Abstract
Alzheimer’s disease (AD) is a neurodegenerative disorder in which oxidative stress is a key hallmark. It occurs early in disease pathogenesis and can exacerbate its progression. Several causes of oxidative stress have been determined over the years. First, mitochondria play an important role in the generation and accumulation of free radicals. In addition to mitochondria, inflammation can also induce oxidative damage, especially via microglia, and microglia are also important for Aβ clearance. In AD, both mitochondrial function and inflammatory response are affected, leading to increased ROS formation and oxidative damage to lipid, proteins and nucleic acids. Some other sources have also been identified.
From these findings, various neuroprotective strategies against ROS-mediated damages have been elaborated in AD research. This review recapitulates some of the major strategies used to prevent oxidative stress and disease progression. Outcomes from in vitro and in vivo studies using models of AD are encouraging. However, only a few clinical trials have provided positive results in terms of slowing down cognitive decline.
Nonetheless, there is still hope for improved compounds that would better target pathways implicated in ROS production. In fact, facilitating the endogenous antioxidant system by modulating transcription has great promise for AD therapy.
Keywords: Alzheimer’s disease, Oxidative stress, Reactive oxygen species, Therapeutic strategies, Neuroprotection, Pathology, Cognitive deficit
Introduction
Alzheimer’s disease (AD) is characterized clinically by progressive cognitive decline and neuropathologically by the presence of amyloid plaques and neurofibrillary tangles. In this neurodegenerative disease, ageing is the most critical risk factor. In addition, oxidative stress is another key feature [1]. Numerous studies have reported the presence of elevated DNA [2, 3], RNA [4, 5], lipid [6, 7] and protein oxidation [8] in brains of subjects with AD and mild cognitive impairment (MCI) [9], suggesting that oxidative stress is an early event in AD pathogenesis [10]. Remarkably, these oxidative stress hallmarks were also observed in transgenic mouse models of AD, in which markers of lipid and protein oxidation are increased, which may precede amyloid deposition [11]. As in human disease, oxidative stress occurs at early stages, prior to the appearance of amyloid plaques [12, 13] and neurofibrillary tangles [11].
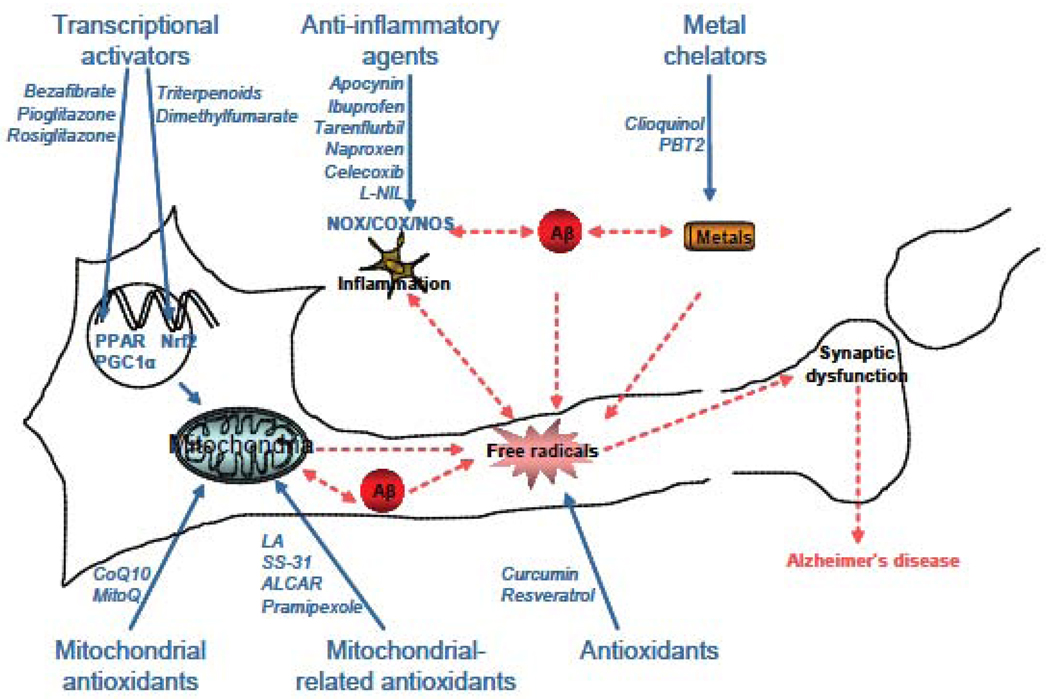
Generation and accumulation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) are detrimental to cells in vitro and in vivo [14], and promote cell death [15]. Therefore, it has been crucial to investigate potential causes of oxidative stress in AD research [16]. There is a large body of evidence demonstrating involvement of mitochondria [17, 18], redox-active metals [19, 20], inflammation via activated microglia [21, 22], and other ROS-mediated pathways (figure 1). These important findings have led to the development of therapeutic strategies to counteract and prevent oxidative damage [23]. Here, we will review some of the major neuroprotective strategies involving ROS in AD, focusing on strategies targeting mitochondria and other potent antioxidant-related pathways.
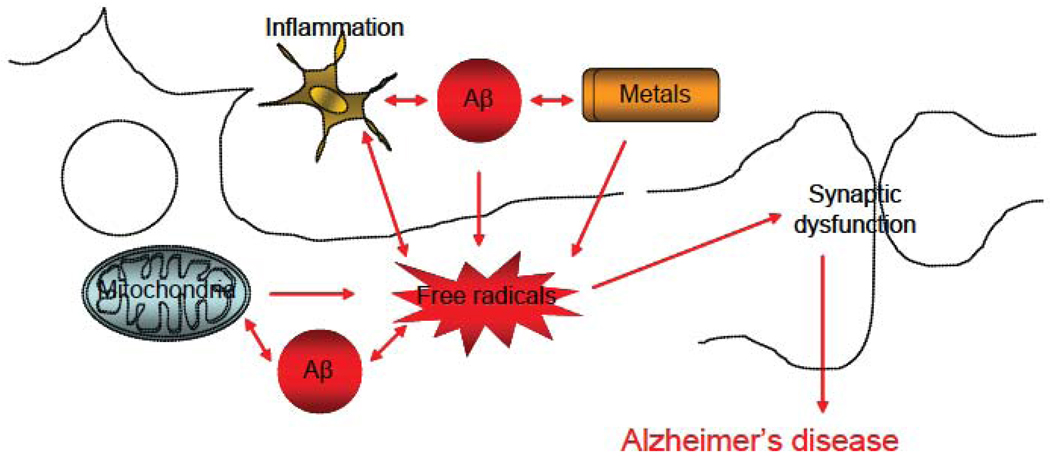
Figure 1. Scheme of the generation and role of free radicals in AD.
In cells, free radicals can be generated by 2 major sources: mitochondria and NAPDH oxidase. Several key players, such as metals and/or Aβ can exacerbate their production. Once accumulating inside the cells, free radicals can cause lipid, protein, DNA and RNA damage that can exacerbate AD pathogenesis.
Neuroprotective strategies targeting mitochondria
Mitochondria are major sources of ROS in the central nervous system (figure 2). They contain redox carriers that can transfer single electrons to oxygen, thus generating the ROS superoxide (O2−). Enzymes of the tricarboxylic acid cycle (TCA), of the electron transport chain (complex I, II and III) (figure 2A), and monoamine oxidases are among the mitochondrial redox carriers generating superoxide (figure 2B). Mitochondria also contain other enzymes able to detoxify ROS. Indeed, superoxide is depleted following a dismutation reaction by superoxide dismutase (SOD) and transformed into hydrogen peroxide (H2O2) (figure 2C). SOD enzymes work in conjunction with catalases and glutathione peroxidases to remove H2O2 within mitochondria. In addition, in the cell, O2− and H2O2 can react with other molecules such as redox-active metals (Fenton’s reaction involving iron) and nitric oxide, leading to the formation of hydroxyl radicals and peroxynitrites respectively (figure 2C). In normal conditions, these chemical events require an accurate balance between ROS production and removal. With ageing and/or AD, this balance is markedly disrupted leading to ROS accumulation and oxidative damage.
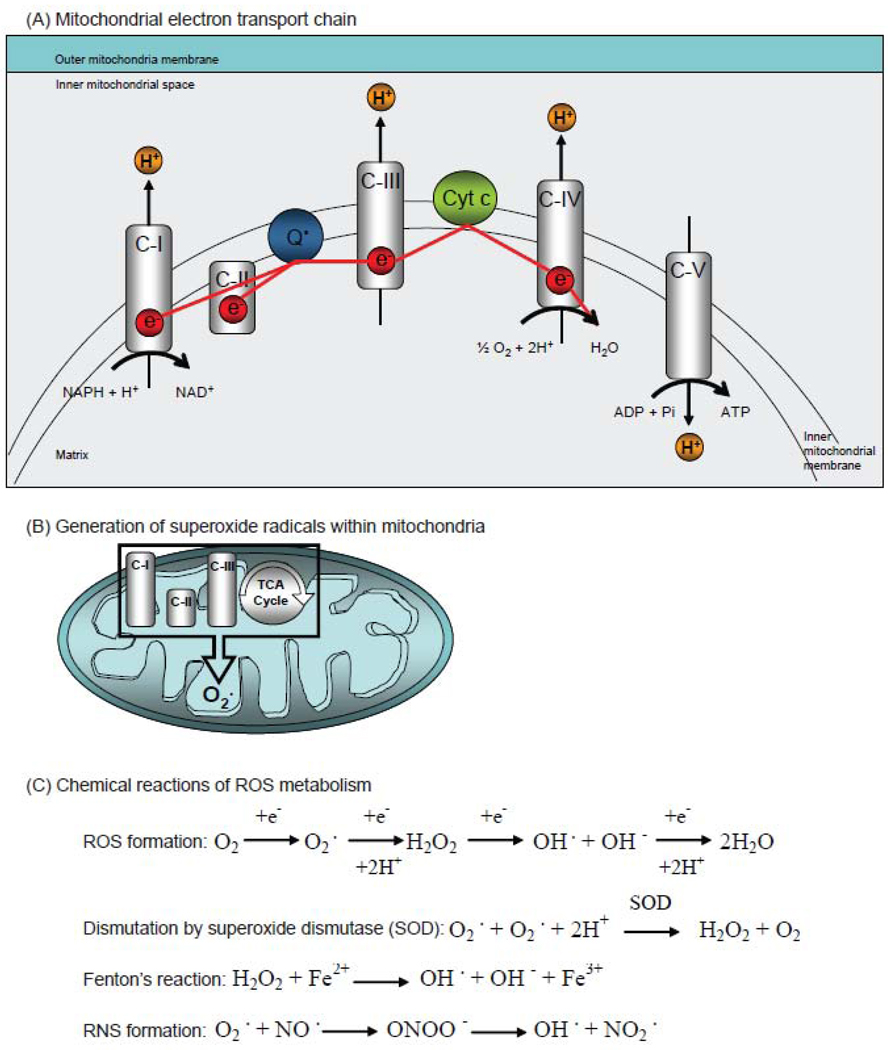
Figure 2. Mitochondria and ROS.
(A) Scheme of the mitochondrial electron transport chain. Electrons are transferred from complex I (C-I) to complex IV (C-IV), including coenzyme Q10 (Q) and cytochrome c (Cyt c). (B) Scheme of mitochondria-mediated ROS from the complex I (C-I), complex II (C-II), and III (C-III) and from the tricarboxylic acid cycle (TCA). (C) Chemical reactions for the generation of reactive oxygen species (ROS) such as superoxide (O2−), hydrogen peroxide (H2O2), reactive nitrogen species (RNS) such as peroxynitrite, and the chemistry of the Fenton reaction, which generates OH− radicals.
Increased numbers of mutations of mitochondrial DNA have been found in AD [24], as have increased concentrations of 8-hydroxy-2-deoxyguanosine, a marker of oxidative damage to DNA [25]. These deletions or point mutations, which may result from oxidative stress, can cause mitochondrial dysfunction and trigger apoptotic cell death [26]. In addition to DNA damage, several mitochondrial key enzymes involved in ROS detoxification are also affected. In human AD brains, levels of the alpha-ketoglutarate dehydrogenase complex (KGDHC) [27, 28], pyruvate dehydrogenase complex (PDHC) [29], and cytochrome oxidase (COX) [30, 31] are markedly reduced. Studies on animal models of AD have also implicated mitochondria in disease pathogenesis.
