Abstract
Background & Aims
In this study, we sought to determine the temporal relationship between hepatic mitochondrial dysfunction, hepatic steatosis and insulin resistance, and to examine their potential role in the natural progression of non-alcoholic fatty liver disease (NAFLD) utilising a sedentary, hyperphagic, obese, Otsuka Long–Evans Tokushima Fatty (OLETF) rat model.
Methods
OLETF rats and their non-hyperphagic control Long–Evans Tokushima Otsuka (LETO) rats were sacrificed at 5, 8, 13, 20, and 40 weeks of age (n = 6–8 per group).
Results
At 5 weeks of age, serum insulin and glucose and hepatic triglyceride (TG) concentrations did not differ between animal groups; however, OLETF animals displayed significant (p < 0.01) hepatic mitochondrial dysfunction as measured by reduced hepatic carnitine palmitoyl-CoA transferase-1 activity, fatty acid oxidation, and cytochrome c protein content compared with LETO rats. Hepatic TG levels were significantly elevated by 8 weeks of age, and insulin resistance developed by 13 weeks in the OLETF rats. NAFLD progressively worsened to include hepatocyte ballooning, perivenular fibrosis, 2.5-fold increase in serum ALT, hepatic mitochondrial ultrastructural abnormalities, and increased hepatic oxidative stress in the OLETF animals at later ages. Measures of hepatic mitochondrial content and function including β-hydroxyacyl-CoA dehydrogenase activity, citrate synthase activity, and immunofluorescence staining for mitochondrial carbamoyl phosphate synthetase-1, progressively worsened and were significantly reduced at 40 weeks in OLETF rats compared to LETO animals.
Conclusions
Our study documents that hepatic mitochondrial dysfunction precedes the development of NAFLD and insulin resistance in the OLETF rats. This evidence suggests that progressive mitochondrial dysfunction contributes to the natural history of obesity-associated NAFLD.
Keywords: Non-alcoholic fatty liver disease, Fatty acid oxidation, Mitochondrial dysfunction, OLETF rat
Introduction
A critical complication of the obesity epidemic experienced by children and adults in Westernized societies is non-alcoholic fatty liver disease (NAFLD). NAFLD affects ~30% of all US adults and 75–100% of obese and morbidly obese individuals [1,2] and is now considered the hepatic representation of the metabolic syndrome [3]. Even more alarming, as the number of overweight and obese children has doubled in the past 2–3 decades in the US, there is an increasing propensity of NAFLD and non-alcoholic steatohepatitis (NASH) development in younger individuals [4]. In fact, it is estimated that 10% of lean and 38% of obese children have fatty livers [5]. Consequently, clinicians warn that the demand for liver transplants may rise as these children become adults if steps are not taken to reverse this trend.
Day and James [6] have proposed the “two-hit hypothesis” to explain the development of hepatic steatosis and the progression to inflammation (NASH), fibrosis, and cirrhosis. This hypothesis states that factors such as insulin resistance and impaired hepatic fatty acid oxidation contribute to NAFLD development [3] and that once steatosis is present, inflammation and oxidative stress are thought to activate stellate cells and increase collagen deposition and fibrogenesis [6]. However, it has been speculated that mitochondrial abnormalities may be involved in the pathogenesis of NAFLD [7–10]. In fact, we previously demonstrated that heterozygosity for mitochondrial β-oxidation defects causes development of NAFLD in aging mice [10], which raises the possibility that NAFLD may be a mitochondrial disease. However, little is known about the spectrum of changes in mitochondrial content and function and their potential role in the natural history of NAFLD.
Otsuka Long–Evans Tokushima Fatty (OLETF) rats are a commonly studied model of obesity and type 2 diabetes [11]. Selectively bred for lack of cholecystokinin-1 receptor expression, OLETF rats exhibit a within meal feedback defect for satiety, resulting in hyperphagia and obesity [12]. OLETF rats display normal glycemic control at a young age [13–15], and we have previously shown they display insulin resistance at 13 and 20 weeks and develop overt type 2 diabetes by 40 weeks of age [16]. In addition, OLETF rats develop hepatic steatosis in the absence of significant liver injury and have hepatic mitochondrial dysfunction at 20 weeks of age [17,18]. However, the causative relationship between hepatic steatosis, hepatic mitochondrial dysfunction, and insulin resistance in this model is unknown. Furthermore, the mechanisms responsible for disease progression in this model, including the potential role of hepatic mitochondrial dysfunction, have not been fully examined. The lack of understanding of early precipitating changes for this disease could restrict the development of optimal therapies to lessen its prevalence.
NAFLD in the OLETF rat resembles that associated with human obesity and occurs in the absence of extreme dietary manipulations. Thus, the OLETF rat represents an excellent model to explore the potential initiating factor in NAFLD development and the role of mitochondrial function in obesity-associated NAFLD, which perhaps mirrors conditions seen in children and through adulthood. We hypothesized that development and progression of NAFLD in the hyperphagic, obese OLETF rat is likely due to early and progressive reductions in hepatic mitochondrial content and function. In this study, we utilised this novel model to assess hepatic mitochondrial content and function in the natural history of NAFLD.
Methods
Animal protocol
The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Missouri. OLETF and LETO male rats at 4 weeks of age were kindly supplied by Otsuka Pharmaceutical (Tokushima, Japan). Cages were in temperature-controlled animal quarters (21 °C) with a 06:00–18:00 h light: 18:00–06:00 h dark cycle, maintained throughout the experimental period. All animals were provided standard rodent chow (Formulab 5008, Purina Mills, St. Louis, MO, USA) in clean cages at the beginning of each week. Body mass and food intake were measured weekly throughout the investigation. At 5, 8, 13, 20, and 40 weeks of age, rats were anaesthetised with sodium pentobarbital (100 mg kg−1) and killed by exsanguination by removal of the heart. All animals were fasted for 5 h prior to sacrifice.
Tissue homogenisation procedure and mitochondrial isolation
Livers were quickly excised from anaesthetised rats and either flash frozen in liquid nitrogen, placed in 10% formalin, or placed in ice-cold buffer (100 mM KCl, 40 mM Tris–HCl, 10 mM Tris–Base, 5 mM MgCl2·6H2O, 1 mM EDTA, and 1 mM ATP; pH 7.4). Fresh tissue hepatic fatty acid oxidation assays were performed as previously described [18]. Mitochondrial suspensions were prepared according to modified methods of Koves et al. [19], as previously described [20].
