Reference | — |
| Second quartile | 3.0 (1.7–5.2) | <.01 |
| Third quartile | 4.1 (2.3–7.4) | <.01 |
| Fourth quartile | 7.1 (3.8–13.3) | <.01 |
| Eczema | 1.5 (1.0–2.3) | .08 |
Additionally adjusted for never tried each food allergen.
Reference group is those with no allergy.
Figure 2 demonstrates the positive effect of multiple food allergies on the prevalence of asthma (χ2 P < .001; Cochrane-Armitage test for linear trend P < .001).
Figure 2.
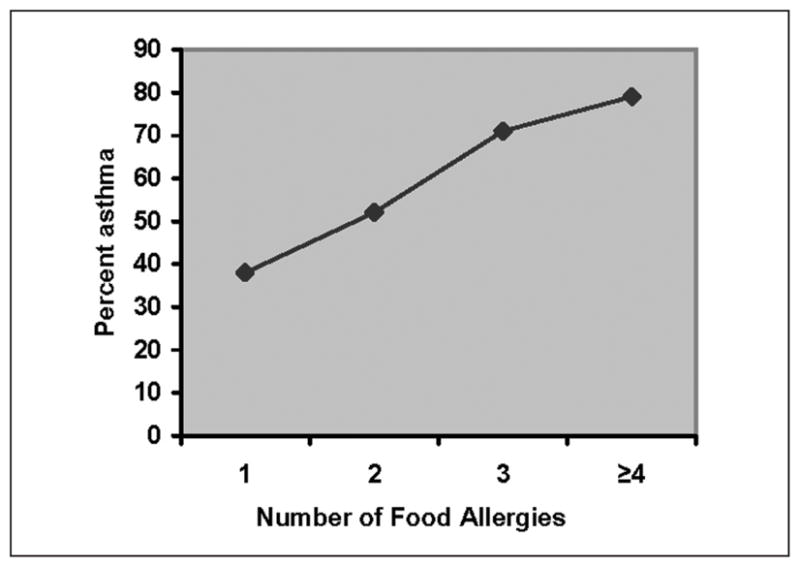
Trend in report of asthma by number of food allergies: number of food allergies limited to peanut, tree nut, seafood, dairy, egg, or sesame seed allergy; χ2 for the distribution, P < .001; Cochrane-Armitage test for linear trend P < .001
Discussion
This study shows that nearly half of children with food allergies report having a diagnosis of asthma. Furthermore, through a very conservative analysis in which each food allergy predictor was adjusted for all the potential food allergies and “not having tried” each of the foods as well as for known asthma risk factors, we demonstrate that a clinical history of egg and tree nut allergy are strongly and significantly associated with asthma.
Several cohorts of asthmatic children have been well characterized for allergic diatheses, and it is clear that food allergy in asthmatic children is highly prevalent.11–14,22,23 Up to 45% of children with asthma have food sensitivity by food sIgE.22 We hypothesized that the prevalence of asthma in this study would be elevated given the shared immunological predisposition to atopic diseases. We found the prevalence of asthma to be 45.6% in our food-allergic pediatric cohort. This is markedly higher than the 29% prevalence of asthma in food-allergic children found by the US National Center for Health Services (NCHS) from data collected by national telephone survey2 in an unselected sample of the general population. This difference likely stems from the different populations sampled. Although both studies rely on self-report for diagnosis of food allergy and asthma, subjects in our study were seen at referral-based allergy practices, and report of allergy was corroborated by food allergy sensitization. It is likely that our subjects had more accurate determination of clinically significant food allergy because the clinical history was validated by objective food allergy tests.
Food allergy has been implicated as a risk factor for asthma,11 a trigger for asthma exacerbations,24 and a factor determining asthma morbidity.13,14 This study shows specific food allergies to be independently associated with asthma in children. In our cohort, egg and tree nut allergy confer significantly higher risk of associated asthma in children with food allergy after adjustment for known risk factors. The statistical model controls for potential confounders such as eczema, report of allergies to common aeroallergens, and family history of atopy, which further strengthens the importance of the interrelationship between egg and tree nut allergy and asthma independently of other atopic risk factors. By including each food allergy in the same model, we account for its independent effect on the outcome, illustrating the strength of the association of egg and tree nut allergy with asthma.