Our group demonstrated that partial genetic deletion of dihydrolipoyl succinyltransferase (one of the KGDHC subunits) increased amyloid pathology, oxidative stress and enhanced memory deficit in transgenic AD female mice [32]. Deficiency of manganese superoxide dismutase (MnSOD) also increased amyloid deposition [33], tau phosphorylation [34] and behavioral deficits [35]. Conversely, overexpression of MnSOD reduced amyloid deposition, oxidative stress, and synaptic and memory deficits in two different transgenic mouse models of AD [36, 37]. It also improved cerebral blood flow and axonal transport in transgenic AD mice [38]. These results indicate that detoxification enzymes localized within mitochondria are essential to prevent free radical accumulation and oxidative stress in AD. Neuroprotection in transgenic AD mice was also reported after genetic deletion of cyclophilin D, a component of the mitochondrial permeability transition pore [39–41]. Fission and fusion of mitochondria are also impaired in AD [42]. Importantly, it has been shown that mitochondrial dysfunction is preferentially located in affected AD brain regions, suggesting that Aβ and mitochondria are linked [43]. As potential mechanisms, recent work showed that both β-amyloid and tau mutations result in mitochondrial dysfunctions, and that there are synergistic effects on mitochondrial dysfunction mediated by the two proteins together [44, 45]. Also, oxidative stress can increase β-secretase expression and tau phosphorylation, through c-Jun amino-terminal kinase/p38 mitogen-activated protein kinase [46] and glycogen synthase kinase 3 [47] respectively. Therefore, several groups have focused their efforts on developing neuroprotective strategies targeting mitochondria.
Some of the major mitochondrial targets used as therapeutics against ROS-mediated damage were members of the quinone family. First, co-enzyme Q10 (CoQ10), also called ubiquinone (figure 3A; table 1), has demonstrated antioxidant and neuroprotective properties both in vitro and in vivo [48], therefore holding great promise in the treatment of neurodegenerative disorders [49]. CoQ10 is localized within mitochondria. It is part of the electron transport chain and acts as electron carrier from complex I and complex II during oxidative phosphorylation and transfers electrons to complex III (figure 2A). Interestingly, administration of CoQ10 in transgenic AD mice reduced amyloid plaque pathology [50, 51]. Unfortunately, CoQ10 has not yet been tested in AD patients. However, another quinone (synthetic analog of CoQ10), idebenone (figure 3B; table 1), has been investigated in clinical trials, for its ability to inhibit lipid peroxidation [52]. Although several smaller studies reported beneficial effects on memory and attention after several months of treatment [53–55], a larger study reported no effect in slowing disease progression [56]. A possible limitation to CoQ10 efficacy is that maintaining the coenzyme Q in its reduced antioxidant form (termed ubiquinol) requires an intact electron transport chain, which is impaired in AD.
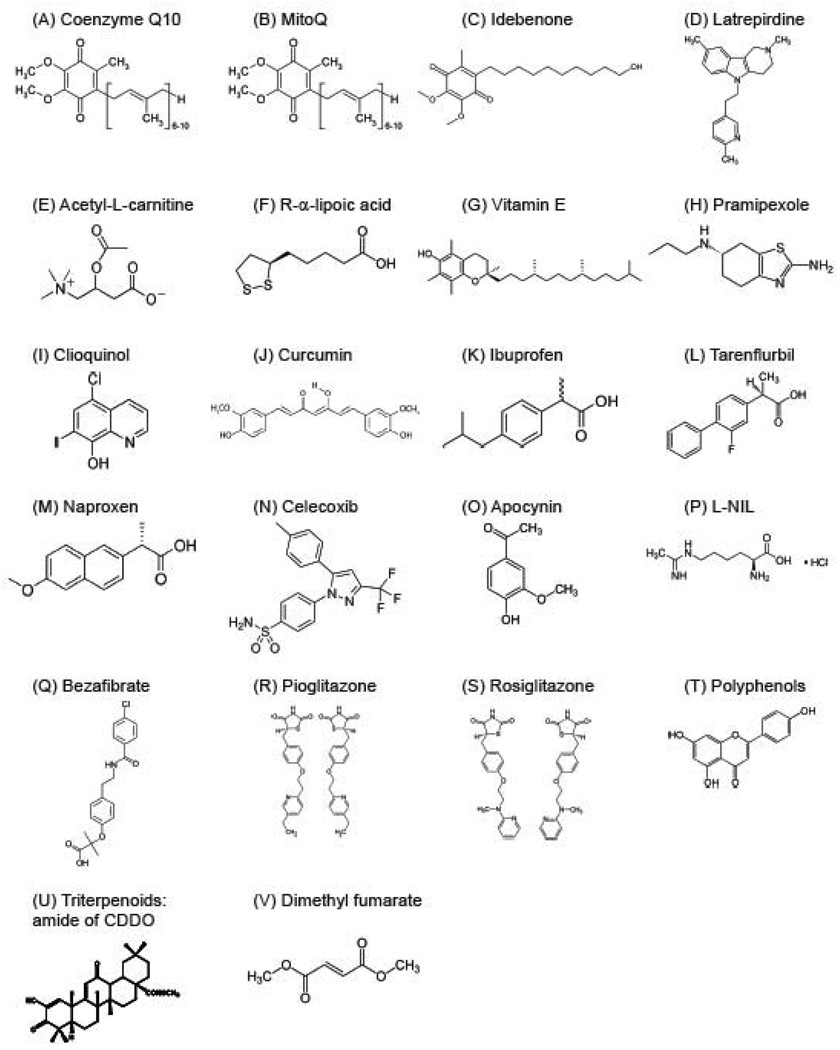
Figure 3. Chemical structures of antioxidants.
Table 1.
Antioxidants and mechanisms of action
| Name | Description | Main target | Mechanisms of action | Results Mouse |
Results Human |
|---|---|---|---|---|---|
| Coenzyme Q10 |
|
Mitochondria |
|
+ | nt |
| Idebenone |
|
Mitochondria |
|
+ | − |
| MitoQ |
|
Mitochondria |
|
nt | |
| Latrepirdine |
|
Neurotransmission Mitochondria |
|
+ | +/− |
| Acetyl-L-carnitine |
|
Mitochondria Nrf2 |
|
+ | + |
| R-α lipoic acid |
|
Mitochondria Nrf2 |
|
+ | nt |
| Vitamin E |
|
|
+ | +/− | |
| Pramipexole |
|
Neurotransmission Mitochondria |
|
+ (PD,ALS) | nt |
| SS-31 |
|
Mitochondria |
|
+ (PD,ALS) | nt |
| Clioquinol |
|
Metals |
|
+ | + |
| PBT2 |
|
Metals |
|
+ | + |
| Curcumin |
|
Inflammation Mitochondria |
|
+ | − |
| Ibuprofen |
|
Inflammation |
|
+ | − |
| Tarenflurbil |
|
Inflammation |
|
+ | − |
| Naproxen |
|
Inflammation |
|
+ | − |
| Celecoxib |
|
Inflammation |
|
+ | − |
| Apocynin |
|
NADPH oxidase |
|
+ (ALS) | nt |
| N6-(1-iminoethyl)-L-lysine |
|
iNOS |
|
+ | nt |
| Bezafibrate |
|
PPAR Mitochondria |
|
+ (PD) | nt |
| Pioglitazone |
|
PPAR Mitochondria |
|
+ | nt |
| Rosiglitazone |
|
PPAR Mitochondria |
|
+ | +/− |
| Polyphenols |
|
Mitochondria Inflammation |
|
+ | nt |
| Triterpenoids |
|
Nrf2/ARE |
|
+ | nt |
| Dimethyl fumarate |
|
Nrf2/ARE |
|
nt | + (MS) |
Legends:
“nt” = not tested
“+” = Beneficial effects on AD progression
“−“ = No beneficial effects on AD progression
“PD” = Parkinson’s disease
“ALS” = Amyotrophic Lateral Sclerosis
“MS” = Multiple Sclerosis
Another ubiquinone derivative, mitoquinone mesylate or mitoQ (figure 3C; table 1), has been used to prevent oxidative damage in AD [57]. MitoQ consists of CoQ10 linked to a triphenylphosphonium ion which has a positive charge. Therefore, it accumulates in mitochondria which have a strongly negative membrane potential (about −120mV). More precisely, mitoQ is adsorbed in the inner mitochondrial membrane facing the matrix. This ROS-enriched region provides a real potency to MitoQ. In addition, MitoQ can function independently of the electron transport chain. In non-neuronal cell cultures and isolated mitochondria, it reduced oxidative stress and cell death [58]. In order to test for its tolerance and potential side effects, wild-type mice were treated with mitoQ for 7 months. Data showed that it was well tolerated and without any adverse effect; in particular, mitoQ was not pro-oxidant [59]. Like CoQ10, mitoQ has not yet been tested in human clinical trials for AD. A clinical trial of mitoQ conducted in patients with Parkinson’s disease (PD) did not show any improvement [60].
Other mitochondrial antioxidants have been selected to determine their potential neuroprotective effects. Latrepirdine or Dimebon (figure 3D; table 1), first used as a nonselective antihistamine, showed promise in vitro for preventing ROS-mediated damage in neurodegenerative diseases [61, 62]. Latrepirdine has several mechanisms of action. First, it can act on a number of neurotransmitter receptors, such as serotoninergic, α-adrenergic [63] and glutamatergic receptors (NMDA and AMPA) [64]. Latrepirdine also blocked Aβ-induced toxicity and L-type calcium channel in cultured neurons [65]. In neuroblasma cells, administration of dimebon enhanced mitochondrial function under normal conditions by increasing succinate dehydrogenase activity, mitochondrial membrane potential, and ATP levels, which may exert indirect effects on ROS generation through mitochondria. It also protected against cell death under stress conditions [66]. Given acutely, dimebon increased Aβ secretion in neuroblastoma N2a cells and levels of Aβ40 in the interstitial fluid of transgenic AD mice [67]. Several clinical trials have been conducted in AD patients. In a phase 2 trial, Dimebon was well tolerated and improved cognition, activities of daily living, behavior, and overall function in MCI and AD patients compared to placebo [68]. However, more recently, the phase 3 CONNECTION trial in AD patients revealed no benefit in any parameter [69, 70].
Acetyl-L-carnitine (ALCAR) (figure 3E; table 1) [71, 72] and R-alpha lipoic acid (LA) (figure 3F; table 1) [73] are also candidates as mitochondrial antioxidants. During exercise and in order to facilitate fatty acid utilization, L-carnitine and acetyl-CoA are converted into ALCAR within mitochondria by carnitine-O-acetyltransferase. Once transported outside mitochondria, the conversion is reversed. LA is an organosulfur compound derived from octanoic acid. LA is primarily a cofactor in aerobic metabolism for PDHC. In cells, LA is reduced to dihydrolipoic acid, its bioactive form providing its full antioxidant properties [74]. In combination with LA, ALCAR decreased ROS-mediated damage, mitochondrial dysfunction due to aging in rats, and improved cognitive and motor functions [75, 76]. Interestingly, in cell models of AD, administration of ALCAR increased alpha-secretase activity and physiological amyloid precursor protein (APP) metabolism, which can enhance the release of non-amyloidogenic fragments of APP [77]. Indeed, ALCAR increased levels of sAPPα and CTF-83, and decreased levels of CTF-99 APP fragments [77]. In addition, ALCAR and LA combined treatment reduced oxidative damage and improved cognitive behavior in normal mice maintained on vitamin-free, iron-enriched, oxidative-challenge diet [78]. The combination also improved mitochondrial structure and memory deficits in apoE4 mice [79]. As an additional possible mechanism of neuroprotection, both ALCAR [80] and LA [81] can restore levels of mitochondrial antioxidant enzymes, and increase nuclear translocation of the nuclear factor erythroid-related factor 2 (Nrf2) that can upregulate transcription of antioxidant genes. In a clinical trial done for one year, AD patients receiving ALCAR (without LA) had slower deterioration of cognition compared to placebo [82]. Thal and colleagues in 1996 found that ALCAR was effective only in early onset of AD compared to placebo [83]. In another study done by the same group, ALCAR did not show any benefits in early onset AD patients [84]. However, more recently, a meta-analysis of ALCAR treatment trials in AD patients slowed clinical scales and psychometric tests of MCI and AD patients [85], giving hope for the use of this drug.