Fatty acid oxidation
Palmitate oxidation was measured with radiolabeled [1-14C]palmitate (American Radiochemicals) in fresh liver homogenate preparations as previously reported [18]. Both 14CO2, representing complete fatty acid oxidation, and 14C labelled acid soluble metabolites (ASMs), representing incomplete oxidation, were collected in the previously described trapping device and then counted on a liquid scintillation counter.
Intrahepatic lipid content, Oil-Red O staining, liver histology, and carbamoylphosphate synthetase-1 (CPS-1) staining
Intrahepatic lipid content, Oil-Red O (neutral lipid), hematoxylin and eosin (H&E), picrosirius red (collagen deposition), CPS-1, and transforming growth factor beta (TGF-β) staining were performed as previously described [18,21,22]. CPS-1 and TGF-β staining were only assessed in 13, 20, and 40-week-old animals. For assessment of hepatic steatosis, histopathologic criteria proposed by Kleiner et al. [23] were adopted. Hepatic steatosis was graded as follows: <5% (score 0), 5–33% (score 1), >33–66% (score 2), or >66% (score 3).
Western blotting
Western blot analyses were performed for the determination of the protein content of total hepatic cytochrome c (Cell Signaling, Beverly, MA, USA) and uncoupling protein-2 (UCP-2, Santa Cruz, Santa Cruz, CA, USA), as previously described [17,18]. Total protein staining (0.1% amido-black) for each lane was used to correct for any differences in protein loading or transfer of all band densities [18]. The intensities of the bands and total protein staining were quantified using Quantity One software (Bio-Rad).
Fat pad collection and serum assays
Retroperitoneal and omental adipose tissue fat pads were removed from exsanguinated animals and weighed. In addition, whole-body composition was measured using a Hologic QDR-1000/w DEXA machine calibrated for rats. Serum glucose, TG, free fatty acids (FFA), insulin, serum alanine aminotransferase (ALT), and hemoglobin A1c (HbA1c) concentrations were measured as previously described [16,18]. Serum ALT and HbA1c were not assessed in 5 and 8 week old rats.
Reduced (GSH) and oxidized glutathione (GSSG)
GSH and GSSG concentrations were determined by fluorometric methods as previously described [24], utilising methods of Hissin and Hilf [25].
Enzyme activity assays
Liver superoxide dismutase (SOD) activity was determined by commercially available methods (Cayman Chemicals, Ann Arbor, MI, USA). Citrate synthase and beta-hydroxyacyl-CoA dehydrogenase (β-HAD) activities were determined in isolated liver mitochondria using the methods of Srere [26] and Bass et al. [27], respectively, as previously described [17,18]. Hepatic carnitine palmitoyl-CoA transferase-1 (CPT-1) activity from isolated liver mitochondria was measured as previously described [20]. Due to technical difficulties, CPT-1 activity was not assessed in the liver of 8 and 13 week old animals and citrate synthase and β-HAD activities were not assessed in 8 week old animals.
Transmission electron microscopy (TEM)
Liver sections were prepared for EM imaging as previously described [10]. EM images (n = 4 animals per group; 13, 20, and 40 week old animals; 5–7 views per animal) were taken at the University of Missouri EM Core using a TEM (1200-EX, Jeol, Ltd., Tokyo, Japan).
Statistics
Each outcome measure was examined in 6–8 animals per age per group. For each outcome measure, a one-way analysis of variance was performed (SPSS/15.0, SPSS, Chicago, IL, USA) for each animal group at each age studied. A significant main effect (p <0.05) was followed-up with Student–Newman–Kuel post hoc comparisons. Values are reported as means ± standard error of the mean (SE), and a p value less than 0.05 denotes a statistically significant difference.
Results
Animal and serum characteristics
Body mass and fat pad mass (omental and retroperitoneal) was significantly greater in the OLETF rats compared with the LETO animals at all ages studied (Table 1). Similar to our previous report [16], percent body fat determined by DEXA was also significantly greater in OLETF compared with LETO animals at 13, 20, and 40 weeks (data not shown). Absolute weekly food consumption increased with age (p <0.05) in both groups and was significantly greater (p <0.01) in OLETF rats at all ages compared with LETO animals (Table 1). Food consumption relative to body weight decreased with age in both groups and was significantly greater at 40 weeks only in the OLETF compared with LETO rats (p <0.01, data not shown). Serum TGs were not significantly elevated until 8 weeks of age and FFAs until 13 weeks in the OLETF animals compared with LETO (Table 2).
Table 1.
Animal characteristics.
| Age | LETO | OLETF | ||||
|---|---|---|---|---|---|---|
| Body weight (g) | Food consumption (g/wk) | Fat pad mass (g) | Body weight (g) | Food consumption (g/wk) | Fat pad mass (g) | |
| 5 wk | 107.6 ± 6.0a | 65.2 ± 2.8a | 0.3 ± 0.03a | 132.4 ± 5.1a* | 88.5 ± 6.5a* | 0.4 ± 0.04a |
| 8 wk | 228.4 ± 6.3b | 135.4 ± 5.3b | 0.9 ± 0.08b | 288.5 ± 5.0b* | 182.9 ± 2.8b* | 3.5 ± 0.19b* |
| 13 wk | 380.8 ± 5.9c | 155.3 ± 1.4c | 4.9 ± 0.3c | 495.4 ± 15.8c* | 222.5 ± 4.8c* | 15.7 ± 1.6c* |
| 20 wk | 477.9 ± 6.6d | 164.0 ± 2.1d | 8.9 ± 0.6d | 607.0 ± 10.5d* | 241.8 ± 7.0c* | 25.8 ± 1.0d* |
| 40 wk | 557.4 ± 15.0e | 166.5 ± 4.6d | 14.6 ± 1.2e | 685.2 ± 34.6e* | 308.5 ± 18.6d* | 55.7 ± 7.5e* |
Values are means ± SE (n = 6–8). Different letter superscripts within an animal group indicate a significant change between ages (p <0.05). Significant age-associated increases were noted in both animals groups for body weight, absolute food consumption, and fat pad mass (omental and retroperitoneal fat pads).
Indicates values at the respective age are significantly different in OLETF compared with LETO rats (p <0.01).
Table 2.