Early food sensitization has been previously identified as a predictor for asthma. Rhodes et al21 demonstrated positive skin prick test to egg or cow’s milk in infancy to be predictive of adult asthma in a cohort at risk for asthma by virtue of family history of atopy. Notably, these were the only food allergens tested in this cohort. Similarly, hen’s egg sensitization predicts later aeroallergen sensitization in infants with eczema25 and, along with wheat sensitization, is associated with development of asthma in wheezy infants.19 Although remarkable, these studies demonstrate sensitization as a risk without explicitly assessing the clinically important variable of symptomatic food allergy.
In children with clinical food allergies, Tariq et al26 found infantile symptomatic egg allergy to be strongly predictive of asthma in a large population-based cohort in the Isle of White. This association held through multiple regression analysis that included important demographic risk factors. In a smaller study, Schroeder et al17 also reported significant associations between several food allergies and asthma in a pediatric cohort in an adjusted model but did not report independent effects of each food allergy in relation to the other food allergens. The current study lends strength to the association between clinical egg allergy, documented by report and sensitization, and asthma in the context of adjusting for other food allergens and aeroallergens. Tree nut allergy or sensitization has infrequently been explored in association with asthma in food allergic children27 and has not been shown to have an independent effect when adjusting for other food allergies. The current analysis demonstrates tree nut allergy to be strongly and significantly associated with asthma independent of other food allergies.
Because this study only recruited children from subspecialty allergy clinics with reported food allergies, it is not unusual that many of the subjects had not yet tried some of the foods in question. This may be a result of general food introduction guidelines,28 which recommended delaying the introduction of common allergens in high-risk children. Additionally, some families may have been instructed to avoid introducing foods to which the child showed laboratory or skin-prick test sensitivity prior to first ingestion. In multivariate analyses, those who had never tried peanut and sesame seed had significant association with asthma, which may be explained by such avoidance. It is difficult to interpret data for subjects who had not tried a food without information regarding why they had not tried it; however, we adjusted for all the “never tried” responses in our multivariate regression analysis. The positive findings of this study take into account this group for whom we have limited information.
Though up to 9% of children may have food-induced wheeze as their only allergic manifestation to a food allergen,29,30 it is unlikely that this accounts for the magnitude and significance of the egg and tree nut effect found here. The foods most often associated with food-induced wheeze in asthmatics are eggs, milk, and fish,29 which would not explain the effect of tree nut in our study. Additionally, several studies have shown that individuals with specific food allergies, particularly to peanut but also to egg and dairy, have greater morbidity, including bronchodilator use, steroid use, and severe asthma exacerbations, independently of concurrent exposure to these allergens.13,31
It has been suggested that the risk for asthma increases with the number of food allergies.17 Our findings support the mounting evidence that greater number of food allergies is also an important factor associated with asthma. This study investigated allergies to 6 food items in a population of children selected for report of any food allergy. When evaluating the cumulative number of food allergies out of the 6 queried, we observed a highly significant positive trend with report of asthma.
Our study is limited by the lack of information on some possible confounding factors such as race and direct indicators of socioeconomic status (SES). Maternal level of education has been correlated with SES in previous asthma studies32 and serves that purpose here. This variable showed a nonsignificant positive effect on increased asthma risk with lower level of maternal education in the univariate analysis, as would be expected from an SES-related variable.32 Parental report as the basis for the primary predictor variables tested allow recall bias, though potential overreporting of allergic reaction was limited by corroborating evidence of sensitization. Double-blind food challenge would be ideal to identify those with true food allergy; however, this was beyond the scope of this study. The report of asthma was not corroborated by objective data and still may fall subject to recall bias.
Conclusions
There is a high prevalence of asthma among the pediatric food-allergic population. We demonstrate that egg and tree nut allergy, independent of other food allergies, are associated with asthma in a large study of food-allergic children. These findings suggest that clinicians should have a low threshold to suspect asthma in children with food allergies, especially if the child is allergic to eggs or tree nuts.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: Funding was received from the Jordan Family Fund for Allergy Research; Dr Gaffin is supported by NIH KL2 RR025757-01, Harvard Clinical and Translational Science Center (KL1) and the American Thoracic Society Fellows Career Development Award; Dr Phipatanakul is funded by NIH/NIAID R-01 grant (AI-073964) and NIH/NHLBI AsthmaNet 1U10HL098102.