Vitamin E (α-tocopherol) has also been used as an antioxidant in AD therapy (figure 3G; table 1). It is a lipid-soluble antioxidant that protects cell membranes from oxidation [86]. Indeed, by reacting with lipid radicals generated from lipid peroxidation, vitamin E inhibits formation of free radical intermediates, thus prevent complete oxidation. Its administration reduced lipid peroxidation in both young and aged transgenic AD mice, but reduced amyloid deposition only when the drug was administered at early ages [87]. Vitamin E slowed down the disease progression in AD patients as measured by an increase in the clinical dementia rating scale or time to institutionalization [88]. In a subsequent trial however, administration of vitamin E to patients with MCI did not prevent the patients from developing AD [89]. The ability of vitamin E to modify the course of AD is therefore controversial, but the results in MCI were disappointing.
Pramipexole (figure 3H; table 1), a dopaminergic agonist, in vitro reduced Aβ-induced caspase activation leading to cell death [90]. Independently of the dopamine, its effects also include reduction of ROS generation from mitochondria, and it has been shown to localize to mitochondria where it may exert its antioxidant effects [91]. By preventing mitochondrial-related cell death, pramipexole can maintain the mitochondrial membrane potential and therefore sustain mitochondrial function. In vivo, this drug was neuroprotective in models of PD by improving motor performances, reducing induced microglial activation and proteasomal inhibition, and by enhancing brain-derived neurotrophic factors and autophagy [92], giving hope for future trials in AD and other neurodegenerative diseases [93]. This drug was also protective in animal models of ALS and it was well tolerated in a phase 2 trial. A phase 3 trial in ALS patients was recently announced as a collaboration between Knopp Pharmaceuticals and Biogen.
We have also tried the effects of the Szeto-Schiller peptides (SS-31) which selectively localize to the inner mitochondrial membrane and produce antioxidant effects within mitochondria. Indeed, in vitro these peptides were able to scavenge H2O2, inhibit oxidation of linoleic acid and low-density lipoprotein (LDL), and diminish mitochondrial swelling [94]. As a mechanism of action, SS31 can target the CD36 pathway where it alters ligand levels, and ligand-receptor interactions. In ischemia-reperfusion, SS31 reduced CD36 expression, and ligand levels by inhibiting LDL peroxidation [95]. These small peptides are neuroprotective against MPTP [96] and in a transgenic mouse model of ALS [97]. SS-31 also protected against amyloid toxicity in vitro and in vivo by increasing neurite outgrowth, rescuing mitochondrial structure and function and decreasing cyclophilin D expression [98].
Antioxidant neuroprotective strategies targeting mitochondria have produced positive outcomes in vitro and in vivo. Most of the interventions produce clear antioxidant and protective effects. Unfortunately, although a few initial trials in MCI and AD patients suggested slowing of disease progression, such results have generally not been confirmed. Therefore, there is a crucial need for improved compounds with increased absorption and solubility, and ability to cross the blood brain barrier and reach mitochondria.
Neuroprotective strategies targeting other ROS-mediated pathways
As mentioned in the introduction, other sources of oxidative stress and free radicals have been identified and have served to elaborate new therapeutic strategies against ROS-mediated damage in AD. In this section, we will review some of the promising antioxidant agents and pathways implicated in ROS production.
In addition to mitochondria, Aβ itself can generate free radicals in the presence of metal ions [99], and methionine 35 is critical for these reactions [100, 101]. Free radicals are generated early in the Aβ aggregation process, when oligomers and protofibrils are formed [102]. One strategy to reduce Aβ-induced free radical generation and metal catalyzed Aβ aggregation would be to chelate the copper and zinc which bind to Aβ, increasing its aggregation. Clioquinol (figure 3I; table 1), a member of the hydroxyquinoline family used as antifungal and antiprotozoal drug, has been considered a potent chelator of copper, zinc and iron. Trials of clioquinol and second generation metal binding compound PBT2 showed improvement in both transgenic AD mice [103], as well as AD patients [104, 105], possibly by inhibiting metal-induced free radical production and by disaggregating metal-induced Aβ assemblies. Interestingly, PBT2 also increased activities of the matrix metalloproteases such as neprilysin, insulin degrading enzyme and tissue plasminogen activator, which lead to increased Aβ clearance [106].
Several natural compounds with potent antioxidant properties, such as spices, green tea, resveratrol, and vitamins, have been evaluated as therapeutic agents for AD [107]. Curcumin, a polyphenol (figure 3J; table 1), can acts as a free radical scavenger and antioxidant which inhibits lipid peroxidation and oxidative DNA damage [108]. It increases the expression of glutathione S-transferase and inhibits cytochrome P450. Curcumin has been used extensively over the years both in vitro and in vivo in transgenic mouse models of AD [109]. In the triple transgenic AD mouse model overexpressing mutant P301L tau, APP and presenilin 1 mutations, curcumin treated mice fed a high fat diet showed improved behavior and reduced tau phosphorylation [109]. In other transgenic mouse models of AD, low and high doses of curcumin reduced levels of oxidized proteins, insoluble and soluble Aβ, amyloid plaques and astrocytosis [110], and restored dystrophic neurites [111]. Curcumin was also able to reduce Aβ aggregation in vitro and in vivo [112]. Interestingly, its effects on amyloid clearance may be due to its ability to bind Aβ and increase Aβ uptake from macrophages [113]. More recently, it was reported that curcumin administration decreased motor dysfunction, neuronal loss and lipid peroxidation present in the spinal cord of old transgenic AD mice [114]. Curcumin was also tested clinically in AD patients during a pilot trial of 6 months comparing 2 formulations, powder and capsule. No differences were found in the curcumin treated group while assessing cognition, levels of isoprostanes and Aβ. However, it should be noted that no cognitive decline was observed in the patients receiving the placebo, which may have biased the results [115]. Furthemore, absorption of curcumin is very poor and better formulations are being developed.
Inflammation plays a key role in AD [116, 117] and in MCI [118]. First, inflammation is involved in Aβ clearance in the brain, in which microglia participate actively by internalizing and degrading soluble [119] and aggregated forms of Aβ [120]. In the triple transgenic AD mice, deficiency in the microglial chemokine receptor Cx3cr1 prevented neuronal loss [121]. In old transgenic AD mice, microglial function is impaired, as shown by the decrease of Aβ-binding scavenger receptors (scavenger receptor A, CD36, and RAGE), and Aβ-degrading enzymes (insulysin, neprilysin, and MMP9) [122]. In the same animals, microglial levels of proinflammatory cytokines interleukin-1β and tumor necrosis factor alpha were markedly increased [122]. Inflammation is also responsible for the expression of cytokines, increasing cellular toxicity and exacerbating AD progression [123]. Therefore, several groups have tested the effects of anti-inflammatory drugs [124, 125]. In relation to oxidative stress, microglia have been identified as an important source of ROS. Indeed, activated microglial cells are able to generate free radicals, specifically superoxide via the NADPH oxidase (NOX) enzyme [126], including in AD [127]. NOX is a transmembrane protein that is activated by the presence of cytosolic elements at the plasma membrane, such as rac, p67phox, or p47phox proteins (figure 4). The NOX assembly can then generate superoxide by reducing O2 via electron transfer. Previous reports showed that the NOX system may be altered in AD, as shown by increased levels of p47phox and p67phox in the membrane fraction of AD brains, suggesting activation of NOX. In MCI brains, NOX activity was markedly increased in comparison with control patients [128]. Consistent with these data, Park and colleagues found that deficiency of NOX2 in transgenic AD mice reduced oxidative stress and improved cerebrovascular function and memory deficits without affecting Aβ levels or amyloid plaques [129]. In addition, in transgenic AD mice treated with ibuprofen, there was a reduction of amyloid plaque burden, microglial activation, and markers of oxidative stress [130]. Importantly, fibrillar Aβ increased ROS generation in microglial cells and stimulated the translocation of Rac (another cytosolic element of the NOX assembly) from the cytosol to the membrane, suggesting that Aβ can affect NOX2-mediated pathways [131]. Conversely, in microglial cells, ibuprofen pretreatment reduced ROS production induced by fibrillar Aβ administration. Indeed, non-steroidal anti-inflammatory drugs such as Ibuprofen (figure 3K; table 1) have been used in the treatment of AD for their ability to inhibit cyclooxygenase 2 (COX2). COX2 converts arachidonic acid to prostaglandin H2, which in turn is converted to prostaglandins and then to thromboxane A2. Ibuprofen was also able to disrupt NOX2-mediated signaling, as evidenced by the inhibition of Rac translocation to the membrane [130]. During clinical trials in AD patients, neither ibuprofen [132], tarenflurbil (figure 3L; table 1) [133], naproxen (figure 3M; table 1) nor celecoxib (figure 3N; table 1) [134] slowed disease progression or cognitive decline.
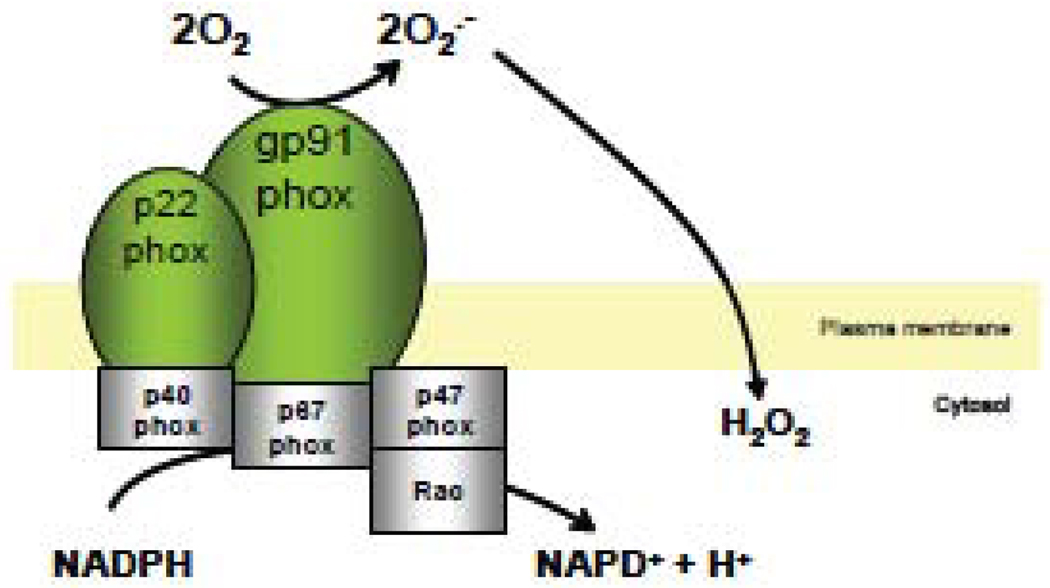
Figure 4. NADPH oxidase and production of ROS.
The assembly of NADPH oxidase subunits (gp91phox/p22phox) with cytolosic subunits p47phox, p40phox, p67phox and rac results in the active enzyme responsible for the generation of superoxide.
Several pharmacological NOX inhibitors are currently available [135]; however, none of them have been tested either in vitro, in vivo or in clinical trials in AD research. In a mouse model of amyotrophic lateral sclerosis, apocynin (figure 3O; table 1), a NOX inhibitor [136], markedly increased survival, reduced ROS production and delayed symptoms induced by the superoxide dismutase 1 mutation [137]. Apocynin, or acetovanillone, blocks NOX assembly and therefore inhibits NOX-mediated superoxide formation. These findings suggest that NOX inhibitors may have potent therapeutic effects in neurodegenerative diseases.
Many groups have studied the implication of COX2 [124] and inducible nitric oxide synthase (iNOS), both involved in inflammation, in the treatment of AD. First, COX2 expression is increased in the frontal cortex of AD patients compared to control patients [138]. The same group also reported that overexpression of COX2 enhanced Aβ pathology in transgenic AD mice [139] and Aβ generation in cells, possibly through activation of the gamma secretase activity [140]. Therefore, inhibitors of COX2 have been considered, such as non-steroidal anti-inflammatory drugs (see paragraph above). In vitro, presenilin 2 mutations induced cell death was reduced by COX2 inhibition [141]. iNOS is another target used in the treatment of AD. Its expression was also increased in neuronal and glial cells of human AD brains, especially in the cortex [142, 143] and the hippocampus [144]. In neuronal and glial cells, iNOS produces nitric oxide (NO) by catalysing a five-electron oxidation of the guanidino nitrogen of L-arginine. In turn, nitric oxide can react with superoxide to generate peroxynitrite. In transgenic AD mice, elevation of iNOS and nitric oxide (NO) expression [145] was also associated with an increase of nitrosative stress at the vicinity of amyloid deposits [146, 147]. To prevent nitrosative damage, several groups have been testing the effect of iNOS inhibition in vitro and in vivo [148]. Deficiency of iNOS by genetic deletion reduced mortality and, at late stage, reduced amyloid plaque burden, microgliosis, astrocytosis, nitrotyrosine levels, and peri-plaque tau phosphorylation in APP/PS1 transgenic mice [149]. Other groups however have found that iNOS inhibition may enhance AD pathology [150, 151]. Nevertheless, it is clear that it plays an important role in AD. The pharmacological inhibitor of iNOS, N6-(1-iminoethyl)-L-lysine (L-NIL) (figure 3P; table 1), acts as an analog of L-arginine. It produces a time-dependent inactivation of citrulline- and NO-forming activity of iNOS in the presence of NADPH and oxygen [152, 153]. Moreover, the inactivation of iNOS is irreversible by displacement of the heme prosthetic group [154, 155]. Interestingly, L-NIL improved behavior and decreased cortical amyloid deposition, as well as microglial activation in transgenic AD mice [156].