Serum and liver characteristics.
| Age | LETO | OLETF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Serum TG (mg/dl) |
Serum FFAs (μmol/L) |
Liver GSH (μmol/μg) |
Liver GSSG (μmol/μg) |
Liver SOD activity (U/ml) |
Serum TG (mg/dl) |
Serum FFAs (μmol/L) |
Liver GSH (μmol/μg) |
Liver GSSG (μmol/μg) |
Liver SOD activity (U/ml) |
|
| 5 wk | 29.5 ± 3.6a | 183.0 ± 51.8 | 2.76 ± 0.18a | 0.30 ± 0.02 | 9.4 ± 0.6a | 34.0 ± 5.2a | 179.1 ± 27.3a | 2.53 ± 0.14a | 0.26 ± 0.02a | 7.3 ± 0.5a* |
| 8 wk | 20.3 ± 2.6a | 139.2 ± 30.7 | 2.62 ± 0.15a | 0.28 ± 0.02 | 8.1 ± 0.3a | 36.2 ± 5.0a* | 176.6 ± 38.9a | 2.35 ± 0.15a | 0.26 ± 0.01a | 6.3 ± 0.5a* |
| 13 wk | 36.8 ± 4.7b | 154.7 ± 43.2 | 2.95 ± 0.06a | 0.31 ± 0.01 | 5.6 ± 0.5b | 114.7 ± 18.2b* | 203.9 ± 20.7a* | 3.16 ± 0.13b | 0.35 ± 0.01b* | 5.9 ± 0.3b |
| 20 wk | 42.7 ± 4.3b | 170.3 ± 23.6 | 3.45 ± 0.06b | 0.35 ± 0.01 | 5.9 ± 0.4b | 177.1 ± 24.8c* | 311.4 ± 46.0b* | 3.54 ± 0.11b | 0.38 ± 0.02b,c* | 4.7 ± 0.9b,c |
| 40 wk | 42.2 ± 3.1b | 187.4 ± 22.0 | 3.21 ± 0.13a,b | 0.35 ± 0.02 | 4.9 ± 0.7b | 253.4 ± 32.8d* | 309.7 ± 56.6b* | 3.74 ± 0.07c* | 0.42 ± 0.02c* | 3.6 ± 0.5c* |
Values are means ± SE (n = 6–8). Different letter superscripts within an animal group indicate a significant change between ages (p <0.05). Significant age-associated increases were noted in both animals groups for serum TG and liver GSH levels and also serum FFAs and liver GSSG concentrations in the OLETF rats only. Significant age-associated decreases were noted in both groups for liver SOD activity.
Indicates values at the respective age are significantly different in OLETF compared with LETO rats (p <0.05).
Liver anti-oxidative status
Liver SOD activity was initially higher at 5 and 8 weeks in the LETO rats and dropped significantly with age in both groups (Table 2), becoming significantly lower in the OLETF animals at 40 weeks compared with LETO animals. OLETF animals displayed an age-associated increase in liver GSH levels, which was significantly higher than LETO animals by 40 weeks. However, GSSG levels were significantly higher in the OLETF animals at 13, 20, and 40 weeks compared with LETO animals (Table 2). Collectively, these data suggest that the liver of the OLETF animals was in a pro-oxidative environment.
Glycemic control
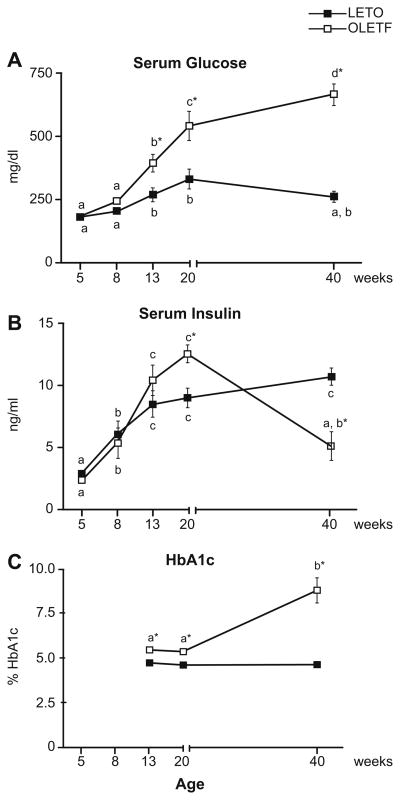
Serum insulin and glucose did not differ between OLETF and LETO rats at 5 or 8 weeks of age (Fig. 1A and B). These findings are consistent with several previous reports indicating normal glycemic control in OLETF rats 10 weeks old and younger [13–15]. In contrast to the LETO groups, the insulin-resistant OLETF animals developed elevated serum glucose (Fig. 1A) at 13 and 20 weeks, followed by the total loss of glycemic control and the development of overt diabetes mellitus as shown by a 70% drop in insulin and doubling in HbA1c between 20 and 40 weeks (Fig. 2B and C).
Fig. 1. Serum glucose (A), serum insulin (B), and HbA1c (C) levels at 5, 8, 13, 20, and 40 weeks of age.
Values (means ± SE, n = 6–8) with different letter superscripts within each animal group are significantly different (p <0.01). *Significantly different from LETO at respective ages (p <0.01).
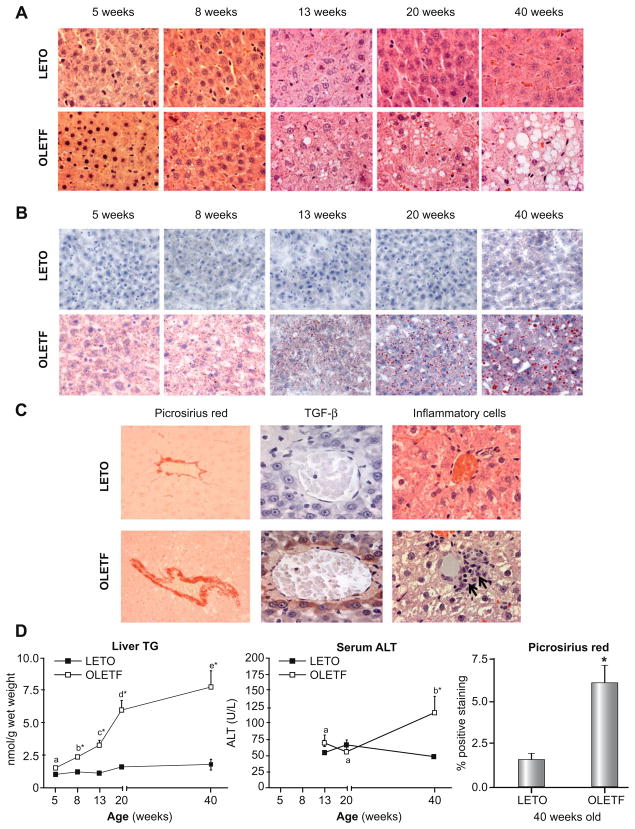
Fig. 2. Representative images of H&E (A), Oil-Red O (B), and picrosirius red staining, TGF-β staining, and inflammatory cell infiltration (C).