Footnotes
Reprints and permission: http://www.sagepub.com/journalsPermissions.nav
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1.Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. J Allergy Clin Immunol. 2003;112:1203–1207. doi: 10.1016/s0091-6749(03)02026-8. [DOI] [PubMed] [Google Scholar]
- 2.Branum AM, Lukacs SL. Food allergy among US children: trends in prevalence and hospitalizations. NCHS Dara Brief. 2008;(10):1–8. [PubMed] [Google Scholar]
- 3.Moorman JE, Rudd RA, Johnson CA, et al. National surveillance for asthma: United States, 1980–2004. MMWR Surveill Summ. 2007;56:1–54. [PubMed] [Google Scholar]
- 4.Rudd RA, Moorman JE. Asthma incidence: data from the National Health Interview Survey, 1980–1996. J Asthma. 2007;44:65–70. doi: 10.1080/02770900601125896. [DOI] [PubMed] [Google Scholar]
- 5.Devereux G. The increase in the prevalence of asthma and allergy: food for thought. Nat Rev Immunol. 2006;6:869–874. doi: 10.1038/nri1958. [DOI] [PubMed] [Google Scholar]
- 6.Osterballe M, Hansen TK, Mortz CG, Host A, Bindslev-Jensen C. The prevalence of food hypersensitivity in an unselected population of children and adults. Pediatr Allergy Immunol. 2005;16:567–573. doi: 10.1111/j.1399-3038.2005.00251.x. [DOI] [PubMed] [Google Scholar]
- 7.Venter C, Pereira B, Grundy J, Clayton CB, Arshad SH, Dean T. Prevalence of sensitization reported and objectively assessed food hypersensitivity amongst six-year-old children: a population-based study. Pediatr Allergy Immunol. 2006;17:356–363. doi: 10.1111/j.1399-3038.2006.00428.x. [DOI] [PubMed] [Google Scholar]
- 8.Venter C, Pereira B, Grundy J, et al. Incidence of parentally reported and clinically diagnosed food hypersensitivity in the first year of life. J Allergy Clin Immunol. 2006;117:1118–1124. doi: 10.1016/j.jaci.2005.12.1352. [DOI] [PubMed] [Google Scholar]
- 9.Pereira B, Venter C, Grundy J, Clayton CB, Arshad SH, Dean T. Prevalence of sensitization to food allergens, reported adverse reaction to foods, food avoidance, and food hypersensitivity among teenagers. J Allergy Clin Immunol. 2005;116:884–892. doi: 10.1016/j.jaci.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 10.Venter C, Pereira B, Voigt K, et al. Prevalence and cumulative incidence of food hypersensitivity in the first 3 years of life. Allergy. 2008;63:354–359. doi: 10.1111/j.1398-9995.2007.01570.x. [DOI] [PubMed] [Google Scholar]
- 11.Leung TF, Lam CW, Chan IH, Li AM, Tang NL. Sensitization to common food allergens is a risk factor for asthma in young Chinese children in Hong Kong. J Asthma. 2002;39:523–529. doi: 10.1081/jas-120004922. [DOI] [PubMed] [Google Scholar]
- 12.Berns SH, Halm EA, Sampson HA, Sicherer SH, Busse PJ, Wisnivesky JP. Food allergy as a risk factor for asthma morbidity in adults. J Asthma. 2007;44:377–381. doi: 10.1080/02770900701364031. [DOI] [PubMed] [Google Scholar]
- 13.Simpson AB, Glutting J, Yousef E. Food allergy and asthma morbidity in children. Pediatr Pulmonol. 2007;42:489–495. doi: 10.1002/ppul.20605. [DOI] [PubMed] [Google Scholar]
- 14.Roberts G, Patel N, Levi-Schaffer F, Habibi P, Lack G. Food allergy as a risk factor for life-threatening asthma in childhood: a case-controlled study. J Allergy Clin Immunol. 2003;112:168–174. doi: 10.1067/mai.2003.1569. [DOI] [PubMed] [Google Scholar]
- 15.Woods RK, Abramson M, Bailey M, Walters EH. International prevalences of reported food allergies and intolerances: comparisons arising from the European Community Respiratory Health Survey (ECRHS) 1991–1994. Eur J Clin Nutr. 2001;55:298–304. doi: 10.1038/sj.ejcn.1601159. [DOI] [PubMed] [Google Scholar]
- 16.Grundy J, Matthews S, Bateman B, Dean T, Arshad SH. Rising prevalence of allergy to peanut in children: data from 2 sequential cohorts. J Allergy Clin Immunol. 2002;110:784–789. doi: 10.1067/mai.2002.128802. [DOI] [PubMed] [Google Scholar]