Another strategy used to prevent ROS-mediated damage is the upregulation of transcriptional factors involved in the antioxidant response. The peroxisome proliferator-activated receptor-γ coactivator 1 alpha (PGC-1α) is an important transcription cofactor involved in energy metabolism [157, 158]. Interestingly, PGC-1α activation is dependent on various insults including the generation of reactive oxygen species. Its expression is reduced in human post-mortem AD brain, and this correlates with the pre-mortem dementia scales (CDR) and numbers of neurofibrillary tangles [159]. The nuclear receptor peroxisome proliferator-activated receptor-γ (PPAR-γ) activates PGC-1α which then interacts with multiple other transcription factors to modulate mitochondrial biogenesis. It has been shown that PPAR-γ may influence gene transcription of BACE1 [160]. Administration of bezafibrate (figure 3Q; table 1), a PPAR pan agonist, reduced behavioral deficit and inflammation in two mouse models of PD, using MPTP and 6-hydroxydopamine [161]. It also prevented the bioenergetic deficit and improved mitochondrial myopathy in mice produced by a deficiency of the nuclear encoded COX10 subunit of cytochrome c oxidase [162]. Pioglitazone (figure 3R; table 1) and rosiglitazone (figure 3S; table 1), two thiazolidinediones (TZDs) that selectively activate PPAR-γ, have been used for the treatment of AD. TZDs modulate the transcription of insulin-sensitive genes involved in glucose and lipid metabolism, especially in muscle, adipose tissue, and liver. These compounds can also bind to the outer mitochondrial membrane protein mitoNEET [163, 164]. In transgenic AD mice, administration of pioglitazone improved cerebrovascular functions and decreased oxidative stress [165], whereas administration of rosiglitazone reduced memory deficit [166]. The latter was also tested in a pilot study on AD patients and its administration had beneficial effects on tests of delayed recall [167]. This finding was not confirmed in a larger trial using 3 different doses of rosiglitazone. It should be noted that apolipoprotein E ε4 noncarrier patients did improve on the highest dose of rosiglitazone [168]. Polyphenols have also been used for their antioxidant properties and their ability to modulate Aβ and tau pathology in transgenic AD mice [169, 170]. Adminitration of polyphenol (grape seed extract) (figure 3T; table 1) also improved cognitive behavior and reduced Aβ oligomerization [171, 172].
The nuclear factor erythroid-related factor 2/antioxidant response element (Nrf2/ARE) pathway has become another promising target in the field of neurodegenerative diseases, including AD therapeutics [173]. Nrf2 is a transcription factor encoded by the NFE2L2 gene in humans [174] and a regulator of the antioxidant response [175, 176]. Its activity is regulated in part by the actin-associated protein Keap1, which binds to Nrf2 and sequesters it in the cytoplasm. Under oxidative stress conditions, the binding of Nrf2 with Keap1 is disrupted and Nrf2 is released. This release then allows the translocation of Nrf2 from the cytoplasm into the nucleus. While in the nucleus, Nrf2 can bind to promoters with AREs, stimulating the expression of genes that coordinate a cytoprotective response, known as a phase 2 response, such as genes encoding for mitochondrial antioxidant enzymes, and heat shock proteins [177]. It also down-regulates inflammatory genes, such as iNOS and COX2. In transgenic AD mice, decreased expression of Nrf2 and Nrf2/ARE regulated genes correlated with increased amyloid deposition in the brain [178]. In this context, the use of Nrf2/ARE activators may represent a promising avenue in the treatment of AD.
In vitro, in hippocampal cells, activation of the Nrf2/ARE pathway via both tert-butylhydroquinone (tBHQ) and overexpression of Nrf2, through adenovirus-mediated gene delivery, was protective by reducing Aβ42 mediated cell death. These beneficial effects were also associated with increased expression of Nr2/ARE related genes [178]. In vivo, overexpression of Nrf2 through adenovirus-mediated gene delivery (injections performed in the hippocampus) improved memory deficits and decreased soluble Aβ levels as well as astrogliosis. Overexpression of Nrf2 also elevated the expression of Nrf2/ARE genes, such as heme oxygenase 1 [179]. Another class of Nrf2/ARE activators, the synthetic triterpenoid derivatives of 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO) (figure 3U; table 1), has shown benefits in mouse models of neurodegeneration, including AD. These compounds can enhance Nrf2/ARE activation, particularly by disrupting Nrf2-Keap1 binding [180, 181]. Administration of CDDO-methylamide to transgenic AD mice improved memory retention, and reduced protein oxidation, microgliosis, amyloid burden and Aβ42 levels [182]. Similar improvements in behavior and reduced oxidative damage were also found in mouse models of Huntington’s disease [183] and of Parkinson’s disease [184], providing hope for future clinical trials. There is extensive preclinical data showing efficacy of antioxidants in both in vitro and in vivo models of AD. Agents stimulating the Nrf2/ARE pathway in human patients have only recently been tested. However, dimethylfumarate (figure 3V; table 1) has shown efficacy in a phase 2 clinical trial in multiple sclerosis [185].
Again, the use of neuroprotective strategies targeting antioxidant-related pathways has brought positive outcomes in vitro and in vivo. However, their effects in human disease have not been extensively studied. Clinical trials using AD and MCI patients may be of great promise.
Concluding remarks
Oxidative stress plays an important role in AD pathogenesis. Generation and accumulation of ROS within cells are detrimental and can exacerbate the disease progression. Therefore, several strategies have been studied to prevent and/or slow down ROS-mediated damages (figure 5). It should be noted that, independently of the strategy, important factors must be considered in the use of antioxidant drugs, such as their bioavailability (absorption, transport, distribution and retention in the targeted area) and reaction kinetics. They must neutralize free radical faster than the radicals can damage their targets. Timing of the treatment is also extremely critical. For most of the drugs discussed above, beneficial effects have been reported in cell culture and partially in animal models. However, success in human clinical trials is much less frequent. One can argue that treatments are started very early in pathogenesis in animals, whereas in humans pathogenesis may already be well advanced by the time diagnosis is made.
Figure 5. Antioxidant strategies in AD.
In human clinical trials, some studies found slowing of disease progression, whereas others did not find any differences between the same drug and placebo. In fact, understanding and assessing antioxidant capacity in vivo is a challenging task and requires further investigations [186]. The antioxidant system forms a complex network, and treating with only a single or even a few may not be sufficient, or may even imbalance the network in a deleterious way. For this reason, upregulation of a coordinated endogenous network may be more successful. Even though discrepancies exist in data from clinical trials, several ROS-mediated neuroprotective strategies continue to provide hope for neuroprotective treatment of AD. For example, the transcriptional facilitation of the endogenous antioxidant system holds great promise.
Acknowledgments
This work was supported by National Institute of Health (NIH) grants RO1-AG20729 and PO1-AG14930. We thank Dr. Michael T. Lin for his review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nunomura A, Castellani RJ, Zhu X, Moreira PI, Perry G, Smith MA. Involvement of oxidative stress in Alzheimer disease. J Neuropathol Exp Neurol. 2006;65:631–641. doi: 10.1097/01.jnen.0000228136.58062.bf. [DOI] [PubMed] [Google Scholar]
- 2.Coppede F, Migliore L. DNA damage and repair in Alzheimer's disease. Curr Alzheimer Res. 2009;6:36–47. doi: 10.2174/156720509787313970. [DOI] [PubMed] [Google Scholar]
- 3.Lovell MA, Markesbery WR. Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer's disease. Nucleic Acids Res. 2007;35:7497–7504. doi: 10.1093/nar/gkm821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lovell MA, Markesbery WR. Oxidatively modified RNA in mild cognitive impairment. Neurobiol Dis. 2008;29:169–175. doi: 10.1016/j.nbd.2007.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nunomura A, Moreira PI, Takeda A, Smith MA, Perry G. Oxidative RNA damage and neurodegeneration. Curr. Med. Chem. 2007;14:2968–2975. doi: 10.2174/092986707782794078. [DOI] [PubMed] [Google Scholar]
- 6.Markesbery WR, Kryscio RJ, Lovell MA, Morrow JD. Lipid peroxidation is an early event in the brain in amnestic mild cognitive impairment. Ann Neurol. 2005;58:730–735. doi: 10.1002/ana.20629. [DOI] [PubMed] [Google Scholar]
- 7.Pratico D. The neurobiology of isoprostanes and Alzheimer's disease. Biochim Biophys Acta. 1801:930–933. doi: 10.1016/j.bbalip.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Sultana R, Perluigi M, Butterfield DA. Oxidatively modified proteins in Alzheimer's disease (AD), mild cognitive impairment and animal models of AD: role of Abeta in pathogenesis. Acta Neuropathol. 2009;118:131–150. doi: 10.1007/s00401-009-0517-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldeiras I, Santana I, Proenca MT, Garrucho MH, Pascoal R, Rodrigues A, Duro D, Oliveira CR. Oxidative Damage and Progression to Alzheimer's Disease in Patients with Mild Cognitive Impairment. J Alzheimers Dis. 2010 doi: 10.3233/jad-2010-091723. [DOI] [PubMed] [Google Scholar]
- 10.Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 11.Resende R, Moreira PI, Proenca T, Deshpande A, Busciglio J, Pereira C, Oliveira CR. Brain oxidative stress in a triple-transgenic mouse model of Alzheimer disease. Free Radic Biol Med. 2008;44:2051–2057. doi: 10.1016/j.freeradbiomed.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Abdul HM, Sultana R, St Clair DK, Markesbery WR, Butterfield DA. Oxidative damage in brain from human mutant APP/PS-1 double knock-in mice as a function of age. Free Radic Biol Med. 2008;45:1420–1425. doi: 10.1016/j.freeradbiomed.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pratico D, Uryu K, Leight S, Trojanoswki JQ, Lee VM. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J. Neurosci. 2001;21:4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- 15.Nunomura A, Moreira PI, Lee HG, Zhu X, Castellani RJ, Smith MA, Perry G. Neuronal death and survival under oxidative stress in Alzheimer and Parkinson diseases. CNS Neurol Disord Drug Targets. 2007;6:411–423. doi: 10.2174/187152707783399201. [DOI] [PubMed] [Google Scholar]