Note the large lipid vacuoles (A) and macro- and micro-vesicular steatosis (B) and the progression from 8 to 40 weeks in the liver of the OLETF rats. Staining for fibrosis, TGF-β, and inflammatory cell infiltration did not differ between OLETF and LETO animals at 5, 8, 13, or 20 weeks of age (data not shown); however by 40 weeks, there was significantly greater staining for picrosirius red and TGF-β (brown staining) and inflammatory cells (black arrows) in the OLETF animals compared with LETO controls (C). Quantification of hepatic TG, serum ALT, and hepatic picrosirius red staining (40 weeks only) are shown in (D). Values (means ± SE, n = 5–8) with different letter superscripts within each animal group are significantly different (p <0.01). No significant differences existed within the LETO groups for any measured parameter (p >0.05). *Significantly different from LETO at respective ages (p <0.01).
NAFLD progression
Significant hepatic lipid accumulation was not present in 5-week-old OLETF or LETO animals (Fig. 2A, B, and D); however, by 8 weeks of age, the OLETF rats exhibited significant hepatic TG accumulation, which progressed with age (Fig. 2A, B, and D). Steatosis scoring and % of hepatocytes with lipids did not differ at 5 weeks between OLETF and LETO animals, but significantly increased from 8 through 40 weeks in the OLETF rats and was significantly greater in OLETF compared with LETO animals (Table 3). The OLETF animals also had hepatocyte ballooning (Fig. 2A, 40 week old OLETFs), nuclear displacement (Fig. 2A, 40 week old OLETFs), and increased fibrosis and collagen deposition in perivenular (around terminal hepatic veins) regions as indicated by picrosirius red and TGF-β staining (Fig. 2C and D; no differences observed at younger ages and data are not shown). Furthermore, significant inflammatory cell infiltration was observed in the liver of 40 week OLETF animals (Fig. 2C; no differences observed at younger ages and data are not shown). Substantial liver injury was further indicated by a 2.5-fold increase in serum ALTs by 40 weeks in the OLETF animals (Fig. 2D), elevations that were absent in the LETO rats.
Table 3.
Liver histology parameters.
| Age | LETO | OLETF | ||
|---|---|---|---|---|
| Steatosis score | Hepatocytes with lipids (%) | Steatosis score | Hepatocytes with lipids (%) | |
| 5 wk | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.13 ± 0.13a | 1.5 ± 1.1a |
| 8 wk | 0.08 ± 0.08 | 4.3 ± 2.5 | 1.06 ± 0.16b* | 19.6 ± 4.5b* |
| 13 wk | 0.42 ± 0.25 | 6.0 ± 4.1 | 1.15 ± 0.10b* | 26.5 ± 3.0b* |
| 20 wk | 0.50 ± 0.10 | 6.3 ± 1.0 | 1.80 ± 0.24c* | 43.4 ± 8.6c* |
| 40 wk | 0.21 ± 0.13 | 1.5 ± 0.9 | 2.83 ± 0.11d* | 83.6 ± 5.8d* |
Values are means ± SE (n = 4–6). Different letter superscripts within an animal group indicate a significant change between ages (p <0.05). Significant age-associated increases were noted for steatosis score and hepatocytes with lipids in the OLETF rats only.
Altered hepatic mitochondrial function, content, and ultrastructure
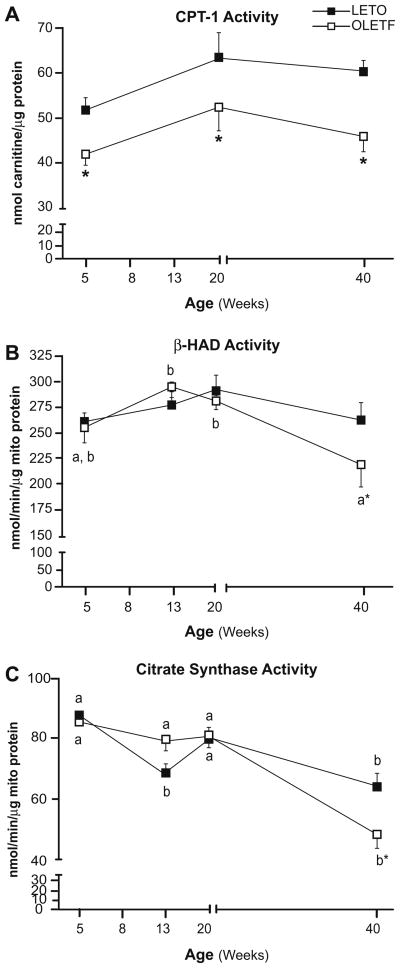
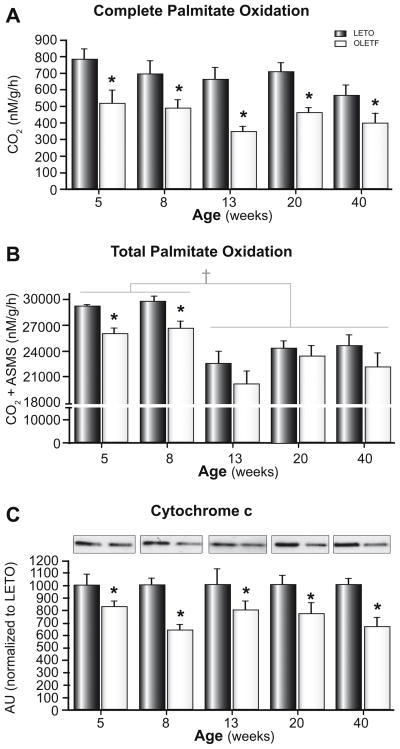
Liver CPT-1 activity was significantly reduced in 5-week-old OLETF rats compared with LETO animals (Fig. 3A) and the reduced activity persisted through 40 weeks of age, suggesting that the OLETF animals have reduced potential for hepatic mitochondrial fatty acid entry prior to the development of hepatic steatosis and insulin resistance and throughout the natural history of the disease. OLETF animals also exhibited an age-associated reduction in hepatic β-HAD (the rate limiting step in mitochondrial β-oxidation) and citrate synthase (rate limiting step in the TCA cycle) activities by 40 weeks. These values were significantly reduced compared with LETO animals (Fig. 3B and C). In addition, complete hepatic palmitate disposal (CO2 production) was reduced by ~50% at all ages studied (Fig. 4A) and total palmitate oxidation (CO2 + acid soluble metabolites) was reduced at 5 and 8 weeks in the OLETF compared with LETO rats (Fig. 4B). In 13, 20, and 40-week animals, total fatty acid oxidation was significantly reduced in both animal groups and did not differ between groups (Fig. 4B). Moreover, hepatic mitochondrial content in OLETF rats, as assessed by total cytochrome c protein content, was reduced at all ages examined (Fig. 4C). Furthermore, reduced CPS-1 staining, a hepatic mitochondrial membrane specific marker, was observed in 40 week old OLETF compared with LETO animals (Fig. 5A; no differences were observed at younger ages, data not shown). By 20 and 40 weeks of age, the OLETF rats also had developed ultrastructural abnormalities of the mitochondria, including apparent disruptions in the cristae, hypodense matrix, and mitochondrial swelling/rounding; these findings were not apparent in the LETO animals (Fig. 5B and C).