- 17.Schroeder A, Kumar R, Pongracic JA, et al. Food allergy is associated with an increased risk of asthma. Clin Exp Allergy. 2009;39:261–270. doi: 10.1111/j.1365-2222.2008.03160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nickel R, Kulig M, Forster J, et al. Sensitization to hen’s egg at the age of twelve months is predictive for allergic sensitization to common indoor and outdoor allergens at the age of three years. J Allergy Clin Immunol. 1997;99:613–617. doi: 10.1016/s0091-6749(97)70021-6. [DOI] [PubMed] [Google Scholar]
- 19.Kotaniemi-Syrjanen A, Reijonen TM, Romppanen J, Korhonen K, Savolainen K, Korppi M. Allergen-specific immunoglobulin E antibodies in wheezing infants: the risk for asthma in later childhood. Pediatrics. 2003;111:e255–e261. doi: 10.1542/peds.111.3.e255. [DOI] [PubMed] [Google Scholar]
- 20.Saarinen KM, Pelkonen AS, Makela MJ, Savilahti E. Clinical course and prognosis of cow’s milk allergy are dependent on milk-specific IgE status. J Allergy Clin Immunol. 2005;116:869–875. doi: 10.1016/j.jaci.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Rhodes HL, Sporik R, Thomas P, Holgate ST, Cogswell JJ. Early life risk factors for adult asthma: a birth cohort study of subjects at risk. J Allergy Clin Immunol. 2001;108:720–725. doi: 10.1067/mai.2001.119151. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Visness CM, Sampson HA. Food allergen sensitization in inner-city children with asthma. J Allergy Clin Immunol. 2005;115:1076–1080. doi: 10.1016/j.jaci.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 23.Kjaer HF, Eller E, Host A, Andersen KE, Bindslev-Jensen C. The prevalence of allergic diseases in an unselected group of 6-year-old children: the DARC birth cohort study. Pediatr Allergy Immunol. 2008;19:737–745. doi: 10.1111/j.1399-3038.2008.00733.x. [DOI] [PubMed] [Google Scholar]
- 24.Roberts G, Lack G. Food allergy and asthma: what is the link? Paediatr Respir Rev. 2003;4:205–212. doi: 10.1016/s1526-0542(03)00058-7. [DOI] [PubMed] [Google Scholar]
- 25.Gustafsson D, Sjoberg O, Foucard T. Development of allergies and asthma in infants and young children with atopic dermatitis: a prospective follow-up to 7 years of age. Allergy. 2000;55:240–245. doi: 10.1034/j.1398-9995.2000.00391.x. [DOI] [PubMed] [Google Scholar]
- 26.Tariq SM, Matthews SM, Hakim EA, Arshad SH. Egg allergy in infancy predicts respiratory allergic disease by 4 years of age. Pediatr Allergy Immunol. 2000;11:162–167. doi: 10.1034/j.1399-3038.2000.00077.x. [DOI] [PubMed] [Google Scholar]
- 27.Schroeder A, Kumar R, Pongracic JA, et al. Food allergy is associated with an increased risk of asthma. Clin Exp Allergy. 2009;39:261–270. doi: 10.1111/j.1365-2222.2008.03160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Academy of Pediatrics. Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics. 2000;106 (2 pt 1):346–349. [PubMed] [Google Scholar]
- 29.Oehling A, Baena Cagnani CE. Food allergy and child asthma. Allergol Immunopathol (Madr) 1980;8:7–14. [PubMed] [Google Scholar]
- 30.Novembre E, de Martino M, Vierucci A. Foods and respiratory allergy. J Allergy Clin Immunol. 1988;81(5 pt 2):1059–1065. doi: 10.1016/0091-6749(88)90181-9. [DOI] [PubMed] [Google Scholar]
- 31.Liang PH, Shyur SD, Huang LH, et al. Risk factors and characteristics of early-onset asthma in Taiwanese children. J Microbiol Immunol Infect. 2006;39:414–421. [PubMed] [Google Scholar]
- 32.Cesaroni G, Farchi S, Davoli M, Forastiere F, Perucci CA. Individual and area-based indicators of socioeconomic status and childhood asthma. Eur Respir J. 2003;22:619–624. doi: 10.1183/09031936.03.00091202. [DOI] [PubMed] [Google Scholar]