- 16.Zhu X, Su B, Wang X, Smith MA, Perry G. Causes of oxidative stress in Alzheimer disease. Cell Mol Life Sci. 2007;64:2202–2210. doi: 10.1007/s00018-007-7218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonda DJ, Wang X, Perry G, Smith MA, Zhu X. Mitochondrial dynamics in Alzheimer's disease: opportunities for future treatment strategies. Drugs Aging. 2010;27:181–192. doi: 10.2165/11532140-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 19.Hung YH, Bush AI, Cherny RA. Copper in the brain and Alzheimer's disease. J Biol Inorg Chem. 15:61–76. doi: 10.1007/s00775-009-0600-y. [DOI] [PubMed] [Google Scholar]
- 20.Smith MA, Zhu X, Tabaton M, Liu G, McKeel DW, Jr, Cohen ML, Wang X, Siedlak SL, Dwyer BE, Hayashi T, Nakamura M, Nunomura A, Perry G. Increased iron and free radical generation in preclinical Alzheimer disease and mild cognitive impairment. J Alzheimers Dis. 19:363–372. doi: 10.3233/JAD-2010-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Block ML. NADPH oxidase as a therapeutic target in Alzheimer's disease. BMC Neurosci. 2008;9 Suppl 2:S8. doi: 10.1186/1471-2202-9-S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 23.Bonda DJ, Wang X, Perry G, Nunomura A, Tabaton M, Zhu X, Smith MA. Oxidative stress in Alzheimer disease: A possibility for prevention. Neuropharmacology. 2010 doi: 10.1016/j.neuropharm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Coskun PE, Beal MF, Wallace DC. Alzheimer's brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proc Natl Acad Sci U S A. 2004;101:10726–10731. doi: 10.1073/pnas.0403649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mecocci P, MacGarvey U, Beal MF. Oxidative damage to mitochondrial DNA is increased in Alzheimer's disease. Ann Neurol. 1994;36:747–751. doi: 10.1002/ana.410360510. [DOI] [PubMed] [Google Scholar]
- 26.Reeve AK, Krishnan KJ, Turnbull D. Mitochondrial DNA mutations in disease, aging, and neurodegeneration. Ann N Y Acad Sci. 2008;1147:21–29. doi: 10.1196/annals.1427.016. [DOI] [PubMed] [Google Scholar]
- 27.Gibson GE, Blass JP, Beal MF, Bunik V. The alpha-ketoglutarate-dehydrogenase complex: a mediator between mitochondria and oxidative stress in neurodegeneration. Mol Neurobiol. 2005;31:43–63. doi: 10.1385/MN:31:1-3:043. [DOI] [PubMed] [Google Scholar]
- 28.Gibson GE, Zhang H, Sheu KF, Bogdanovich N, Lindsay JG, Lannfelt L, Vestling M, Cowburn RF. Alpha-ketoglutarate dehydrogenase in Alzheimer brains bearing the APP670/671 mutation. Ann Neurol. 1998;44:676–681. doi: 10.1002/ana.410440414. [DOI] [PubMed] [Google Scholar]
- 29.Sorbi S, Bird ED, Blass JP. Decreased pyruvate dehydrogenase complex activity in Huntington and Alzheimer brain. Ann Neurol. 1983;13:72–78. doi: 10.1002/ana.410130116. [DOI] [PubMed] [Google Scholar]
- 30.Cardoso SM, Proenca MT, Santos S, Santana I, Oliveira CR. Cytochrome c oxidase is decreased in Alzheimer's disease platelets. Neurobiol Aging. 2004;25:105–110. doi: 10.1016/s0197-4580(03)00033-2. [DOI] [PubMed] [Google Scholar]
- 31.Pickrell AM, Fukui H, Moraes CT. The role of cytochrome c oxidase deficiency in ROS and amyloid plaque formation. J Bioenerg Biomembr. 2009;41:453–456. doi: 10.1007/s10863-009-9245-3. [DOI] [PubMed] [Google Scholar]
- 32.Dumont M, Ho DJ, Calingasan NY, Xu H, Gibson G, Beal MF. Mitochondrial dihydrolipoyl succinyltransferase deficiency accelerates amyloid pathology and memory deficit in a transgenic mouse model of amyloid deposition. Free Radic Biol Med. 2009;47:1019–1027. doi: 10.1016/j.freeradbiomed.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li F, Calingasan NY, Yu F, Mauck WM, Toidze M, Almeida CG, Takahashi RH, Carlson GA, Flint Beal M, Lin MT, Gouras GK. Increased plaque burden in brains of APP mutant MnSOD heterozygous knockout mice. J. Neurochem. 2004;89:1308–1312. doi: 10.1111/j.1471-4159.2004.02455.x. [DOI] [PubMed] [Google Scholar]
- 34.Melov S, Adlard PA, Morten K, Johnson F, Golden TR, Hinerfeld D, Schilling B, Mavros C, Masters CL, Volitakis I, Li QX, Laughton K, Hubbard A, Cherny RA, Gibson B, Bush AI. Mitochondrial oxidative stress causes hyperphosphorylation of tau. PLoS ONE. 2007;2:e536. doi: 10.1371/journal.pone.0000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Esposito L, Raber J, Kekonius L, Yan F, Yu GQ, Bien-Ly N, Puolivali J, Scearce-Levie K, Masliah E, Mucke L. Reduction in mitochondrial superoxide dismutase modulates Alzheimer's disease-like pathology and accelerates the onset of behavioral changes in human amyloid precursor protein transgenic mice. J. Neurosci. 2006;26:5167–5179. doi: 10.1523/JNEUROSCI.0482-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dumont M, Wille E, Stack C, Calingasan NY, Beal MF, Lin MT. Reduction of oxidative stress, amyloid deposition, and memory deficit by manganese superoxide dismutase overexpression in a transgenic mouse model of Alzheimer's disease. Faseb J. 2009;23:2459–2466. doi: 10.1096/fj.09-132928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massaad CA, Washington TM, Pautler RG, Klann E. Overexpression of SOD-2 reduces hippocampal superoxide and prevents memory deficits in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2009;106:13576–13581. doi: 10.1073/pnas.0902714106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Massaad CA, Amin SK, Hu L, Mei Y, Klann E, Pautler RG. Mitochondrial superoxide contributes to blood flow and axonal transport deficits in the Tg2576 mouse model of Alzheimer's disease. PLoS One. 5:e10561. doi: 10.1371/journal.pone.0010561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Du H, Guo L, Zhang W, Rydzewska M, Yan S, Cyclophilin D. deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du H, Yan SS. Mitochondrial permeability transition pore in Alzheimer's disease: cyclophilin D and amyloid beta. Biochim Biophys Acta. 1802:198–204. doi: 10.1016/j.bbadis.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X. Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. J Neurosci. 2009;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pavlov PF, Petersen CH, Glaser E, Ankarcrona M. Mitochondrial accumulation of APP and Abeta: Significance for Alzheimer disease pathogenesis. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2009.00892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eckert A, Schulz KL, Rhein V, Gotz J. Convergence of amyloid-beta and tau pathologies on mitochondria in vivo. Mol Neurobiol. 41:107–114. doi: 10.1007/s12035-010-8109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rhein V, Song X, Wiesner A, Ittner LM, Baysang G, Meier F, Ozmen L, Bluethmann H, Drose S, Brandt U, Savaskan E, Czech C, Gotz J, Eckert A. Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer's disease mice. Proc Natl Acad Sci U S A. 2009;106:20057–20062. doi: 10.1073/pnas.0905529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tamagno E, Parola M, Bardini P, Piccini A, Borghi R, Guglielmotto M, Santoro G, Davit A, Danni O, Smith MA, Perry G, Tabaton M. Beta-site APP cleaving enzyme up-regulation induced by 4-hydroxynonenal is mediated by stress-activated protein kinases pathways. J Neurochem. 2005;92:628–636. doi: 10.1111/j.1471-4159.2004.02895.x. [DOI] [PubMed] [Google Scholar]
- 47.Lovell MA, Xiong S, Xie C, Davies P, Markesbery WR. Induction of hyperphosphorylated tau in primary rat cortical neuron cultures mediated by oxidative stress and glycogen synthase kinase-3. J Alzheimers Dis. 2004;6:659–671. doi: 10.3233/jad-2004-6610. discussion 673–681. [DOI] [PubMed] [Google Scholar]
- 48.Wadsworth TL, Bishop JA, Pappu AS, Woltjer RL, Quinn JF. Evaluation of coenzyme Q as an antioxidant strategy for Alzheimer's disease. J Alzheimers Dis. 2008;14:225–234. doi: 10.3233/jad-2008-14210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spindler M, Beal MF, Henchcliffe C. Coenzyme Q10 effects in neurodegenerative disease. Neuropsychiatr Dis Treat. 2009;5:597–610. doi: 10.2147/ndt.s5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang X, Dai G, Li G, Yang ES. Coenzyme Q10 reduces beta-amyloid plaque in an APP/PS1 transgenic mouse model of Alzheimer's disease. J Mol Neurosci. 41:110–113. doi: 10.1007/s12031-009-9297-1. [DOI] [PubMed] [Google Scholar]
- 51.Yang X, Yang Y, Li G, Wang J, Yang ES. Coenzyme Q10 attenuates beta-amyloid pathology in the aged transgenic mice with Alzheimer presenilin 1 mutation. J Mol Neurosci. 2008;34:165–171. doi: 10.1007/s12031-007-9033-7. [DOI] [PubMed] [Google Scholar]
- 52.Suno M, Nagaoka A. [Effect of idebenone and various nootropic drugs on lipid peroxidation in rat brain homogenate in the presence of succinate] Nippon Yakurigaku Zasshi. 1988;91:295–299. doi: 10.1254/fpj.91.295. [DOI] [PubMed] [Google Scholar]
- 53.Gutzmann H, Kuhl KP, Hadler D, Rapp MA. Safety and efficacy of idebenone versus tacrine in patients with Alzheimer's disease: results of a randomized, double-blind, parallel-group multicenter study. Pharmacopsychiatry. 2002;35:12–18. doi: 10.1055/s-2002-19833. [DOI] [PubMed] [Google Scholar]
- 54.Senin U, Parnetti L, Barbagallo-Sangiorgi G, Bartorelli L, Bocola V, Capurso A, Cuzzupoli M, Denaro M, Marigliano V, Tammaro AE, Fioravanti M. Idebenone in senile dementia of Alzheimer type: a multicentre study. Arch Gerontol Geriatr. 1992;15:249–260. doi: 10.1016/0167-4943(92)90060-h. [DOI] [PubMed] [Google Scholar]
- 55.Weyer G, Babej-Dolle RM, Hadler D, Hofmann S, Herrmann WM. A controlled study of 2 doses of idebenone in the treatment of Alzheimer's disease. Neuropsychobiology. 1997;36:73–82. doi: 10.1159/000119366. [DOI] [PubMed] [Google Scholar]
- 56.Thal LJ, Grundman M, Berg J, Ernstrom K, Margolin R, Pfeiffer E, Weiner MF, Zamrini E, Thomas RG. Idebenone treatment fails to slow cognitive decline in Alzheimer's disease. Neurology. 2003;61:1498–1502. doi: 10.1212/01.wnl.0000096376.03678.c1. [DOI] [PubMed] [Google Scholar]
- 57.Smith RA, Murphy MP. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann N Y Acad Sci. 1201:96–103. doi: 10.1111/j.1749-6632.2010.05627.x. [DOI] [PubMed] [Google Scholar]
- 58.Tauskela JS. MitoQ--a mitochondria-targeted antioxidant. IDrugs. 2007;10:399–412. [PubMed] [Google Scholar]
- 59.Rodriguez-Cuenca S, Cocheme HM, Logan A, Abakumova I, Prime TA, Rose C, Vidal-Puig A, Smith AC, Rubinsztein DC, Fearnley IM, Jones BA, Pope S, Heales SJ, Lam BY, Neogi SG, McFarlane I, James AM, Smith RA, Murphy MP. Consequences of long-term oral administration of the mitochondria-targeted antioxidant MitoQ to wild-type mice. Free Radic Biol Med. 48:161–172. doi: 10.1016/j.freeradbiomed.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 60.Snow BJ, Rolfe FL, Lockhart MM, Frampton CM, O'Sullivan JD, Fung V, Smith RA, Murphy MP, Taylor KM. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson's disease. Mov Disord. doi: 10.1002/mds.23148. [DOI] [PubMed] [Google Scholar]
- 61.Doody RS. Dimebon as a potential therapy for Alzheimer's disease. CNS Spectr. 2009;14:14–16. doi: 10.1017/s1092852900024913. discussion 16–18. [DOI] [PubMed] [Google Scholar]
- 62.Okun I, Tkachenko SE, Khvat A, Mitkin O, Kazey V, Ivachtchenko AV. From anti-allergic to anti-Alzheimer's: Molecular pharmacology of Dimebon. Curr Alzheimer Res. 7:97–112. doi: 10.2174/156720510790691100. [DOI] [PubMed] [Google Scholar]