Fig. 3. Hepatic mitochondrial CPT-1 activity (A), β-HAD activity (B), and citrate synthase activity (C).
Values (means ± SE, n = 5–8) with different letter superscripts within each animal group are significantly different (p <0.05). *Significantly different from LETO at respective ages (p <0.05).
Fig. 4. Complete hepatic palmitate oxidation (CO2 production; A), total hepatic palmitate oxidation (CO2 + ASMs; B), and total cytochrome c protein content (C).
Values are means ± SE, n = 6–8. *Significantly different from LETO at respective ages (p <0.01). †Significantly reduced in the 13, 20, and 40 week old compared with 5 and 8 week old animals (p <0.05).
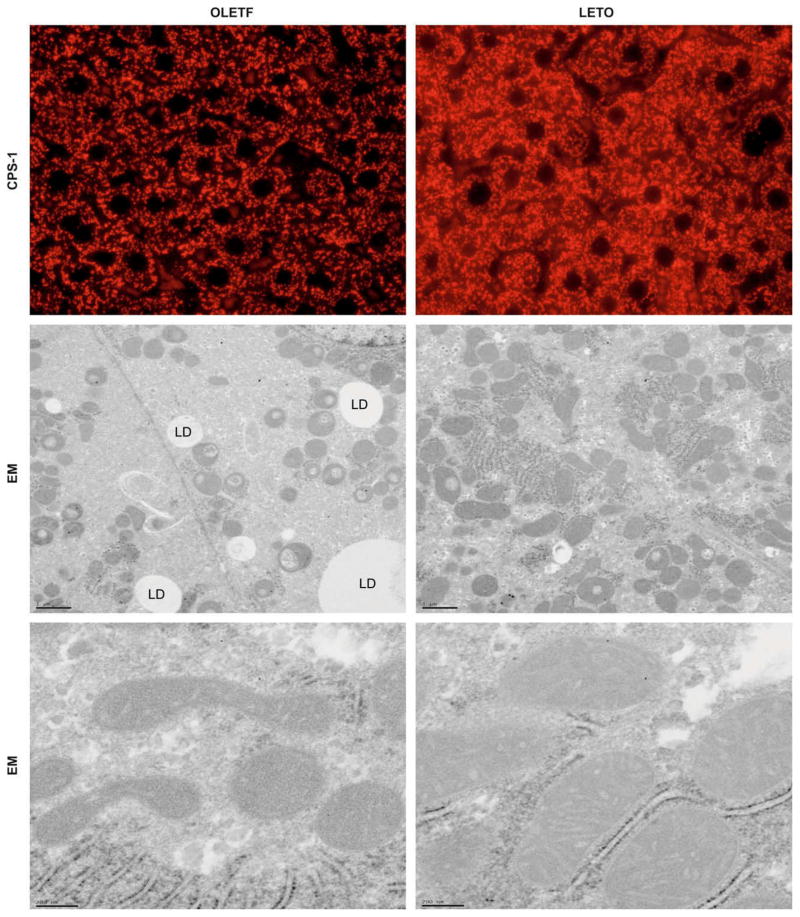
Fig. 5. Representative immunofluorescent photomicrographs from liver for mitochondrial marker CPS-1 (red) shows granular staining patterns in liver sections (A).
While there were no differences between animal groups at 5, 8, 13, and 20 weeks of age (not shown), the OLETF livers (left) had visibly decreased CPS-1 staining compared to the LETO animals (right) at 40 weeks of age. Also shown are representative electron micrographs (EM) from liver of OLETF (left) and LETO (right) rats at low (B) and high (C) magnifications. While no apparent differences were observed at 5, 8, or 13 weeks of age (data not shown), the mitochondria in the OLETF animals at 20 and 40 weeks show signs of rounding (B) and disruptions in cristae and outer and inner mitochondrial membranes (C). LD = lipid droplet.
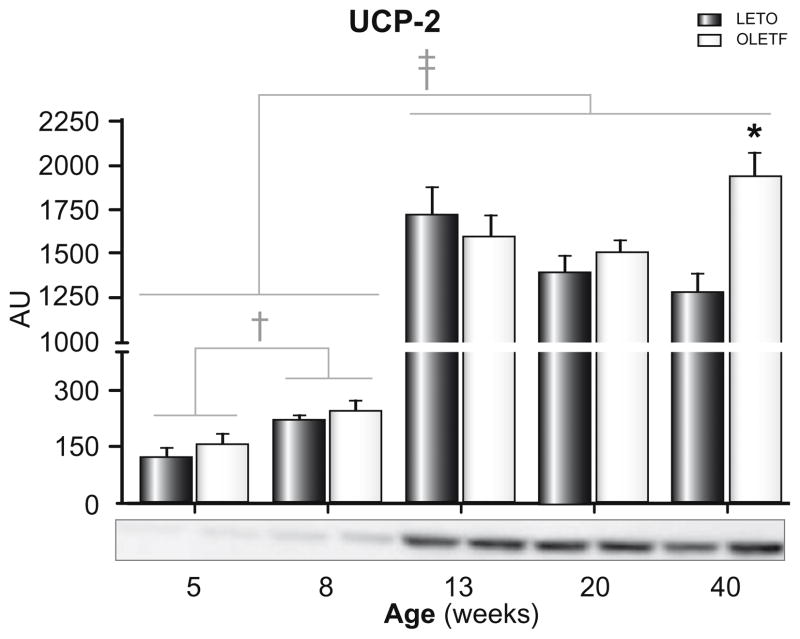
In order to examine the possible role of mitochondrial uncoupling in NAFLD development and progression in OLETF rats, hepatic UCP-2 protein content was determined (Fig. 6). Hepatic UCP-2 protein content significantly increased from 5 to 8 weeks (two-fold, p <0.05) and from 8 to 13 weeks of age (10-fold; p <0.001) in both groups; however, hepatic UCP-2 protein content did not differ between groups until 40 weeks of age, where content was significantly greater in OLETF compared with LETO animals (Fig. 6, p <0.05).
Fig. 6. Hepatic UCP-2 protein content in LETO and OLETF rats.
Values are means ± SE, n = 6–8. †Significantly greater in 8 week old compared with 5 week old animals (p < 0.05). ‡Significantly greater in the 13, 20, and 40 week old compared with 5 and 8 week old animals (p <0.001). *Significantly greater in OLETF compared with LETO animals (p <0.05).
Discussion
Overnutrition is considered the most common cause of NAFLD, with an estimated incidence of 15–20% of Western populations [28]. Here we report several novel findings related to the development and natural history of NAFLD in the hyperphagic, obese OLETF rat, an animal model that we liken to overeating, sedentary, obese humans. Reduced hepatic fatty acid oxidation and mitochondrial enzyme activity preceded NAFLD development and insulin resistance; reduced hepatic mitochondrial function was present at 5 weeks of age, followed by hepatic micro-vesicular steatosis by 8 weeks, and then the development of systemic insulin resistance by 13 weeks of age in the OLETF rats. NAFLD progressed to include hepatocyte ballooning and nuclear displacement, perivenular fibrosis, inflammation, and elevated serum ALTs by 40 weeks of age. These changes occurred in conjunction with a worsening in glycemic control and a loss of hepatic anti-oxidative capacity, increased hepatic oxidative stress, reduced hepatic mitochondrial content and function, and disruption in hepatic mitochondria ultrastructure. In addition, these findings occurred in the absence of extreme dietary manipulations, such as methionine and choline-deficient diets, which are known to exacerbate the disease condition in this rodent model [29,30], but do not induce insulin resistance or obesity, both hallmark features of human NAFLD. Furthermore, rats were fed low-fat chow, diminishing the contribution of dietary fat consumption. These novel findings suggest that reduced mitochondrial function precedes insulin resistance and may thus be the primary event that triggers NAFLD development, at least in OLETF rats. Secondly, the natural progression pattern observed in OLETF rats in the current report closely resembles that observed in both overweight children and adults, suggesting its application as an excellent model for further mechanistic insight into NAFLD pathogenesis.
It has been proposed that insulin resistance serves as the “first hit” in hepatic steatosis development [6], and that once steatosis is present, inflammation and oxidative stress are thought to activate stellate cells and increase collagen deposition and fibrogenesis [6]. However, others have recognized the potential importance of impaired hepatic fatty acid oxidation and speculated that mitochondrial abnormalities may be involved in NAFLD [3,7–10,31]. Mitochondrial oxidative capacity is decreased in the liver tissue of patients and animal models with hepatic steatosis [32,33], but not always in NASH patients [31]. These studies raise the possibility that mitochondrial dysfunction can be a cause, effect or a concurrent feature in development of NAFLD. In the current report, we have conducted a longitudinal assessment of mitochondrial content and function, and NAFLD to provide insights into the causative relationship between mitochondrial dysfunction and development of NAFLD in our model. We have noted that hepatic CPT-1 activity (the rate limiting step in mitochondrial fatty acid entry), complete disposal of fatty acids, and total fatty acid oxidation were reduced in the livers of 5-week-old OLETF rats prior to development of NAFLD or insulin resistance. Importantly, these differences also were observed before the presence of hyperlipidemia or significant differences in abdominal fat pad mass. Based on our previous work [17] and the preliminary observations of increased hepatic acetyl-CoA carboxylase protein content in the OLETF compared with LETO animals (RSR, preliminary results), it is likely that excess food consumption in the OLETF rats leads to substrate driven malonyl-CoA formation, which inhibits hepatic CPT-1 in the OLETF animals, reducing mitochondrial fatty acid entry and fatty acid oxidation, and thereby increasing liver TG accumulation. These findings are in agreement with previous work where the overexpression of CPT-1 increases fatty acid oxidation and reduces TG accumulation in hepatocytes [34], and collectively suggest that deficiencies in mitochondrial fatty acid entry and oxidative capacity play a role in hepatic steatosis development in this hyperphagic animal model.
Complementing the reduced capacity to oxidize fatty acids at 5 weeks in the OLETF animals was decreased electron transport protein cytochrome c per unit of liver mass. Our study also documents a progressive loss of hepatic mitochondrial content and function in the OLETF rat, which likely contributed to the progression of NAFLD in this animal model. By 40 weeks of age, all measures of mitochondrial function and content were reduced by 30–50% in the OLETF compared with LETO controls. This collectively demonstrates the inability to maintain normal levels of hepatic mitochondrial content in this obese rodent model of NAFLD. These findings are in support of previous work showing that depletion of hepatic mitochondrial DNA impairs mitochondrial function and causes hepatic steatosis and liver injury [35]. Interestingly, the progression of hepatic mitochondrial dysfunction and NAFLD was associated with worsening of insulin resistance and the development of type 2 diabetes in OLETF rats at 40 weeks of age. While loss of glycemic control does not appear to be the primary cause of NAFLD in this model, insulin resistance and type 2 diabetes development certainly play a role in disease progression. Further mechanistic examination of this relationship is warranted in future studies.
Hepatic activity of the free radical scavenger SOD was significantly reduced in 5 and 8 week old OLETF animals, with a subsequent increase in GSSG levels at 13, 20, and 40 weeks of age. This negative imbalance between increased oxidative stress and reduced anti-oxidant defence systems likely predisposes hepatocytes and hepatic mitochondria to progressive injury [31]. One possibility for the increase in oxidative stress was reduced complete hepatic fatty acid oxidation in the OLETF rats, which likely lead to the accumulation of mitochondrial and peroxisomal derived lipotoxic lipid moieties, factors known to activate hepatic mitochondrial oxidative stress [36]. Furthermore, the findings that abnormal reduction of oxygen by the electron transport chain lead to additional reactive oxygen species formation [31], the loss of mitochondrial content in the current study likely contributed to the elevated levels of serum ALT, perivenular fibrosis, and ultrastructural abnormalities seen in the mitochondria (commonly seen in NASH patients) [8,9] in livers from the OLETF animals.