- 63.Wu J, Li Q, Bezprozvanny I. Evaluation of Dimebon in cellular model of Huntington's disease. Mol Neurodegener. 2008;3:15. doi: 10.1186/1750-1326-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grigorev VV, Dranyi OA, Bachurin SO. Comparative study of action mechanisms of dimebon and memantine on AMPA- and NMDA-subtypes glutamate receptors in rat cerebral neurons. Bull Exp Biol Med. 2003;136:474–477. doi: 10.1023/b:bebm.0000017097.75818.14. [DOI] [PubMed] [Google Scholar]
- 65.Lermontova NN, Redkozubov AE, Shevtsova EF, Serkova TP, Kireeva EG, Bachurin SO. Dimebon and tacrine inhibit neurotoxic action of beta-amyloid in culture and block L-type Ca(2+) channels. Bull Exp Biol Med. 2001;132:1079–1083. doi: 10.1023/a:1017972709652. [DOI] [PubMed] [Google Scholar]
- 66.Zhang S, Hedskog L, Petersen CA, Winblad B, Ankarcrona M. Dimebon (Latrepirdine) Enhances Mitochondrial Function and Protects Neuronal Cells from Death. J Alzheimers Dis. doi: 10.3233/JAD-2010-100174. [DOI] [PubMed] [Google Scholar]
- 67.Steele JW, Kim SH, Cirrito JR, Verges DK, Restivo JL, Westaway D, Fraser P, Hyslop PS, Sano M, Bezprozvanny I, Ehrlich ME, Holtzman DM, Gandy S. Acute dosing of latrepirdine (Dimebon), a possible Alzheimer therapeutic, elevates extracellular amyloid-beta levels in vitro and in vivo. Mol Neurodegener. 2009;4:51. doi: 10.1186/1750-1326-4-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Doody RS, Gavrilova SI, Sano M, Thomas RG, Aisen PS, Bachurin SO, Seely L, Hung D. Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer's disease: a randomised, double-blind, placebo-controlled study. Lancet. 2008;372:207–215. doi: 10.1016/S0140-6736(08)61074-0. [DOI] [PubMed] [Google Scholar]
- 69.AlzforumDimebonDisappoints Alzheimer Research Forum. Dimebon Disappoints in Phase 3 Trial [Google Scholar]
- 70.CTdotgovdimebon ClinicalTrials.gov study NCT00675623. A Safety and Efficacy Study of Oral Dimebon in Patients With Mild-To-Moderate Alzheimer's Disease (CONNECTION) [Google Scholar]
- 71.Acetyl-L-carnitine. Monograph. Altern Med Rev. 15:76–83. [PubMed] [Google Scholar]
- 72.Ames BN, Liu J. Delaying the mitochondrial decay of aging with acetylcarnitine. Ann N Y Acad Sci. 1033:108–116. doi: 10.1196/annals.1320.010. [DOI] [PubMed] [Google Scholar]
- 73.Maczurek A, Hager K, Kenklies M, Sharman M, Martins R, Engel J, Carlson DA, Munch G. Lipoic acid as an anti-inflammatory and neuroprotective treatment for Alzheimer's disease. Adv Drug Deliv Rev. 2008;60:1463–1470. doi: 10.1016/j.addr.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 74.Haenen GR, Bast A. Scavenging of hypochlorous acid by lipoic acid. Biochem Pharmacol. 1991;42:2244–2246. doi: 10.1016/0006-2952(91)90363-a. [DOI] [PubMed] [Google Scholar]
- 75.Aliev G, Liu J, Shenk JC, Fischbach K, Pacheco GJ, Chen SG, Obrenovich ME, Ward WF, Richardson AG, Smith MA, Gasimov E, Perry G, Ames BN. Neuronal mitochondrial amelioration by feeding acetyl-L-carnitine and lipoic acid to aged rats. J Cell Mol Med. 2009;13:320–333. doi: 10.1111/j.1582-4934.2008.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Long J, Gao F, Tong L, Cotman CW, Ames BN, Liu J. Mitochondrial decay in the brains of old rats: ameliorating effect of alpha-lipoic acid and acetyl-Lcarnitine. Neurochem Res. 2009;34:755–763. doi: 10.1007/s11064-008-9850-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Epis R, Marcello E, Gardoni F, Longhi A, Calvani M, Iannuccelli M, Cattabeni F, Canonico PL, Di Luca M. Modulatory effect of acetyl-L-carnitine on amyloid precursor protein metabolism in hippocampal neurons. Eur J Pharmacol. 2008;597:51–56. doi: 10.1016/j.ejphar.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 78.Suchy J, Chan A, Shea TB. Dietary supplementation with a combination of alpha-lipoic acid, acetyl-L-carnitine, glycerophosphocoline, docosahexaenoic acid, and phosphatidylserine reduces oxidative damage to murine brain and improves cognitive performance. Nutr Res. 2009;29:70–74. doi: 10.1016/j.nutres.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 79.Shenk JC, Liu J, Fischbach K, Xu K, Puchowicz M, Obrenovich ME, Gasimov E, Alvarez LM, Ames BN, Lamanna JC, Aliev G. The effect of acetyl-L-carnitine and R-alpha-lipoic acid treatment in ApoE4 mouse as a model of human Alzheimer's disease. J Neurol Sci. 2009;283:199–206. doi: 10.1016/j.jns.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barhwal K, Hota SK, Jain V, Prasad D, Singh SB, Ilavazhagan G. Acetyl-l-carnitine (ALCAR) prevents hypobaric hypoxia-induced spatial memory impairment through extracellular related kinase-mediated nuclear factor erythroid 2-related factor 2 phosphorylation. Neuroscience. 2009;161:501–514. doi: 10.1016/j.neuroscience.2009.02.086. [DOI] [PubMed] [Google Scholar]
- 81.Petersen Shay K, Moreau RF, Smith EJ, Hagen TM. Is alpha-lipoic acid a scavenger of reactive oxygen species in vivo? Evidence for its initiation of stress signaling pathways that promote endogenous antioxidant capacity. IUBMB Life. 2008;60:362–367. doi: 10.1002/iub.40. [DOI] [PubMed] [Google Scholar]
- 82.Pettegrew JW, Klunk WE, Panchalingam K, Kanfer JN, McClure RJ. Clinical and neurochemical effects of acetyl-L-carnitine in Alzheimer's disease. Neurobiol Aging. 1995;16:1–4. doi: 10.1016/0197-4580(95)80001-8. [DOI] [PubMed] [Google Scholar]
- 83.Thal LJ, Carta A, Clarke WR, Ferris SH, Friedland RP, Petersen RC, Pettegrew JW, Pfeiffer E, Raskind MA, Sano M, Tuszynski MH, Woolson RF. A 1-year multicenter placebo-controlled study of acetyl-L-carnitine in patients with Alzheimer's disease. Neurology. 1996;47:705–711. doi: 10.1212/wnl.47.3.705. [DOI] [PubMed] [Google Scholar]
- 84.Thal LJ, Calvani M, Amato A. Carta, A. A 1-year controlled trial of acetyl-l-carnitine in early-onset AD. Neurology. 2000;55:805–810. doi: 10.1212/wnl.55.6.805. [DOI] [PubMed] [Google Scholar]
- 85.Montgomery SA, Thal LJ, Amrein R. Meta-analysis of double blind randomized controlled clinical trials of acetyl-L-carnitine versus placebo in the treatment of mild cognitive impairment and mild Alzheimer's disease. Int Clin Psychopharmacol. 2003;18:61–71. doi: 10.1097/00004850-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 86.Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic Biol Med. 2007;43:4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sung S, Yao Y, Uryu K, Yang H, Lee VM, Trojanowski JQ, Pratico D. Early vitamin E supplementation in young but not aged mice reduces Abeta levels and amyloid deposition in a transgenic model of Alzheimer's disease. Faseb J. 2004;18:323–325. doi: 10.1096/fj.03-0961fje. [DOI] [PubMed] [Google Scholar]
- 88.Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, Woodbury P, Growdon J, Cotman CW, Pfeiffer E, Schneider LS, Thal LJ. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer's disease. The Alzheimer's Disease Cooperative Study. N Engl J Med. 1997;336:1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 89.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, Galasko D, Jin S, Kaye J, Levey A, Pfeiffer E, Sano M, van Dyck CH, Thal LJ. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 90.Abramova NA, Cassarino DS, Khan SM, Painter TW, Bennett JP., Jr Inhibition by R(+) or S(−) pramipexole of caspase activation and cell death induced by methylpyridinium ion or beta amyloid peptide in SH-SY5Y neuroblastoma. J Neurosci Res. 2002;67:494–500. doi: 10.1002/jnr.10127. [DOI] [PubMed] [Google Scholar]
- 91.Ferrari-Toninelli G, Maccarinelli G, Uberti D, Buerger E, Memo M. Mitochondria-targeted antioxidant effects of S(−) and R(+) pramipexole. BMC Pharmacol. 10:2. doi: 10.1186/1471-2210-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li C, Guo Y, Xie W, Li X, Janokovic J, Le W. Neuroprotection of Pramipexole in UPS Impairment Induced Animal Model of Parkinson's Disease. Neurochem Res. doi: 10.1007/s11064-010-0214-3. [DOI] [PubMed] [Google Scholar]
- 93.Gribkoff VK, Bozik ME. KNS-760704 [(6R)-4,5,6,7-tetrahydro-N6-propyl-2, 6-benzothiazole-diamine dihydrochloride monohydrate] for the treatment of amyotrophic lateral sclerosis. CNS Neurosci Ther. 2008;14:215–226. doi: 10.1111/j.1755-5949.2008.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhao K, Luo G, Giannelli S, Szeto HH. Mitochondria-targeted peptide prevents mitochondrial depolarization and apoptosis induced by tert-butyl hydroperoxide in neuronal cell lines. Biochem Pharmacol. 2005;70:1796–1806. doi: 10.1016/j.bcp.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 95.Cho S, Szeto HH, Kim E, Kim H, Tolhurst AT, Pinto JT. A novel cell-permeable antioxidant peptide, SS31, attenuates ischemic brain injury by down-regulating CD36. J Biol Chem. 2007;282:4634–4642. doi: 10.1074/jbc.M609388200. [DOI] [PubMed] [Google Scholar]
- 96.Yang L, Zhao K, Calingasan NY, Luo G, Szeto HH, Beal MF. Mitochondria targeted peptides protect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity. Antioxid Redox Signal. 2009;11:2095–2104. doi: 10.1089/ars.2009.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Petri S, Kiaei M, Damiano M, Hiller A, Wille E, Manfredi G, Calingasan NY, Szeto HH, Beal MF. Cell-permeable peptide antioxidants as a novel therapeutic approach in a mouse model of amyotrophic lateral sclerosis. J Neurochem. 2006;98:1141–1148. doi: 10.1111/j.1471-4159.2006.04018.x. [DOI] [PubMed] [Google Scholar]
- 98.Manczak M, Mao P, Calkins MJ, Cornea A, Reddy AP, Murphy MP, Szeto HH, Park B, Reddy PH. Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer's disease neurons. J Alzheimers Dis. 20 Suppl 2:S609–S631. doi: 10.3233/JAD-2010-100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang X, Atwood CS, Hartshorn MA, Multhaup G, Goldstein LE, Scarpa RC, Cuajungco MP, Gray DN, Lim J, Moir RD, Tanzi RE, Bush AI. The A beta peptide of Alzheimer's disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry. 1999;38:7609–7616. doi: 10.1021/bi990438f. [DOI] [PubMed] [Google Scholar]
- 100.Varadarajan S, Yatin S, Kanski J, Jahanshahi F, Butterfield DA. Methionine residue 35 is important in amyloid beta-peptide-associated free radical oxidative stress. Brain Res Bull. 1999;50:133–141. doi: 10.1016/s0361-9230(99)00093-3. [DOI] [PubMed] [Google Scholar]
- 101.Butterfield DA, Galvan V, Lange MB, Tang H, Sowell RA, Spilman P, Fombonne J, Gorostiza O, Zhang J, Sultana R, Bredesen DE. In vivo oxidative stress in brain of Alzheimer disease transgenic mice: Requirement for methionine 35 in amyloid beta-peptide of APP. Free Radic Biol Med. 2010;48:136–144. doi: 10.1016/j.freeradbiomed.2009.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tabner BJ, El-Agnaf OM, Turnbull S, German MJ, Paleologou KE, Hayashi Y, Cooper LJ, Fullwood NJ, Allsop D. Hydrogen peroxide is generated during the very early stages of aggregation of the amyloid peptides implicated in Alzheimer disease and familial British dementia. J Biol Chem. 2005;280:35789–35792. doi: 10.1074/jbc.C500238200. [DOI] [PubMed] [Google Scholar]