UCP-2 is a mitochondrial inner membrane protein that mediates protein leak by uncoupling fuel oxidation from ATP synthesis [37]. Although it is upregulated in models of obesity and NAFLD [38], the precise role of UCP-2 in fatty liver disease is not completely understood. Increased UCP-2 expression is thought to help promote substrate disposal and limit mitochondrial ROS production [39] by decreasing the redox pressure on the electron transport chain [40]. However, it has also been found that obesity-related fatty liver disease is unchanged in UCP-2 deficient mice [41]. In the current study, hepatic UCP-2 protein expression dramatically increased with age in both the non-hyperphagic LETO and the hyperphagic OLETF rats, and it was not until 40 weeks of age that OLETF rats had higher hepatic UCP-2 protein content compared with LETO rats. These findings would collectively suggest the lack of a role of hepatic UCP-2 in the initiation of NAFLD in the OLETF rat, but perhaps a potential factor in the progression of NAFLD in the OLETF rat. This possibility warrants future investigation.
In summary, compelling evidence is provided that hepatic mitochondrial dysfunction precedes the development of NAFLD and insulin resistance in the hyperphagic, obese OLETF rat. In addition, our study documents that a progressive loss of mitochondrial function in conjunction with the transition from insulin resistance to type 2 diabetes likely contributes to NAFLD progression. Mitochondrial function represents an attractive therapeutic target in the prevention and treatment of NAFLD.
Acknowledgments
This work was partially supported by the College of Veterinary Medicine and Department of Internal Medicine at University of Missouri and NIH Grants HL-36088 (M.H.L.) and F32 DK-83182 (R.S.R.). The OLETF and LETO rats were a generous gift of the Tokushima Research Institute, Otsuka Pharmaceutical (Tokushima, Japan). The authors would like to thank Suzie Ridenhour and Craig Meers for excellent technical assistance and Whitney Collins and Aaron Bunker for help with animal husbandry. The authors also would like to thank the Veterinary Medicine Diagnostics Laboratory at the University of Missouri for help with the histological sections and serum ALT measurements and the Diabetes Diagnostics Lab at the University of Missouri for help with the hemoglobin A1c measurements. In addition, we would like to thank the University of Missouri Electron Microscopy Core for help with the EM images. This work was supported with resources and the use of facilities at the Harry S. Truman Memorial Veterans Hospital in Columbia, MO.
Abbreviations
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- OLETF
Otsuka Long–Evans Tokushima Fatty rats
- ASM
acid soluble metabolite
- CPS-1
carbamoylphosphate synthetase-1
- H&E
hematoxylin and eosin
- TGF-β
transforming growth factor-β
- TG
triglycerides
- FFA
free fatty acids
- ALT
alanine aminotransferase
- HbA1c
hemoglobin A1c
- GSSG
oxidized glutathione
- SOD
superoxide dismutase
- β-HAD
beta-hydroxyacyl-CoA dehydrogenase
- CPT-1
carnitine palmitoyl-CoA transferase-1
- TEM
transmission electron microscopy
- SE
standard error of the mean
- UCP-2
uncoupling protein-2
Footnotes
The authors who have taken part in this study declared that they do not have anything to declare regarding funding from industry or conflict of interest with respect to this manuscript.
References
- 1.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 2.Bellentani S, Saccoccio G, Masutti F, Croce LS, Brandi G, Sasso F, et al. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112–117. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- 3.Farrell GC, Larter CZ. Non-alcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 4.Chiarelli F, Marcovecchio ML. Insulin resistance and obesity in childhood. Eur J Endocrinol. 2008;159:S67–S74. doi: 10.1530/EJE-08-0245. [DOI] [PubMed] [Google Scholar]
- 5.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 6.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 7.Pessayre D, Fromenty B. NASH: a mitochondrial disease. J Hepatol. 2005;42:928–940. doi: 10.1016/j.jhep.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Caldwell SH, Swerdlow RH, Khan EM, Iezzoni JC, Hespenheide EE, Parks JK, et al. Mitochondrial abnormalities in non-alcoholic steatohepatitis. J Hepatol. 1999;31:430–434. doi: 10.1016/s0168-8278(99)80033-6. [DOI] [PubMed] [Google Scholar]
- 9.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, et al. Non-alcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 10.Ibdah JA, Perlegas P, Zhao Y, Angdisen J, Borgerink H, Shadoan MK, et al. Mice heterozygous for a defect in mitochondrial trifunctional protein develop hepatic steatosis and insulin resistance. Gastroenterology. 2005;128:1381–1390. doi: 10.1053/j.gastro.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long–Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992;41:1422–1428. doi: 10.2337/diab.41.11.1422. [DOI] [PubMed] [Google Scholar]
- 12.Moran TH, Bi S. Hyperphagia and obesity of OLETF rats lacking CCK1 receptors: developmental aspects. Dev Psychobiol. 2006;48:360–367. doi: 10.1002/dev.20149. [DOI] [PubMed] [Google Scholar]
- 13.Man ZW, Hirashima T, Mori S, Kawano K. Decrease in triglyceride accumulation in tissues by restricted diet and improvement of diabetes in Otsuka Long–Evans Tokushima fatty rats, a non-insulin-dependent diabetes model. Metabolism. 2000;49:108–114. doi: 10.1016/s0026-0495(00)90913-2. [DOI] [PubMed] [Google Scholar]
- 14.Park SY, Choi GH, Choi HI, Ryu J, Jung CY, Lee W. Calorie restriction improves whole-body glucose disposal and insulin resistance in association with the increased adipocyte-specific GLUT4 expression in Otsuka Long–Evans Tokushima fatty rats. Arch Biochem Biophys. 2005;436:276–284. doi: 10.1016/j.abb.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Bajotto G, Murakami T, Nagasaki M, Tamura T, Tamura N, Harris RA, et al. Downregulation of the skeletal muscle pyruvate dehydrogenase complex in the Otsuka Long–Evans Tokushima Fatty rat both before and after the onset of diabetes mellitus. Life Sci. 2004;75:2117–2130. doi: 10.1016/j.lfs.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Laye MJ, Rector RS, Warner SO, Naples SP, Perretta AL, Uptergrove GM, et al. Changes in visceral adipose tissue mitochondrial content with type 2 diabetes and daily voluntary wheel running in OLETF rats. J Physiol. 2009;587:3729–3739. doi: 10.1113/jphysiol.2009.172601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rector RS, Thyfault JP, Laye MJ, Morris RT, Borengasser SJ, Uptergrove GM, et al. Cessation of daily exercise dramatically alters precursors of hepatic steatosis in Otsuka Long–Evans Tokushima Fatty (OLETF) rats. J Physiol. 2008;586:4241–4249. doi: 10.1113/jphysiol.2008.156745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, et al. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long–Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol. 2008;294:G619–G626. doi: 10.1152/ajpgi.00428.2007. [DOI] [PubMed] [Google Scholar]
- 19.Koves TR, Noland RC, Bates AL, Henes ST, Muoio DM, Cortright RN. Subsarcolemmal and intermyofibrillar mitochondria play distinct roles in regulating skeletal muscle fatty acid metabolism. Am J Physiol Cell Physiol. 2005;288:C1074–C1082. doi: 10.1152/ajpcell.00391.2004. [DOI] [PubMed] [Google Scholar]
- 20.Laye MJ, Rector RS, Borengasser SJ, Naples SP, Uptergrove GM, Ibdah JA, et al. Cessation of daily wheel running differentially alters fat oxidation capacity in liver, muscle, and adipose tissue. J Appl Physiol. 2009;106:161–168. doi: 10.1152/japplphysiol.91186.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei Y, Clark SE, Morris EM, Thyfault JP, Uptergrove GM, Whaley-Connell AT, et al. Angiotensin II-induced non-alcoholic fatty liver disease is mediated by oxidative stress in transgenic TG(mRen2)27(Ren2) rats. J Hepatol. 2008;49:417–428. doi: 10.1016/j.jhep.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thyfault JP, Rector RS, Uptergrove GM, Borengasser SJ, Morris EM, Wei Y, et al. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J Physiol. 2009;587:1805–1816. doi: 10.1113/jphysiol.2009.169060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for non-alcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 24.Rivera H, Shibayama M, Tsutsumi V, Perez-Alvarez V, Muriel P. Resveratrol and trimethylated resveratrol protect from acute liver damage induced by CCl4 in the rat. J Appl Toxicol. 2008;28:147–155. doi: 10.1002/jat.1260. [DOI] [PubMed] [Google Scholar]
- 25.Hissin PJ, Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem. 1976;74:214–226. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- 26.Srere PA. Citrate synthase. Methods Enzymol. 1969;13:3–5. [Google Scholar]
- 27.Bass A, Brdiczka D, Eyer P, Hofer S, Pette D. Metabolic differentiation of distinct muscle types at the level of enzymatic organization. Eur J Biochem. 1969;10:198–206. doi: 10.1111/j.1432-1033.1969.tb00674.x. [DOI] [PubMed] [Google Scholar]
- 28.Anderson N, Borlak J. Molecular mechanisms and therapeutic targets in steatosis and steatohepatitis. Pharmacol Rev. 2008;60:311–357. doi: 10.1124/pr.108.00001. [DOI] [PubMed] [Google Scholar]
- 29.Ota T, Takamura T, Kurita S, Matsuzawa N, Kita Y, Uno M, et al. Insulin resistance accelerates a dietary rat model of non-alcoholic steatohepatitis. Gastroenterology. 2007;132:282–293. doi: 10.1053/j.gastro.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 30.Kurita S, Takamura T, Ota T, Matsuzawa-Nagata N, Kita Y, Uno M, et al. Olmesartan ameliorates a dietary rat model of non-alcoholic steatohepatitis through its pleiotropic effects. Eur J Pharmacol. 2008;588:316–324. doi: 10.1016/j.ejphar.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 31.Begriche K, Igoudjil A, Pessayre D, Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1–28. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Carreras M, Del Hoyo P, Martin MA, Rubio JC, Martin A, Castellano G, et al. Defective hepatic mitochondrial respiratory chain in patients with non-alcoholic steatohepatitis. Hepatology. 2003;38:999–1007. doi: 10.1053/jhep.2003.50398. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Ruiz I, Rodriguez-Juan C, Diaz-Sanjuan T, del Hoyo P, Colina F, Munoz-Yague T, et al. Uric acid and anti-TNF antibody improve mitochondrial dysfunction in ob/ob mice. Hepatology. 2006;44:581–591. doi: 10.1002/hep.21313. [DOI] [PubMed] [Google Scholar]
- 34.Stefanovic-Racic M, Perdomo G, Mantell BS, Sipula IJ, Brown NF, O’Doherty RM. A moderate increase in carnitine palmitoyltransferase 1a activity is sufficient to substantially reduce hepatic triglyceride levels. Am J Physiol Endocrinol Metab. 2008;294:E969–E977. doi: 10.1152/ajpendo.00497.2007. [DOI] [PubMed] [Google Scholar]
- 35.Demeilliers C, Maisonneuve C, Grodet A, Mansouri A, Nguyen R, Tinel M, et al. Impaired adaptive resynthesis and prolonged depletion of hepatic mitochondrial DNA after repeated alcohol binges in mice. Gastroenterology. 2002;123:1278–1290. doi: 10.1053/gast.2002.35952. [DOI] [PubMed] [Google Scholar]
- 36.Muoio DM, Koves TR. Skeletal muscle adaptation of fatty acid depends on coordinated actions of the PPARs and PGC1a: implications for metabolic disease. Appl Physiol Nutr Metab. 2007;32:874–883. doi: 10.1139/H07-083. [DOI] [PubMed] [Google Scholar]
- 37.Boss O, Hagen T, Lowell BB. Uncoupling proteins 2 and 3: potential regulators of mitochondrial energy metabolism. Diabetes. 2000;49:143–156. doi: 10.2337/diabetes.49.2.143. [DOI] [PubMed] [Google Scholar]
- 38.Yang S, Zhu H, Li Y, Lin H, Gabrielson K, Trush MA, et al. Mitochondrial adaptations to obesity-related oxidant stress. Arch Biochem Biophys. 2000;378:259–268. doi: 10.1006/abbi.2000.1829. [DOI] [PubMed] [Google Scholar]
- 39.Cortez-Pinto H, Zhi Lin H, Qi Yang S, Odwin Da Costa S, Diehl AM. Lipids up-regulate uncoupling protein 2 expression in rat hepatocytes. Gastroenterology. 1999;116:1184–1193. doi: 10.1016/s0016-5085(99)70022-3. [DOI] [PubMed] [Google Scholar]
- 40.Negre-Salvayre A, Hirtz C, Carrera G, Cazenave R, Troly M, Salvayre R, et al. A role for uncoupling protein-2 as a regulator of mitochondrial hydrogen peroxide generation. FASEB J. 1997;11:809–815. [PubMed] [Google Scholar]
- 41.Baffy G, Zhang CY, Glickman JN, Lowell BB. Obesity-related fatty liver is unchanged in mice deficient for mitochondrial uncoupling protein 2. Hepatology. 2002;35:753–761. doi: 10.1053/jhep.2002.32028. [DOI] [PubMed] [Google Scholar]