- 103.Adlard PA, Cherny RA, Finkelstein DI, Gautier E, Robb E, Cortes M, Volitakis I, Liu X, Smith JP, Perez K, Laughton K, Li QX, Charman SA, Nicolazzo JA, Wilkins S, Deleva K, Lynch T, Kok G, Ritchie CW, Tanzi RE, Cappai R, Masters CL, Barnham KJ, Bush AI. Rapid restoration of cognition in Alzheimer's transgenic mice with 8-hydroxy quinoline analogs is associated with decreased interstitial Abeta. Neuron. 2008;59:43–55. doi: 10.1016/j.neuron.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 104.Ritchie CW, Bush AI, Mackinnon A, Macfarlane S, Mastwyk M, MacGregor L, Kiers L, Cherny R, Li QX, Tammer A, Carrington D, Mavros C, Volitakis I, Xilinas M, Ames D, Davis S, Beyreuther K, Tanzi RE, Masters CL. Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Abeta amyloid deposition and toxicity in Alzheimer disease: a pilot phase 2 clinical trial. Arch Neurol. 2003;60:1685–1691. doi: 10.1001/archneur.60.12.1685. [DOI] [PubMed] [Google Scholar]
- 105.Lannfelt L, Blennow K, Zetterberg H, Batsman S, Ames D, Harrison J, Masters CL, Targum S, Bush AI, Murdoch R, Wilson J, Ritchie CW. Safety, efficacy, and biomarker findings of PBT2 in targeting Abeta as a modifying therapy for Alzheimer's disease: a phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2008;7:779–786. doi: 10.1016/S1474-4422(08)70167-4. [DOI] [PubMed] [Google Scholar]
- 106.Bandyopadhyay S, Huang X, Lahiri DK, Rogers JT. Novel drug targets based on metallobiology of Alzheimer's disease. Expert Opin Ther Targets. 14:1177–1197. doi: 10.1517/14728222.2010.525352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim J, Lee HJ, Lee KW. Naturally occurring phytochemicals for the prevention of Alzheimer's disease. J Neurochem. 112:1415–1430. doi: 10.1111/j.1471-4159.2009.06562.x. [DOI] [PubMed] [Google Scholar]
- 108.Shukla PK, Khanna VK, Khan MY, Srimal RC. Protective effect of curcumin against lead neurotoxicity in rat. Hum Exp Toxicol. 2003;22:653–658. doi: 10.1191/0960327103ht411oa. [DOI] [PubMed] [Google Scholar]
- 109.Begum AN, Jones MR, Lim GP, Morihara T, Kim P, Heath DD, Rock CL, Pruitt MA, Yang F, Hudspeth B, Hu S, Faull KF, Teter B, Cole GM, Frautschy SA. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer's disease. J Pharmacol Exp Ther. 2008;326:196–208. doi: 10.1124/jpet.108.137455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21:8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Garcia-Alloza M, Borrelli LA, Rozkalne A, Hyman BT, Bacskai BJ. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J Neurochem. 2007;102:1095–1104. doi: 10.1111/j.1471-4159.2007.04613.x. [DOI] [PubMed] [Google Scholar]
- 112.Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 113.Zhang L, Fiala M, Cashman J, Sayre J, Espinosa A, Mahanian M, Zaghi J, Badmaev V, Graves MC, Bernard G, Rosenthal M. Curcuminoids enhance amyloid-beta uptake by macrophages of Alzheimer's disease patients. J Alzheimers Dis. 2006;10:1–7. doi: 10.3233/jad-2006-10101. [DOI] [PubMed] [Google Scholar]
- 114.Seo JS, Leem YH, Lee KW, Kim SW, Lee JK, Han PL. Severe motor neuron degeneration in the spinal cord of the Tg2576 mouse model of Alzheimer disease. J Alzheimers Dis. 21:263–276. doi: 10.3233/JAD-2010-091528. [DOI] [PubMed] [Google Scholar]
- 115.Baum L, Lam CW, Cheung SK, Kwok T, Lui V, Tsoh J, Lam L, Leung V, Hui E, Ng C, Woo J, Chiu HF, Goggins WB, Zee BC, Cheng KF, Fong CY, Wong A, Mok H, Chow MS, Ho PC, Ip SP, Ho CS, Yu XW, Lai CY, Chan MH, Szeto S, Chan IH, Mok V. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J Clin Psychopharmacol. 2008;28:110–113. doi: 10.1097/jcp.0b013e318160862c. [DOI] [PubMed] [Google Scholar]
- 116.Galasko D, Montine TJ. Biomarkers of oxidative damage and inflammation in Alzheimer's disease. Biomark Med. 4:27–36. doi: 10.2217/bmm.09.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schwartz M, Shechter R. Systemic inflammatory cells fight off neurodegenerative disease. Nat Rev Neurol. 6:405–410. doi: 10.1038/nrneurol.2010.71. [DOI] [PubMed] [Google Scholar]
- 118.McGeer EG, McGeer PL. Neuroinflammation in Alzheimer's disease and mild cognitive impairment: a field in its infancy. J Alzheimers Dis. 19:355–361. doi: 10.3233/JAD-2010-1219. [DOI] [PubMed] [Google Scholar]
- 119.Mandrekar S, Jiang Q, Lee CY, Koenigsknecht-Talboo J, Holtzman DM, Landreth GE. Microglia mediate the clearance of soluble Abeta through fluid phase macropinocytosis. J Neurosci. 2009;29:4252–4262. doi: 10.1523/JNEUROSCI.5572-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee CY, Landreth GE. The role of microglia in amyloid clearance from the AD brain. J Neural Transm. 117:949–960. doi: 10.1007/s00702-010-0433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fuhrmann M, Bittner T, Jung CK, Burgold S, Page RM, Mitteregger G, Haass C, LaFerla FM, Kretzschmar H, Herms J. Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer's disease. Nat Neurosci. 13:411–413. doi: 10.1038/nn.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer's disease mice. J Neurosci. 2008;28:8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Holmes C, Cunningham C, Zotova E, Woolford J, Dean C, Kerr S, Culliford D, Perry VH. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2009;73:768–774. doi: 10.1212/WNL.0b013e3181b6bb95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cole GM, Frautschy SA. Mechanisms of action of non-steroidal anti-inflammatory drugs for the prevention of Alzheimer's disease. CNS Neurol Disord Drug Targets. 9:140–148. doi: 10.2174/187152710791011991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Szekely CA, Zandi PP. Non-steroidal anti-inflammatory drugs and Alzheimer's disease: the epidemiological evidence. CNS Neurol Disord Drug Targets. 9:132–139. doi: 10.2174/187152710791012026. [DOI] [PubMed] [Google Scholar]
- 126.Sorce S, Krause KH. NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal. 2009;11:2481–2504. doi: 10.1089/ars.2009.2578. [DOI] [PubMed] [Google Scholar]
- 127.Wilkinson BL, Landreth GE. The microglial NADPH oxidase complex as a source of oxidative stress in Alzheimer's disease. J Neuroinflammation. 2006;3:30. doi: 10.1186/1742-2094-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bruce-Keller AJ, Gupta S, Parrino TE, Knight AG, Ebenezer PJ, Weidner AM, LeVine H, 3rd, Keller JN, Markesbery WR. NOX activity is increased in mild cognitive impairment. Antioxid Redox Signal. 2010;12:1371–1382. doi: 10.1089/ars.2009.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Park L, Zhou P, Pitstick R, Capone C, Anrather J, Norris EH, Younkin L, Younkin S, Carlson G, McEwen BS, Iadecola C. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc. Natl. Acad. Sci. U S A. 2008;105:1347–1352. doi: 10.1073/pnas.0711568105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wilkinson BL, Cramer PE, Varvel NH, Reed-Geaghan E, Jiang Q, Szabo A, Herrup K, Lamb BT, Landreth GE. Ibuprofen attenuates oxidative damage through NOX2 inhibition in Alzheimer's disease. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wilkinson B, Koenigsknecht-Talboo J, Grommes C, Lee CY, Landreth G. Fibrillar beta-amyloid-stimulated intracellular signaling cascades require Vav for induction of respiratory burst and phagocytosis in monocytes and microglia. J Biol Chem. 2006;281:20842–20850. doi: 10.1074/jbc.M600627200. [DOI] [PubMed] [Google Scholar]
- 132.Pasqualetti P, Bonomini C, Dal Forno G, Paulon L, Sinforiani E, Marra C, Zanetti O, Rossini PM. A randomized controlled study on effects of ibuprofen on cognitive progression of Alzheimer's disease. Aging Clin Exp Res. 2009;21:102–110. doi: 10.1007/BF03325217. [DOI] [PubMed] [Google Scholar]
- 133.Green RC, Schneider LS, Amato DA, Beelen AP, Wilcock G, Swabb EA, Zavitz KH. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. JAMA. 2009;302:2557–2564. doi: 10.1001/jama.2009.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Martin BK, Szekely C, Brandt J, Piantadosi S, Breitner JC, Craft S, Evans D, Green R, Mullan M. Cognitive function over time in the Alzheimer's Disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Arch Neurol. 2008;65:896–905. doi: 10.1001/archneur.2008.65.7.nct70006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jaquet V, Scapozza L, Clark RA, Krause KH, Lambeth JD. Small-molecule NOX inhibitors: ROS-generating NADPH oxidases as therapeutic targets. Antioxid Redox Signal. 2009;11:2535–2552. doi: 10.1089/ars.2009.2585. [DOI] [PubMed] [Google Scholar]
- 136.Stefanska J, Pawliczak R. Apocynin: molecular aptitudes. Mediators Inflamm. 2008;2008:106507. doi: 10.1155/2008/106507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Harraz MM, Marden JJ, Zhou W, Zhang Y, Williams A, Sharov VS, Nelson K, Luo M, Paulson H, Schoneich C, Engelhardt JF. SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J Clin Invest. 2008;118:659–670. doi: 10.1172/JCI34060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pasinetti GM, Aisen PS. Cyclooxygenase-2 expression is increased in frontal cortex of Alzheimer's disease brain. Neuroscience. 1998;87:319–324. doi: 10.1016/s0306-4522(98)00218-8. [DOI] [PubMed] [Google Scholar]
- 139.Xiang Z, Ho L, Yemul S, Zhao Z, Qing W, Pompl P, Kelley K, Dang A, Teplow D, Pasinetti GM. Cyclooxygenase-2 promotes amyloid plaque deposition in a mouse model of Alzheimer's disease neuropathology. Gene Expr. 2002;10:271–278. doi: 10.3727/000000002783992352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Qin W, Ho L, Pompl PN, Peng Y, Zhao Z, Xiang Z, Robakis NK, Shioi J, Suh J, Pasinetti GM. Cyclooxygenase (COX)-2 and COX-1 potentiate beta-amyloid peptide generation through mechanisms that involve gamma-secretase activity. J Biol Chem. 2003;278:50970–50977. doi: 10.1074/jbc.M307699200. [DOI] [PubMed] [Google Scholar]
- 141.Qin W, Peng Y, Ksiezak-Reding H, Ho L, Stetka B, Lovati E, Pasinetti GM. Inhibition of cyclooxygenase as potential novel therapeutic strategy in N141I presenilin-2 familial Alzheimer's disease. Mol Psychiatry. 2006;11:172–181. doi: 10.1038/sj.mp.4001773. [DOI] [PubMed] [Google Scholar]
- 142.Fernandez-Vizarra P, Fernandez AP, Castro-Blanco S, Encinas JM, Serrano J, Bentura ML, Munoz P, Martinez-Murillo R, Rodrigo J. Expression of nitric oxide system in clinically evaluated cases of Alzheimer's disease. Neurobiol Dis. 2004;15:287–305. doi: 10.1016/j.nbd.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 143.Vodovotz Y, Lucia MS, Flanders KC, Chesler L, Xie QW, Smith TW, Weidner J, Mumford R, Webber R, Nathan C, Roberts AB, Lippa CF, Sporn MB. Inducible nitric oxide synthase in tangle-bearing neurons of patients with Alzheimer's disease. J Exp Med. 1996;184:1425–1433. doi: 10.1084/jem.184.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lee SC, Zhao ML, Hirano A, Dickson DW. Inducible nitric oxide synthase immunoreactivity in the Alzheimer disease hippocampus: association with Hirano bodies, neurofibrillary tangles, and senile plaques. J Neuropathol Exp Neurol. 1999;58:1163–1169. doi: 10.1097/00005072-199911000-00006. [DOI] [PubMed] [Google Scholar]
- 145.Rodrigo J, Fernandez-Vizarra P, Castro-Blanco S, Bentura ML, Nieto M, Gomez-Isla T, Martinez-Murillo R, MartInez A, Serrano J, Fernandez AP. Nitric oxide in the cerebral cortex of amyloid-precursor protein (SW) Tg2576 transgenic mice. Neuroscience. 2004;128:73–89. doi: 10.1016/j.neuroscience.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 146.Luth HJ, Holzer M, Gartner U, Staufenbiel M, Arendt T. Expression of endothelial and inducible NOS-isoforms is increased in Alzheimer's disease, in APP23 transgenic mice and after experimental brain lesion in rat: evidence for an induction by amyloid pathology. Brain Res. 2001;913:57–67. doi: 10.1016/s0006-8993(01)02758-5. [DOI] [PubMed] [Google Scholar]
- 147.Luth HJ, Munch G, Arendt T. Aberrant expression of NOS isoforms in Alzheimer's disease is structurally related to nitrotyrosine formation. Brain Res. 2002;953:135–143. doi: 10.1016/s0006-8993(02)03280-8. [DOI] [PubMed] [Google Scholar]
- 148.Malinski T. Nitric oxide and nitroxidative stress in Alzheimer's disease. J Alzheimers Dis. 2007;11:207–218. doi: 10.3233/jad-2007-11208. [DOI] [PubMed] [Google Scholar]
- 149.Nathan C, Calingasan N, Nezezon J, Ding A, Lucia MS, La Perle K, Fuortes M, Lin M, Ehrt S, Kwon NS, Chen J, Vodovotz Y, Kipiani K, Beal MF. Protection from Alzheimer's-like disease in the mouse by genetic ablation of inducible nitric oxide synthase. J Exp Med. 2005;202:1163–1169. doi: 10.1084/jem.20051529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Colton CA, Vitek MP, Wink DA, Xu Q, Cantillana V, Previti ML, Van Nostrand WE, Weinberg JB, Dawson H. NO synthase 2 (NOS2) deletion promotes multiple pathologies in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2006;103:12867–12872. doi: 10.1073/pnas.0601075103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Colton CA, Wilcock DM, Wink DA, Davis J, Van Nostrand WE, Vitek MP. The Effects of NOS2 Gene Deletion on Mice Expressing Mutated Human AbetaPP. J Alzheimers Dis. 2008;15:571–587. doi: 10.3233/jad-2008-15405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Moore WM, Webber RK, Jerome GM, Tjoeng FS, Misko TP, Currie MG. L-N6-(1-iminoethyl)lysine: a selective inhibitor of inducible nitric oxide synthase. J Med Chem. 1994;37:3886–3888. doi: 10.1021/jm00049a007. [DOI] [PubMed] [Google Scholar]
- 153.Wolff DJ, Lubeskie A, Gauld DS, Neulander MJ. Inactivation of nitric oxide synthases and cellular nitric oxide formation by N6-iminoethyl-L-lysine and N5-iminoethyl-L-ornithine. Eur J Pharmacol. 1998;350:325–334. doi: 10.1016/s0014-2999(98)00267-2. [DOI] [PubMed] [Google Scholar]
- 154.Bryk R, Wolff DJ. Mechanism of inducible nitric oxide synthase inactivation by aminoguanidine and L-N6-(1-iminoethyl)lysine. Biochemistry. 1998;37:4844–4852. doi: 10.1021/bi972065t. [DOI] [PubMed] [Google Scholar]
- 155.Bryk R, Wolff DJ. Pharmacological modulation of nitric oxide synthesis by mechanism-based inactivators and related inhibitors. Pharmacol Ther. 1999;84:157–178. doi: 10.1016/s0163-7258(99)00030-3. [DOI] [PubMed] [Google Scholar]
- 156.Dumont M, Wille E, Calingasan NY, Nathan C, Flint Beal M, Lin MT. N-iminoethyl-L-lysine improves memory and reduces amyloid pathology in a transgenic mouse model of amyloid deposition. Neurochem Int. 56:345–351. doi: 10.1016/j.neuint.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 157.Spiegelman BM. Transcriptional control of energy homeostasis through the PGC1 coactivators. Novartis Found Symp. 2007;286:3–6. discusssion 6–12, 162–163, 196–203. [PubMed] [Google Scholar]
- 158.Spiegelman BM. Transcriptional control of mitochondrial energy metabolism through the PGC1 coactivators. Novartis Found Symp. 2007;287:60–63. discussion 63–69. [PubMed] [Google Scholar]
- 159.Qin W, Haroutunian V, Katsel P, Cardozo CP, Ho L, Buxbaum JD, Pasinetti GM. PGC-1alpha expression decreases in the Alzheimer disease brain as a function of dementia. Arch Neurol. 2009;66:352–361. doi: 10.1001/archneurol.2008.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rossner S, Sastre M, Bourne K, Lichtenthaler SF. Transcriptional and translational regulation of BACE1 expression--implications for Alzheimer's disease. Prog Neurobiol. 2006;79:95–111. doi: 10.1016/j.pneurobio.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 161.Kreisler A, Duhamel A, Vanbesien-Mailliot C, Destee A, Bordet R. Differing short-term neuroprotective effects of the fibrates fenofibrate and bezafibrate in MPTP and 6-OHDA experimental models of Parkinson's disease. Behav Pharmacol. 21:194–205. doi: 10.1097/FBP.0b013e32833a5c81. [DOI] [PubMed] [Google Scholar]
- 162.Wenz T, Diaz F, Spiegelman BM, Moraes CT. Activation of the PPAR/PGC-1alpha pathway prevents a bioenergetic deficit and effectively improves a mitochondrial myopathy phenotype. Cell Metab. 2008;8:249–256. doi: 10.1016/j.cmet.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 163.Colca JR, McDonald WG, Waldon DJ, Leone JW, Lull JM, Bannow CA, Lund ET, Mathews WR. Identification of a novel mitochondrial protein ("mitoNEET") cross-linked specifically by a thiazolidinedione photoprobe. Am J Physiol Endocrinol Metab. 2004;286:E252–E260. doi: 10.1152/ajpendo.00424.2003. [DOI] [PubMed] [Google Scholar]
- 164.Paddock ML, Wiley SE, Axelrod HL, Cohen AE, Roy M, Abresch EC, Capraro D, Murphy AN, Nechushtai R, Dixon JE, Jennings PA. MitoNEET is a uniquely folded 2Fe 2S outer mitochondrial membrane protein stabilized by pioglitazone. Proc Natl Acad Sci U S A. 2007;104:14342–14347. doi: 10.1073/pnas.0707189104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nicolakakis N, Aboulkassim T, Ongali B, Lecrux C, Fernandes P, Rosa-Neto P, Tong XK, Hamel E. Complete rescue of cerebrovascular function in aged Alzheimer's disease transgenic mice by antioxidants and pioglitazone, a peroxisome proliferator-activated receptor gamma agonist. J Neurosci. 2008;28:9287–9296. doi: 10.1523/JNEUROSCI.3348-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Escribano L, Simon AM, Perez-Mediavilla A, Salazar-Colocho P, Del Rio J, Frechilla D. Rosiglitazone reverses memory decline and hippocampal glucocorticoid receptor down-regulation in an Alzheimer's disease mouse model. Biochem Biophys Res Commun. 2009;379:406–410. doi: 10.1016/j.bbrc.2008.12.071. [DOI] [PubMed] [Google Scholar]
- 167.Watson GS, Cholerton BA, Reger MA, Baker LD, Plymate SR, Asthana S, Fishel MA, Kulstad JJ, Green PS, Cook DG, Kahn SE, Keeling ML, Craft S. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry. 2005;13:950–958. doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]
- 168.Risner ME, Saunders AM, Altman JF, Ormandy GC, Craft S, Foley IM, Zvartau-Hind ME, Hosford DA, Roses AD. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer's disease. Pharmacogenomics J. 2006;6:246–254. doi: 10.1038/sj.tpj.6500369. [DOI] [PubMed] [Google Scholar]
- 169.Ho L, Chen LH, Wang J, Zhao W, Talcott ST, Ono K, Teplow D, Humala N, Cheng A, Percival SS, Ferruzzi M, Janle E, Dickstein DL, Pasinetti GM. Heterogeneity in red wine polyphenolic contents differentially influences Alzheimer's disease-type neuropathology and cognitive deterioration. J Alzheimers Dis. 2009;16:59–72. doi: 10.3233/JAD-2009-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ho L, Yemul S, Wang J, Pasinetti GM. Grape seed polyphenolic extract as a potential novel therapeutic agent in tauopathies. J Alzheimers Dis. 2009;16:433–439. doi: 10.3233/JAD-2009-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ono K, Condron MM, Ho L, Wang J, Zhao W, Pasinetti GM, Teplow DB. Effects of grape seed-derived polyphenols on amyloid beta-protein self-assembly and cytotoxicity. J Biol Chem. 2008;283:32176–32187. doi: 10.1074/jbc.M806154200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang J, Ho L, Zhao W, Ono K, Rosensweig C, Chen L, Humala N, Teplow DB, Pasinetti GM. Grape-derived polyphenolics prevent Abeta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer's disease. J Neurosci. 2008;28:6388–6392. doi: 10.1523/JNEUROSCI.0364-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.van Muiswinkel FL, Kuiperij HB. The Nrf2-ARE Signalling pathway: promising drug target to combat oxidative stress in neurodegenerative disorders. Curr. Drug Targets CNS Neurol. Disord. 2005;4:267–281. doi: 10.2174/1568007054038238. [DOI] [PubMed] [Google Scholar]
- 174.Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci U S A. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li W, Kong AN. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog. 2009;48:91–104. doi: 10.1002/mc.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Greco T, Fiskum G. Neuroprotection through stimulation of mitochondrial antioxidant protein expression. J Alzheimers Dis. 20 Suppl 2:S427–S437. doi: 10.3233/JAD-2010-100519. [DOI] [PubMed] [Google Scholar]
- 178.Kanninen K, Malm TM, Jyrkkanen HK, Goldsteins G, Keksa-Goldsteine V, Tanila H, Yamamoto M, Yla-Herttuala S, Levonen AL, Koistinaho J. Nuclear factor erythroid 2-related factor 2 protects against beta amyloid. Mol Cell Neurosci. 2008;39:302–313. doi: 10.1016/j.mcn.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 179.Kanninen K, Heikkinen R, Malm T, Rolova T, Kuhmonen S, Leinonen H, Yla-Herttuala S, Tanila H, Levonen AL, Koistinaho M, Koistinaho J. Intrahippocampal injection of a lentiviral vector expressing Nrf2 improves spatial learning in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2009;106:16505–16510. doi: 10.1073/pnas.0908397106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Liby K, Hock T, Yore MM, Suh N, Place AE, Risingsong R, Williams CR, Royce DB, Honda T, Honda Y, Gribble GW, Hill-Kapturczak N, Agarwal A, Sporn MB. The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer Res. 2005;65:4789–4798. doi: 10.1158/0008-5472.CAN-04-4539. [DOI] [PubMed] [Google Scholar]
- 181.Liby KT, Yore MM, Sporn MB. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat. Rev. Cancer. 2007;7:357–369. doi: 10.1038/nrc2129. [DOI] [PubMed] [Google Scholar]
- 182.Dumont M, Wille E, Calingasan NY, Tampellini D, Williams C, Gouras GK, Liby K, Sporn M, Nathan C, Flint Beal M, Lin MT. Triterpenoid CDDO-methylamide improves memory and decreases amyloid plaques in a transgenic mouse model of Alzheimer's disease. J Neurochem. 2009;109:502–512. doi: 10.1111/j.1471-4159.2009.05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Stack C, Ho D, Wille E, Calingasan NY, Williams C, Liby K, Sporn M, Dumont M, Beal MF. Triterpenoids CDDO-ethyl amide and CDDO-trifluoroethyl amide improve the behavioral phenotype and brain pathology in a transgenic mouse model of Huntington's disease. Free Radic Biol Med. 49:147–158. doi: 10.1016/j.freeradbiomed.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yang L, Calingasan NY, Thomas B, Chaturvedi RK, Kiaei M, Wille EJ, Liby KT, Williams C, Royce D, Risingsong R, Musiek ES, Morrow JD, Sporn M, Beal MF. Neuroprotective effects of the triterpenoid, CDDO methyl amide, a potent inducer of Nrf2-mediated transcription. PLoS One. 2009;4:e5757. doi: 10.1371/journal.pone.0005757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Schimrigk S, Brune N, Hellwig K, Lukas C, Bellenberg B, Rieks M, Hoffmann V, Pohlau D, Przuntek H. Oral fumaric acid esters for the treatment of active multiple sclerosis: an open-label, baseline-controlled pilot study. Eur J Neurol. 2006;13:604–610. doi: 10.1111/j.1468-1331.2006.01292.x. [DOI] [PubMed] [Google Scholar]
- 186.Niki E. Assessment of antioxidant capacity in vitro and in vivo. Free Radic Biol Med. 49:503–515. doi: 10.1016/j.freeradbiomed.2010.04.016. [DOI] [PubMed] [Google Scholar]